About 313 million major surgical procedures are performed worldwide every year.1 Since the 1970s, vascular surgery has been identified as a subpopulation with disproportionately elevated risk.2,3 This risk persists today. For example, in the Vascular Events in Noncardiac Surgery Patients Cohort Evaluation (VISION) study of over 15,000 individuals undergoing major noncardiac surgery, vascular surgery was a predictor of 30-day mortality [adjusted hazard ratio (HR)=2.38].4 An analysis of about 123,500 cases in the National Surgical Quality Improvement Program (NSQIP) database from 2007 to 2010 revealed the absolute risk for death within 30 days after major vascular surgery to be 3%.5 Importantly, procedure-specific risks do vary considerably on the basis of the operative site, approach employed, and urgency of the procedure.6
An important reason for the elevated risk in vascular surgery patients is their burden of atherosclerotic disease. Many vascular surgical procedures address complications of peripheral arterial disease (PAD) or cerebrovascular disease (CVD). Atherosclerosis is the pathophysiological mechanism for both entities, as well as for coronary artery disease (CAD). Atherosclerosis involves plaques forming dense deposits in arterial walls and irreversibly expanding, subsequently leading to a narrowed vessel lumen and decreased compliance. Although atherosclerosis is not a regional process, it is defined in such terms; hence, the distinction between CAD, CVD, and PAD. Nonetheless, given their shared pathophysiology, there is a high association between CAD, CVD, and PAD.7 In the nonoperative setting, asymptomatic PAD is associated with a 3- to 4-fold increase in the risk of CAD and CVD.8 Further, the presence of PAD is a predictor of worse outcomes after both myocardial infarction (MI) and stroke.9,10 Thus, atherosclerosis in multiple organ systems is indicative of both disease breadth and severity.
Because of the high risks of vascular surgery and the complex phenotype of patients undergoing these operations, it is imperative that anesthesiologists perform a thorough preoperative evaluation. The purpose of the preoperative assessment is to identify potential problems, ensure the patient is willing and able to tolerate the procedure with associated risks, mitigate perioperative risks, and facilitate appropriate postoperative monitoring. Given the influence of cardiovascular disease on outcomes in vascular surgery, it is important to identify individuals at risk to ensure appropriate management. Moreover, in individuals identified as being at very high risk, a frank discussion between the patient, surgeon, and anesthesiologist may be needed regarding the risks of the procedure, as well as possible alternatives to planned surgery.
The goal of this chapter is to present an evidence-based approach to preoperative cardiac evaluation and management of patients undergoing major vascular surgery. Where possible, we focus on relevant current evidence and guidelines. The purpose is to present an approach to evaluate patients for common cardiac diseases and inform preoperative management. Thus, we will not discuss relatively uncommon cardiac diseases (eg, pulmonary hypertension, congenital heart disease), although these conditions are still important for perioperative care. Finally, we will assume sufficient time to complete a formal evaluation and permit management; however, we recognize this is often not the case. When discussing the urgency of cases, we use the definitions in the 2014 American College of Cardiology (ACC) and American Heart Association (AHA) perioperative guidelines. An emergency procedure is defined as threatening life or limb and requiring the patient to be in the operating room within <6 hours; an urgent procedure is defined as requiring an operation within 6 to 12 hours; a time-sensitive procedure is one that should be performed within 1 to 6 weeks, and an elective procedure allows time up to 1 year.11
Preoperative Evaluation
Approach
Preoperative evaluation is a key component of the preparation of patients before major surgery,12 especially for higher-risk patients and complex procedures.13 It enables identification of patients with elevated risk for complications, in turn facilitating appropriate individualized management. A thorough assessment can help identify comorbidities, assess disease severity, and determine the need for further testing. The purpose should be to inform perioperative management. For example, the evaluation can help alter medications, communicate risks accurately to patients, change intraoperative care, modify surgical procedures, or influence postoperative level of care. A systematic approach is best for capturing all relevant details. Thus, the classic approach should be adopted by first characterizing the presenting illness (with planned surgery, including indication), followed by studying the past medical history, conducting a focused physical examination, and reviewing previous investigations.
Clinical History and Assessment
Indications for Surgery: PAD and CVD
The clinical assessment of the vascular surgery patient begins with the planned procedure and its indication. The indications for most vascular surgery procedures include symptomatic PAD, limb ischemia, central aneurysm, or prior stroke or transient ischemic attack. Thus, the presence of atherosclerosis should always be suspected. The main atherosclerotic diseases prompting vascular surgery are PAD and CVD, although many patients have concomitant CAD.
Although the classic symptom of PAD is exertional claudication, it is present in only 10% of individuals. Symptoms typically appear with an ankle brachial index (ABI) below 0.9. Nonetheless, one quarter of asymptomatic patients with PAD have an ABI below 0.7.14 Thus, test results should be reviewed to assess disease severity and extent. Interestingly, an abnormal ABI is also a risk factor for postoperative cardiac complications. In a cohort study of 242 patients undergoing noncardiac surgery, an ABI below 0.9 or absence of all 4 pedal pulses was a predictor of cardiac complications [adjusted odds ratio (OR)=10.2].15 A focused physical examination for a patient with PAD should include measurement of blood pressure in both arms, auscultation for bruits (carotid, renal, and femoral), and assessment of vascular access sites.
With respect to CVD, a history should ascertain any previous stroke or transient ischemic attack, as well as detail the associated presentation and deficits. It is important to document the etiology to distinguish carotid stenosis (atherosclerosis) from cardioembolic disease. Causes of cardiac emboli include stasis (atrial fibrillation, severe cardiomyopathy, ventricular aneurysm), thrombogenic (valvular heart disease, prosthetic heart valve), and paradoxical venous source (patent foramen ovale). The physical examination should include a brief neurological exam to identify any preexisting deficits, auscultation for carotid bruits, and a precordial assessment for murmurs or extra heart sounds.
The presence of PAD and CVD has implications for perioperative risk assessment and management. PAD and CVD are significant risk factors for postoperative death,4 major adverse cardiac events (MACE),16 and myocardial injury after major noncardiac surgery.17 Further, CVD is associated with a 2- to 3-fold increase in the risk of acute stroke after major noncardiac surgery18,19 and vascular surgery.20 As acute stroke is a devastating complication of perioperative β-blockade (see the Pharmacologic therapy section—β-Blockers),18,21 delineation of any CVD helps inform the risk-benefit balance of β-blockade in surgical patients.
CAD
The overall prevalence of CAD among American adults is 6.2%.22 The prevalence in vascular surgery patients is difficult to determine given the selection bias within individual studies, and varying sensitivity of different screening tests (eg, cardiac stress testing vs. coronary angiography). For example, a systematic review in the nonoperative setting found the prevalence of CAD in individuals with PAD to range from 15% to 90% on the basis of the screening method.7 Its prevalence was about 20% to 45% when CAD was ascertained using clinical history plus an electrocardiogram (ECG), about 60% when stress testing was employed, and 90% when angiography was used. The prevalence is likely even higher among patients with PAD who require vascular surgery. This prevalence was first quantified in 1984 by Hertzer and colleagues who performed preoperative coronary angiograms on 1000 vascular surgery patients. Only 8% had normal coronary arteries, whereas 60% had severe disease (>70% stenosis in ≥1 major coronary artery).23 Consistent with this observation, a randomized controlled trial (RCT) of routine preoperative coronary angiography for major vascular surgery found significant stenosis of a major coronary artery in 62% of individuals allocated to routine screening.24 Thus, a high index of suspicion for undiagnosed CAD is needed in vascular surgery patients.
The primary concern regarding CAD stems from it being a risk factor for postoperative death,4 MI,16 and myocardial injury after noncardiac surgery.17 Perioperative MI after vascular surgery has a mortality of 10% to 25%.25,26 Further, it is a strong predictor of elevated early (in-hospital or 30-d) and long-term mortality.25–31 The newer clinical entity of myocardial injury after noncardiac surgery (ie, significant troponin elevations after noncardiac surgery) is also associated with elevated 30-day mortality. Notably, cardiovascular complications are the leading cause of death after major vascular surgery.32–34
When evaluating a patient with known or suspected CAD, the preoperative assessment should begin with a history regarding specific symptoms of CAD, prior events, diagnostic tests, previous interventions, and treatment. It is important to characterize the presence, frequency, precipitants, and duration of angina. Communication between clinicians is often facilitated by standardized scales such as the Canadian Cardiovascular Society grading system for rating the severity of angina.35 Further, it is essential to document any temporal change in symptoms to differentiate stable angina from unstable angina.
As a prior MI is a risk factor for perioperative cardiac morbidity, it is important to document the date of any cardiac events and revascularization procedures. The impact of a prior MI on subsequent noncardiac surgery depends on the interval since the MI and any revascularization procedures. The impact of the interval from a prior MI on perioperative risk is informed by a large retrospective cohort study of 563,842 surgical procedures; abdominal aortic aneurysm (AAA) repair surgery was among the 5 procedures included in the study.36 A previous MI within 30 days of surgery was associated with a dramatic increase in 30-day MI after AAA repair [adjusted relative risk (RR)=15.36]; the risk was still elevated, albeit not to the same degree, for a previous MI within 31 to 60 days before surgery (adjusted RR=4.5). These data support current guideline recommendations to delay nonurgent major noncardiac surgery for at least 60 days after an MI.11 As indicated above, it is important to document the timing of any prior revascularization procedure with percutaneous coronary interventions (PCI) or coronary artery bypass graft (CABG) surgery. If PCI was performed, it is imperative to determine whether a bare metal stent (BMS) or drug eluting stent (DES) was inserted. The distinction between stent types determines the recommended minimum duration of dual antiplatelet therapy with aspirin plus a P2Y12 inhibitor (eg, clopidogrel, prasugrel). The concern regarding prior PCI stems from the potential need to interrupt dual antiplatelet therapy, which along with the prothrombotic state generated from the stress of surgery increases the risk for catastrophic acute stent thrombosis. Recent practice guidelines recommend that time-sensitive and elective noncardiac surgery be delayed for at least 30 days after insertion of BMS, after which patients can proceed to surgery on aspirin alone.11,37 These recommendations are supported by recent large multicenter retrospective cohort studies.38,39 Conversely, evidence regarding the minimum safe interval from DES insertion to noncardiac surgery continues to evolve. The 2009 ACC/AHA guidelines on perioperative cardiovascular evaluation recommended a minimum 1-year interval from DES insertion to surgery.37 Several subsequent large cohort studies then found that noncardiac surgery can be performed safely once >180 days had elapsed from DES insertion.38–40 Combined with other nonrandomized studies,39,41,42 these data support the current new guideline recommendation to ideally delay elective noncardiac surgery until at least 365 days after DES, with the provision that surgery can proceed after 180 days in selected cases.11
Another important predictor of outcomes in CAD is exercise capacity. As early as 1989, a study of exercise testing in 100 patients undergoing major vascular surgery found that preoperative exercise tolerance was inversely proportional to the risk for MACE.43 Other studies have shown that the ability to perform >4 to 6 metabolic equivalents (METs) on objective exercise testing was associated with low perioperative cardiovascular risk.44,45 The key challenge in clinical practice is identifying individuals with poor exercise capacity using clinical evaluation alone, as opposed to formal exercise testing. The conventional assessment of exercise capacity involves physicians making a subjective estimate based on patients’ self-reported history. The data supporting the prognostic accuracy of subjective estimates of exercise capacity are relatively weak. A single-center study of 600 patients undergoing noncardiac surgery did show patients’ self-reported inability to climb 2 flights of stairs or walk 4 blocks to be a risk factor for perioperative cardiovascular complications (adjusted OR=1.9).46 Nonetheless, when expressed as a likelihood ratio (LR), self-reported poor exercise capacity had a positive LR of 1.3 and negative LR of 0.62 for predicting complications. LR values >2 or <0.5 are recommended for providing even minimal additional information.47 Further, another single-center study of 5939 surgical patients found physicians’ subjective estimates of exercise capacity to have minimal to poor accuracy at predicting mortality or cardiac complications.48 A potential improvement encouraged by guidelines is objective scales with correlation to objectively measured exercise capacity,11 such as the Duke Activity Status Index (DASI).49 The use of a questionnaire, as opposed to subjective assessment, can lead to different estimates of exercise capacity. A single-center study of 74 surgical patients found poor agreement between subjective physician assessment and the DASI, with a tendency of the former to underestimate capacity.50 Despite these limitations, it remains important to evaluate exercise capacity, especially to inform decisions regarding the need for further investigation.
