Abstract
Objective
Convergent evidence indicates that apathy affects cognitive behavior in different neurological and psychiatric conditions. Studies of clinical populations have also suggested the primary involvement of the prefrontal cortex and the basal ganglia in apathy. These brain regions are interconnected at both the structural and functional levels and are deeply involved in cognitive processes, such as working memory and attention. However, it is unclear how apathy modulates brain processing during cognition and whether such a modulation occurs in healthy young subjects. To address this issue, we investigated the link between apathy and prefrontal and basal ganglia function in healthy young individuals. We hypothesized that apathy may be related to sub-optimal activity and connectivity in these brain regions.
Methods
Three hundred eleven healthy subjects completed an apathy assessment using the Starkstein’s Apathy Scale and underwent fMRI during working memory and attentional performance tasks. Using an ROI approach, we investigated the association of apathy with activity and connectivity in the DLPFC and the basal ganglia.
Results
Apathy scores correlated positively with prefrontal activity and negatively with prefrontal-basal ganglia connectivity during both working memory and attention tasks. Furthermore, prefrontal activity was inversely related to attentional behavior.
Conclusions
These results suggest that in healthy young subjects, apathy is a trait associated with inefficient cognitive-related prefrontal activity, i.e., it increases the need for prefrontal resources to process cognitive stimuli. Furthermore, apathy may alter the functional relationship between the prefrontal cortex and the basal ganglia during cognition.
Introduction
Apathy is defined as reduced motivation towards goal-directed behavior, a flattened affect, emotional indifference and a restricted response to important life events [1–2]. It implies diminished motivation and effort to perform personal and social everyday activities [1, 3]. In addition to being associated with a broad range of brain disorders [4–7], apathy is also present in elderly and young healthy subjects [8–12].
The brain circuitry that sustains apathy has been investigated primarily in clinical and elderly populations. Many of these studies have demonstrated an association between apathy and structural anomalies, such as lesions of lateral or medial areas of the prefrontal cortex (PFC) [13–14] and decreased PFC volume [15–18]. Furthermore, functional imaging studies in dementia and in post-stroke patients corroborate the relationship between frontal alterations and apathy. In particular, previous reports have indicated associations between apathy and reduced glucose metabolism and frontal rCBF perfusion [19–20], as well as a negative correlation between rCBF perfusion and apathy scores [21]. Other studies also suggested a link between apathy and abnormalities in the basal ganglia (BG). In particular, this trait has been related to focal lesions of the globus pallidus, the caudate and the putamen [22–24], as well as to BG hypoperfusion [25–26] and reduced BG volume [27–28]. Moreover, apathy is a frequent symptom in clinical conditions that are characterized by BG impairment [4, 29–30]. A small number of studies have suggested the involvement of other brain regions, such as the cingulate cortex, the insula or the premotor cortex [31–32], in apathy, but these results require replication. Overall, the current evidence is consistent in suggesting a role for both the PFC and the BG in apathy in clinical and elderly populations. Accordingly, it has been hypothesized that apathy may be subtended by a dysfunction in the neuronal circuit that includes both BG and PFC [33–35], which are interconnected at both the structural and functional levels [36–38].
The loop between the BG and the dorsolateral prefrontal portion of the PFC (DLPFC) is deeply involved in cognitive processing, as indicated repeatedly by studies that focused on working memory and attentional processing [39–41]. Apathy has also been correlated with anomalies in cognitive functions, as revealed by findings in clinical populations; these findings highlighted a relationship between high levels of apathy and poorer performance on tests for working memory and attention [42–46]. Similarly, apathy has been associated with cognitive impairment in elderly individuals who are not affected by neurological or psychiatric conditions [9, 47–49].
Given that most of studies of apathy have been performed in elderly or clinical populations, it is important to investigate this trait in healthy young subjects, thus nullifying the possible impact of other illness-related variables or aging on brain physiology. To our knowledge, only one recent study has addressed the relationship between apathy and brain imaging phenotypes in healthy young individuals. In that study [32], the authors investigated behavioral apathy (i.e., a sub-domain of apathy that is characterized by a lack of physical engagement, productivity and initiative). The results of this study indicated that behavioral apathy is associated with greater recruitment of the supplemental motor area and the motor cingulate, as well as lower functional connectivity between these brain regions during a reward task. However, to our knowledge, no studies have been performed to investigate the relationship between apathy and brain correlates of high order cognitive processing, such as working memory and attention, in healthy young subjects.
Here, we aimed to investigate the association of apathy with brain activity and functional connectivity during working memory and attentional processing in healthy young individuals. Given our strong a priori hypothesis concerning the role of the basal ganglia and the PFC in apathy, we focused our analyses on those regions. Based on current knowledge, we hypothesized that greater levels of apathy would be associated with sub-optimal activity and connectivity in the DLPFC and the BG during these cognitive processes.
Methods
Subjects
Three hundred eleven healthy subjects (146 males, mean age + SD = 27.3 + 6.79 years) were included in the study. All subjects were evaluated using the Structured Clinical Interview [50] for the Diagnostic and Statistical Manual of Mental Disorders to exclude any actual or past psychiatric disorder. A standard MRI procedure was used to exclude brain structural alterations or illness. Other exclusion criteria were a history of drug or alcohol abuse, active drug use in the past year, head trauma with loss of consciousness and any significant medical condition. IQ (WAIS-R [51]), handedness (Edinburgh Inventory [52]), and socio-economic status (Hollingshead Four Factor Index, [53]) were also measured (Table 1). Handedness was determined by using the Edinburgh Inventory [52], which assesses the dominance of a person's right or left hand in everyday activities. The scores ranged from -1 (totally left handed) to 1 (totally right-handed). Socio-economic status was determined based on reports of paternal and maternal education and occupation by using the Hollingshead Four Factor Index [53]. This index ranges from 8 to 66 (8 = lowest level of occupational status and education). Furthermore, all subjects completed an apathy assessment and underwent one or more of the fMRI procedures described below.
Table 1. Demographic data and Apathy Scale (AS) data for the whole sample (All) and for the subsamples performing the working memory (N-Back) and attentional control (VAC) tasks.
| Demographic Data | Apathy Scale | ||||||
|---|---|---|---|---|---|---|---|
| N | Age | Handedness | Socio-economic status | IQ | |||
| All | 311 | mean | 27.3 | 0.72 | 41.13 | 108.3 | 9.46 |
| 146 ♂ | sd | 6.79 | 0.48 | 16.29 | 12.15 | 3.83 | |
| N-Back | 247 | mean | 27.2 | 0.73 | 42.42 | 108.49 | 9.53 |
| 119 ♂ | sd | 6.96 | 0.47 | 16.18 | 12.40 | 3.85 | |
| VAC | 201 | mean | 27 | 0.72 | 42.42 | 109.645 | 9.16 |
| 94 ♂ | sd | 5.4 | 0.48 | 16.7 | 11.81 | 3.25 | |
The present study was approved by the local institutional review board, i.e. the “Independent Ethical Committee” at the University of Bari ‘Aldo Moro,’ and written informed consent was obtained from all subjects after a full explanation of all procedures was provided.
