Abstract
Th1 pro-inflammatory cytokines, i.e., TNF-α and IFN-γ, in combination are known to induce cell death in several cell types, including oligodendrocytes, but the mechanism of their synergistic cytotoxicity is unclear. Although ceramide (Cer) has been implicated in cytokine- and stress-induced cell death, its intracellular levels alone cannot explain cytokine synergy. We considered the possibility that Cer released as part of extracellular vesicles may contribute to cytokine-induced synergistic cell death. Using a human oligodendroglioma (HOG) cell line as a model, here we show that exosomes derived from TNF-α-treated “donor” cells, while being mildly toxic to fresh cultures (similar to individual cytokines), induce enhanced cell death when added to IFN-γ-primed target cultures in a fashion resembling the effect of cytokine combination. Further, the sphingolipid profiles of secreted exosomes, as determined by HPLC-MS/MS, revealed that the treatment with the cytokines time-dependently induced the formation and exosomal release, in particular of C16-, C24-, and C24:1-Cer species; C16-, C24-, and C24:1-dihydroCer species; and C16-, C24-, and C24:1-SM species. Finally, exogenous C6-Cer or C16-Cer mimicked and enhanced the cytotoxic effects of the cytokines upon HOG cells, thereby supporting the cell death-signaling role of extracellular Cer.
Keywords: cell signaling, vesicles, lipidomics, multiple sclerosis, oligodendrocyte, sphingolipids
The Th1 pro-inflammatory cytokines, TNF-α and IFN-γ, are known to act synergistically to induce cell death in different types of target cells, including pancreatic β cells (1–3), hepatoma cells (4), human salivary gland cells (5), myeloblastic leukemic (ML)-1 cells (6, 7), and oligodendrocyte/oligodendroglioma cells (8, 9). Thus, in the oligodendrocyte cell system (primary and cell lines), while TNF-α by itself exerts minimal cytotoxic effects, in combination with IFN-γ, it induces marked apoptotic death that is developmentally regulated (8). In an attempt to clarify the mechanism(s) of synergistic cytotoxic effects of TNF-α and IFN-γ in oligodendrocytes and other target cells, studies have focused on intracellular signals, including kinases, caspases, transcription factors, etc. (8–12). The studies with oligodendrocyte lineage cells in particular, have indicated the potential role of ceramide (Cer) as an intracellular regulator of cytokine- as well as stressor-induced cell death signaling. Thus, while direct addition of cell-permeable C2-Cer induces cell death in cultured oligodendrocytes (13), there is evidence that different forms of stressors, including oxidative stress, glutamate, ionizing radiation, and β-amyloid, induce the production of Cer that potentially mediates a cell death response (14–17). Elevated intracellular Cer is also implicated in oligodendrocyte cell death induced by cytokines, i.e., TNF-α (10, 11) and nerve growth factor (18).
Recent findings show that Cer participates in the formation of secreted vesicles that are being increasingly recognized for their roles in intercellular communications, both under physiological and pathological conditions. Most cells secrete small extracellular vesicles (EVs) by two membrane inversion events (19–22). This process includes the budding of intraluminal vesicles at endosomes during multivesicular body maturation and subsequent vesicle secretion upon their fusion with the plasma membrane. Although many details remain to be delineated, the general process of exosome budding involves the participation of the endosomal sorting complex required for transport (ESCRT) machinery in a cell type-dependent manner (23, 24). Alternative mechanisms have been characterized as ESCRT independent, including a Cer-mediated process first described in an oligodendroglial cell line (25). While exosome secretion serves several physiological functions, including disposal of unneeded cellular components, signaling to neighboring cells involving the horizontal transfer of biomolecules, and regulation of immune response (26–30), recent studies also indicate pathophysiological roles of exosomes in inflammatory and neurodegenerative diseases (31–35). There is evidence that EVs can modulate the effects of cytokines in inflammatory diseases as well (33). Because it is likely that they may also transmit apoptotic signals extracellularly, in the present study, we tested the effects of cytotoxic cytokines, i.e., TNF-α and IFN-γ, on the secretion of exosomes in a susceptible human oligodendroglioma (HOG) cell line. In addition, we determined the sphingolipid (SL) profiles, including Cer species of exosomes in the context of synergistic cytotoxic effects elicited by the cytokine combination.
MATERIALS AND METHODS
Cell culture
The HOG cell line used in this study was kindly donated by Dr. R. Yu (Georgia Regents University, Augusta, GA). This cell line was originally derived from a HOG and was characterized to express low levels of myelin-specific proteins and lipids (36). The cells were maintained under standard cell culture conditions and grown as a monolayer in DMEM supplemented with 10% FBS (Atlanta Biologicals, Norcross, GA) and 1% penicillin/streptomycin at 37°C in a humidified atmosphere containing 5% CO2. The cells were subcultured into either 60 mm dishes (0.8 × 106/2 ml) or 24-well plates (0.3 × 105/0.5 ml/well). After 2 days, the cultures were switched to serum-reduced medium (0.05% FBS) and exposed to the cytokines, TNF-α, IFN-γ (100 ng/ml each), or their combination, in order to induce apoptosis as described previously (9).
In parallel experiments, HOG cells were incubated for indicated times with exogenous C6-Cer (appropriately diluted from 20 mM stock in DMSO; Lipidomic Shared Resource) or C16-Cer [diluted from 10 mM stock in ethanol/dodecane (98/2%, v/v); Cayman Chemical, Ann Arbor, MI], with or without added cytokines.
Analysis of cell survival/death
Cell viability was determined by 3-[4,5-dimethylthiazol-2-yl]2,5-diphenyltetrazolium bromide (MTT) assay as described by Andrews, Zhang, and Bhat (8). Briefly, MTT was dissolved in PBS at the concentration 5 mg/ml. The MTT solution was added to the culture medium at a dilution of 1:10. The plates were incubated at 37°C for 2 h. The dark brown formazan crystals formed by the reduction of tetrazolium ring by the mitochondria of living cells were dissolved in acidified (40 mM HCl) isopropanol and the optical density of the supernatant was read at 570 nm. Cell death was quantified based on the measurement of intracellular lactate dehydrogenase (LDH) activity released into the medium by using the LDH cytotoxicity assay kit from Cayman Chemicals according to the manufacturer’s instructions. The percentage of LDH released was calculated by dividing the activity recovered in the medium by the sum of the cellular and released activity. Additional cell death analysis was performed by fluorescent staining with Hoechst 33342 (Sigma-Aldrich, St. Louis, MO).
Exosome preparation
Exosomes were isolated by sequential centrifugation steps (37). The culture supernatant of HOG cells after various treatments in medium containing 0.05% FBS was collected. The medium was centrifuged for 20 min at 10,000 g at 4°C in order to remove dead cell debris. The small endosome-derived vesicles remaining in the supernatant were pelleted by ultracentrifugation for 1 h at 100,000 g at 4°C. The isolated pellets were kept at −80°C before use/analysis. It has been shown previously, using an oligodendroglial cell line, that the pellets obtained under these conditions contain small membrane vesicles with a diameter of about 50–100 nm (25). Hence, we refer to the 100,000 g membrane fractions as exosomes.