In addition to the routine cardiac examination, the physical examination for a patient with known or suspected CAD should assess for other cardiovascular disease. The pulse should be examined to assess for regular sinus rhythm. Particular attention should be paid to signs of heart failure (HF) and valvular disease (see the HF section and valvular heart disease section). Baseline vital signs, particularly blood pressure, should be recorded to inform perioperative hemodynamic management and assess therapeutic control in hypertensive patients. The main utility of documenting the baseline blood pressure is to guide anesthesiologists in maintaining hemodynamics within an individual’s normal physiological range. This goal is increasingly important given accumulating evidence that intraoperative hypotension is associated with major morbidity after noncardiac surgery.51–53
Heart Failure (HF)
HF can result from many different etiologies, each with specific implications. During preoperative evaluation, HF is best characterized with respect to associated symptoms and the nature of ventricular impairment. Classically, HF was divided into systolic HF, when ventricular systolic dysfunction was present, and diastolic HF, when ventricular filling is impaired. Diastolic HF has generally not received much attention in the perioperative literature, despite being much more common than previously understood and accounting for half of all cases of HF.54 After recent investigations showed the pathophysiology of diastolic HF to involve a much broader range of factors than impaired ventricular filling, there has been a shift in the terminology of HF. Diastolic HF is now described as HF with preserved ejection fraction (EF). A distinction is made from HF with systolic dysfunction, which is now termed HF with reduced EF.54 The type of HF is important for management and has implications for life expectancy. In an individual patient meta-analysis of 41,927 patients, survival was significantly better for HF with preserved versus reduced EF (adjusted HR=0.68). Nonetheless, it should be emphasized that the absolute mortality for HF with preserved EF was still elevated.55 This terminology is still highly debated and may represent an ongoing evolution in the understanding of HF.56,57 Although the distinction between HF with preserved versus reduced EF has facilitated targeted therapeutic intervention, its relevance for perioperative management remains to be thoroughly investigated.
HF has been recognized as a risk factor for perioperative MACE for almost 40 years.3 Goldman et al3 reported it as one of 9 major risk factors for perioperative MACE, with the presence of HF defined by a third heart sound (S3) or jugular venous distension. Further, HF represents one of the 6 risk factors included in the commonly used Revised Cardiac Risk Index (RCRI), where the definition was broadened to include physical findings (bilateral rales, S3 gallop), radiographic evidence (pulmonary vascular redistribution), and a history of HF, pulmonary edema, or paroxysmal nocturnal dyspnea.16 Symptomatic HF continues to be identified as a risk factor for adverse perioperative outcomes in multiple studies. For example, in a retrospective cohort study of about 47,800 Medicare beneficiaries in the United States, a history of HF was associated with a doubling in the risk for 30-day death after noncardiac surgery (adjusted OR=2.19).58 A subsequent larger study also showed a history of HF to be associated with a qualitatively similar increase in the risks for 30-day mortality in about 159,300 Medicare beneficiaries.59 Most recently, a matched cohort study using the NSQIP registry showed new or worsened HF within 30 days before surgery to be associated with increased risk for 30-day mortality (adjusted RR=2.08) or major morbidity (adjusted RR=1.54).60 The stability of patients’ HF status immediately before surgery may also have prognostic importance. In a pragmatic study of 567 patients with HF who underwent elective noncardiac surgery, Xu-Cai and colleagues61 evaluated the impact of a specialized preoperative clinic intended to stabilize patients before surgery. The results suggest that preoperative medical management to achieve stability is important, as HF patients and propensity-matched controls did not have significant differences in 30-day mortality. Nonetheless, hospital length of stay and readmission rates remained higher for the HF patients.
Although symptomatic HF is clearly a marker of increased perioperative morbidity, the impact of reduced left ventricular EF is less clear. To study the impact of left ventricular EF on perioperative outcomes, Healy and colleagues studied 174 patients with HF undergoing noncardiac surgery, of whom 47% had vascular surgery. An EF<30% was associated with the composite outcome of 30-day death, MI, and HF (adjusted OR=4.88).62 Conversely, in another cohort study of 339 individuals undergoing noncardiac surgery, a reduced EF was associated with increased cardiac morbidity, but this information did not improve risk prediction beyond that achieved with clinical risk factors.63 In another cohort study of 570 individuals undergoing noncardiac surgery, a reduced EF had prognostic importance only in individuals with at least 2 risk factors from the RCRI.64 In summary, the prognostic relevance of asymptomatic left ventricular dysfunction in the perioperative setting is unclear.
A preoperative assessment for HF should include a history to clarify its type, etiology, prior exacerbations, and recent investigations (eg, prior ventricular function measurements). The severity of and recent changes in HF symptoms should be documented, including paroxysmal nocturnal dyspnea, orthopnea, and lower extremity edema. Current therapy should be assessed, with particular attention paid to drugs with perioperative implications (see the pharmacologic therapy section). Potential therapies may include cardiac resynchronization therapy, which will require appropriate perioperative management. Functional limitation due to HF should be characterized using the New York Heart Association classification system to provide a standardized measure.65 If a patient cannot exercise because of noncardiopulmonary reasons (eg, arthritis), left ventricular EF is not a proxy measure of exercise capacity.66,67
The findings of HF on physical examination may be subtle, but some signs are useful in making the diagnosis. On precordial exam, a S3 gallop is the strongest predictor of HF with a positive LR of 11. If there is uncertainty regarding the presence of HF, a chest radiograph may provide further guidance. In dyspneic patients, both pulmonary vascular redistribution (LR=12) and interstitial edema (LR=12) increase the likelihood of HF.68 If the cause for dyspnea still remains unclear, B-type natriuretic peptide (BNP) levels can be useful for differentiating between cardiac and noncardiac etiologies.68
Valvular Heart Disease
During the preoperative evaluation, it is important to ascertain any known cardiac valvular disease through a history and precordial examination. In individuals with known disease, HF symptoms, exercise capacity, echocardiographic findings (ie, valvular lesions, ventricular function, pulmonary hypertension), and therapy (eg, anticoagulants) should be documented. It is especially important to identify stenotic valvular lesions that limit the ability of patients to compensate for the vasodilating effects of general anesthesia. Of particular concern is aortic stenosis, which is the most common stenotic valvular lesion in North America.69
Significant aortic stenosis has been a recognized risk factor for perioperative morbidity for almost 40 years.3 These risks were confirmed in a contemporary single-center cohort of about 630 patients who underwent noncardiac surgery with unrepaired moderate-to-severe aortic stenosis.70 Compared with matched controls, individuals with aortic stenosis experienced significantly higher risk for death or MI within 30 days after surgery. Conversely, 2 other smaller single-center studies (74 patients in total) demonstrated that carefully selected patients with unrepaired aortic stenosis could undergo noncardiac surgery with acceptable rates of morbidity and mortality.71,72 Thus, the decision to perform preoperative aortic valve replacement versus proceeding directly to vascular surgery with unrepaired aortic stenosis must consider the severity of the valvular lesion, symptoms, level of available perioperative care (eg, cardiac anesthesiologists, intraoperative transesophageal echocardiography, critical care monitoring), as well as the urgency and invasiveness of the planned vascular procedure.11
In an individual without known valvular heart disease, a systolic ejection murmur on precordial examination should always raise the suspicion of undiagnosed aortic stenosis. Although such individuals should ideally undergo echocardiography for definitive diagnostic evaluation, physical examination can help rule out moderate to severe aortic stenosis. Specifically, a clinical decision rule found that the absence of a murmur radiating below the right clavicle has a negative LR of 0.05 to 0.1 for ruling out significant disease.73
Arrhythmias
The preoperative assessment should include a history of prior significant arrhythmia. It should be noted whether the arrhythmia is chronic or paroxysmal; in the latter case, any precipitating factors, hemodynamic compromise, and effective treatment should be documented. Paroxysmal arrhythmias can be classified as ventricular or atrial. Atrial fibrillation is associated with increased cardiac risk in noncardiac surgery.74,75 For example, in the VISION study, preoperative atrial fibrillation was associated with increased risk for MACE (adjusted OR=1.58).75 It is important to establish the presence of medical therapy (ie, antiarrhythmics, rate-control medication, anticoagulants) and any cardiac implantable electronic device (CIED). The CIED may be a defibrillator or a pacemaker. The details and location of any CIED should be obtained, along with the current settings, arrhythmia response, and response to a magnet. Further details on perioperative CIED management are presented in guidelines from the American Society of Anesthesiologists and Heart Rhythm Society.76 Physical examination should assess pulses, signs of HF (see the HF section), and murmurs suggestive of valvular disease.
Among patients with atrial fibrillation, the CHADS2 index has modest performance in discriminating risks for postoperative stroke or death.75 This information has the potential to inform selection of individuals requiring perioperative bridging anticoagulation. Nonetheless, the value of bridging anticoagulation has been called into question by the recent Bridging Anticoagulation in Patients who Require Temporary Interruption of Warfarin Therapy for an Elective Invasive Procedure or Surgery (BRIDGE) trial, which showed perioperative withdrawal of anticoagulation to be noninferior to bridging therapy in 1884 participants.77
Preoperative Risk Assessment
Given the disproportionally elevated morbidity and mortality associated with vascular surgery, risk assessment is a critical step in the preoperative assessment. Accurately prognosticating the risk of the surgical procedure is essential to allow clear communication between clinicians and patients regarding the risks versus benefits of the planned surgery, thereby making it integral to an informed consent. Further, if a high-risk patient can be identified before surgery, appropriate precaution, monitoring, and care can be applied during the intraoperative period, and the correct level of postoperative care can be specified after surgery.
Predictive Risk Indices
As there is enormous potential utility of an accurate preoperative risk index, it is not surprising that so many have been published. The fundamental structure is generally the same; these indices comprise several variables identified as independently predicting the outcome of interest. These predictive factors can be more broadly categorized into patient (eg, CAD) and procedure (eg, vascular surgery) factors.
It has been almost 40 years since the original Goldman Cardiac Risk Index was published, transforming the preoperative evaluation process and providing the impetus for a new field of research.3 Although many more indices have been published subsequently,2,78–80 arguably the most widely used cardiac risk index in contemporary practice is the RCRI. This straightforward scoring method consists of 6 equally weighted components: high-risk surgery (intraperitoneal, intrathoracic, or suprainguinal vascular procedures), history of CAD, history of HF, history of CVD, diabetes mellitus requiring treatment with insulin, and preoperative renal insufficiency (serum creatinine exceeding 2.0 mg/dL).16 The RCRI performs moderately well in discriminating based on risk for postoperative cardiac complications, with preserved performance in validation studies based on a pooled area under the receiver operating characteristic curve (AUROC) of 0.75.81 However, it has several limitations. First, the RCRI has poor accuracy for predicting an individual’s absolute cardiac risk. For example, it underestimated cardiac event rates by 6- to 7-fold in 2 validation studies.79,82 Second, 2 components of the RCRI have not shown consistent predictive value in validation. Specifically, the value of retaining diabetes mellitus requiring treatment with insulin80,83 and preoperative serum creatinine exceeding 2.0 mg/dL in the index is unclear, although replacing the latter with reduced estimated glomerular filtration rate (eGFR) does improve performance.83 Finally, the RCRI has shown poor predictive performance in vascular surgery, particularly AAA repair surgery.16,79–81 Despite these limitations, RCRI does remain a useful predictive index in vascular surgery, with its main strengths remaining its simplicity and extensive external validation.