All the procedures were performed according to the Declaration of Helsinki. The data of this study are available at the 4TU.ResearchData repository; http://dx.doi.org/10.4121/uuid:d5ec1a8a-6c61-4f19-8af1-b728bb07e8c0.
Apathy Scale
Apathy was measured in all individuals using the self-administered form of the Starkstein’s Apathy Scale (AS) [54], which is a reduced version of the Marin’s Apathy Evaluation Scale [55]. The AS was developed by removing redundant items from the Marin’s Apathy Evaluation Scale based on a factor analysis and included a total of 14 questions (e.g., “Do you have plans and goals for the future?”). Each item is rated on a four-point scale. The total score ranged from 0 to 42, with higher scores indicating greater apathy levels. The AS has adequate reliability, as well as good one-week test-retest and inter-rater reliability [54].
The Shapiro-Wilk normality test was used to assess the normality of the distribution for demographic characteristics and AS scores (p<0.05). Considering that the AS scores, age, handedness and socio-economic status were not normally distributed (see below), we used Spearman’s correlations and Kruskal-Wallis tests as needed to investigate putative relationships between demographic characteristics and AS scores. The statistical threshold was set at p<0.05.
fMRI tasks
Two hundred forty-seven participants (119 males; mean age 27.20 + 6.96 years; Table 1) performed the N-Back task, which is a paradigm that has been used extensively to evaluate brain activity during working memory (WM) tasks [56–58]. ‘‘N-Back” refers to how far back in a sequence of stimuli the subject can recall. The stimuli consisted of numbers (1–4) shown in random sequence and displayed at the points of a diamond-shaped box. A non-memory-guided control condition (0-back) simply required the subjects to identify the stimulus currently seen. In the working memory condition, the task required the recollection of a number seen one (1-Back), two (2-Back) or three stimuli (3-Back) beforehand, while continuing to encode additional incoming stimuli. The stimuli were arranged in a block design, consisting of eight 30 sec blocks: four blocks of the control condition alternating with four blocks of each WM condition. Each run lasted 4 min 8 sec.
To extend our findings to another cognitive domain, 201 subjects (94 males; mean age 27 ± 5.4 years; Table 1) performed the Variable Attentional Control (VAC) task, which is a paradigm that has been used in several previous investigations [57, 59–63] and was designed to elicit increasing demands for attentional control processing. The stimuli were composed of arrows of three different sizes pointing either to the right or to the left; small arrows were embedded in medium-sized arrows, which were in turn embedded in a large arrow. The subjects were instructed by a cue word (big, medium or small) displayed above each stimulus to press a button corresponding to the direction of the large, medium or small arrows (right or left). To increase the level of attentional control required, the direction of the arrows was congruent or incongruent across all three sizes. This approach resulted in the following conditions: low level of attentional control (Low), for which all 3 sizes of arrows were congruent in direction, with the cue word BIG; intermediate level of attentional control (Int), for which two stimuli used, with the big arrow incongruent in direction to the small and the medium arrows in both stimuli and cue words of BIG in one stimulus and SMALL in the other stimulus; and high level of attentional control (High), for which two stimuli were used, with the medium-sized arrows incongruent in direction to the big and the small arrows in both stimuli and cue words of SMALL in one stimulus and MEDIUM in the other stimulus. A simple bold arrow pointing either to the left or right was used as a sensorimotor control condition.
The total number of stimuli was 241: 50 High (25 of each of the two stimuli that required a high degree of attentional control), 68 Int (34 of each of the two stimuli that required an intermediate degree of attentional control), 57 Low and 66 stimuli for the control condition. A fixation cross-hair was presented during the interstimulus interval, which ranged from 2 to 6 sec. The total duration of the task was 10 min 8 sec. The subjects were instructed to respond to task stimuli with the right hand using a button box (right button for ‘right’ response, left button for ‘left’ response) and to press the response button as rapidly and accurately as possible.
For both the N-Back and VAC tasks, the stimuli were presented via a back-projection system. The responses were recorded through a fiber optic response box, allowing measurement of the percent accuracy and the reaction time (in milliseconds). All subjects were trained to perform the task prior to the fMRI session. One hundred forty-one subjects performed both of the tasks used in this study.
fMRI Data Acquisition
Blood oxygen level-dependent (BOLD) fMRI was performed on a GE Signa 3T scanner (GE Healthcare) equipped with a standard quadrature head coil. A gradient-echo planar imaging sequence (repetition time, 2000 ms; echo time, 30 ms; thickness, 4 mm; gap, 1 mm; flip angle, 90°; field of view, 24 cm; and matrix, 64 × 64) was used to acquire images while the subjects performed the tasks (N-back: 120 volumes for each run, 20 interleaved axial slices; VAC: 300 volumes, 26 interleaved axial slices). The first four scans were discarded to allow for T1 equilibration effects.
fMRI
BOLD response
The analysis of the fMRI data was completed using Statistical Parametric Mapping 8 (SPM8; http://www.fil.ion.ucl.ac.uk/spm). Images of each subject were pre-processed and slice timing corrected using the centrally acquired slice as the reference slice. In particular, standard procedures for realignment to the mean image were performed using the Realign and Unwarp algorithm provided in SPM8 in order to compensate for non-linear signal distortions that may be induced by head motion. Furthermore, movement parameters were extracted to eventually exclude subjects with excessive head motion (>2 mm translation, > 2° rotation). The realigned images were resliced to a 2 mm isotropic voxel size, spatially normalized into a standard space (Montreal Neurological Institute template) with a 12 parameter affine model and smoothed using a 6 mm full-width half-maximum isotropic Gaussian kernel to minimize noise and to account for residual inter-subject differences.
In the N-Back first-level analysis, a box car model convolved with the hemodynamic response function at each voxel was used. Linear contrasts were then computed, producing a t statistical map for the 1-, 2- and 3-Back conditions, assuming the 0-Back condition as a baseline. Thus, a multiple regression analysis of the N-Back-related brain activity was then performed at the group level, using apathy as the continuous predictor, WM load (1-, 2-, and 3-Back) as the repeated-measures factor and age and gender as covariates of no interest (see below).
In the VAC task, the fMRI responses were modeled using a canonical hemodynamic response function. Vectors were created for each condition using the timing of correct responses. Using a t statistic, linear contrasts were computed for the three levels of attentional control (High, Int, and Low). Thus, a multiple regression analysis was performed on VAC-related brain activity, using apathy as the continuous predictor, attentional control load (High, Int, and Low) as the repeated-measures factor and age and gender as covariates of no interest. Because of our strong a priori hypothesis about the involvement of the DLPFC and of BG in working memory and attentional control processing [60, 64–66], as well as the putative involvement of these regions in apathy [34, 67], we used a statistical threshold of p<0.05, family-wise error (FWE) small-volume corrected within a region of interest that included DLPFC Brodmann’s areas 9, 10 and 46, as well as the BG, as defined by the WFU_PickAtlas [68].