Sample preparation, lipid extraction, and HPLC-MS/MS analysis
After various treatments, the medium was collected, exosome fractions prepared as above and kept frozen at −80°C until used for lipid analysis. The adherent cells were gently washed twice with cold PBS, harvested, and sedimented by low-speed centrifugation. The pellets were lysed in RIPA buffer and an aliquot used for protein estimation by BCA method (38) and the remainder kept frozen at −80°C until used for sphingolipidomic analysis. A HPLC-MS/MS analysis of endogenous Cer, dihydroceramide (dhCer), and SM components was performed on a ThermoFisher TSQ Quantum or SCIEX Q-Trap triple-stage quadrupole mass spectrometer, operating in a multiple reaction monitoring positive ionization mode, as previously described (39, 40). Briefly, test samples were fortified with the internal standards (ISs) [17C base D-erythro-sphingosine (17Sph), 17C base D-erythro-sphingosine 1-phosphate (17S1P), 17C base D-erythro-dihydrosphingosine (17dhSph), D-erythro-N-palmitoyl-13C-D-erythro-sphingosine (13C/C16-Cer), N-heptadecanoyl-D-erythro-sphingosine (18C/C17-Cer), N-heptadecanoyl-D-erythro- dihydrosphingosine (18C/C17-dhCer), D-erythro-N-palmitoyl-17C-D-erythro-sphingosine (17C/C16-Cer), D-erythro-N-nervonoyl-17C-D-erythro-sphingosine (17C/C24:1-Cer), D-erythro-C6-SM (18C/C6-SM), D-erythro-C17-SM (18C/C17-SM)], and extracted into a one-phase solvent system with ethyl acetate/isopropanol/water (60/30/10%; v/v). The lipid extract was divided into two portions: A and B. Part A was used for analysis of Cers and dhCers. Part B was subjected to base hydrolysis (to remove glycerolipids interfering with SM analysis) and used for analysis of SM species. Both extracts, A and B, after evaporation and reconstitution in 150 μl of acidified (0.2% formic acid) methanol, were injected on the HP1100/TSQ-Quantum LC/MS system and gradient-eluted from the BDS Hypersil C8, 150 × 3.2 mm, 3 μm particle size column, with 1.0 mM methanolic ammonium formate/2 mM aqueous ammonium formate mobile phase system. Peaks corresponding to the target analytes and ISs were collected and processed using the Xcalibur software system. Quantitative analysis was based on calibration curves generated by spiking an artificial matrix with known amounts of the target analyte synthetic standards and an equal amount of the IS. The target analyte peak area ratios from the samples were similarly normalized to their respective ISs and compared with the calibration curves using a linear regression model.
Statistical analysis
The data are expressed as raw values, the percentage of control or the difference from control, where control values were set to 100%. The treatment groups are expressed as mean ± SEM and statistical significance was assessed with one-way ANOVA followed by Tukey’s multiple comparison test or two-way ANOVA followed by Bonferroni’s posttest for cells and exosomes, respectively. A value of P < 0.05 was considered statistically significant.
RESULTS
Exosome-like vesicles released from cytokine-treated cultures induce cell death in fresh (naïve) HOG cells
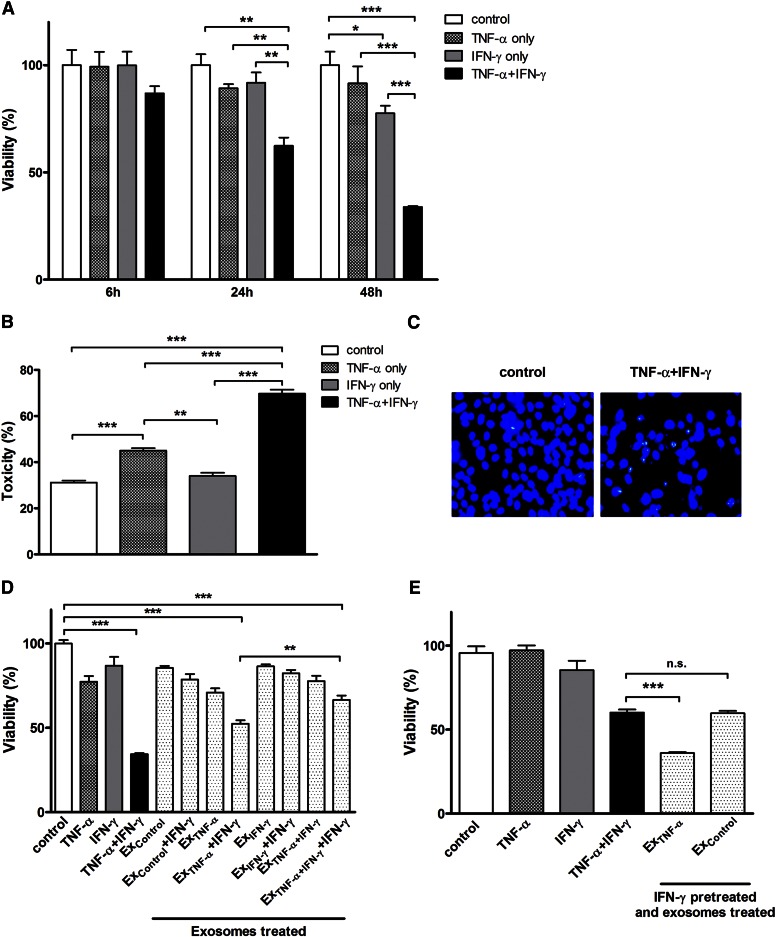
In this study, we used the HOG cell line as an in vitro model to investigate the synergistic cytotoxic effects of the two Th1 cytokines, TNF-α and IFN-γ. Cell death-inducing effects of the cytokines were examined individually and in combination. Quantitative analysis of cell survival was determined by MTT assay at 6, 24, and 48 h after cytokine treatment. As shown in Fig. 1A, cell viability was reduced time-dependently to 86.8 ± 5.8%, 56.4 ± 4.3%, and 33.8 ± 0.9%, respectively. As reported previously (8, 9), the cytokines acted synergistically to elicit maximal cytotoxic effects. The pattern of cytokine-induced cell death was further confirmed by LDH release assay (Fig. 1B). Figure 1C depicts the occurrence of apoptosis in cytokine combination-treated cultures as evidenced by Hoechst staining of fragmented DNA. However, because apoptotic cells can undergo secondary necrosis in the absence of phagocytic cells under in vitro conditions (41), it is likely that both types of cell death co-occur and contribute to the observed cytokine toxicity.
Fig. 1.
Cytotoxic effects of Th1 cytokines (i.e., TNF-α and IFN-γ) and exosomes derived from cytokine-treated cultures on HOG cells. HOG cells were treated with TNF-α (100 ng/ml), IFN-γ (100 ng/ml), or both in combination under serum-free conditions. A: Cell viability of the cytokine-treated cultures after 6, 24, and 48 h was determined by MTT assay, as described in the Materials and Methods. The results expressed as average values are presented as percent of control (untreated cultures) set at 100% ± SEM; *P < 0.05, **P < 0.01, ***P < 0.001; N = 3. B: Cytotoxic effect of the cytokines on HOG cells treated as above was quantified by LDH assay. The results expressed as average values are presented as percent of LDH released from control cultures lysed with Triton X-100 ± SEM; **P < 0.01, ***P < 0.001; N = 3. C: Morphological evidence for apoptotic cells, i.e., Hoechst-stained fragmented DNA in cultures exposed to cytokine combination. D: Exosomes prepared from the media derived from untreated cultures (Excontrol) as well as those treated with TNF-α, IFN-γ, and their combination (i.e., ExTNF-α, ExIFN-γ, and ExTNF-α+IFN-γ, respectively) were used for testing their effects on fresh cultures of HOG cells in the presence and absence of simultaneously added IFN-γ. Cell viability was determined by MTT assay after 48 h and the results are expressed as above; ***P < 0.001; N = 3. E: Another fresh set of HOG cultures were pretreated with IFN-γ (100 ng/ml) for 6 h, washed, and then exposed to either ExTNF-α or Excontrol for 24 h. Cell viability was determined by MTT assay and the results expressed as above; ***P < 0.001; n.s., not significant; N = 3.