In 2010, a new score-based index was developed for vascular surgery patients: the Vascular Surgery Group of New England Cardiac Risk Index (VSG-CRI). The index was derived in a cohort that included carotid endarterectomy, lower extremity bypass, aortobifemoral bypass, open AAA repair, and endovascular aneurysm repair (EVAR). The factors in the index were age, smoking, CAD, HF, chronic obstructive pulmonary disease, diabetes mellitus requiring insulin therapy, creatinine exceeding 1.8 mg/dL, preoperative β-blocker therapy, and prior CABG or PCI. Temporal validation in a sequential cohort of 1873 patients showed only modest discriminative performance (AUROC=0.71), which was not significantly improved with procedure-specific models.79 Further, the calculation of MACE risk using the VSG-CRI can be cumbersome; a Web-based calculator is now available to help address this issue (http://www.vsgne.org).
More recently, Gupta and colleagues developed a preoperative risk calculator using the NSQIP registry. The derivation cohort involved 211,410 patients having major noncardiac surgery, and the primary outcome was a composite of MI or cardiac arrest (MICA).80 The 5 factors included in the predictive model are age, dependent functional status, American Society of Anesthesiologists Physical Status (ASA-PS), preoperative creatinine concentration >1.5 mg/dL, and surgery type. The model was temporally validated using a sequential cohort of another 257,385 individuals in the NSQIP registry. Uniquely, the calculator is not a score-based index; rather, the relevant information is entered into a Web-based calculator that implements the full predictive model to report the predicted risk for MICA. The authors argue that, with the advent of hand-held computing devices, it is not necessary to simplify risk calculators (http://www.surgicalriskcalculator.com/miorcardiacarrest). The overall discriminative performance was excellent (AUROC=0.87) in the validation cohort, but performance was diminished in the vascular surgery subgroup (AUROC=0.75). Several issues pertaining to the risk calculator merit discussion. Of significance is its inclusion of ASA-PS class, which has variable interrater reliability.84 In addition, as with the VSG-CRI, the NSQIP risk calculator has not been externally validated. Finally, ascertainment of postoperative MI in the NSQIP registry does not involve routine surveillance with troponin assays. This lack of standardized surveillance can lead to underestimation of cardiac event rates, especially as only 30% of postoperative MIs are accompanied by typical symptoms such as angina or dyspnea.17 Consistent with this possibility, Gupta and colleagues observed a MICA rate of only 0.65%. In contrast, the VISION study implemented routine postoperative troponin monitoring in a large sample of unselected major noncardiac surgical patients, and found an MI rate of 3.3%.17 Despite these limitations, the risk calculator of Gupta and colleagues is a reasonably straightforward and accurate method to estimate perioperative cardiac risk. In addition, this model has been incorporated into the universal American College of Surgeons (ACS) NSQIP risk calculators (http://www.riskcalculator.facs.org), allowing clinicians to estimate risks for several morbid events (eg, pneumonia, surgical site infection) simultaneously with cardiac risk estimation.85
Influence of Procedure-specific Risk
A major component of risk indices is procedure-specific risk, which can vary widely within a broad category of surgical procedures. Indeed, between the lowest and highest risk surgical procedures, risks for 30-day mortality can vary by 250-fold, whereas risks for MACE can vary by 25-fold.86 Variable procedure-specific risk is especially relevant in vascular surgery where endovascular repair of many arteries is now possible. For example, CVD can be treated with carotid stenting, AAA treated with EVAR, and PAD treated with peripheral arterial stenting. Given the reduced degree of tissue injury, blood loss, and surgical stress from these new surgical approaches, there is potential for substantial improvement in 30-day mortality. The evidence for improved perioperative mortality is most evident in comparisons of EVAR versus open AAA repair. Three randomized comparisons of endovascular versus open repair that together randomized 2484 patients consistently showed reduced 30-day mortality and morbidity with EVAR.87–89 Importantly, over longer follow-up, the survival advantage of EVAR is abolished within 2 to 4 years, likely because of the increased need for reinterventions in patients who underwent EVAR, as well as their unchanged burden of chronic medical disease.88–90
Given the rapid evolution in this field, the question that remains is how can preoperative physicians best determine procedure-specific risk. A potential solution is provided by the universal ACS-NSQIP Surgical Risk Calculator mentioned above. This calculator was developed in a derivation cohort of 1,414,006 patients undergoing 1557 different procedure types. In total, 21 preoperative factors (demographics, comorbidities, procedure) are used to predict 8 different outcomes, including 30-day mortality.85 At present, the Universal Risk Calculator is likely the most convenient and perhaps most accurate method for estimating procedure-specific risk. Its major limitations include the paucity of external validation studies and underestimation of cardiac event rates due to the absence of routine troponin surveillance in the derivation cohort.
Biomarkers: Natriuretic Peptides and High-sensitivity Troponins
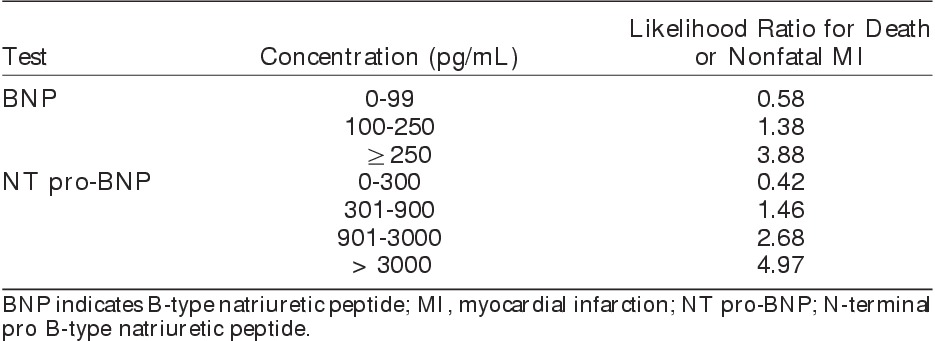
B-type (brain) natriuretic peptide (BNP) and N-terminal-pro-BNP (NT pro-BNP) are neurohormones secreted by the ventricles in response to stretch or ischemia of the atrial and ventricular walls.91 The biomarkers were first studied as a screening and diagnostic test in the setting of acute HF. Subsequently, elevated BNP and NT pro-BNP levels were recognized as powerful markers of cardiovascular risk in individuals who are at risk for CAD, have CAD, or have HF.92 There are now promising data pointing to the role of BNP and NT pro-BNP for preoperative risk stratification. Specifically, cohort studies and meta-analyses of these studies indicate that preoperative levels of these biomarkers are associated with postoperative cardiac death or MI. In an individual patient data meta-analysis of 6 studies involving 850 vascular surgery patients, elevated preoperative BNP levels were associated with postoperative MACE, MI, and cardiac death (ORs=7.9, 7.5, and 4.3, respectively). Moreover, BNP further improved risk prediction when used in combination with RCRI.93 A subsequent individual patient data meta-analysis of 18 studies (2051 patients) in mixed noncardiac surgery showed that both low and elevated preoperative natriuretic peptide levels can help identify patients with differing cardiac risks (Table 1).94 Despite these encouraging results, the evidence is not yet conclusive, with the ideal screening thresholds and the target population yet to be defined. These issues will be better clarified with the publication of several completed or ongoing large cohort studies of preoperative natriuretic peptide testing. An example is a subinvestigation of the VISION study that will evaluate NT pro-BNP in 8000 to 10,000 patients undergoing major noncardiac surgery (Clinicaltrials.gov NCT00512109).
Table 1.
Prediction of 30-Day Death or Nonfatal MI After Noncardiac Surgery Based on Preoperative Concentrations of Natriuretic Peptide94
Measurement of troponin is integral to the diagnosis of MI. The development of high-sensitivity troponin assays has enabled detection of subtle resting elevations of cardiac troponin levels in individuals without other manifestations of acute coronary syndromes. In the nonoperative setting, resting elevations predict mortality as well as development of CAD and HF.95,96 Notably, a substudy of 325 patients in the VISION study found that 20% of patients have elevated troponin levels before major noncardiac surgery.97 Thus, measurement of preoperative troponin levels is essential in determining whether an elevated postoperative troponin level reflects new acute injury. Emerging data also suggest that preoperative troponin elevations may aid in risk stratification for noncardiac surgery.98,99 In a single-center study of 608 patients with CAD or associated risk factors who were undergoing major noncardiac surgery, preoperative elevations in high-sensitivity troponin T (>14 ng/L) were associated with increased postoperative MI (OR=3.67). Similarly, in a multicenter cohort study of 979 patients with CAD or associated risk factors who were undergoing major noncardiac surgery, preoperative high-sensitivity troponin T levels exceeding 14 ng/L were also associated with increased in-hospital death and cardiac morbidity (adjusted HR=2.60).98 Further research remains to confirm these initial results, establish ideal screening thresholds, and define the target population.
Investigations
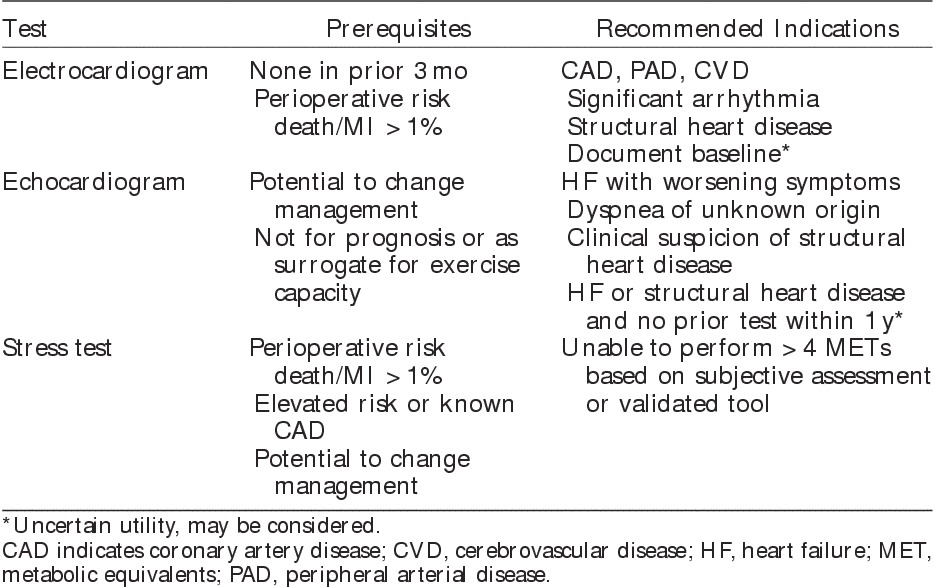
Before any test is ordered, it is important to consider its utility—namely, whether the information provided will significantly change the likelihood of disease being present or occurring in the future, and whether it will change management. The following section will focus on the utility of preoperative investigations in the vascular surgery population, highlighting current evidence and guidelines to inform appropriate testing (Table 2).
Table 2.
Recommendations for Preoperative Cardiac Investigations for Vascular Surgery11
Laboratory Investigations
Although multiple laboratory investigations have the potential to influence perioperative clinical decision making (eg, elevated blood glucose concentration prompting an endocrinology consultation), we will focus on 2 tests with the most evidence: hemoglobin and creatinine.