To explore the association of brain activity with behavior, BOLD parameter estimates were extracted for both the N-back and VAC from the cluster that showed a significant association with apathy using MarsBaR (http://marsbar.sourceforge.net/) (see below).
Psychophysiological interactions (PPI)
A psychophysiological interaction (PPI) analysis [69] was performed to evaluate the association of apathy scores with DLPFC functional connectivity. For both the N-Back and VAC tasks, 5 mm ROIs centered on the clusters whose activity was associated with apathy (see results) were used as seed regions. PPI was calculated using the first eigenvariate of individual raw activation time courses, which were extracted by using a singular value decomposition method from a volume of interest (VOI) centered on the subject-specific peak cluster within the seed regions. These time courses were then mean-centered, high-pass filtered and deconvolved. A general linear model was computed using three regressors: a physiological regressor (the time course response in the VOI), a psychological regressor (task design) and a PPI term, which was calculated as the cross-product of the previous two terms. Thus, subject-specific statistical PPI contrast images were entered in second-level random effects multiple regressions, using apathy as the continuous predictor, task load as the repeated-measures factor and age and gender as covariates of no interest. Based on the relevance of the DLPFC/BG functional loop for both apathy and cognitive processing [34], we focused our investigation on the BG, which included the caudate, the putamen and the globus pallidus, as identified using the WFU Pickatlas. A statistical threshold of p<0.05, family-wise error (FWE) small-volume corrected within this ROI, was used for these analyses.
To further explore the association of brain connectivity with behavior, PPI values were extracted for both the N-back and VAC tasks from the cluster that showed a significant association with apathy using MarsBaR (http://marsbar.sourceforge.net/) (see below).
Correlation analysis
To explore the relationship between apathy and cognitive behavior, we calculated a parametric cognitive efficiency (PCE) score for both the N-Back and the VAC tasks, which takes into account the increase in cognitive load that is elicited by the working memory and attentional control tasks. In particular, we obtained an efficiency index as the ratio between the percent accuracy and the reaction time for each of the three loads of the tasks (1-, 2- and 3-Back for the N-back and Low, Int and High for the VAC). Then, we ranked each cognitive load (1-Back = 1; 2-Back = 2; 3-Back = 3 for the N-back; Low = 1; Int = 2; High = 3 for the VAC) and multiplied the efficiency index and the rank of each load. Thus, we summed the scores obtained for each cognitive load in order to obtain an individual PCE for each task. This procedure is simplified by the following formulas:
The Shapiro-Wilk normality test was used to assess the normality of the distribution of the PCE scores (p<0.05). Considering that the N-Back PCE score was not distributed normally (see below), for each task, we performed separate Spearman’s correlation analyses between the PCE score and the BOLD parameter estimates or the PPI values extracted from significant clusters obtained in the activity and connectivity analyses. A statistical threshold of p<0.05 was used for these analyses.
To assess the relationship between PCE and AS scores, we used robust regression models, which allowed us to control for age and gender (see below) without being affected by violations of parametric assumptions. The statistical threshold was set at p<0.05.
Results
Apathy scores and Demographics
The Shapiro-Wilk normality test indicated that the AS scores, age, handedness and socio-economic status were not distributed normally (AS score W = 0.95, p<0.001; Age W = 0.83, p<0.001; handedness W = 0.97, p<0.001; socio-economic status W = 0.59, p<0.001). The AS scores (range = 2 to 26, mean ± SD = 9.46 ± 3.83; N-Back sample = 9.53 ± 3.85; VAC sample = 9.16 + 3.25) were greater in males than in females (Kruskal-Wallis Chi-squared = 7.29; p = 0.006) and correlated with age (Spearman’s rho = -0.18; p = 0.002) but not with handedness, socio-economic status and IQ (all p>0.05). Age and gender were thus used as covariates in all fMRI and behavioral analyses that included apathy.
fMRI
Regional activity
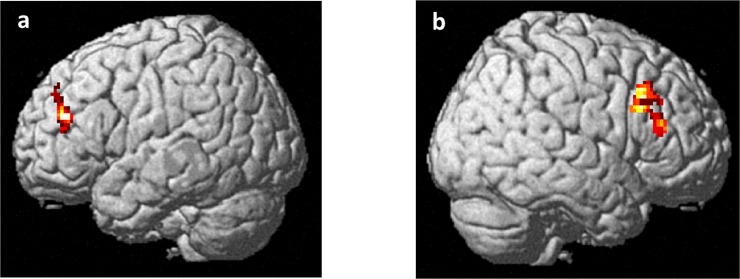
Analysis of the N-back data indicated a positive correlation between apathy scores and left middle frontal gyrus BOLD responses during the N-back task (BA 46; x -42, y 40, z 24; Z = 3.98; k = 145; p = 0.03 FWE-corrected) (Fig 1A). No interaction between apathy and working memory load was found. Similarly, analysis of the VAC data indicated a positive correlation between apathy scores and right middle frontal gyrus activity (BA 46; x 42, y 30, z 18; Z = 3.83; k = 228; p = 0.047 FWE-corrected) (Fig 1B). Again, no interaction was observed between apathy scores and attentional load.
Fig 1.
Rendered image of the brain depicting the dorsolateral prefrontal clusters whose activity correlated positively with apathy scores during (a) working memory and (b) attentional control tasks. See the text for statistics.
PPI
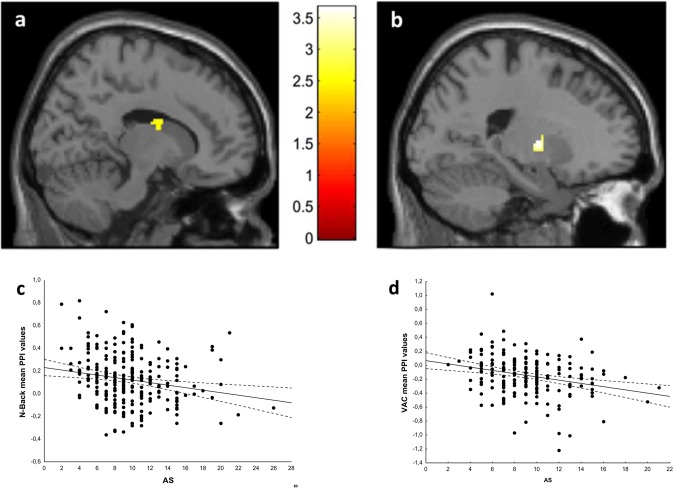
A PPI analysis was performed using the DLPFC clusters associated with apathy scores during N-Back and VAC performance as seeds. A PPI analysis of the N-Back data revealed that apathy scores correlated negatively with functional connectivity between the DLPFC and the right BG (x18,y -2,z 22; Z = 3.71; k = 68; p = 0.036 FWE-corrected) (Fig 2A and 2C). No interaction between working memory load and apathy scores was present. Similarly, a PPI analysis of the VAC data revealed a negative correlation between apathy scores and functional connectivity between the DLPFC and the left BG (x -20, y -6, z 4; Z = 3.64; k = 70; p = 0.044 FWE-corrected) (Fig 2B and 2D). No interaction was observed between attentional load and apathy scores.