Previous studies, including our own (8, 9), aimed at defining the mechanism of cytokine synergy have analyzed changes in intracellular signaling pathways and mediators, but with no clear explanation for the synergistic effects of the cytokines. Hence, we decided to “look outside the cell” with a particular focus on EVs released from cytokine-exposed cells. Specifically, we wondered whether cytokine-exposed HOG cells released exosomes and whether they contributed to the observed synergistic cytotoxic effects. To test this possibility, exosomes were isolated and their aliquots representing one-half the original volume of the medium were added to fresh HOG cultures and their effects monitored 1–2 days later. No significant changes in cell viability were observed in HOG cells exposed to exosomes derived from control cultures (Excontrol; Fig. 1D, bar 5) or those derived from cultures exposed to TNF-α (ExTNF-α), FN-γ (ExIFN-γ), or their combination (ExTNF-α+IFN-γ) (Fig. 1D, bars 7, 9, and 11, respectively). However, cotreatment with IFN-γ (but not with TNF-α, data not shown) and exosomes led to substantial cell death (Fig. 1D; bars 8 and 12). Thus, ExTNF-α (Fig. 1D, bar 8) or ExTNF-α+IFN-γ (Fig. 1D, bar 12), when added simultaneously with IFN-γ, induced target cell death in a fashion resembling the direct effect of the cytokine combination, i.e., TNF-α plus IFN-γ (Fig. 1D, bar 4). Interestingly, ExTNF-α + IFN-γ decreased cell viability even more than ExTNF-α+IFN-γ + IFN-γ (Fig. 1D, bar 8 vs. bar 12; P < 0.01). Figure 1 also shows that pre-exposure of HOG cells to IFN-γ for 6 h prior to the addition of ExTNF-α resulted in a cell death response that was more pronounced than that elicited by the cytokine combination (Fig. 1E, bar 5 vs. bar 4). These results suggest that pre-exposure to IFN-γ renders the cells particularly sensitive to TNF-α-inducible exosome-mediated cell death. Because extracellular exosomes can target multiple IFN-γ-primed cells, this phenomenon could form the basis for the synergistic effects of the two cytokines. Although the nature of the cytotoxic molecules associated with the exosomes is unknown, it is possible that SLs, particularly Cer, may participate in this process directly or indirectly as pro-apoptotic components of exosomal cargo carrier. To address this possibility, we undertook a detailed analysis of the SL profile of HOG cells treated with the cytokines as well as the extracellularly released exosomes.
SL profile of HOG cells and the effects of TNF-α and IFN-γ
We used a HPLC-MS/MS approach for determination of a basic lipidomic profile (18:1, 18:0 sphingoid backbone, C(14)-C(26) N-acyl-part) of SLs in HOG cells. SLs analyzed included molecular species of Cer, dhCer, and SM. The major Cer species were determined to be: C24:1-Cer (37.4 ± 1.2%), C24-Cer (17.5 ± 0.7%), C18-Cer (12.3 ± 1.8%), C16-Cer (11.0 ± 0.9%), C22-Cer (7.9 ± 0.3%), and C18:1-Cer (3.8 ± 0.4%). The minor Cers were: C22:1-Cer (2.9 ± 0.3%), C20-Cer (2.8 ± 0.3%), C14-Cer (2.3 ± 0.4%), C26:1-Cer (1.1 ± 0.03%), C20:1-Cer (0.4 ± 0.04%), and C26-Cer (0.4 ± 0.07%) (supplemental Fig. S1A). Their major dihydro-homologs, dhC16-Cer and dhC24:1-Cer, constituted 37.4 ± 0.4% and 16.1 ± 0.2%, respectively (supplemental Fig. S1B). The cellular SM analysis in HOG cells indicated the most abundant components to be: C16-SM (36.4 ± 1.9%), C24:1-SM (34.8 ± 2.4%), C18-SM (11.8 ± 1.2%), C18:1-SM (4.6 ± 0.8%), and C24-SM (3.4 ± 0.3%), while C14-SM (3.7 ± 0.3%), C22-SM (2.2 ± 0.3%), C22:1-SM (1.7 ± 0.3%), C20-SM (1.0 ± 0.2%), C20:1-SM (0.2 ± 0.03%), and C26:1-SM (0.06 ± 0.03%) were determined as minor species (supplemental Fig. S1C).
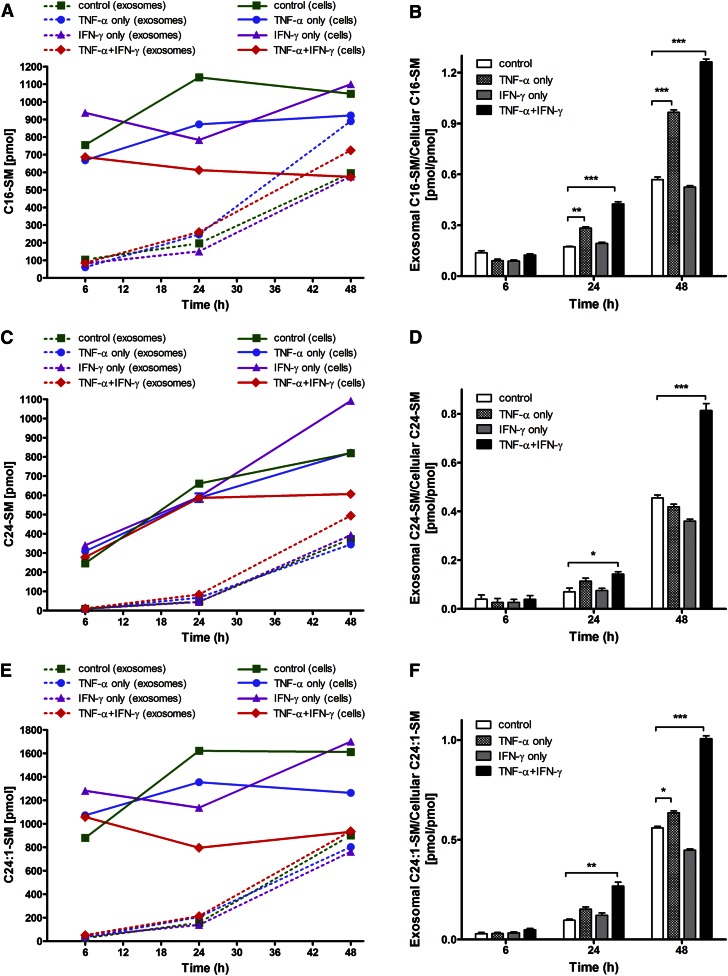
We next determined the SL profile in cells exposed to the two cytokines, TNF-α and IFN-γ, individually and in combination (supplemental Figs. S2–S4). The most significant increases (about 2-fold compared with control) could be seen in the major Cer species by 48 h (Table 1, supplemental Fig. S2). Also, the major dhCer species showed time-dependent changes with their robust elevation at 48 h (Table 2, supplemental Fig. S3). Analyses of the various SM species revealed that TNF-α/IFN-γ exposure elicited no statistically significant effect on the major SM components, C16-SM and C24:1-SM (supplemental Fig. S4).
TABLE 1.
Effect by Th1 pro-inflammatory cytokines, TNF-α and IFN-γ, upon HOG cells major Cer species
| Treatment | Time (h) | Cer Species | ||||
| C16:0-Cer | C18:0-Cer | C24:0-Cer | C24:1-Cer | Total Cer | ||
| Control | 6 | 4.9 ± 0.3 | 5.6 ± 1.2 | 7.7 ± 0.2 | 16.6 ± 1.0 | 44.5 ± 3.0 |
| TNF-α + IFN-γ | 7.0 ± 0.7 | 9.0 ± 1.3 | 12.3 ± 1.9 | 24.4 ± 3.6 | 66.8 ± 9.0 | |
| Control | 24 | 3.1 ± 0.4 | 5.8 ± 0.5 | 10.2 ± 1.3 | 19.9 ± 1.9 | 47.6 ± 5.1 |
| TNF-α + IFN-γ | 4.8 ± 0.8 | 6.7 ± 0.9 | 13.8 ± 2.1 | 21.9 ± 3.5 | 57.8 ± 8.4 | |
| Control | 48 | 1.7 ± 0.1 | 2.9 ± 0.04 | 6.0 ± 0.2 | 12.8 ± 0.5 | 28.7 ± 1.2 |
| TNF-α + IFN-γ | 4.2 ± 0.4a | 6.0 ± 0.9c | 16.9 ± 1.7c | 22.3 ± 3.3b | 59.6 ± 7.5c | |
Values are mean (expressed as pmol/mg of protein) ± SEM (N = 3).
P < 0.05 compared with control.
P < 0.01 compared with control.
P < 0.001 compared with control.
TABLE 2.