A key purpose of measuring preoperative hemoglobin concentration is to identify anemia. The relationship between preoperative anemia and adverse postoperative outcomes has been extensively studied with 24 studies (949,445 patients) identified by a recent systematic review.100 On the basis of the World Health Organization definitions (<13 g/dL in male patients and <12 g/dL in female patients),101 about 30% of surgical patients are anemic before surgery. In noncardiac surgery, preoperative anemia is associated with considerably increased perioperative mortality (pooled OR=2.87).100 These adverse consequences are also seen in vascular surgery. In a retrospective cohort of 31,857 vascular surgery patients in the NSQIP registry, preoperative anemia was present in 48% of patients and was associated with more than a doubling in risks for MICA.102 Although anemia is clearly a marker of poor prognosis, its appropriate management is unclear. Clinical circumstances often dictate that anemic patients receive red cell transfusions; however, transfusion itself is also associated with adverse outcomes.103 Further research is therefore needed to evaluate other approaches to treat preoperative anemia, such as intravenous iron or erythropoietin injections. Importantly, these interventions have risks and can delay surgery. Further, anemia has relevance for the risk-benefit balance of perioperative β-blockade. Two prior cohort studies suggest that β-blockers are associated with net harm in the setting of low hematocrits and increased red cell transfusion during surgery.104,105 These data suggest that caution is needed when using β-blockers in anemic surgical patients.
Preoperative renal impairment is a well-recognized predictor of mortality and cardiac morbidity after noncardiac surgery. In a systematic review of 31 cohort studies (153,885 patients), patients with chronic kidney disease experienced more than a 2-fold increase in death and cardiovascular events after noncardiac surgery.106 In another systematic review that evaluated preoperative renal function expressed using eGFR, postoperative mortality increased considerably once eGFR fell below 60 mL/min/1.73 m2.107 Chronic kidney disease is also a component of several cardiac risk indices, including the RCRI, NSQIP risk calculator, and VSG-CRI.16,79,85 Importantly, there is uncertainty as to how best to define preoperative renal insufficiency. While the RCRI, NSQIP risk calculator, and VSG-CRI define renal insufficiency based on serum creatinine concentrations, recent research suggests that expressing renal function using eGFR results in improved predictive performance.83
Electrocardiogram (ECG)
ECGs are simple and inexpensive tests with diagnostic utility in well-defined settings. ECGs remain an important component of diagnostic criteria for acute MI and can reveal the presence of a prior MI (pathologic Q-wave).108 Further, in a patient with known or suspected arrhythmias, an ECG helps diagnose and characterize the underlying abnormality. Conversely, its utility for preoperative risk assessment or screening is controversial. Although some findings, such as nonsinus rhythm (eg, atrial fibrillation), Q-waves, ST depression, voltage criteria for left ventricular hypertrophy, and bundle-branch block, may be associated with increased perioperative risk,109–111 ECG findings do not improve the accuracy of risk prediction beyond that achieved with clinical risk factors alone.111
On the basis of ACC/AHA perioperative guidelines, a new preoperative ECG is reasonable if the patient has no other recent ECG (ie, within prior 3 mo), is undergoing intermediate-risk to high-risk surgery, and has known CAD, CVD, PAD, significant arrhythmia, or structural heart disease.11 By these criteria, almost all vascular surgery patients qualify for an ECG. Perhaps the most common clinical basis for obtaining a recent ECG is to provide a baseline for comparison in the event of suspected ischemia during the perioperative period.
Echocardiography
In the preoperative setting, echocardiography has clear diagnostic utility. For example, it can help determine the basis for dyspnea of unknown etiology (eg, systolic dysfunction, severe mitral regurgitation, pulmonary hypertension), recent worsening of HF symptoms, or suspicious systolic ejection murmur. When echocardiograms are performed to help address such specific clinical questions, they have important diagnostic yield and inform clinical management.112,113 Outside these situations, the value of preoperative echocardiography to estimate cardiovascular risk is uncertain. In an individual without a history or symptoms of HF, measurement of left ventricular function does not improve the accuracy of risk prediction beyond that achieved with the usual clinical risk factors.63 Furthermore, in individuals unable to exercise because of noncardiopulmonary reasons (eg, arthritis), left ventricular function is not a proxy measure of exercise capacity.66,67 Finally, fixed wall motion abnormalities, although indicative of a prior MI, are not indicative of increased perioperative risk.114 Consistent with these data, a population-based cohort study of about 71,000 patients undergoing major noncardiac surgery found no improvement in postoperative survival in individuals who underwent preoperative echocardiography in comparison with propensity-matched controls.115
Cardiac Stress Testing
Cardiac stress testing can have both diagnostic and prognostic roles in the preoperative assessment of vascular surgery patients. If a patient has a history suspicious for undiagnosed CAD (eg, chest pain), it is reasonable to perform stress testing to help diagnose CAD. Stress testing can also help inform preoperative risk assessment by stratifying individuals by their expected rates of cardiac morbidity. Importantly, testing should be performed only if the results can impact clinical care. Thus, stress testing has little value if the results are unlikely to influence preoperative management (eg, medications, revascularization), intraoperative care, postoperative disposition, or decision to proceed with the planned procedure.
Current ACC/AHA guidelines recommend a stepwise approach when determining whether cardiac stress testing should be performed to inform perioperative risk assessment.11 Initially, testing should be considered only in individuals with expected risks for postoperative death or nonfatal MI exceeding 1% based on usual clinical risk factors. The importance of selecting a correct target population for testing is highlighted by a population-based study that assessed the association of preoperative cardiac stress testing with survival in a propensity-matched cohort of about 48,000 patients undergoing major elective noncardiac surgery.116 Testing was associated with harm in individuals with RCRI scores of zero (HR=1.35), whereas it was associated with moderate benefit in individuals with scores of ≥3 (HR=0.80).
Further, the guidelines recommend against testing in individuals whose exercise capacity exceeds 4 METs, as individuals capable of >4 to 6 METs on objective exercise testing have low perioperative cardiovascular risk.44,45 A key challenge is determining how best to identify these individuals with moderate-to-good exercise capacity, especially given the limitations of current usual clinical assessment.48 Pending further research, clinicians should consider using a standardized questionnaire (eg, DASI) to help make this assessment.
Once the decision to perform stress testing has been made, a testing modality and stress technique must be selected. In general, exercise stress should be used whenever possible as it allows for simultaneous quantification of exercise capacity, which is useful for predicting perioperative risk.43–45 If individuals cannot exercise, pharmacologic stress can be used. Although some data suggest that stress echocardiography performs better than nuclear perfusion imaging, the choice of testing should largely be based on local availability and expertise.117 Reversible defects are the key important prognostic findings in stress imaging, with greater extent of reversibility being indicative of higher postoperative risk for death or MI.114 Notably, fixed defects in isolation are not associated with elevated postoperative cardiac risk.114
Coronary Angiography
As coronary angiography can definitively diagnose CAD and as vascular surgery patients have a high burden of CAD, it has been investigated as a first-line preoperative test for vascular surgery. In a single-center study Monaco and colleagues24 randomized 208 moderate-risk to high-risk (RCRI of ≥2) vascular surgery patients to either routine angiography or initial assessment with stress testing. Individuals randomized to routine angiography experienced higher rates of preoperative revascularizations (58% vs. 41%). Routine angiography did not result in any significant improvement in 30-day mortality or MACE, but there was a statistically significant survival benefit at 4 years (87% vs. 70%) that persisted up to 8 years. Although this evidence is encouraging, caution is warranted, given the small sample size and single-center design. Most importantly, as the benefits were over the long term, these data do not prove that angiography improves short-term perioperative risk. Current guidelines therefore recommend against routine screening coronary angiography.11 Rather, angiography should be used if there is sufficient time to safely delay the planned surgery, the information gained could inform perioperative care, and the patient meets usual standard indications for angiography.
Computed tomography coronary angiography (CTCA) uses advanced imaging technology to facilitate a less invasive assessment of coronary anatomy. An initial prospective cohort study of 239 patients undergoing intermediate-risk noncardiac surgery found that CTCA improved risk predication, especially in high-risk groups.118 More recently, Sheth and colleagues reported the results of a larger multicenter prospective cohort study of preoperative CTCA in 955 patients undergoing noncardiac surgery. Addition of CTCA resulted in an improvement, albeit still inadequate, in discrimination (AUROC increase from 0.62 to 0.66) for cardiac death or MI. Further, it performed poorly when evaluated using risk reclassification measures. When added to the RCRI, information from CTCA was 5 times more likely to overestimate risk in low-risk individuals than identify a previously misclassified high-risk individual.119 Thus, current data do not support CTCA as a first-line preoperative screening test for CAD.
Preoperative Therapy
Coronary Revascularization
Given the burden of CAD in vascular surgery patients, and the strong association of prior CAD with postoperative MACE, it seems plausible that preoperative revascularization should decrease perioperative cardiac risk. To date, 2 RCTs have evaluated preoperative revascularization in vascular surgery,120,121 but the validity of one of them121 is in doubt.122 The largest trial—namely, the Coronary Artery Revascularization Prophylaxis (CARP) trial—randomized 501 individuals, who had been referred for coronary angiography before elective major vascular surgery, to either prophylactic revascularization or medical management.120 Revascularization consisted of either PCI or CABG, at the discretion of the local care team. There was no difference in 30-day MI, 30-day death, or long-term survival between groups. In addition, the time to vascular surgery was significantly longer in the revascularization group. Notably, the CARP trial excluded individuals with very high-risk coronary anatomy, including left main disease, severe aortic stenosis, or a left ventricular EF<20%. In a subsequent analysis of patients screened for the CARP trial, revascularization was associated with benefit in unprotected left main disease.123 Consistent with these data, current ACC/AHA perioperative guidelines recommend preoperative revascularization only in circumstances where it would be indicated even in the nonoperative setting.11 In general, CABG is recommended for left main disease, triple-vessel disease, complex anatomy, or high-risk comorbidities (eg, diabetes).124 Given the uncertain benefits of preoperative PCI for improving outcome after noncardiac surgery, current guidelines suggest consideration of PCI only for patients with left main disease whose comorbidities preclude CABG and for patients with unstable CAD (eg, ST-elevation MI, non–ST-elevation acute coronary syndrome) who are appropriate candidates for emergency or urgent revascularization. If revascularization by PCI is considered, BMS is preferred over DES for vascular surgery patients given the time pressure to proceed with vascular surgery (Table 3).
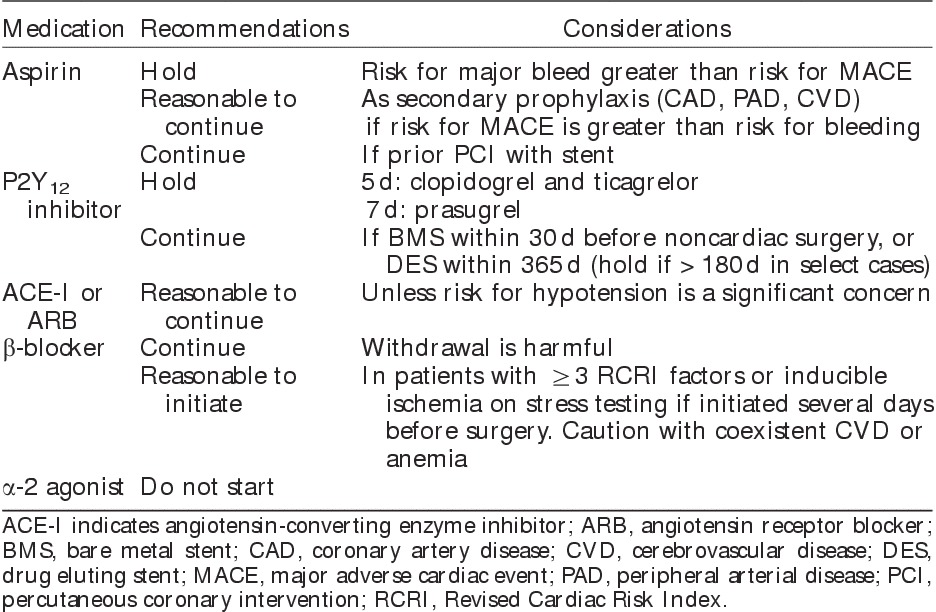
Table 3.