Fig 2.
Sections of the brain depicting the basal ganglia clusters whose functional connections with the dorsolateral prefrontal cortex correlated negatively with apathy scores during (a) the working memory task and (b) the attentional control task. Scatterplots depicting the negative association between PPI values and apathy during (c) the working memory task and (d) the attentional control task. See the text for statistics.
Correlation analysis
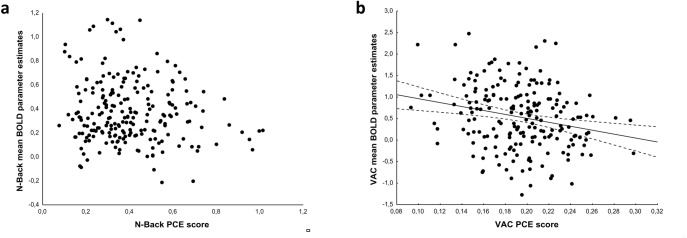
Behavioral data concerning the subjects who performed the N-Back task and the VAC task are reported in Table 2. The Shapiro-Wilk normality test indicated that the N-Back PCE score was not distributed normally (N-back PCE W = 0.95, p<0.001). Spearman’s correlations indicated a negative correlation between PCE scores and BOLD activity during the VAC task (Spearman’s Rho = -0.22; p = 0.002) (Fig 3B) but not during the N-back task (Spearman’s Rho = -0.08; p = 0.2) (Fig 3A). Spearman’s correlations between PPI and behavioral data revealed no significant results (p>0.05). Robust regression models did not indicate a relationship between apathy and PCE scores during the N-back task or the VAC task (all p> 0.05).
Table 2. Behavioral data for the subsamples performing the working memory (N-Back) and attentional control (VAC) tasks.
| N-BACK | VAC | ||||||
|---|---|---|---|---|---|---|---|
| 1 Back | 2 Back | 3 Back | Low | Medium | High | ||
| % Accuracy | mean | 95.5 | 85.3 | 77.9 | 99 | 89.9 | 83.5 |
| sd | 8.1 | 14.7 | 16.2 | 4.3 | 9.5 | 13.7 | |
| Reaction Time | mean | 527.9 | 536.2 | 492.9 | 781.9 | 931.2 | 1059.5 |
| sd | 218.8 | 225.7 | 238.9 | 175 | 183.7 | 206.7 | |
| PCE | mean | 0.40 | 0.19 | ||||
| sd | 0.18 | 0.03 | |||||
Fig 3.
Scatterplots of Spearman’s test on cognitive behavior as indexed by a parametric cognitive efficiency score (PCE) and BOLD parameter estimates extracted from the dorsolateral prefrontal region associated with apathy, depicting (a) absence of correlation during working memory task and (b) negative correlation during attentional control. See text for statistics.
Discussion
Here we investigated whether apathy is associated with DLPFC and BG activity and functional connectivity during cognitive processing in healthy individuals. We found a positive correlation between apathy scores and prefrontal responses during working memory tasks, such that subjects with greater apathy also had greater DLPFC activity during this cognitive skill. Notably, we found a similar relationship when investigating the domain of attentional control processing. Moreover, greater levels of apathy were also associated with lower functional connectivity between the DLPFC and the BG during both cognitive processes. These results suggest a relationship between patterns of brain cognitive processing and apathy in healthy subjects.
The observed association between dorsolateral prefrontal activity and AS scores in healthy subjects is consistent with previous results obtained in clinical populations, suggesting that apathy may be subtended by damage that occurs primarily in the lateral prefrontal cortex [13]. The DLPFC is a key region for working memory and attention processing [70–72], and previous models suggested that for between-group comparisons, greater DLPFC activity despite worse or unaffected behavior, may be an index of inefficient prefrontal processing during cognition [58, 60, 70, 73]. In light of this model, our results that demonstrate a positive correlation between AS and DLPFC activity suggest that greater apathy in healthy subjects is linked with less efficient prefrontal processing of working memory and attentional stimuli. Consistent with this finding, our correlation analysis indicates that greater DLPFC activity linked with greater apathy predicts poorer behavioral performance in the attentional control task used in our study. Indeed, we found no significant correlation between the PCE score and DLPFC activity during working memory tasks. A possible explanation for this lack of a relationship is that the region that we found to be associated with apathy during working memory is less modulated by task load but is relevant for task execution [74]. Overall, our findings in healthy subjects strengthen previous evidence of an association between apathy and impaired cognition [42–45, 75–78], further suggesting a biological basis for this phenomenon and uncoupling this relationship from the effects of confounding factors related to disease or aging. Moreover, our findings highlight that the role of cognitive processing in apathy flanks those of emotion processing and mood [79–80].
Another finding of the present study is the relationship between apathy and DLPFC-BG connectivity during both working memory and attentional control tasks. In particular, we found that greater levels of apathy are associated with a weaker functional connection between inefficient clusters of prefrontal activity during cognitive processing and BG. These results are consistent with previous models that posited a role for reduced DLPFC-BG connectivity in apathy [33–35, 67] and with previous findings that indicated that bilateral lesions of the BG are associated with a severe form of apathy (auto-activation deficit) that is characterized by a complete loss of self-initiated goal-directed behavior [81]. The DLPFC-BG loop plays a crucial role in high-order cognitive processes, such as working memory and attentional control [64],and participates in a so-called “associative pathway,” in which information that arises from several associative areas is transmitted to the caudate and the anterior putamen and subsequently reaches the DLPFC through the thalamus [66, 82]. This pathway is involved in several cognitive processes and is crucial for the generation of context-dependent and goal-directed patterns of behavior [66, 82–83]. Indeed, previous models postulated that a neurobiological mechanism subtending apathy may imply a failure of the BG to engage the DLPFC, thus lowering the ability of the DLPFC to support goal-directed cognitive processing [35, 67].
In our PPI analysis, we found that AS scores for both tasks are correlated with the functional connection between the DLPFC and the contralateral BG. Previous findings indicated the existence of a bilateral interconnection between the DLPFC and the BG [84–85]. Furthermore, by lowering the statistical threshold of our analysis to an uncorrected threshold of p<0.005, we found that AS scores in the N-Back and VAC tasks also correlated with the functional connections between the DLPFC and the homolateral BG. Thus, it is possible that our PPI finding at the corrected p value is related to the statistical threshold used.
A potential limitation of the present study is that apathy is considered to be an aspect of depression, and correlations between apathy and depression scales have been reported [2]. Even if we excluded from the study all of the subjects with a current or past diagnosis of depression, as well as individuals with first-degree relatives affected by a psychiatric disorder, it remains possible that subclinical levels of depression might have affected our results. However, previous studies have suggested good discriminability between apathy and depression scores in both clinical [86–87] and non-clinical populations [11–12], as well as an adequate discriminant validity between apathy scales and depression scales [88]. Thus, it is possible that our results are not strongly impacted by subclinical levels of depression. Further studies should address this issue.