Effect by Th1 pro-inflammatory cytokines, TNF-α and IFN-γ, upon HOG cells major dhCer species
| Treatment | Time (h) | dhCer Species | ||||
| C16:0-dhCer | C18:0-dhCer | C24:0-dhCer | C24:1-dhCer | Total dhCer | ||
| Control | 6 | 1.8 ± 0.1 | 0.3 ± 0.03 | 0.3 ± 0.02 | 0.8 ± 0.1 | 4.9 ± 0.4 |
| TNF-α + IFN-γ | 2.4 ± 0.4 | 0.6 ± 0.1 | 0.4 ± 0.1 | 1.1 ± 0.2 | 6.8 ± 0.7 | |
| Control | 24 | 1.5 ± 0.2 | 0.3 ± 0.03 | 0.3 ± 0.02 | 0.9 ± 0.1 | 4.8 ± 0.5 |
| TNF-α + IFN-γ | 2.7 ± 0.3a | 0.6 ± 0.05b | 0.9 ± 0.1b | 1.5 ± 0.2 | 8.3 ± 1.0a | |
| Control | 48 | 0.9 ± 0.02 | 0.2 ± 0.01 | 0.3 ± 0.03 | 0.7 ± 0.03 | 3.6 ± 0.1 |
| TNF-α + IFN-γ | 3.1 ± 0.4c | 0.6 ± 0.04c | 1.3 ± 0.1c | 2.2 ± 0.3c | 9.9 ± 1.3c | |
Values are mean (expressed as pmol/mg of protein) ± SEM (N = 3).
P < 0.05 compared with control.
P < 0.01 compared with control.
P < 0.001 compared with control.
SL profile of exosomes and effects of TNF-α and IFN-γ
We next determined the SL composition of exosomes released from cytokine-treated HOG cultures (supplemental Figs. S5–S7; Tables 3, 4). The cultures were treated with the cytokine combination for 6, 24, and 48 h and the media from control and treated cultures were individually collected for exosome isolation followed by SL analysis. The major Cer components released from HOG cells to extracellular compartment were: C16-, C24-, and C24:1-Cer (supplemental Fig. S5). Their levels increased upon cytokine treatment displaying the most significant changes by 48 h (Table 3). These changes reflected the synergistic effects of TNF-α and IFN-γ as early as 6 h of treatment (Table 3), unlike cell-associated Cers at this time interval (Table 1) when only a trend toward an increase in their levels was apparent.
TABLE 3.
Effect by Th1 pro-inflammatory cytokines, TNF-α and IFN-γ, upon major exosomal Cer species released from cytokine-treated HOG cultures
| Treatment | Time (h) | Cer Species | |||
| C16:0-Cer | C24:0-Cer | C24:1-Cer | Total Cer | ||
| Control | 6 | 1.6 ± 0.8 | 0.9 ± 0.5 | 1.1 ± 0.7 | 7.9 ± 2.5 |
| TNF-α + IFN-γ | 3.1 ± 0.1 | 2.3 ± 0.3 | 5.7 ± 1.3a | 19.0 ± 2.8c | |
| Control | 24 | 2.3 ± 0.1 | 4.0 ± 0.6 | 6.1 ± 1.1 | 21.4 ± 2.4 |
| TNF-α + IFN-γ | 8.5 ± 2.5 | 14.6 ± 3.4 | 29.1 ± 3.5a | 78.0 ± 2.4c | |
| Control | 48 | 3.1 ± 0.1 | 6.2 ± 0.3 | 12.0 ± 0.7 | 33.7 ± 2.6 |
| TNF-α + IFN-γ | 10.7 ± 0.5b | 26.0 ± 1.9c | 44.8 ± 2.6c | 122.3 ± 3.7c | |
Values are mean (pmol) ± SEM (N = 2).
P < 0.05 compared with control.
P < 0.01 compared with control.
P < 0.001 compared with control.
TABLE 4.
Effect by Th1 pro-inflammatory cytokines, TNF-α and IFN-γ, upon major exosomal dhCer species released from cytokine-treated HOG cultures
| Treatment | Time (h) | dhCer Species | |||
| C16:0-dhCer | C24:0-dhCer | C24:1-dhCer | Total dhCer | ||
| Control | 6 | 0.1 ± 0.1 | 0.5 ± 0.3 | 0.3 ± 0.3 | 3.8 ± 0.4 |
| TNF-α + IFN-γ | 0.4 ± 0.2 | 1.4 ± 0.2 | 0.3 ± 0.1 | 11.7 ± 3.7b | |
| Control | 24 | 0.6 ± 0.2 | 1.5 ± 0.1 | 0.5 ± 0.1 | 12.9 ± 2.5 |
| TNF-α + IFN-γ | 3.8 ± 0.0 | 2.9 ± 0.1 | 2.8 ± 0.8 | 21.2 ± 4.2a | |
| Control | 48 | 1.6 ± 0.4 | 1.3 ± 0.4 | 1.7 ± 0.5 | 9.9 ± 3.5 |
| TNF-α + IFN-γ | 12.1 ± 1.5b | 8.5 ± 1.7b | 12.3 ± 0.3b | 52.5 ± 0.3b | |
Values are mean (pmol) ± SEM (N = 2).
P < 0.01 compared with control.
P < 0.001 compared with control.
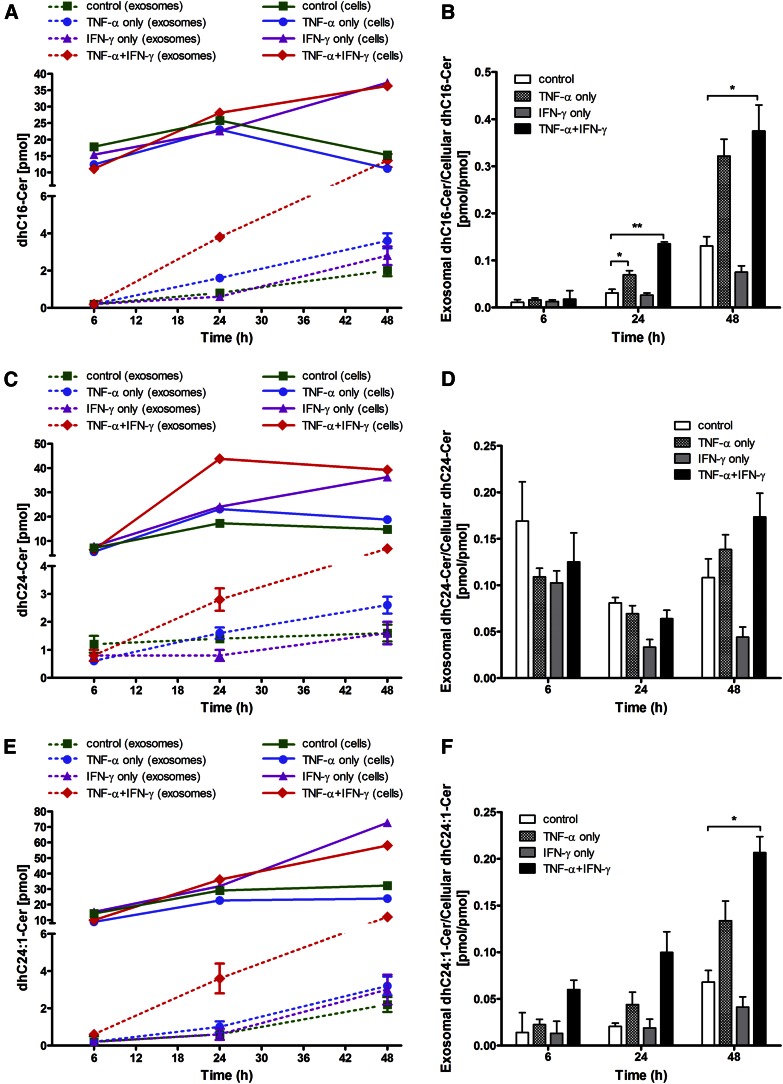
The combination of cytokines time-dependently elevated the levels of total dhCer at 6 and 24 h so that by 48 h the levels of all major dhCer species showed significant increases (Table 4, supplemental Fig. S6), likely contributing to de novo production of corresponding Cer species. The increases in the most abundant SM species, C16-SM and C24:1-SM (supplemental Fig. S7), also correlated well with increased dhC16-Cer and dhC24:1-Cer (Table 4, supplemental Fig. S6).