Preoperative Management of Medications for Vascular Surgery11
Pharmacologic Therapy
Aspirin
By inhibiting platelet aggregation and reducing thrombus formations, aspirin reduces MACE in patients with atherosclerotic disease. An individual patient-data meta-analysis of over 100,000 patients in 22 trials showed that aspirin was effective in reducing MACE for secondary prevention indications, with uncertain benefit for primary prevention.125 Current practice guidelines recommend aspirin for secondary prevention in patients with CAD126 and PAD.127 In noncardiac surgery, 40% of patients are on aspirin preoperatively,128 with the prevalence likely to be even higher in vascular surgery. Despite the benefits of aspirin for secondary prevention, perioperative aspirin (ie, either starting treatment de novo or continuing chronic therapy perioperatively) has not been shown to prevent perioperative MACE. Further, even low doses of aspirin can increase the risk for major surgical bleeding. Specifically, the PeriOperative ISchemic Evaluation (POISE) 2 study of 10,010 patients undergoing major noncardiac surgery found no significant effect of aspirin (100 mg/d) on death or MI within 30 days after surgery.128 Conversely, the risk for major bleeding was increased up to 7 days after surgery. In assessing the generalizability of these results, only 5% of the POISE-2 sample underwent major vascular surgery, 9% had PAD, and <5% underwent prior PCI. Therefore, caution must be exercised in extrapolating these results to a high-risk vascular surgery population. A reasonable approach is to continue chronic aspirin therapy in vascular surgery patients with atherosclerotic disease, with the caveat that implications of increased bleeding must also be weighed.
P2Y12 Inhibitors
In patients taking P2Y12 inhibitors (clopidogrel, ticagrelor, and prasugrel) it is important to clarify the indication. They are often used in combination with aspirin (ie, dual antiplatelet therapy) after PCI to prevent stent thrombosis. They are also indicated in patients with PAD who are unable to tolerate aspirin,127 and may be used in individuals with previous stroke. A prior systematic review found that most studies that compared continuation versus preoperative withdrawal of P2Y12 inhibitors were in cardiac surgery.129 These data supported the withdrawal of thienopyridines before cardiac surgery, but there was insufficient evidence to make firm conclusions for noncardiac surgery. As mentioned above, the recent ACC/AHA perioperative guidelines recommend waiting at least 30 days after PCI with BMS and at least 180 days (ideally 365) after DES before stopping dual antiplatelet therapy.11 Should surgery have to occur inside this time frame, a discussion between the interventional cardiologist, vascular surgeon, and the patient is required to balance the risks of surgical delay, bleeding from continuation of dual antiplatelet therapy, and stent thrombosis if dual antiplatelet therapy is discontinued. If surgery is time sensitive and the risk for bleeding outweighs the risk for stent thrombosis, P2Y12 inhibitors can be temporarily discontinued and restarted as soon as possible while aspirin should be continued. Guidelines recommend that clopidogrel and ticagrelor be held for 5 days, whereas prasugrel be held for 7 days before surgery.124 Importantly, interim administration of heparin does not substitute for withdrawal of antiplatelet agents. Prior data on vascular surgery patients showed heparin to paradoxically reduce the antiplatelet action of aspirin.130
Angiotensin-converting Enzyme (ACE) Inhibitors and Angiotensin Receptor Blockers (ARB)
ACE inhibitors and ARBs are among first-line treatments for primary hypertension.131 Further, they improve survival in HF, prevent progression of chronic kidney disease, and prevent cardiovascular events in CAD.132 The current ACC/AHA guidelines state that it is reasonable to continue these medications throughout the perioperative period.11 Nonetheless, administration of these drugs shortly before surgery has risks. Episodes of profound intraoperative hypotension associated with ACE inhibitors taken on the morning of surgery have been reported.133 Thus, some centers routinely instruct patients to withhold chronic ACE inhibitor and ARB therapy for 12 to 24 hours before elective surgery. A systematic review of 5 studies (343 patients) that compared continuation versus withdrawal of chronic therapy showed that continuation of therapy was associated with a significant increase in hypotension requiring vasopressors shortly after induction of anesthesia.133 It is less clear that this generally treatable hypotension translates into other important clinical events, or whether acute withdrawal of chronic cardiovascular therapy has unrecognized adverse effects. Furthermore, the failure to restart ACE inhibitors or ARBs after preoperative withdrawal is itself associated with poor postoperative outcomes.134,135 Given the long-term benefits of these medications, and the paucity of evidence to suggest significant perioperative harm, a reasonable approach is to generally continue these medications up to surgery. Nonetheless, in individual circumstances, if hypotension is a substantial concern because of low preoperative blood pressure, planned use of epidural analgesia, or potential for significant blood loss, hemodynamic instability, and large fluid shifts, it is reasonable to hold ACE inhibitors or ARBs on the morning of surgery, with the goal to resume treatment when these threats have passed.
β-Blockers
Perioperative prophylactic β-blockers showed early promise for decreasing perioperative cardiac risk after noncardiac surgery. This enthusiasm was largely based on 2 small RCTs that demonstrated substantial reductions in risks for MI and death.136,137 These benefits were not replicated in several subsequent intermediate-sized RCTs.138,139 The 2008 publication of the POISE-1 trial further challenged the enthusiastic view of β-blockade in noncardiac surgery. In over 8000 patients randomized to perioperative metoprolol or placebo,18 β-blockers reduced rates of perioperative MI, but at the cost of significantly increased risks for hypotension, bradycardia, stroke, and death. More recently, concerns have been raised about the scientific validity of the 2 earlier RCTs that showed significant benefits from perioperative β-blockade.137,140 To help clarify the overall evidence base surrounding perioperative β-blockade, the recent ACC/AHA guidelines were accompanied by a comprehensive systematic review.21 After excluding the 2 trials with uncertain validity, the review identified 16 RCTs including 12,043 participants. When the pooled results were applied to a population of 1000 individuals, prophylactic β-blocker use resulted in 17 fewer MIs, at the cost of 4 excess strokes and 6 deaths.21 Importantly, the adverse effects of increased stroke and hypotension were also observed in trials that did not use the high-dose metoprolol succinate protocol utilized in the POISE-2 trial. Further, the review identified major limitations in the current data; for example, there were no valid trials assessing β-blockade that was started >24 hours before surgery, and very few trials evaluated agents aside from metoprolol.
Consistent with these findings, the ACC/AHA guidelines recommend against starting β-blocker therapy <24 hours before surgery. On the basis of observational data, initiation of therapy might be considered in individuals with RCRI scores of ≥3,141,142 or with reversible ischemia on stress testing. Caution should be exercised when initiating therapy in individuals with preoperative anemia104 or CVD. If a decision is made to start therapy, it should be initiated several days before surgery,143 with the preferred agents being bisoprolol or atenolol.144,145
Importantly, the entire discussion above pertains to initiating β-blockers de novo in a treatment-naive individual. Many vascular surgery patients are on preexisting chronic β-blocker therapy. As withdrawal of chronic therapy is associated with increased MACE and death,146,147 chronic β-blocker therapy should be continued perioperatively.11 Treatment may need titration perioperatively to address changing clinical circumstances such as hypotension, bradycardia, or massive blood loss.11
α-2 Agonists
α-2 agonists (eg, clonidine, dexmedetomidine) have also been studied for preventing postoperative cardiovascular complications. A Cochrane review identified 35 RCTs with 4500 participants, with most studies being small and of insufficient quality.148 α-2 agonists reduced postoperative mortality but with associated increases in bradycardia and hypotension. Interestingly, the largest benefits for mortality and MI were reported in vascular surgery. More recently, the POISE-2 study evaluated clonidine for preventing MACE in 10,010 randomized participants undergoing major noncardiac surgery. There was no difference in rates of death or nonfatal MI, which was also confirmed in the vascular surgery subgroup.149 Clonidine also increased risks for significant hypotension, but had no significant effect on postoperative stroke. These data suggest that α-2 agonists do not prevent cardiac complications, although they may have other benefits such as improved postoperative analgesia.150
Conclusions
Patients undergoing vascular surgery constitute a unique population with an elevated risk for perioperative death and major cardiac events. These risks are partly due to the overwhelming prevalence of diffuse atherosclerosis and associated comorbidities. This context underscores the importance of a thorough cardiac evaluation to assess for CAD, HF, valvular heart disease, and arrhythmias. The patient, surgeon, and anesthesiologist can be initially informed about the risk of surgery using modern preoperative risk indices. Modern biomarkers, such as natriuretic peptides and high-sensitivity troponin assays, are likely to play a more substantial role in preoperative assessment in the future. It is important to consider the utility of any additional preoperative specialized testing, with particular attention to whether it has potential to change management. In general, preoperative coronary revascularization has a very limited role, being largely reserved for the same indications as in routine circumstances. For the most part, chronic cardiovascular medications, such as aspirin, ACE inhibitors, ARBs, and β-blockers, should be continued, but the decision should be individualized to each patient’s circumstances. Ideally, P2Y12 inhibitors should be held before surgery, aside from cases of recent coronary stenting, where expert opinion should be sought. Finally, there are no compelling data indicating that starting new cardiovascular medications before surgery can decrease perioperative risk, although there may be a role for perioperative β-blockade in specific circumstances.
Footnotes
D.N.W. is supported in part by a New Investigator Award from the Canadian Institutes of Health Research and a Merit Award from the Department of Anesthesia at the University of Toronto.
The authors declare that they have nothing to disclose.