In conclusion, we provided evidence for a relationship between inefficient brain processing during cognition and apathy in healthy subjects in the absence of confounding factors, such as pathophysiological conditions or pharmacological treatment. These findings shed new light on our understanding of the link between apathy and brain processes that may be relevant to neurological and psychiatric conditions for which apathy is a central feature.
Acknowledgments
We are grateful to Dr. Raffaella Romano and Dr. Annamaria Porcelli, for making data acquisition possible. Moreover, we gratefully acknowledge the work by Ivan Abbrescia, Catia D’Agostino, Nunzio Langiulli, Pierluigi Selvaggi and Aldo Tomasicchio for their help with data processing. Finally, we would like to acknowledge all people who participated to this study.
Data Availability
The data of this study are available at the 4TU.ResearchData repository; http://dx.doi.org/10.4121/uuid:d5ec1a8a-6c61-4f19-8af1-b728bb07e8c0.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Marin RS. Differential diagnosis and classification of apathy. The American journal of psychiatry. 1990;147(1):22–30. Epub 1990/01/01. 10.1176/ajp.147.1.22 [DOI] [PubMed] [Google Scholar]
- 2.Marin RS. Apathy: a neuropsychiatric syndrome. J Neuropsychiatry Clin Neurosci. 1991;3(3):243–54. Epub 1991/01/01. 10.1176/jnp.3.3.243 . [DOI] [PubMed] [Google Scholar]
- 3.Starkstein SE, Leentjens AF. The nosological position of apathy in clinical practice. Journal of neurology, neurosurgery, and psychiatry. 2008;79(10):1088–92. 10.1136/jnnp.2007.136895 . [DOI] [PubMed] [Google Scholar]
- 4.Leentjens AF, Dujardin K, Marsh L, Martinez-Martin P, Richard IH, Starkstein SE, et al. Apathy and anhedonia rating scales in Parkinson's disease: critique and recommendations. Movement disorders: official journal of the Movement Disorder Society. 2008;23(14):2004–14. 10.1002/mds.22229 . [DOI] [PubMed] [Google Scholar]
- 5.Roth RM, Koven NS, Pendergrass JC, Flashman LA, McAllister TW, Saykin AJ. Apathy and the processing of novelty in schizophrenia. Schizophrenia research. 2008;98(1–3):232–8. 10.1016/j.schres.2007.08.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bentley SM, Pagalilauan GL, Simpson SA. Major Depression. The Medical clinics of North America. 2014;98(5):981–1005. Epub 2014/08/20. 10.1016/j.mcna.2014.06.013 . [DOI] [PubMed] [Google Scholar]
- 7.Starkstein SE, Petracca G, Chemerinski E, Kremer J. Syndromic validity of apathy in Alzheimer's disease. The American journal of psychiatry. 2001;158(6):872–7. Epub 2001/06/01. 10.1176/appi.ajp.158.6.872 [DOI] [PubMed] [Google Scholar]
- 8.Pardini M, Cordano C, Guida S, Grafman J, Krueger F, Sassos D, et al. Prevalence and cognitive underpinnings of isolated apathy in young healthy subjects. J Affect Disord. 2015;189:272–5. Epub 2015/10/12. S0165-0327(15)30617-0 [pii] 10.1016/j.jad.2015.09.062 . [DOI] [PubMed] [Google Scholar]
- 9.Okura T, Plassman BL, Steffens DC, Llewellyn DJ, Potter GG, Langa KM. Prevalence of neuropsychiatric symptoms and their association with functional limitations in older adults in the United States: the aging, demographics, and memory study. J Am Geriatr Soc. 2010;58(2):330–7. Epub 2010/04/09. JGS2680 [pii] 10.1111/j.1532-5415.2009.02680.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Onyike CU, Sheppard JM, Tschanz JT, Norton MC, Green RC, Steinberg M, et al. Epidemiology of apathy in older adults: the Cache County Study. Am J Geriatr Psychiatry. 2007;15(5):365–75. Epub 2007/04/28. 15/5/365 [pii] 10.1097/01.JGP.0000235689.42910.0d . [DOI] [PubMed] [Google Scholar]
- 11.Spalletta G, Fagioli S, Caltagirone C, Piras F. Brain microstructure of subclinical apathy phenomenology in healthy individuals. Hum Brain Mapp. 2013;34(12):3193–203. Epub 2012/07/19. 10.1002/hbm.22137 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bonnelle V, Veromann KR, Burnett Heyes S, Lo Sterzo E, Manohar S, Husain M. Characterization of reward and effort mechanisms in apathy. J Physiol Paris. 2015;109(1–3):16–26. 10.1016/j.jphysparis.2014.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paradiso S, Chemerinski E, Yazici KM, Tartaro A, Robinson RG. Frontal lobe syndrome reassessed: comparison of patients with lateral or medial frontal brain damage. J Neurol Neurosurg Psychiatry. 1999;67(5):664–7. Epub 1999/10/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barrash J, Tranel D, Anderson SW. Acquired personality disturbances associated with bilateral damage to the ventromedial prefrontal region. Dev Neuropsychol. 2000;18(3):355–81. Epub 2001/06/02. 10.1207/S1532694205Barrash . [DOI] [PubMed] [Google Scholar]
- 15.Roth RM, Flashman LA, Saykin AJ, McAllister TW, Vidaver R. Apathy in schizophrenia: reduced frontal lobe volume and neuropsychological deficits. Am J Psychiatry. 2004;161(1):157–9. Epub 2004/01/02. 10.1176/appi.ajp.161.1.157 [DOI] [PubMed] [Google Scholar]
- 16.Reijnders JS, Scholtissen B, Weber WE, Aalten P, Verhey FR, Leentjens AF. Neuroanatomical correlates of apathy in Parkinson's disease: A magnetic resonance imaging study using voxel-based morphometry. Movement disorders: official journal of the Movement Disorder Society. 2010;25(14):2318–25. 10.1002/mds.23268 . [DOI] [PubMed] [Google Scholar]
- 17.Grool AM, Geerlings MI, Sigurdsson S, Eiriksdottir G, Jonsson PV, Garcia ME, et al. Structural MRI correlates of apathy symptoms in older persons without dementia: AGES-Reykjavik Study. Neurology. 2014;82(18):1628–35. Epub 2014/04/18. 10.1212/WNL.0000000000000378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stanton BR, Leigh PN, Howard RJ, Barker GJ, Brown RG. Behavioural and emotional symptoms of apathy are associated with distinct patterns of brain atrophy in neurodegenerative disorders. Journal of neurology. 2013;260(10):2481–90. 10.1007/s00415-013-6989-9 . [DOI] [PubMed] [Google Scholar]