Increased shedding of Cer, dhCer, and SM species from cytokine-treated HOG cells
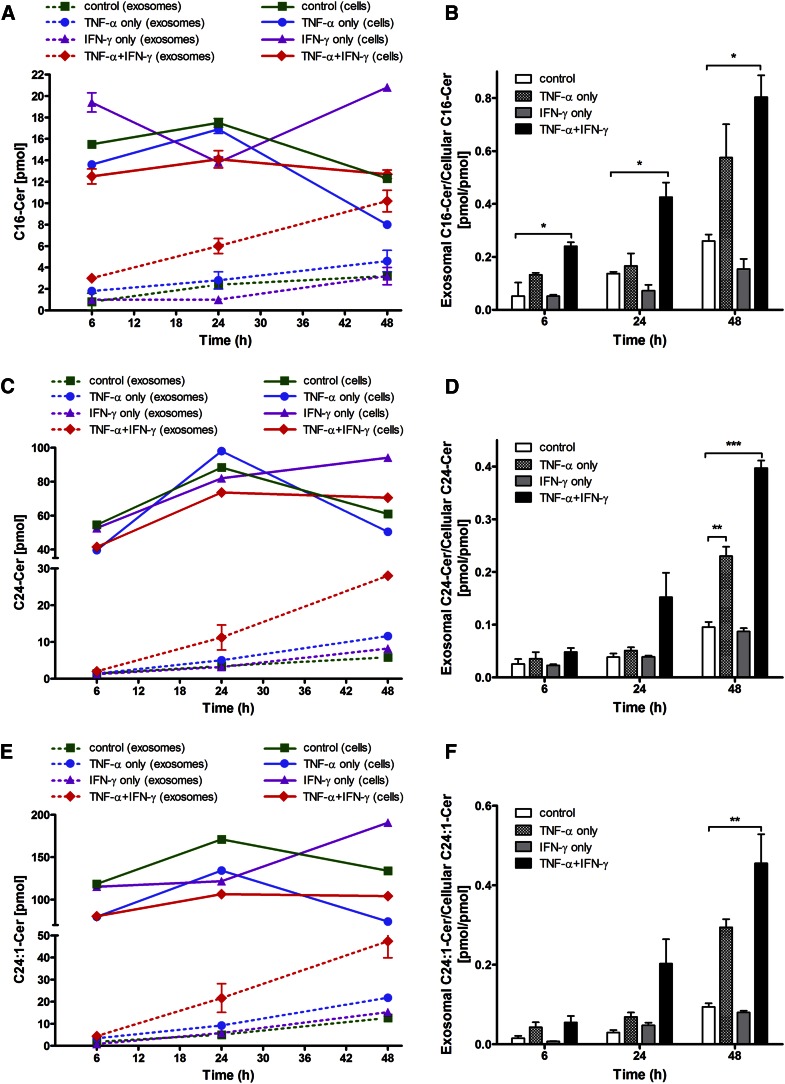
In the next experiment, we compared the relative levels of cell-associated and extracellularly released SL species, Cer (Fig. 2), dhCer (Fig. 3), and SM (Fig. 4), and the effects of the two cytokines, individually and in combination, on this distribution. It is clear that increased amounts of these SLs were released extracellularly with increasing time (i.e., from 6 to 24 to 48 h) following cytokine exposure, as depicted for the most prominent SL species: C16-Cer, C24-Cer, and C24:1-Cer (Fig. 2A, C, E); dhC16-Cer, dhC24-Cer, and dhC24:1-Cer (Fig. 3A, C, E); and C16-SM, C24-SM, and C24:1-SM (Fig. 4A, C, E). A common pattern of changes was noted for each of the SL species when expressed as “exosomal to cell-associated” ratios: an upregulation by TNF-α as well as by the two cytokines in combination and a downregulation by IFN-γ (Figs. 2–4). In general, most significant increases in the release of Cer, dhCer, and SM species were observed by 48 h with some subspecies showing significant increases even earlier, i.e., C16-Cer (6 h, Fig. 2B); dhC16-Cer (24 h, Fig. 3B); and C16-SM, C24-SM, and C24:1-SM (24 h, Fig. 4B, D, F, respectively). Overall however, the shedding process was not selective to Cer species, but rather reflected their cellular content.
Fig. 2.
Levels of major Cer species released from exosomes relative to their cellular content as a function of time of Th1 cytokine treatment. HOG cells were either left untreated or treated with TNF-α (100 ng/ml), IFN-γ (100 ng/ml), or both under serum-free conditions. After the specified time of treatment (6, 24, and 48 h), the harvested cells and exosomes isolated were subjected to Cer analysis by HPLC-MS/MS. A, C, E: Exosomal levels of major Cer species: C16:0-, C24:0-, and C24:1-Cer (dashed lines) relative to their cellular level (solid lines). B, D, F: The corresponding exosomal-to-cellular ratios of the same Cer species. *P < 0.05, **P < 0.01, ***P < 0.001; N = 2.
Fig. 3.
Levels of major dhCer species released in exosomes relative to their cellular content as a function of time of Th1 cytokine treatment. HOG cells were either left untreated or treated with TNF-α (100 ng/ml), IFN-γ (100 ng/ml), or both under serum-free conditions. After the specified time of treatment (6, 24, and 48 h), the harvested cells and isolated exosomes were subjected to dhCer analysis by HPLC-MS/MS. A, C, E: Exosomal levels of major dhCer species: dhC16:0-, dhC24:0-, and dhC24:1-Cer (dashed lines) relative to their cellular level (solid lines). B, D, F: The corresponding exosomal-to-cellular ratios of the same dhCer species. *P < 0.05, **P < 0.01; N = 2.
Fig. 4.
Levels of major SM species released in exosomes relative to their cellular content as a function of time of Th1 cytokine treatment. HOG cells were either left untreated or treated with TNF-α (100 ng/ml), IFN-γ (100 ng/ml), or both under serum-free conditions. After the specified time of treatment (6, 24, and 48 h), the harvested cells and isolated exosomes were subjected to SM analysis by HPLC-MS/MS. A, C, E: Exosomal levels of major SM species: C16:0-, C24:0-, and C24:1-SM (dashed lines) relative to their cellular level (solid lines). B, D, F: The corresponding exosomal-to-cellular ratios of the same SM species. *P < 0.05, **P < 0.01, ***P < 0.001; N = 2.
Next, we determined the relative abundance of cell-associated and extracellularly released Cer species with different fatty acid chain lengths. The shorter chain fatty acid containing Cer species (i.e., 16:0 and 18:0) comprised about one-tenth (13.2%) of the total cellular Cers, while representing about one-fifth of the total exosomal Cers (22.1%), implying a relatively greater amount released into the extracellular compartment for cytokine-treated sets (supplemental Table S1) as well as control sets (not shown). These results are in agreement with a previous report using a neuroblastoma cell line showing that SLs with shorter fatty acid species are present to a greater amount in the shed lipids than in the cellular lipids (42).