References
- 1.Weiser TG, Haynes AB, Molina G, et al. Estimate of the global volume of surgery in 2012: an assessment supporting improved health outcomes. Lancet. 2015;385(suppl 2):S11. [DOI] [PubMed] [Google Scholar]
- 2.Detsky AS, Abrams HB, Forbath N, et al. Cardiac assessment for patients undergoing noncardiac surgery. A multifactorial clinical risk index. Arch Intern Med. 1986;146:2131–2134. [PubMed] [Google Scholar]
- 3.Goldman L, Caldera DL, Nussbaum SR, et al. Multifactorial index of cardiac risk in noncardiac surgical procedures. N Engl J Med. 1977;297:845–850. [DOI] [PubMed] [Google Scholar]
- 4.Vascular Events In Noncardiac Surgery Patients Cohort Evaluation (VISION) Study Investigators. Association between postoperative troponin levels and 30-day mortality among patients undergoing noncardiac surgery. JAMA. 2012;307:2295–2304. [DOI] [PubMed] [Google Scholar]
- 5.Siracuse JJ, Meltzer EC, Gill HL, et al. Outcomes and risk factors of cardiac arrest after vascular surgery procedures. J Vasc Surg. 2015;61:197–202. [DOI] [PubMed] [Google Scholar]
- 6.Beiles CB, Bourke B, Thomson I. Results from the Australasian Vascular Surgical Audit: the inaugural year. ANZ J Surg. 2012;82:105–111. [DOI] [PubMed] [Google Scholar]
- 7.Golomb BA, Dang TT, Criqui MH. Peripheral arterial disease: morbidity and mortality implications. Circulation. 2006;114:688–699. [DOI] [PubMed] [Google Scholar]
- 8.Stoffers HE, Rinkens PE, Kester AD, et al. The prevalence of asymptomatic and unrecognized peripheral arterial occlusive disease. Int J Epidemiol. 1996;25:282–290. [DOI] [PubMed] [Google Scholar]
- 9.Eagle KA, Rihal CS, Foster ED, et al. Long-term survival in patients with coronary artery disease: importance of peripheral vascular disease. J Am Coll Cardiol. 1994;23:1091–1095. [DOI] [PubMed] [Google Scholar]
- 10.Tonelli C, Finzi G, Catamo A, et al. Prevalence and prognostic value of peripheral arterial disease in stroke patients. Int Angiol. 1993;12:342–343. [PubMed] [Google Scholar]
- 11.Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: executive summary. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;130:2215–2245. [DOI] [PubMed] [Google Scholar]
- 12.Kluger MT, Tham EJ, Coleman NA, et al. Inadequate pre-operative evaluation and preparation: a review of 197 reports from the Australian incident monitoring study. Anaesthesia. 2000;55:1173–1178. [DOI] [PubMed] [Google Scholar]
- 13.Vazirani S, Lankarani-Fard A, Liang LJ, et al. Perioperative processes and outcomes after implementation of a hospitalist-run preoperative clinic. J Hosp Med. 2012;7:697–701. [DOI] [PubMed] [Google Scholar]
- 14.Centers for Disease Control and Prevention. Lower extremity disease among persons aged> or =40 years with and without diabetes—United States, 1999-2002. MMWR Morb Mortal Wkly Rep. 2005;54:1158–1160. [PubMed] [Google Scholar]
- 15.Fisher BW, Ramsay G, Majumdar SR, et al. The ankle-to-arm blood pressure index predicts risk of cardiac complications after noncardiac surgery. Anesth Analg. 2008;107:149–154. [DOI] [PubMed] [Google Scholar]
- 16.Lee TH, Marcantonio ER, Mangione CM, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation. 1999;100:1043–1049. [DOI] [PubMed] [Google Scholar]
- 17.Botto F, Alonso-Coello P, Chan MT, et al. Myocardial injury after noncardiac surgery: a large, international, prospective cohort study establishing diagnostic criteria, characteristics, predictors, and 30-day outcomes. Anesthesiology. 2014;120:564–578. [DOI] [PubMed] [Google Scholar]
- 18.POISE Study Group. Effects of extended-release metoprolol succinate in patients undergoing non-cardiac surgery (POISE trial): a randomised controlled trial. Lancet. 2008;371:1839–1847. [DOI] [PubMed] [Google Scholar]
- 19.Mashour GA, Shanks AM, Kheterpal S. Perioperative stroke and associated mortality after noncardiac, nonneurologic surgery. Anesthesiology. 2011;114:1289–1296. [DOI] [PubMed] [Google Scholar]
- 20.Sharifpour M, Moore LE, Shanks AM, et al. Incidence, predictors, and outcomes of perioperative stroke in noncarotid major vascular surgery. Anesth Analg. 2013;116:424–434. [DOI] [PubMed] [Google Scholar]
- 21.Wijeysundera DN, Duncan D, Nkonde-Price C, et al. Perioperative beta blockade in noncardiac surgery: a systematic review for the 2014 ACC/AHA Guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;130:2246–2264. [DOI] [PubMed] [Google Scholar]
- 22.Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics—2015 update: a report from the American Heart Association. Circulation. 2015;131:e29–e322. [DOI] [PubMed] [Google Scholar]
- 23.Hertzer NR, Beven EG, Young JR, et al. Coronary artery disease in peripheral vascular patients. A classification of 1000 coronary angiograms and results of surgical management. Ann Surg. 1984;199:223–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Monaco M, Stassano P, Di Tommaso L, et al. Systematic strategy of prophylactic coronary angiography improves long-term outcome after major vascular surgery in medium- to high-risk patients: a prospective, randomized study. J Am Coll Cardiol. 2009;54:989–996. [DOI] [PubMed] [Google Scholar]
- 25.Landesberg G, Mosseri M, Zahger D, et al. Myocardial infarction after vascular surgery: the role of prolonged stress-induced, ST depression-type ischemia. J Am Coll Cardiol. 2001;37:1839–1845. [DOI] [PubMed] [Google Scholar]
- 26.Le Manach Y, Perel A, Coriat P, et al. Early and delayed myocardial infarction after abdominal aortic surgery. Anesthesiology. 2005;102:885–891. [DOI] [PubMed] [Google Scholar]
- 27.Badner NH, Knill RL, Brown JE, et al. Myocardial infarction after noncardiac surgery. Anesthesiology. 1998;88:572–578. [DOI] [PubMed] [Google Scholar]
- 28.Landesberg G, Shatz V, Akopnik I, et al. Association of cardiac troponin, CK-MB, and postoperative myocardial ischemia with long-term survival after major vascular surgery. J Am Coll Cardiol. 2003;42:1547–1554. [DOI] [PubMed] [Google Scholar]
- 29.Li SL, Wang DX, Wu XM, et al. Perioperative acute myocardial infarction increases mortality following noncardiac surgery. J Cardiothorac Vasc Anesth. 2013;27:1277–1281. [DOI] [PubMed] [Google Scholar]
- 30.McFalls EO, Ward HB, Santilli S, et al. The influence of perioperative myocardial infarction on long-term prognosis following elective vascular surgery. Chest. 1998;113:681–686. [DOI] [PubMed] [Google Scholar]
- 31.Simons JP, Baril DT, Goodney PP, et al. The effect of postoperative myocardial ischemia on long-term survival after vascular surgery. J Vasc Surg. 2013;58:1600–1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Golden MA, Whittemore AD, Donaldson MC, et al. Selective evaluation and management of coronary artery disease in patients undergoing repair of abdominal aortic aneurysms. A 16-year experience. Ann Surg. 1990;21:415–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kertai MD, Boersma E, Klein J, et al. Optimizing the prediction of perioperative mortality in vascular surgery by using a customized probability model. Arch Intern Med. 2005;165:898–904. [DOI] [PubMed] [Google Scholar]
- 34.Ultee KH, Rouwet EV, Hoeks SE, et al. Coronary revascularization induces a shift from cardiac toward noncardiac mortality without improving survival in vascular surgery patients. J Vasc Surg. 2015;61:1543–1549. [DOI] [PubMed] [Google Scholar]
- 35.Campeau L. Grading of angina pectoris. Circulation. 1976;54:522–523. [PubMed] [Google Scholar]
- 36.Livhits M, Ko CY, Leonardi MJ, et al. Risk of surgery following recent myocardial infarction. Ann Surg. 2011;253:857–864. [DOI] [PubMed] [Google Scholar]
- 37.Fleisher LA, Beckman JA, Brown KA, et al. 2009ACCF/AHA focused update on perioperative beta blockade incorporated into the ACC/AHA 2007 guidelines on perioperative cardiovascular evaluation and care for noncardiac surgery: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2009;120:e169–e276. [DOI] [PubMed] [Google Scholar]
- 38.Wijeysundera DN, Wijeysundera HC, Yun L, et al. Risk of elective major noncardiac surgery after coronary stent insertion: a population-based study. Circulation. 2012;126:1355–1362. [DOI] [PubMed] [Google Scholar]
- 39.Hawn MT, Graham LA, Richman JSI, et al. Risk of major adverse cardiac events following noncardiac surgery in patients with coronary stents. JAMA. 2013;310:1462–1472. [DOI] [PubMed] [Google Scholar]
- 40.Holcomb CN, Graham LA, Richman JS, et al. The incremental risk of noncardiac surgery on adverse cardiac events following coronary stenting. J Am Coll Cardiol. 2014;64:2730–2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cruden NL, Harding SA, Flapan AD, et al. Previous coronary stent implantation and cardiac events in patients undergoing noncardiac surgery. Circ Cardiovasc Interv. 2010;3:236–242. [DOI] [PubMed] [Google Scholar]
- 42.van Kuijk JP, Flu WJ, Schouten O, et al. Timing of noncardiac surgery after coronary artery stenting with bare metal or drug-eluting stents. Am J Cardiol. 2009;104:1229–1234. [DOI] [PubMed] [Google Scholar]
- 43.McPhail N, Calvin JE, Shariatmadar A, et al. The use of preoperative exercise testing to predict cardiac complications after arterial reconstruction. J Vasc Surg. 1988;7:60–68. [PubMed] [Google Scholar]
- 44.James S, Jhanji S, Smith A, et al. Comparison of the prognostic accuracy of scoring systems, cardiopulmonary exercise testing, and plasma biomarkers: a single-centre observational pilot study. Br J Anaesth. 2014;112:491–497. [DOI] [PubMed] [Google Scholar]
- 45.Sgura FA, Kopecky SL, Grill JP, et al. Supine exercise capacity identifies patients at low risk for perioperative cardiovascular events and predicts long-term survival. Am J Med. 2000;108:334–336. [DOI] [PubMed] [Google Scholar]
- 46.Reilly DF, McNeely MJ, Doerner D, et al. Self-reported exercise tolerance and the risk of serious perioperative complications. Arch Intern Med. 1999;159:2185–2192. [DOI] [PubMed] [Google Scholar]
- 47.Jaeschke R, Guyatt GH, Sackett DL. Users’ guides to the medical literature. III. How to use an article about a diagnostic test. B. What are the results and will they help me in caring for my patients? JAMA. 1994;271:703–707. [DOI] [PubMed] [Google Scholar]
- 48.Wiklund RA, Stein HD, Rosenbaum SH. Activities of daily living and cardiovascular complications following elective, noncardiac surgery. Yale J Biol Med. 2001;74:75–87. [PMC free article] [PubMed] [Google Scholar]
- 49.Hlatky MA, Boineau RE, Higginbotham MB, et al. A brief self-administered questionnaire to determine functional capacity (the Duke Activity Status Index). Am J Cardiol. 1989;64:651–654. [DOI] [PubMed] [Google Scholar]
- 50.Melon CC, Eshtiaghi P, Luksun WJ, et al. Validated questionnaire vs. physicians’ judgment to estimate preoperative exercise capacity. JAMA Intern Med. 2014;174:1507–1508. [DOI] [PubMed] [Google Scholar]
- 51.Monk TG, Bronsert MR, Henderson WG, et al. Association between intraoperative hypotension and hypertension and 30-day postoperative mortality in noncardiac surgery. Anesthesiology. 2015;123:307–319. [DOI] [PubMed] [Google Scholar]