- 19.Marshall GA, Monserratt L, Harwood D, Mandelkern M, Cummings JL, Sultzer DL. Positron emission tomography metabolic correlates of apathy in Alzheimer disease. Arch Neurol. 2007;64(7):1015–20. Epub 2007/07/11. 64/7/1015 [pii] 10.1001/archneur.64.7.1015 . [DOI] [PubMed] [Google Scholar]
- 20.Okada K, Kobayashi S, Yamagata S, Takahashi K, Yamaguchi S. Poststroke apathy and regional cerebral blood flow. Stroke; a journal of cerebral circulation. 1997;28(12):2437–41. Epub 1997/12/31. . [DOI] [PubMed] [Google Scholar]
- 21.Benoit M, Clairet S, Koulibaly PM, Darcourt J, Robert PH. Brain perfusion correlates of the apathy inventory dimensions of Alzheimer's disease. International journal of geriatric psychiatry. 2004;19(9):864–9. Epub 2004/09/08. 10.1002/gps.1163 . [DOI] [PubMed] [Google Scholar]
- 22.Strub RL. Frontal lobe syndrome in a patient with bilateral globus pallidus lesions. Arch Neurol. 1989;46(9):1024–7. Epub 1989/09/01. . [DOI] [PubMed] [Google Scholar]
- 23.Bhatia KP, Marsden CD. The behavioural and motor consequences of focal lesions of the basal ganglia in man. Brain. 1994;117 (Pt 4):859–76. Epub 1994/08/01. . [DOI] [PubMed] [Google Scholar]
- 24.Mendez MF, Adams NL, Lewandowski KS. Neurobehavioral changes associated with caudate lesions. Neurology. 1989;39(3):349–54. Epub 1989/03/01. . [DOI] [PubMed] [Google Scholar]
- 25.Onoda K, Kuroda Y, Yamamoto Y, Abe S, Oguro H, Nagai A, et al. Post-stroke apathy and hypoperfusion in basal ganglia: SPECT study. Cerebrovasc Dis. 2011;31(1):6–11. Epub 2010/10/29. 000319771 [pii] 10.1159/000319771 . [DOI] [PubMed] [Google Scholar]
- 26.Lopez OL, Zivkovic S, Smith G, Becker JT, Meltzer CC, DeKosky ST. Psychiatric symptoms associated with cortical-subcortical dysfunction in Alzheimer's disease. The Journal of neuropsychiatry and clinical neurosciences. 2001;13(1):56–60. Epub 2001/02/24. 10.1176/jnp.13.1.56 [DOI] [PubMed] [Google Scholar]
- 27.Josephs KA, Whitwell JL, Eggers SD, Senjem ML, Jack CR, Jr. Gray matter correlates of behavioral severity in progressive supranuclear palsy. Mov Disord. 2011;26(3):493–8. Epub 2011/04/05. 10.1002/mds.23471 . [DOI] [PubMed] [Google Scholar]
- 28.Bruen PD, McGeown WJ, Shanks MF, Venneri A. Neuroanatomical correlates of neuropsychiatric symptoms in Alzheimer's disease. Brain. 2008;131(Pt 9):2455–63. Epub 2008/08/02. awn151 [pii] 10.1093/brain/awn151 . [DOI] [PubMed] [Google Scholar]
- 29.Craufurd D, Thompson JC, Snowden JS. Behavioral changes in Huntington Disease. Neuropsychiatry, neuropsychology, and behavioral neurology. 2001;14(4):219–26. Epub 2001/11/29. . [PubMed] [Google Scholar]
- 30.Litvan I, Mega MS, Cummings JL, Fairbanks L. Neuropsychiatric aspects of progressive supranuclear palsy. Neurology. 1996;47(5):1184–9. Epub 1996/11/01. . [DOI] [PubMed] [Google Scholar]
- 31.Yuen GS, Gunning-Dixon FM, Hoptman MJ, AbdelMalak B, McGovern AR, Seirup JK, et al. The salience network in the apathy of late-life depression. Int J Geriatr Psychiatry. 2014;29(11):1116–24. Epub 2014/07/06. 10.1002/gps.4171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bonnelle V, Manohar S, Behrens T, Husain M. Individual Differences in Premotor Brain Systems Underlie Behavioral Apathy. Cereb Cortex. 2016;26(2):807–19. 10.1093/cercor/bhv247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brown RG, Pluck G. Negative symptoms: the 'pathology' of motivation and goal-directed behaviour. Trends in neurosciences. 2000;23(9):412–7. Epub 2000/08/15. . [DOI] [PubMed] [Google Scholar]
- 34.Levy R, Dubois B. Apathy and the functional anatomy of the prefrontal cortex-basal ganglia circuits. Cereb Cortex. 2006;16(7):916–28. 10.1093/cercor/bhj043 . [DOI] [PubMed] [Google Scholar]
- 35.Levy R. Apathy: a pathology of goal-directed behaviour: a new concept of the clinic and pathophysiology of apathy. Revue neurologique. 2012;168(8–9):585–97. 10.1016/j.neurol.2012.05.003 . [DOI] [PubMed] [Google Scholar]
- 36.Middleton FA, Strick PL. Basal-ganglia 'projections' to the prefrontal cortex of the primate. Cereb Cortex. 2002;12(9):926–35. Epub 2002/08/17. . [DOI] [PubMed] [Google Scholar]
- 37.Lehericy S, Ducros M, Van de Moortele PF, Francois C, Thivard L, Poupon C, et al. Diffusion tensor fiber tracking shows distinct corticostriatal circuits in humans. Annals of neurology. 2004;55(4):522–9. Epub 2004/03/30. 10.1002/ana.20030 . [DOI] [PubMed] [Google Scholar]
- 38.Kemp JM, Powell TP. The cortico-striate projection in the monkey. Brain: a journal of neurology. 1970;93(3):525–46. Epub 1970/01/01. . [DOI] [PubMed] [Google Scholar]
- 39.Lindgren HS, Wickens R, Tait DS, Brown VJ, Dunnett SB. Lesions of the dorsomedial striatum impair formation of attentional set in rats. Neuropharmacology. 2013;71:148–53. Epub 2013/04/16. S0028-3908(13)00130-5 [pii] 10.1016/j.neuropharm.2013.03.034 . [DOI] [PubMed] [Google Scholar]
- 40.McNab F, Klingberg T. Prefrontal cortex and basal ganglia control access to working memory. Nat Neurosci. 2008;11(1):103–7. Epub 2007/12/11. nn2024 [pii] 10.1038/nn2024 . [DOI] [PubMed] [Google Scholar]
- 41.Baier B, Karnath HO, Dieterich M, Birklein F, Heinze C, Muller NG. Keeping memory clear and stable—the contribution of human basal ganglia and prefrontal cortex to working memory. J Neurosci. 2010;30(29):9788–92. Epub 2010/07/28. 30/29/9788 [pii] 10.1523/JNEUROSCI.1513-10.2010 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Andersson S, Bergedalen AM. Cognitive correlates of apathy in traumatic brain injury. Neuropsychiatry, neuropsychology, and behavioral neurology. 2002;15(3):184–91. . [PubMed] [Google Scholar]
- 43.McPherson S, Fairbanks L, Tiken S, Cummings JL, Back-Madruga C. Apathy and executive function in Alzheimer's disease. J Int Neuropsychol Soc. 2002;8(3):373–81. Epub 2002/04/10. . [DOI] [PubMed] [Google Scholar]