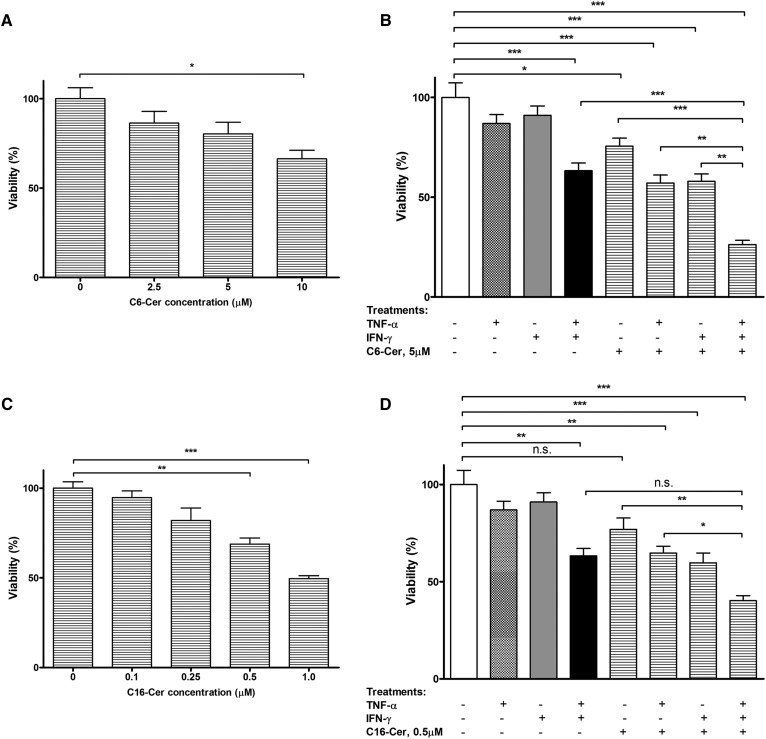
Exogenous Cer enhances the effect of Th1 cytokine(s)
We postulated that Cer might represent a pro-apoptotic component of exosomes and thus account for the cytotoxic effects of the cytokines observed. To test the effect of exogenous Cer, we treated the cells with C6-Cer, which is known to generate long fatty acyl-containing Cers, e.g., C16-Cer, C24-Cer, and C24:1-Cer (43), the species found in HOG cell-derived exosomes. As shown in Fig. 5A, C6-Cer exerted a dose-dependent cytotoxic effect, as indicated by reduced cell viability that reached 66.4% (P < 0.05) at the concentration of 10 μM. We then tested the effects of a sub-optimal dose (5 μM) of C6-Cer in cultures exposed to the two cytokines individually or in combination. As shown in Fig. 5B, C6-Cer was able to induce significant cell death in cultures exposed to either TNF-α (Fig. 5B, bar 6 vs. bar 1; P < 0.001) or IFN-γ (Fig. 5B, bar 7 vs. bar 1; P < 0.001), which on their own were unable to do so (Fig. 5B, bar 2 vs. bar 1 and bar 3 vs. bar 1), while its addition to cultures exposed to cytokine combination resulted in a further increase in cell death (Fig. 5B, bar 8 vs. bar 4; P < 0.001).
Fig. 5.
The effects of exogenous Cers on HOG cells in the presence or absence of Th1 cytokines. The cultures were exposed to different doses of C6-Cer (A) or 16-Cer (C) for 48 h and cell viability determined by MTT assay. Sets of cultures were also exposed to a sub-optimal concentration of C6-Cer [5 μM (B)] or C16-Cer [0.5 μM (D)] in the presence of the cytokines (100 ng/ml each), individually or in combination, and after 48 h, analyzed for cell viability. The first four bars in (B) and (D) show the effects of individual cytokines and their combination for comparison. The results are expressed as average values (set as percent of control) ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001; n.s., not significant; N = 3.
We also performed the experiment using a representative of the major Cer species present in the exosomes, i.e., C16-Cer. As shown in Fig. 5C, there was a dose-dependent cytotoxic effect with a maximum (about 50%, P < 0.001) at 1 μM, no further effect being observed at higher doses. Again, as in the case of C6-Cer, its addition at a sub-optimal dose (0.5 μM) to cultures exposed to either of TNF-α (Fig. 5D, bar 6 vs. bar 1; P < 0.05) or IFN-γ (Fig. 5D, bar 7 vs. bar 1; P < 0.001) resulted in a significantly increased cell death response relative to cytokine alone-treated cultures.
DISCUSSION
The main findings of our current studies are that HOG cells respond to cytotoxic cytokines, TNF-α and IFN-γ, the two Th1 pro-inflammatory cytokines known to accumulate in multiple sclerosis brain, by increased release of Cer-enriched exosomes that carry cell death signal. The results suggest an exosome-mediated new mechanism of synergistic cytotoxicity of the two cytokines. Thus, while either of the cytokines induces minimal cytotoxicity individually, TNF-α-inducible exosomes induce robust cell death in IFN-γ-primed target cells (Fig. 1D). The results further suggest that pre-exposure to IFN-γ renders the HOG cells sensitive to TNF-α-inducible exosomes (Fig. 1E), thereby implying a synergistic mechanism by which the combination of the two cytokines would efficiently propagate the cell death signal.
The nature of the exosome-associated cell death signal(s) emanating from Th1 cytokine-exposed HOG cells is unclear and forms the subject of further investigation. However, our observations that exogenous C6-Cer or C16-Cer mimic and further enhance the cytotoxic effects of the cytokines are in support of the role of Cer per se as a contributor to cell death response. This is consistent with previous studies showing that exogenous Cer could induce apoptosis in the same time frame and kill HOG cells as effectively as TNF-α (11) and that an upregulation of Cer was linked to oligodendrocyte cell injury (44, 45). It is also known that many of the toxic effects of TNF-α in diverse cell types occur through generation of C16:0-Cer, and that these effects can be reversed by a reduction in this Cer (46, 47). It is very likely, however, that HOG cell-released exosomes may carry other cell death mediators similar to membrane vesicles shed by another oligodendroglioma cell line, G26/24, which were shown to carry Fas-L, Nogo-B, and other cell death-inducing molecules (48).
Experimental studies over the past decade have clearly shown that changes in lipid metabolism commonly underlie pathological processes of neurodegeneration and inflammation, thereby suggesting that the multiple targets/steps identified in the complex network of lipid classes are worth detailed study (49, 50). A particular strength of the present study is the state-of-the-art sphingolipidomics approach for SL profiling of an oligodendroglioma cell type and derived exosomes. The treatment of HOG cells with the pro-apoptotic cytokines, TNF-α and IFN-γ, resulted in specific changes in cell-associated versus exosomal SL profiles. There was an upregulation of cellular dhC16-, dhC24-, and dhC24:1-Cer species suggesting that the main pathway for Cer generation de novo was active. However, contrary to our expectation, Cer levels in the intracellular compartment (Table 1) did not increase as much as those for dhCer (∼3-fold) (Table 2). One possibility is that there was, in fact, an increased generation of Cer, but it was immediately exported out of the cells by exosomes. The situation was completely different in the extracellular compartment, where time-dependent increases, particularly in C16-, C24-, and C24:1-Cer (Fig. 2) and dhC16-, dhC24-, and dhC24:1-Cer (Fig. 3) as well as C16-, C24-, and C24:1-SM species (Fig. 4) were observed, suggesting that the Cer originating from de novo synthesis was processed to SM by SM synthase. Overall, the pattern of extracellular rather than intracellular Cer dynamics correlated well with the synergistic cytokine toxicity.
Our observations suggest that the cytokine-induced release of Cer-enriched exosomes likely involves a non-ESCRT Cer-dependent mechanism first reported in another oligodendroglial cell line (Oli-neu) (25). With respect to the intracellular source of Cer, previous studies with cytokine-treated HOG cells have indicated the major role of raft-associated neutral SMase in SM hydrolysis without the participation of the more abundant acid SMase (10). This is consistent with the ascribed role of neutral SMase in Cer-dependent exosome biogenesis (25). However, the task of elucidating the origin(s) of the Cer species from a broad range of cellular SLs, i.e., cerebrosides, sulfatides, and gangliosides, besides SM, as well as the contribution of the de novo pathway of Cer synthesis to exosome formation in response to cytokines, are particularly challenging and require comprehensive molecular approaches (i.e., siRNA, anti-sense oligonucleotides).
Finally, shedding of HOG cell Cers may have important pathophysiological implications because it is likely that exosomes released from stressed or cytokine-targeted oligodendrocytes and/or their precursors in vivo may similarly “broadcast” the cell death signal. We envision that shedding of Cers may promote the autoimmune response that occurs under demyelinating conditions in the central nervous system. There is evidence for an accumulation of Cer species, including C16:0-, C18:0-, C18:1-, C24:0-, and C24:1-Cer, in multiple sclerosis plaques and/or animal models of the disease (44, 45, 51), as well as increased levels of C16:0- and C24:0-Cer in the multiple sclerosis cerebrospinal fluid (52). However, with respect to such in vivo significance of our present findings, it would be important to first verify the phenomenon of exosome-mediated cell death signaling in primary oligodendrocytes prior to its in vivo validation. A related point of interest is the possibility of a “lipid signature” of exosomes released by different cell types in normal versus pathological conditions akin to exosome-associated proteins (and miRNA) (34, 53, 54). Such information should further contribute to the development of “engineered” exosomes as efficient vehicles for delivery of therapeutic agents across the blood-brain barrier (55).