- 52.Sun LY, Wijeysundera DN, Tait GA, et al. Association of intraoperative hypotension with acute kidney injury after elective noncardiac surgery. Anesthesiology. 2015;123:515–523. [DOI] [PubMed] [Google Scholar]
- 53.Walsh M, Devereaux PJ, Garg AX, et al. Relationship between intraoperative mean arterial pressure and clinical outcomes after noncardiac surgery: toward an empirical definition of hypotension. Anesthesiology. 2013;119:507–515. [DOI] [PubMed] [Google Scholar]
- 54.Udelson JE. Heart failure with preserved ejection fraction. Circulation. 2011;124:e540–e543. [DOI] [PubMed] [Google Scholar]
- 55.Meta-analysis Global Group in Chronic Heart Failure (MAGGIC). The survival of patients with heart failure with preserved or reduced left ventricular ejection fraction: an individual patient data meta-analysis. Eur Heart J. 2012;33:1750–1757. [DOI] [PubMed] [Google Scholar]
- 56.Lam CS. What is normal in HFNEF?: the case for HFpEF. JACC Heart Fail. 2014;2:541–543. [DOI] [PubMed] [Google Scholar]
- 57.Sanderson JE. HFNEF, HFpEF, HF-PEF, or DHF: what is in an acronym? JACC Heart Fail. 2014;2:93–94.24622122 [Google Scholar]
- 58.Hernandez AF, Whellan DJ, Stroud S, et al. Outcomes in heart failure patients after major noncardiac surgery. J Am Coll Cardiol. 2004;44:1446–1453. [DOI] [PubMed] [Google Scholar]
- 59.Hammill BG, Curtis LH, Bennett-Guerrero E, et al. Impact of heart failure on patients undergoing major noncardiac surgery. Anesthesiology. 2008;108:559–567. [DOI] [PubMed] [Google Scholar]
- 60.Maile MD, Engoren MC, Tremper KKJ, et al. Worsening preoperative heart failure is associated with mortality and noncardiac complications, but not myocardial infarction after noncardiac surgery: a retrospective cohort study. Anesth Analg. 2014;119:522–532. [DOI] [PubMed] [Google Scholar]
- 61.Xu-Cai YO, Brotman DJ, Phillips CO, et al. Outcomes of patients with stable heart failure undergoing elective noncardiac surgery. Mayo Clin Proc. 2008;83:280–288. [DOI] [PubMed] [Google Scholar]
- 62.Healy KO, Waksmonski CA, Altman RK, et al. Perioperative outcome and long-term mortality for heart failure patients undergoing intermediate- and high-risk noncardiac surgery: impact of left ventricular ejection fraction. Congest Heart Fail. 2010;16:45–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Halm EA, Browner WS, Tubau JF, et al. Echocardiography for assessing cardiac risk in patients having noncardiac surgery. Ann Intern Med. 1996;125:433–441. [DOI] [PubMed] [Google Scholar]
- 64.Rohde LE, Polanczyk CA, Goldman L, et al. Usefulness of transthoracic echocardiography as a tool for risk stratification of patients undergoing major noncardiac surgery. Am J Cardiol. 2001;87:505–509. [DOI] [PubMed] [Google Scholar]
- 65.The Criteria Committee of the New York Heart Association. Nomenclature and Criteria for Diagnosis of Diseases of the Heart and Great Vessels, 9th ed Boston, MA: Little, Brown & Co; 1994. [Google Scholar]
- 66.Benge W, Litchfield RL, Marcus ML. Exercise capacity in patients with severe left ventricular dysfunction. Circulation. 1980;61:955–959. [DOI] [PubMed] [Google Scholar]
- 67.Franciosa JA, Park M, Levine TB. Lack of correlation between exercise capacity and indexes of resting left ventricular performance in heart failure. Am J Cardiol. 1981;47:33–39. [DOI] [PubMed] [Google Scholar]
- 68.Wang CS, FitzGerald JM, Schulzer M, et al. Does this dyspneic patient in the emergency department have congestive heart failure? JAMA. 2005;294:1944–1956. [DOI] [PubMed] [Google Scholar]
- 69.Nkomo VT, Gardin JM, Skelton TN, et al. Burden of valvular heart diseases: a population-based study. Lancet. 2006;368:1005–1011. [DOI] [PubMed] [Google Scholar]
- 70.Agarwal S, Rajamanickam A, Bajaj NS, et al. Impact of aortic stenosis on postoperative outcomes after noncardiac surgeries. Circ Cardiovasc Qual Outcomes. 2013;6:193–200. [DOI] [PubMed] [Google Scholar]
- 71.Raymer K, Yang H. Patients with aortic stenosis: cardiac complications in non-cardiac surgery. Can J Anaesth. 1998;45:855–859. [DOI] [PubMed] [Google Scholar]
- 72.Torsher LC, Shub C, Rettke SR, et al. Risk of patients with severe aortic stenosis undergoing noncardiac surgery. Am J Cardiol. 1998;81:448–452. [DOI] [PubMed] [Google Scholar]
- 73.Etchells E, Glenns V, Shadowitz S, et al. A bedside clinical prediction rule for detecting moderate or severe aortic stenosis. J Gen Intern Med. 1998;13:699–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.van Diepen S, Bakal JA, McAlister FA, et al. Mortality and readmission of patients with heart failure, atrial fibrillation, or coronary artery disease undergoing noncardiac surgery: an analysis of 38 047 patients. Circulation. 2011;124:289–296. [DOI] [PubMed] [Google Scholar]
- 75.McAlister FA, Jacka M, Graham M, et al. The prediction of postoperative stroke or death in patients with preoperative atrial fibrillation undergoing non-cardiac surgery: a VISION sub-study. J Thromb. 2015;13:1768–1775. [DOI] [PubMed] [Google Scholar]
- 76.Crossley GH, Poole JE, Rozner MA, et al. The Heart Rhythm Society (HRS)/American Society of Anesthesiologists (ASA) Expert Consensus Statement on the perioperative management of patients with implantable defibrillators, pacemakers and arrhythmia monitors. Heart Rhythm. 2011;8:1114–1154. [DOI] [PubMed] [Google Scholar]
- 77.Douketis JD, Spyropoulos AC, Kaatz S, et al. Perioperative bridging anticoagulation in patients with atrial fibrillation. N Engl J Med. 2015;373:823–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Eagle KA, Coley CM, Newell JB, et al. Combining clinical and thallium data optimizes preoperative assessment of cardiac risk before major vascular surgery. Ann Intern Med. 1989;110:859–866. [DOI] [PubMed] [Google Scholar]
- 79.Bertges DJ, Goodney PP, Zhao Y, et al. The Vascular Study Group of New England Cardiac Risk Index (VSG-CRI) predicts cardiac complications more accurately than the Revised Cardiac Risk Index in vascular surgery patients. J Vasc Surg. 2010;52:674–683. [DOI] [PubMed] [Google Scholar]
- 80.Gupta PK, Gupta H, Sundaram A, et al. Development and validation of a risk calculator for prediction of cardiac risk after surgery. Circulation. 2011;124:381–387. [DOI] [PubMed] [Google Scholar]
- 81.Ford MK, Beattie WS, Wijeysundera DN. Systematic review: prediction of perioperative cardiac complications and mortality by the Revised Cardiac Risk Index. Ann Intern Med. 2010;152:26–35. [DOI] [PubMed] [Google Scholar]
- 82.Vision Pilot Study Investigators. An international prospective cohort study evaluating major vascular complications among patients undergoing noncardiac surgery: the VISION Pilot Study. Open Med. 2011;5:e193–e200. [PMC free article] [PubMed] [Google Scholar]
- 83.Davis C, Tait G, Carroll J, et al. The Revised Cardiac Risk Index in the new millennium: a single-centre prospective cohort re-evaluation of the original variables in 9,519 consecutive elective surgical patients. Can J Anaesth. 2013;60:855–863. [DOI] [PubMed] [Google Scholar]
- 84.Sankar A, Johnson SR, Beattie WS, et al. Reliability of the American Society of Anesthesiologists Physical Status scale in clinical practice. Br J Anaesth. 2014;113:424–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bilimoria KY, Liu Y, Paruch JL, et al. Development and evaluation of the universal ACS NSQIP surgical risk calculator: a decision aid and informed consent tool for patients and surgeons. J Am Coll Surg. 2013;217:833–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Biccard B. Proposed research plan for the derivation of a new Cardiac Risk Index. Anesth Analg. 2015;120:543–553. [DOI] [PubMed] [Google Scholar]
- 87.De Bruin JL, Baas AF, Buth J, et al. Long-term outcome of open or endovascular repair of abdominal aortic aneurysm. N Engl J Med. 2010;362:1881–1889. [DOI] [PubMed] [Google Scholar]
- 88.Lederle FA, Freischlag JA, Kyriakides TC, et al. Long-term comparison of endovascular and open repair of abdominal aortic aneurysm. N Engl J Med. 2012;367:1988–1997. [DOI] [PubMed] [Google Scholar]
- 89.United Kingdom EVAR Trial Investigators. Endovascular versus open repair of abdominal aortic aneurysm. N Engl J Med. 2010;362:1863–1871. [DOI] [PubMed] [Google Scholar]
- 90.Schermerhorn ML, Buck DB, O’Malley AJ, et al. Long-term outcomes of abdominal aortic aneurysm in the Medicare population. N Engl J Med. 2015;373:328–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chen HH, Burnett JC., Jr The natriuretic peptides in heart failure: diagnostic and therapeutic potentials. Proc Assoc Am Physicians. 1999;111:406–416. [DOI] [PubMed] [Google Scholar]
- 92.Balion C, Santaguida PL, Hill S, et al. Testing for BNP and NT-proBNP in the diagnosis and prognosis of heart failure. Evid Rep Technol Assess (Full Rep). 2006;142:1–147. [PMC free article] [PubMed] [Google Scholar]
- 93.Rodseth RN, Lurati Buse GA, Bolliger D, et al. The predictive ability of pre-operative B-type natriuretic peptide in vascular patients for major adverse cardiac events: an individual patient data meta-analysis. J Am Coll Cardiol. 2011;58:522–529. [DOI] [PubMed] [Google Scholar]
- 94.Rodseth RN, Biccard BM, Le Manach Y, et al. The prognostic value of pre-operative and post-operative B-type natriuretic peptides in patients undergoing noncardiac surgery: B-type natriuretic peptide and N-terminal fragment of pro-B-type natriuretic peptide. A systematic review and individual patient data meta-analysis. J Am Coll Cardiol. 2014;63:170–180. [DOI] [PubMed] [Google Scholar]
- 95.de Lemos JA, Drazner MH, Omland T, et al. Association of troponin T detected with a highly sensitive assay and cardiac structure and mortality risk in the general population. JAMA. 2010;304:2503–2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.deFilippi CR, de Lemos JA, Christenson RH, et al. Association of serial measures of cardiac troponin T using a sensitive assay with incident heart failure and cardiovascular mortality in older adults. JAMA. 2010;304:2494–2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kavsak PA, Walsh M, Srinathan S, et al. High sensitivity troponin T concentrations in patients undergoing noncardiac surgery: a prospective cohort study. Clin Biochem. 2011;44:1021–1024. [DOI] [PubMed] [Google Scholar]
- 98.Weber M, Luchner A, Seeberger M, et al. Incremental value of high-sensitive troponin T in addition to the Revised Cardiac Index for peri-operative risk stratification in non-cardiac surgery. Eur Heart J. 2013;34:853–862. [DOI] [PubMed] [Google Scholar]
- 99.Nagele P, Brown F, Gage BF, et al. High-sensitivity cardiac troponin T in prediction and diagnosis of myocardial infarction and long-term mortality after noncardiac surgery. Am Heart J. 2013;166:325–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Fowler AJ, Ahmad T, Phull MK, et al. Meta-analysis of the association between preoperative anaemia and mortality after surgery. Br J Surg. 2015;102:1314–1324. [DOI] [PubMed] [Google Scholar]
- 101.WHO. Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity. Vitamin and Mineral Nutrition Information System. Geneva, World Health Organization, 2011. (WHO/NMH/NHD/MNM/11.1). Available at: http://www.who.int/vmnis/indicators/haemoglobin.pdf. Accessed September 2015.