- 44.Zgaljardic DJ, Borod JC, Foldi NS, Rocco M, Mattis PJ, Gordon MF, et al. Relationship between self-reported apathy and executive dysfunction in nondemented patients with Parkinson disease. Cognitive and behavioral neurology: official journal of the Society for Behavioral and Cognitive Neurology. 2007;20(3):184–92. 10.1097/WNN.0b013e318145a6f6 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Konstantakopoulos G, Ploumpidis D, Oulis P, Patrikelis P, Soumani A, Papadimitriou GN, et al. Apathy, cognitive deficits and functional impairment in schizophrenia. Schizophrenia research. 2011;133(1–3):193–8. Epub 2011/07/27. 10.1016/j.schres.2011.07.003 . [DOI] [PubMed] [Google Scholar]
- 46.Varanese S, Perfetti B, Ghilardi MF, Di Rocco A. Apathy, but not depression, reflects inefficient cognitive strategies in Parkinson's disease. PLoS One. 2011;6(3):e17846 Epub 2011/03/26. 10.1371/journal.pone.0017846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yamashita K, Iijima K, Kobayashi S. Relationship among activities of daily living, apathy, and subjective well-being in elderly people living alone in a rural town. Gerontology. 1999;45(5):279–82. Epub 1999/08/26. doi: ger45279 [pii]. . [DOI] [PubMed] [Google Scholar]
- 48.Clarke DE, Ko JY, Lyketsos C, Rebok GW, Eaton WW. Apathy and cognitive and functional decline in community-dwelling older adults: results from the Baltimore ECA longitudinal study. International psychogeriatrics / IPA. 2010;22(5):819–29. Epub 2010/05/19. 10.1017/S1041610209991402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brodaty H, Heffernan M, Draper B, Reppermund S, Kochan NA, Slavin MJ, et al. Neuropsychiatric symptoms in older people with and without cognitive impairment. Journal of Alzheimer's disease: JAD. 2012;31(2):411–20. Epub 2012/05/11. 10.3233/JAD-2012-120169 . [DOI] [PubMed] [Google Scholar]
- 50.First MB, Gibbon M, Spitzer RL, Williams JBW. Guide for the structured clinical interview for DSM-IV axis I disorders-Research version New York: Biometrics Research; 1996. [Google Scholar]
- 51.Orsini A, Laicardi C. Factor structure of the Italian version of the WAIS-R compared with the American standardization. Percept Mot Skills. 2000;90(3 Pt 2):1091–100. Epub 2000/08/12. 10.2466/pms.2000.90.3c.1091 . [DOI] [PubMed] [Google Scholar]
- 52.Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9(1):97–113. Epub 1971/03/01. . [DOI] [PubMed] [Google Scholar]
- 53.Hollingshead AA. Four-factor index of social status (Unpublished Manuscript) Yale University, New Haven, CT: 1975. [Google Scholar]
- 54.Starkstein SE, Mayberg HS, Preziosi TJ, Andrezejewski P, Leiguarda R, Robinson RG. Reliability, validity, and clinical correlates of apathy in Parkinson's disease. The Journal of neuropsychiatry and clinical neurosciences. 1992;4(2):134–9. Epub 1992/01/01. 10.1176/jnp.4.2.134 [DOI] [PubMed] [Google Scholar]
- 55.Marin RS, Biedrzycki RC, Firinciogullari S. Reliability and validity of the Apathy Evaluation Scale. Psychiatry research. 1991;38(2):143–62. Epub 1991/08/01. . [DOI] [PubMed] [Google Scholar]
- 56.Bertolino A, Caforio G, Blasi G, De Candia M, Latorre V, Petruzzella V, et al. Interaction of COMT (Val(108/158)Met) genotype and olanzapine treatment on prefrontal cortical function in patients with schizophrenia. The American journal of psychiatry. 2004;161(10):1798–805. Epub 2004/10/07. 10.1176/appi.ajp.161.10.1798 . [DOI] [PubMed] [Google Scholar]
- 57.Blasi G, Napolitano F, Ursini G, Di Giorgio A, Caforio G, Taurisano P, et al. Association of GSK-3beta genetic variation with GSK-3beta expression, prefrontal cortical thickness, prefrontal physiology, and schizophrenia. Am J Psychiatry. 2013;170(8):868–76. Epub 2013/04/20. 1680035 [pii] 10.1176/appi.ajp.2012.12070908 . [DOI] [PubMed] [Google Scholar]
- 58.Gelao B, Fazio L, Selvaggi P, Di Giorgio A, Taurisano P, Quarto T, et al. DRD2 genotype predicts prefrontal activity during working memory after stimulation of D2 receptors with bromocriptine. Psychopharmacology (Berl). 2014;231(11):2361–70. Epub 2014/01/16. 10.1007/s00213-013-3398-9 . [DOI] [PubMed] [Google Scholar]
- 59.Blasi G, Taurisano P, Papazacharias A, Caforio G, Romano R, Lobianco L, et al. Nonlinear response of the anterior cingulate and prefrontal cortex in schizophrenia as a function of variable attentional control. Cereb Cortex. 2010;20(4):837–45. Epub 2009/07/28. bhp146 [pii] 10.1093/cercor/bhp146 . [DOI] [PubMed] [Google Scholar]
- 60.Blasi G, Goldberg TE, Elvevag B, Rasetti R, Bertolino A, Cohen J, et al. Differentiating allocation of resources and conflict detection within attentional control processing. Eur J Neurosci. 2007;25(2):594–602. Epub 2007/02/08. EJN5283 [pii] 10.1111/j.1460-9568.2007.05283.x . [DOI] [PubMed] [Google Scholar]
- 61.Rasetti R, Mattay VS, Stankevich B, Skjei K, Blasi G, Sambataro F, et al. Modulatory effects of modafinil on neural circuits regulating emotion and cognition. Neuropsychopharmacology. 2010;35(10):2101–9. Epub 2010/06/18. npp201083 [pii] 10.1038/npp.2010.83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Blasi G, De Virgilio C, Papazacharias A, Taurisano P, Gelao B, Fazio L, et al. Converging evidence for the association of functional genetic variation in the serotonin receptor 2a gene with prefrontal function and olanzapine treatment. JAMA Psychiatry. 2013;70(9):921–30. Epub 2013/07/12. 1710489 [pii] 10.1001/jamapsychiatry.2013.1378 . [DOI] [PubMed] [Google Scholar]
- 63.Blasi G, Selvaggi P, Fazio L, Antonucci LA, Taurisano P, Masellis R, et al. Variation in Dopamine D2 and Serotonin 5-HT2A Receptor Genes is Associated with Working Memory Processing and Response to Treatment with Antipsychotics. Neuropsychopharmacology. 2015;40(7):1600–8. Epub 2015/01/08. npp20155 [pii] 10.1038/npp.2015.5 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.O'Reilly RC. Biologically based computational models of high-level cognition. Science. 2006;314(5796):91–4. Epub 2006/10/07. 314/5796/91 [pii] 10.1126/science.1127242 . [DOI] [PubMed] [Google Scholar]