Supplementary Material
Acknowledgments
The authors are grateful to Barbara Rembiesa, Jason S. Pierce, and Sangeeta Mohanty for their skillful technical assistance and to Professor Kumar Sambamurti and Professor Jolanta Zakrzewska-Czerwinska for access to the ultracentrifuge.
Footnotes
Abbreviations:
- Cer
- ceramide
- dhCer
- dihydroceramide
- ESCRT
- endosomal sorting complex required for transport
- EV
- extracellular vesicle
- HOG
- human oligodendroglioma
- IS
- internal standard
- LDH
- lactate dehydrogenase
- MTT
- 3-[4,5-dimethylthiazol-2-yl]2,5-diphenyltetrazolium bromide
- SL
- sphingolipid
This research was supported by Foundation for the National Institutes of Health Grant 5P30GM103339-03, the Lipidomics Shared Resource, Hollings Cancer Center, Medical University of South Carolina (P30 CA138313), and the Lipidomics Core in the South Carolina Lipidomics and Pathobiology COBRE (P20 RR017677) to A.B.; a pilot research funding from the COBRE in Lipidomics and Pathobiology (Grant 5P30GM103339-03) at the Medical University of South Carolina to N.R.B.; and the Multiple Sclerosis International Federation (Du Pré Grant), and the National Science Centre, Krakow, Poland (UMO-2013/08/M/NZ3/01040) to M.P. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
The online version of this article (available at http://www.jlr.org) contains a supplement.
REFERENCES
- 1.Suk K., Kim S., Kim Y. H., Kim K. A., Chang I., Yagita H., Shong M., and Lee M. S.. 2001. IFN-gamma/TNF-alpha synergism as the final effector in autoimmune diabetes: a key role for STAT1/IFN regulatory factor-1 pathway in pancreatic beta cell death. J. Immunol. 166: 4481–4489. [DOI] [PubMed] [Google Scholar]
- 2.Barthson J., Germano C. M., Moore F., Maida A., Drucker D. J., Marchetti P., Gysemans C., Mathieu C., Nunez G., Jurisicova A., et al. 2011. Cytokines tumor necrosis factor-alpha and interferon-gamma induce pancreatic beta-cell apoptosis through STAT1-mediated Bim protein activation. J. Biol. Chem. 286: 39632–39643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kiaer C., and Thams P.. 2009. Serum albumin protects from cytokine-induced pancreatic beta cell death by a phosphoinositide 3-kinase-dependent mechanism. Endocrine. 35: 325–332. [DOI] [PubMed] [Google Scholar]
- 4.Sasagawa T., Hlaing M., and Akaike T.. 2000. Synergistic induction of apoptosis in murine hepatoma Hepa1–6 cells by IFN-gamma and TNF-alpha. Biochem. Biophys. Res. Commun. 272: 674–680. [DOI] [PubMed] [Google Scholar]
- 5.Kamachi M., Kawakami A., Yamasaki S., Hida A., Nakashima T., Nakamura H., Ida H., Furuyama M., Nakashima K., Shibatomi K., et al. 2002. Regulation of apoptotic cell death by cytokines in a human salivary gland cell line: distinct and synergistic mechanisms in apoptosis induced by tumor necrosis factor alpha and interferon gamma. J. Lab. Clin. Med. 139: 13–19. [DOI] [PubMed] [Google Scholar]
- 6.Takuma T., Takeda K., and Konno K.. 1987. Synergism of tumor necrosis factor and interferon-gamma in induction of differentiation of human myeloblastic leukemic ML-1 cells. Biochem. Biophys. Res. Commun. 145: 514–521. [DOI] [PubMed] [Google Scholar]
- 7.Craig R. W., and Buchan H. L.. 1989. Differentiation-inducing and cytotoxic effects of tumor necrosis factor and interferon-gamma in myeloblastic ML-1 cells. J. Cell. Physiol. 141: 46–52. [DOI] [PubMed] [Google Scholar]
- 8.Andrews T., Zhang P., and Bhat N. R.. 1998. TNFalpha potentiates IFNgamma-induced cell death in oligodendrocyte progenitors. J. Neurosci. Res. 54: 574–583. [DOI] [PubMed] [Google Scholar]
- 9.Buntinx M., Moreels M., Vandenabeele F., Lambrichts I., Raus J., Steels P., Stinissen P., and Ameloot M.. 2004. Cytokine-induced cell death in human oligodendroglial cell lines: I. Synergistic effects of IFN-gamma and TNF-alpha on apoptosis. J. Neurosci. Res. 76: 834–845. [DOI] [PubMed] [Google Scholar]
- 10.Testai F. D., Landek M. A., and Dawson G.. 2004. Regulation of sphingomyelinases in cells of the oligodendrocyte lineage. J. Neurosci. Res. 75: 66–74. [DOI] [PubMed] [Google Scholar]
- 11.Scurlock B., and Dawson G.. 1999. Differential responses of oligodendrocytes to tumor necrosis factor and other pro-apoptotic agents: role of ceramide in apoptosis. J. Neurosci. Res. 55: 514–522. [DOI] [PubMed] [Google Scholar]
- 12.Buntinx M., Gielen E., Van Hummelen P., Raus J., Ameloot M., Steels P., and Stinissen P.. 2004. Cytokine-induced cell death in human oligodendroglial cell lines. II: alterations in gene expression induced by interferon-gamma and tumor necrosis factor-alpha. J. Neurosci. Res. 76: 846–861. [DOI] [PubMed] [Google Scholar]
- 13.Larocca J. N., Farooq M., and Norton W. T.. 1997. Induction of oligodendrocyte apoptosis by C2-ceramide. Neurochem. Res. 22: 529–534. [DOI] [PubMed] [Google Scholar]
- 14.Haimovitz-Friedman A., Kolesnick R. N., and Fuks Z.. 1997. Ceramide signaling in apoptosis. Br. Med. Bull. 53: 539–553. [DOI] [PubMed] [Google Scholar]
- 15.Lee J. T., Xu J., Lee J. M., Ku G., Han X., Yang D. I., Chen S., and Hsu C. Y.. 2004. Amyloid-beta peptide induces oligodendrocyte death by activating the neutral sphingomyelinase-ceramide pathway. J. Cell Biol. 164: 123–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jana A., Hogan E. L., and Pahan K.. 2009. Ceramide and neurodegeneration: susceptibility of neurons and oligodendrocytes to cell damage and death. J. Neurol. Sci. 278: 5–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Novgorodov S. A., Chudakova D. A., Wheeler B. W., Bielawski J., Kindy M. S., Obeid L. M., and Gudz T. I.. 2011. Developmentally regulated ceramide synthase 6 increases mitochondrial Ca2+ loading capacity and promotes apoptosis. J. Biol. Chem. 286: 4644–4658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yoon S. O., Casaccia-Bonnefil P., Carter B., and Chao M. V.. 1998. Competitive signaling between TrkA and p75 nerve growth factor receptors determines cell survival. J. Neurosci. 18: 3273–3281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ge R., Tan E., Sharghi-Namini S., and Asada H. H.. 2012. Exosomes in cancer microenvironment and beyond: have we overlooked these extracellular messengers? Cancer Microenviron. 5: 323–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kowal J., Tkach M., and Thery C.. 2014. Biogenesis and secretion of exosomes. Curr. Opin. Cell Biol. 29: 116–125. [DOI] [PubMed] [Google Scholar]
- 21.Raposo G., and Stoorvogel W.. 2013. Extracellular vesicles: exosomes, microvesicles, and friends. J. Cell Biol. 200: 373–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Colombo M., Raposo G., and Thery C.. 2014. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu. Rev. Cell Dev. Biol. 30: 255–289. [DOI] [PubMed] [Google Scholar]
- 23.Simons M., and Raposo G.. 2009. Exosomes–vesicular carriers for intercellular communication. Curr. Opin. Cell Biol. 21: 575–581. [DOI] [PubMed] [Google Scholar]
- 24.Bobrie A., Colombo M., Raposo G., and Thery C.. 2011. Exosome secretion: molecular mechanisms and roles in immune responses. Traffic. 12: 1659–1668. [DOI] [PubMed] [Google Scholar]