- 102.Gupta PK, Sundaram A, Mactaggart JN, et al. Preoperative anemia is an independent predictor of postoperative mortality and adverse cardiac events in elderly patients undergoing elective vascular operations. Ann Surg. 2013;258:1096–1102. [DOI] [PubMed] [Google Scholar]
- 103.Marik PE, Corwin HL. Efficacy of red blood cell transfusion in the critically ill: a systematic review of the literature. Crit Care Med. 2008;36:2667–2674. [DOI] [PubMed] [Google Scholar]
- 104.Beattie WS, Wijeysundera DN, Karkouti K, et al. Acute surgical anemia influences the cardioprotective effects of beta-blockade: a single-center, propensity-matched cohort study. Anesthesiology. 2010;112:25–33. [DOI] [PubMed] [Google Scholar]
- 105.Le Manach Y, Collins GS, Ibanez C, et al. Impact of perioperative bleeding on the protective effect of beta-blockers during infrarenal aortic reconstruction. Anesthesiology. 2012;117:1203–1211. [DOI] [PubMed] [Google Scholar]
- 106.Mathew A, Devereaux PJ, O’Hare A, et al. Chronic kidney disease and postoperative mortality: a systematic review and meta-analysis. Kidney Int. 2008;73:1069–1081. [DOI] [PubMed] [Google Scholar]
- 107.Mooney JF, Ranasinghe I, Chow CK, et al. Preoperative estimates of glomerular filtration rate as predictors of outcome after surgery: a systematic review and meta-analysis. Anesthesiology. 2013;118:809–824. [DOI] [PubMed] [Google Scholar]
- 108.Thygesen K, Alpert JS, Jaffe AS, et al. Third universal definition of myocardial infarction. J Am Coll Cardiol. 2012;60:1581–1598. [DOI] [PubMed] [Google Scholar]
- 109.Noordzij PG, Boersma E, Bax JJ, et al. Prognostic value of routine preoperative electrocardiography in patients undergoing noncardiac surgery. Am J Cardiol. 2006;97:1103–1106. [DOI] [PubMed] [Google Scholar]
- 110.Landesberg G, Einav S, Christopherson R, et al. Perioperative ischemia and cardiac complications in major vascular surgery: importance of the preoperative twelve-lead electrocardiogram. J Vasc Surg. 1997;26:570–578. [DOI] [PubMed] [Google Scholar]
- 111.van Klei WA, Bryson GL, Yang H, et al. The value of routine preoperative electrocardiography in predicting myocardial infarction after noncardiac surgery. Ann Surg. 2007;246:165–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Canty DJ, Royse CF. Audit of anaesthetist-performed echocardiography on perioperative management decisions for non-cardiac surgery. Br J Anaesth. 2009;103:352–358. [DOI] [PubMed] [Google Scholar]
- 113.Cowie B. Three years’ experience of focused cardiovascular ultrasound in the peri-operative period. Anaesthesia. 2011;66:268–273. [DOI] [PubMed] [Google Scholar]
- 114.Etchells E, Meade M, Tomlinson G, et al. Semiquantitative dipyridamole myocardial stress perfusion imaging for cardiac risk assessment before noncardiac vascular surgery: a meta-analysis. J Vasc Surg. 2002;36:534–540. [DOI] [PubMed] [Google Scholar]
- 115.Wijeysundera DN, Beattie WS, Karkouti K, et al. Association of echocardiography before major elective non-cardiac surgery with postoperative survival and length of hospital stay: population based cohort study. BMJ. 2011;342:d3695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wijeysundera DN, Beattie WS, Austin PC, et al. Non-invasive cardiac stress testing before elective major non-cardiac surgery: population based cohort study. BMJ. 2010;340:b5526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Beattie WS, Abdelnaem E, Wijeysundera DN, et al. A meta-analytic comparison of preoperative stress echocardiography and nuclear scintigraphy imaging. Anesth Analg. 2006;102:8–16. [DOI] [PubMed] [Google Scholar]
- 118.Ahn JH, Park JR, Min JH, et al. Risk stratification using computed tomography coronary angiography in patients undergoing intermediate-risk noncardiac surgery. J Am Coll Cardiol. 2013;61:661–668. [DOI] [PubMed] [Google Scholar]
- 119.Sheth T, Chan M, Butler C, et al. Prognostic capabilities of coronary computed tomographic angiography before non-cardiac surgery: prospective cohort study. BMJ. 2015;350:h1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.McFalls EO, Ward HB, Moritz TE, et al. Coronary-artery revascularization before elective major vascular surgery. N Engl J Med. 2004;351:2795–2804. [DOI] [PubMed] [Google Scholar]
- 121.Poldermans D, Schouten O, Vidakovic R, et al. A clinical randomized trial to evaluate the safety of a noninvasive approach in high-risk patients undergoing major vascular surgery: the DECREASE-V Pilot Study. J Am Coll Cardiol. 2007;49:1763–1769. [DOI] [PubMed] [Google Scholar]
- 122.Bouri S, Shun-Shin MJ, Cole GD, et al. Meta-analysis of secure randomised controlled trials of beta-blockade to prevent perioperative death in non-cardiac surgery. Heart. 2014;100:456–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Garcia S, Moritz TE, Ward HB, et al. Usefulness of revascularization of patients with multivessel coronary artery disease before elective vascular surgery for abdominal aortic and peripheral occlusive disease. Am J Cardiol. 2008;102:809–813. [DOI] [PubMed] [Google Scholar]
- 124.Hillis LD, Smith PK, Anderson JL, et al. 2011 ACCF/AHA Guideline for Coronary Artery Bypass Graft Surgery. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2011;58:e123–e210. [DOI] [PubMed] [Google Scholar]
- 125.Antithrombotic Trialists Collaboration. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet. 2009;373:1849–1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wright RS, Anderson JL, Adams CD, et al. 2011 ACCF/AHA focused update of the guidelines for the management of patients with unstable angina/non-ST-elevation myocardial infarction (updating the 2007 Guideline): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2011;123:2022–2060. [DOI] [PubMed] [Google Scholar]
- 127.Rooke TW, Hirsch AT, Misra S, et al. 2011 ACCF/AHA focused update of the guideline for the management of patients with peripheral artery disease (updating the 2005 guideline): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2011;58:2020–2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Devereaux PJ, Mrkobrada M, Sessler DI, et al. Aspirin in patients undergoing noncardiac surgery. N Engl J Med. 2014;370:1494–1503. [DOI] [PubMed] [Google Scholar]
- 129.Au AG, Majumdar SR, McAlister FA. Preoperative thienopyridine use and outcomes after surgery: a systematic review. Am J Med. 2012;125:87–99. [DOI] [PubMed] [Google Scholar]
- 130.Webster SE, Payne DA, Jones CI, et al. Anti-platelet effect of aspirin is substantially reduced after administration of heparin during carotid endarterectomy. J Vasc Surg. 2004;40:463–468. [DOI] [PubMed] [Google Scholar]
- 131.James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311:507–520. [DOI] [PubMed] [Google Scholar]
- 132.Rosendorff C, Lackland DT, Allison M, et al. Treatment of hypertension in patients with coronary artery disease: a scientific statement from the American Heart Association, American College of Cardiology, and American Society of Hypertension. Circulation. 2015;131:e435–e470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Rosenman DJ, McDonald FS, Ebbert JO, et al. Clinical consequences of withholding versus administering renin-angiotensin-aldosterone system antagonists in the preoperative period. J Hosp Med. 2008;3:319–325. [DOI] [PubMed] [Google Scholar]
- 134.Mudumbai SC, Takemoto S, Cason BA, et al. Thirty-day mortality risk associated with the postoperative nonresumption of angiotensin-converting enzyme inhibitors: a retrospective study of the Veterans Affairs Healthcare System. J Hosp Med. 2014;9:289–296. [DOI] [PubMed] [Google Scholar]
- 135.Lee SM, Takemoto S, Wallace AW. Association between withholding angiotensin receptor blockers in the early postoperative period and 30-day mortality: a cohort study of the Veterans Affairs Healthcare System. Anesthesiology. 2015;123:288–306. [DOI] [PubMed] [Google Scholar]
- 136.Mangano DT, Layug EL, Wallace A, et al. Effect of atenolol on mortality and cardiovascular morbidity after noncardiac surgery. N Engl J Med. 1996;335:1713–1720. [DOI] [PubMed] [Google Scholar]
- 137.Poldermans D, Boersma E, Bax JJ, et al. The effect of bisoprolol on perioperative mortality and myocardial infarction in high-risk patients undergoing vascular surgery. N Engl J Med. 1999;341:1789–1794. [DOI] [PubMed] [Google Scholar]
- 138.Yang H, Raymer K, Butler R, et al. The effects of perioperative beta-blockade: results of the Metoprolol after Vascular Surgery (MaVS) study, a randomized controlled trial. Am Heart J. 2006;152:983–990. [DOI] [PubMed] [Google Scholar]
- 139.Juul AB, Wetterslev J, Gluud C, et al. Effect of perioperative beta blockade in patients with diabetes undergoing major non-cardiac surgery: randomised placebo controlled, blinded multicentre trial. BMJ. 2006;332:1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Dunkelgrun M, Boersma E, Schouten O, et al. Bisoprolol and fluvastatin for the reduction of perioperative cardiac mortality and myocardial infarction in intermediate-risk patients undergoing noncardiovascular surgery: a randomized controlled trial (DECREASE-IV). Ann Surg. 2009;249:921–926. [DOI] [PubMed] [Google Scholar]
- 141.London MJ, Hur K, Schwartz GG, et al. Association of perioperative beta-blockade with mortality and cardiovascular morbidity following major noncardiac surgery. JAMA. 2013;309:1704–1713. [DOI] [PubMed] [Google Scholar]
- 142.Lindenauer PK, Pekow P, Wang K, et al. Perioperative beta-blocker therapy and mortality after major noncardiac surgery. N Engl J Med. 2005;353:349–361. [DOI] [PubMed] [Google Scholar]
- 143.Wijeysundera DN, Beattie WS, Wijeysundera HC, et al. Duration of preoperative beta-blockade and outcomes after major elective noncardiac surgery. Can J Cardiol. 2014;30:217–223. [DOI] [PubMed] [Google Scholar]
- 144.Ashes C, Judelman S, Wijeysundera DN, et al. Selective beta 1-antagonism with bisoprolol is associated with fewer postoperative strokes than atenolol or metoprolol: a single-center cohort study of 44,092 consecutive patients. Anesthesiology. 2013;119:777–787. [DOI] [PubMed] [Google Scholar]
- 145.Mashour GA, Sharifpour M, Freundlich RE, et al. Perioperative metoprolol and risk of stroke after noncardiac surgery. Anesthesiology. 2013;119:1340–1346. [DOI] [PubMed] [Google Scholar]
- 146.Hoeks SE, Scholte Op Reimer WJ, van Urk H, et al. Increase of 1-year mortality after perioperative beta-blocker withdrawal in endovascular and vascular surgery patients. Eur J Vasc Endovasc Surg. 2007;33:13–19. [DOI] [PubMed] [Google Scholar]
- 147.Shammash JB, Trost JC, Gold JM, et al. Perioperative beta-blocker withdrawal and mortality in vascular surgical patients. Am Heart J. 2001;141:148–153. [DOI] [PubMed] [Google Scholar]
- 148.Wijeysundera DN, Bender JS, Beattie WS. Alpha-2 adrenergic agonists for the prevention of cardiac complications among patients undergoing surgery. Cochrane Database Syst Rev. 2009;4:CD004126. [DOI] [PubMed] [Google Scholar]
- 149.Devereaux PJ, Sessler DI, Leslie K, et al. Clonidine in patients undergoing noncardiac surgery. N Engl J Med. 2014;370:1504–1513. [DOI] [PubMed] [Google Scholar]
- 150.Blaudszun G, Lysakowski C, Elia N, et al. Effect of perioperative systemic alpha2 agonists on postoperative morphine consumption and pain intensity: systematic review and meta-analysis of randomized controlled trials. Anesthesiology. 2012;116:1312–1322. [DOI] [PubMed] [Google Scholar]