- 65.Bertolino A, Taurisano P, Pisciotta NM, Blasi G, Fazio L, Romano R, et al. Genetically determined measures of striatal D2 signaling predict prefrontal activity during working memory performance. PLoS One. 2010;5(2):e9348 Epub 2010/02/25. 10.1371/journal.pone.0009348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Arsalidou M, Duerden EG, Taylor MJ. The centre of the brain: topographical model of motor, cognitive, affective, and somatosensory functions of the basal ganglia. Hum Brain Mapp. 2013;34(11):3031–54. Epub 2012/06/20. 10.1002/hbm.22124 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Levy R, Czernecki V. Apathy and the basal ganglia. J Neurol. 2006;253 Suppl 7:VII54–61. 10.1007/s00415-006-7012-5 . [DOI] [PubMed] [Google Scholar]
- 68.Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19(3):1233–9. Epub 2003/07/26. S1053811903001691 [pii]. . [DOI] [PubMed] [Google Scholar]
- 69.Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ. Psychophysiological and modulatory interactions in neuroimaging. Neuroimage. 1997;6(3):218–29. 10.1006/nimg.1997.0291 [DOI] [PubMed] [Google Scholar]
- 70.Callicott JH, Mattay VS, Bertolino A, Finn K, Coppola R, Frank JA, et al. Physiological characteristics of capacity constraints in working memory as revealed by functional MRI. Cereb Cortex. 1999;9(1):20–6. [DOI] [PubMed] [Google Scholar]
- 71.Fuster JM, Alexander GE. Neuron activity related to short-term memory. Science. 1971;173(997):652–4. Epub 1971/08/13. . [DOI] [PubMed] [Google Scholar]
- 72.Goldman-Rakic PS. Cellular and circuit basis of working memory in prefrontal cortex of nonhuman primates. Prog Brain Res. 1990;85:325–35. [DOI] [PubMed] [Google Scholar]
- 73.Taurisano P, Romano R, Mancini M, Giorgio AD, Antonucci LA, Fazio L, et al. Prefronto-striatal physiology is associated with schizotypy and is modulated by a functional variant of DRD2. Front Behav Neurosci. 2014;8:235 Epub 2014/07/30. 10.3389/fnbeh.2014.00235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Callicott JH, Mattay VS, Bertolino A, Finn K, Coppola R, Frank JA, et al. Physiological characteristics of capacity constraints in working memory as revealed by functional MRI. Cereb Cortex. 1999;9(1):20–6. Epub 1999/02/18. . [DOI] [PubMed] [Google Scholar]
- 75.Baudic S, Maison P, Dolbeau G, Boisse MF, Bartolomeo P, Dalla Barba G, et al. Cognitive impairment related to apathy in early Huntington's disease. Dementia and geriatric cognitive disorders. 2006;21(5–6):316–21. 10.1159/000091523 . [DOI] [PubMed] [Google Scholar]
- 76.Pluck GC, Brown RG. Apathy in Parkinson's disease. Journal of neurology, neurosurgery, and psychiatry. 2002;73(6):636–42. 10.1136/jnnp.73.6.636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Isella V, Melzi P, Grimaldi M, Iurlaro S, Piolti R, Ferrarese C, et al. Clinical, neuropsychological, and morphometric correlates of apathy in Parkinson's disease. Movement disorders: official journal of the Movement Disorder Society. 2002;17(2):366–71. . [DOI] [PubMed] [Google Scholar]
- 78.Faerden A, Vaskinn A, Finset A, Agartz I, Ann Barrett E, Friis S, et al. Apathy is associated with executive functioning in first episode psychosis. BMC psychiatry. 2009;9:1 10.1186/1471-244X-9-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Robert G, Le Jeune F, Dondaine T, Drapier S, Peron J, Lozachmeur C, et al. Apathy and impaired emotional facial recognition networks overlap in Parkinson's disease: a PET study with conjunction analyses. J Neurol Neurosurg Psychiatry. 2014;85(10):1153–8. Epub 2014/01/10. jnnp-2013-307025 [pii] 10.1136/jnnp-2013-307025 . [DOI] [PubMed] [Google Scholar]
- 80.Bayard S, Jacus JP, Raffard S, Gely-Nargeot MC. Apathy and emotion-based decision-making in amnesic mild cognitive impairment and Alzheimer's disease. Behav Neurol. 2014;2014:231469 Epub 2014/07/23. 10.1155/2014/231469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Laplane D, Dubois B. Auto-Activation deficit: a basal ganglia related syndrome. Movement disorders: official journal of the Movement Disorder Society. 2001;16(5):810–4. Epub 2001/12/18. . [DOI] [PubMed] [Google Scholar]
- 82.Parent A, Hazrati LN. Functional anatomy of the basal ganglia. I. The cortico-basal ganglia-thalamo-cortical loop. Brain Res Brain Res Rev. 1995;20(1):91–127. Epub 1995/01/01. 016501739400007C [pii]. . [DOI] [PubMed] [Google Scholar]
- 83.Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9:357–81. Epub 1986/01/01. 10.1146/annurev.ne.09.030186.002041 . [DOI] [PubMed] [Google Scholar]
- 84.Postuma RB, Dagher A. Basal ganglia functional connectivity based on a meta-analysis of 126 positron emission tomography and functional magnetic resonance imaging publications. Cereb Cortex. 2006;16(10):1508–21. Epub 2005/12/24. bhj088 [pii] 10.1093/cercor/bhj088 . [DOI] [PubMed] [Google Scholar]
- 85.Jarbo K, Verstynen TD. Converging structural and functional connectivity of orbitofrontal, dorsolateral prefrontal, and posterior parietal cortex in the human striatum. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2015;35(9):3865–78. Epub 2015/03/06. 35/9/3865 [pii] 10.1523/JNEUROSCI.2636-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Levy ML, Cummings JL, Fairbanks LA, Masterman D, Miller BL, Craig AH, et al. Apathy is not depression. J Neuropsychiatry Clin Neurosci. 1998;10(3):314–9. Epub 1998/08/26. 10.1176/jnp.10.3.314 . [DOI] [PubMed] [Google Scholar]
- 87.Starkstein SE, Ingram L, Garau ML, Mizrahi R. On the overlap between apathy and depression in dementia. J Neurol Neurosurg Psychiatry. 2005;76(8):1070–4. Epub 2005/07/19. 76/8/1070 [pii] 10.1136/jnnp.2004.052795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Clarke DE, Ko JY, Kuhl EA, van Reekum R, Salvador R, Marin RS. Are the available apathy measures reliable and valid? A review of the psychometric evidence. J Psychosom Res. 2011;70(1):73–97. Epub 2011/01/05. S0022-3999(10)00017-6 [pii] 10.1016/j.jpsychores.2010.01.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data of this study are available at the 4TU.ResearchData repository; http://dx.doi.org/10.4121/uuid:d5ec1a8a-6c61-4f19-8af1-b728bb07e8c0.