- 25.Trajkovic K., Hsu C., Chiantia S., Rajendran L., Wenzel D., Wieland F., Schwille P., Brugger B., and Simons M.. 2008. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science. 319: 1244–1247. [DOI] [PubMed] [Google Scholar]
- 26.Simons M., and Trajkovic K.. 2006. Neuron-glia communication in the control of oligodendrocyte function and myelin biogenesis. J. Cell Sci. 119: 4381–4389. [DOI] [PubMed] [Google Scholar]
- 27.Trajkovic K., Dhaunchak A. S., Goncalves J. T., Wenzel D., Schneider A., Bunt G., Nave K. A., and Simons M.. 2006. Neuron to glia signaling triggers myelin membrane exocytosis from endosomal storage sites. J. Cell Biol. 172: 937–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Frühbeis C., Fröhlich D., and Krämer-Albers E. M.. 2012. Emerging roles of exosomes in neuron-glia communication. Front. Physiol. 3: 119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Turola E., Furlan R., Bianco F., Matteoli M., and Verderio C.. 2012. Microglial microvesicle secretion and intercellular signaling. Front. Physiol. 3: 149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Robbins P. D., and Morelli A. E.. 2014. Regulation of immune responses by extracellular vesicles. Nat. Rev. Immunol. 14: 195–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Candelario K. M., and Steindler D. A.. 2014. The role of extracellular vesicles in the progression of neurodegenerative disease and cancer. Trends Mol. Med. 20: 368–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Record M., Carayon K., Poirot M., and Silvente-Poirot S.. 2014. Exosomes as new vesicular lipid transporters involved in cell-cell communication and various pathophysiologies. Biochim. Biophys. Acta. 1841: 108–120. [DOI] [PubMed] [Google Scholar]
- 33.Buzas E. I., Gyorgy B., Nagy G., Falus A., and Gay S.. 2014. Emerging role of extracellular vesicles in inflammatory diseases. Nat. Rev. Rheumatol. 10: 356–364. [DOI] [PubMed] [Google Scholar]
- 34.Chen W. X., Liu X. M., Lv M. M., Chen L., Zhao J. H., Zhong S. L., Ji M. H., Hu Q., Luo Z., Wu J. Z., et al. 2014. Exosomes from drug-resistant breast cancer cells transmit chemoresistance by a horizontal transfer of microRNAs. PLoS One. 9: e95240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rajendran L., Bali J., Barr M. M., Court F. A., Kramer-Albers E. M., Picou F., Raposo G., van der Vos K. E., van Niel G., Wang J., et al. 2014. Emerging roles of extracellular vesicles in the nervous system. J. Neurosci. 34: 15482–15489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Post G. R., and Dawson G.. 1992. Characterization of a cell line derived from a human oligodendroglioma. Mol. Chem. Neuropathol. 16: 303–317. [DOI] [PubMed] [Google Scholar]
- 37.Bakhti M., Winter C., and Simons M.. 2011. Inhibition of myelin membrane sheath formation by oligodendrocyte-derived exosome-like vesicles. J. Biol. Chem. 286: 787–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., and Klenk D. C.. 1985. Measurement of protein using bicinchoninic acid. Anal. Biochem. 150: 76–85. [DOI] [PubMed] [Google Scholar]
- 39.Bielawski J., Pierce J. S., Snider J., Rembiesa B., Szulc Z. M., and Bielawska A.. 2009. Comprehensive quantitative analysis of bioactive sphingolipids by high-performance liquid chromatography-tandem mass spectrometry. Methods Mol. Biol. 579: 443–467. [DOI] [PubMed] [Google Scholar]
- 40.Bielawski J., Pierce J. S., Snider J., Rembiesa B., Szulc Z. M., and Bielawska A.. 2010. Sphingolipid analysis by high performance liquid chromatography-tandem mass spectrometry (HPLC-MS/MS). Adv. Exp. Med. Biol. 688: 46–59. [DOI] [PubMed] [Google Scholar]
- 41.Krysko D. V., Vanden Berghe T., D’Herde K., and Vandenabeele P.. 2008. Apoptosis and necrosis: detection, discrimination and phagocytosis. Methods. 44: 205–221. [DOI] [PubMed] [Google Scholar]
- 42.Li R. X., and Ladisch S.. 1991. Shedding of human neuroblastoma gangliosides. Biochim. Biophys. Acta. 1083: 57–64. [DOI] [PubMed] [Google Scholar]
- 43.Kjellberg M. A., Lonnfors M., Slotte J. P., and Mattjus P.. 2015. Metabolic conversion of ceramides in HeLa cells - a cholesteryl phosphocholine delivery approach. PLoS One. 10: e0143385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Qin J., Berdyshev E., Goya J., Natarajan V., and Dawson G.. 2010. Neurons and oligodendrocytes recycle sphingosine 1-phosphate to ceramide: significance for apoptosis and multiple sclerosis. J. Biol. Chem. 285: 14134–14143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim S., Steelman A. J., Zhang Y., Kinney H. C., and Li J.. 2012. Aberrant upregulation of astroglial ceramide potentiates oligodendrocyte injury. Brain Pathol. 22: 41–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Osawa Y., Uchinami H., Bielawski J., Schwabe R. F., Hannun Y. A., and Brenner D. A.. 2005. Roles for C16-ceramide and sphingosine 1-phosphate in regulating hepatocyte apoptosis in response to tumor necrosis factor-alpha. J. Biol. Chem. 280: 27879–27887. [DOI] [PubMed] [Google Scholar]
- 47.White-Gilbertson S., Mullen T., Senkal C., Lu P., Ogretmen B., Obeid L., and Voelkel-Johnson C.. 2009. Ceramide synthase 6 modulates TRAIL sensitivity and nuclear translocation of active caspase-3 in colon cancer cells. Oncogene. 28: 1132–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.D’Agostino S., Salamone M., Di Liegro I., and Vittorelli M. L.. 2006. Membrane vesicles shed by oligodendroglioma cells induce neuronal apoptosis. Int. J. Oncol. 29: 1075–1085. [PubMed] [Google Scholar]
- 49.Merrill A. H., Jr 2011. Sphingolipid and glycosphingolipid metabolic pathways in the era of sphingolipidomics. Chem. Rev. 111: 6387–6422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mencarelli C., and Martinez-Martinez P.. 2013. Ceramide function in the brain: when a slight tilt is enough. Cell. Mol. Life Sci. 70: 181–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wheeler D., Bandaru V. V., Calabresi P. A., Nath A., and Haughey N. J.. 2008. A defect of sphingolipid metabolism modifies the properties of normal appearing white matter in multiple sclerosis. Brain. 131: 3092–3102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vidaurre O. G., Haines J. D., Katz Sand I., Adula K. P., Huynh J. L., McGraw C. A., Zhang F., Varghese M., Sotirchos E., Bhargava P., et al. 2014. Cerebrospinal fluid ceramides from patients with multiple sclerosis impair neuronal bioenergetics. Brain. 137: 2271–2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mathivanan S., and Simpson R. J.. 2009. ExoCarta: A compendium of exosomal proteins and RNA. Proteomics. 9: 4997–5000. [DOI] [PubMed] [Google Scholar]
- 54.Mathivanan S., Fahner C. J., Reid G. E., and Simpson R. J.. 2012. ExoCarta 2012: database of exosomal proteins, RNA and lipids. Nucleic Acids Res. 40: D1241–D1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lakhal S., and Wood M. J.. 2011. Exosome nanotechnology: an emerging paradigm shift in drug delivery: exploitation of exosome nanovesicles for systemic in vivo delivery of RNAi heralds new horizons for drug delivery across biological barriers. BioEssays. 33: 737–741. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.