Abstract
Atherogenic mixed dyslipidemia associates with oxidative stress and defective HDL antioxidative function in metabolic syndrome (MetS). The impact of statin treatment on the capacity of HDL to inactivate LDL-derived, redox-active phospholipid hydroperoxides (PCOOHs) in MetS is indeterminate. Insulin-resistant, hypertriglyceridemic, hypertensive, obese males were treated with pitavastatin (4 mg/day) for 180 days, resulting in marked reduction in plasma TGs (−41%) and LDL-cholesterol (−38%), with minor effects on HDL-cholesterol and apoAI. Native plasma LDL (baseline vs. 180 days) was oxidized by aqueous free radicals under mild conditions in vitro either alone or in the presence of the corresponding pre- or poststatin HDL2 or HDL3 at authentic plasma mass ratios. Lipidomic analyses revealed that statin treatment i) reduced the content of oxidizable polyunsaturated phosphatidylcholine (PUPC) species containing DHA and linoleic acid in LDL; ii) preferentially increased the content of PUPC species containing arachidonic acid (AA) in small, dense HDL3; iii) induced significant elevation in the content of phosphatidylcholine and phosphatidylethanolamine (PE) plasmalogens containing AA and DHA in HDL3; and iv) induced formation of HDL3 particles with increased capacity to inactivate PCOOH with formation of redox-inactive phospholipid hydroxide. Statin action attenuated LDL oxidability Concomitantly, the capacity of HDL3 to inactivate redox-active PCOOH was enhanced relative to HDL2, consistent with preferential enrichment of PE plasmalogens and PUPC in HDL3.
Keywords: antioxidative activity, low density lipoprotein, high density lipoprotein 3, lipidomics, metabolic syndrome disease, pitavastatin, oxidative stress, phospholipid hydroxides
By virtue of efficacious lowering of circulating levels of LDL-cholesterol (LDL-C), statins, or HMG-CoA inhibitors, stabilize vulnerable, lipid-rich atherosclerotic plaques, an effect that translates into a decrease in cardiovascular morbi-mortality (1–3). Statins primarily target hepatic cholesterol production, with upregulation of LDL receptor expression subsequent to reduction in the cholesterol content of hepatic cellular membranes and activation of the sterol regulatory element-binding protein-2 transcription factor pathway (4). These agents equally reduce plasma concentrations of atherogenic TG-rich lipoproteins and remnants in dyslipidemic patients; furthermore, they induce a variable degree of elevation (typically <10%) in levels of HDL-cholesterol (HDL-C) and equally of apoAI, which is manifested primarily in large, cholesteryl ester (CE)-rich α-migrating HDL particles (5–9). Such minor statin-mediated increment in HDL-C appears, however, to contribute to plaque regression (10).
Several mechanisms potentially underlie statin-mediated elevation in HDL-C and apoAI, encompassing hepatic and intestinal assembly of nascent HDL particles, modulation of intravascular particle remodeling mediated by lipid transfer proteins and lipases, lipoprotein lipid transfer and exchange with circulating cells and tissues, and finally, tissue catabolism of these particles (11). Statins equally impact circulating levels of VLDL, IDL, and LDL; furthermore, they may differentially impact key pathways of cellular lipid metabolism in addition to that of cholesterol synthesis in metabolic syndrome (MetS) (4, 12–15). As a consequence, modification of cellular lipid metabolism by statins may directly or indirectly modulate the molecular lipid profile of HDL (11–17). The multiplicity of molecular components in the HDL lipidome, of which there are >250 species (18–20), adds a further level of complexity to these processes, however.
Importantly, emerging evidence suggests that the molecular composition of HDL particles is of immediate relevance to their biological function and thus to their capacity to exert atheroprotective effects; prominent among these atheroprotective effects are antioxidative activities (18, 20, 21). By contrast, it is now established that HDL particles display defective biological activities across a wide range of dyslipidemic states associated with premature atherosclerotic vascular disease, and that such dysfunction is intimately linked to alterations in their lipidomic and proteomic profiles (22–27). With respect to the lipidome, alterations in content of both neutral core [triacylglycerols and CEs] and electrostatically charged surface lipids [sphingolipids and phospholipids (PLs)] are implicated in HDL dysfunction in dyslipidemia (22–27). To what degree then might statin-mediated modulation of HDL metabolism and particle composition impact the HDL lipidome, and might such modulation contribute to potential statin-mediated normalization of defective HDL function in dyslipidemic states?
Atherogenic mixed dyslipidemia occurs frequently in the general population, typically presenting in subjects with MetS or type 2 diabetes in association with a cluster of cardiovascular risk factors, among which visceral obesity and insulin resistance are preponderant (6, 22–25, 27, 28). Moreover, mixed dyslipidemias involve elevated levels of TG-rich lipoproteins and small, dense LDL, together with subnormal levels of HDL-C and apoAI (17, 22, 23, 25, 28, 29). Furthermore, substantial evidence attests to the defective antioxidative activity of HDL in these cardiometabolic disorders, which are intimately linked to the prevalence of oxidative stress (22–25, 27, 29).
HDL particles may protect against oxidative stress via several mechanisms, central among which appears to be the capacity to accept phospholipid hydroperoxides (PCOOHs) derived from polyunsaturated phosphatidylcholine (PUPC) species in LDL and to reduce them to inactive hydroxides [phospholipid hydroxide (PCOH)] via oxidation of methionine residues in apoAI and apoAII (30–32). Equally, however, evidence is emerging that HDL-associated plasmalogens, PLs containing a vinyl-ether bond of high oxidative susceptibility, are implicated in this potentially antiatherogenic process (26, 33–35). Indeed, it is of immediate relevance that the oxidative products of plasmalogens do not propagate lipid peroxidation, thereby attenuating formation of proatherogenic secondary oxidation products such as aldehydes (33, 35, 36). In clinical studies, plasmalogen levels were correlated not only with the risk of coronary artery disease (CAD), but also with the antiapoptotic activity of HDL (34). Furthermore, as oxidative modification of LDL particles is central to the pathophysiology of atherosclerotic vascular disease, it is relevant that LDL enrichment in plasmalogens leads to prolongation of the lag phase for conjugated diene (CD) formation during copper-mediated oxidation (37, 38).
The CAPITAIN study (An Open Label Study of the Chronic and Acute Effects of Pitavastatin on Monocyte Phenotype, Endothelial Dysfunction, and HDL Atheroprotective Function in Subjects with Metabolic Syndrome) was designed to provide insight into mechanisms whereby statin treatment might impact dyslipidemia and atheroprotective HDL function in subjects with key risk factors of the MetS (i.e., visceral obesity, insulin resistance, and mixed dyslipidemia) (14, 28, 39). We presently evaluate the question as to whether statin treatment impacts the defective antioxidative activity of HDL in MetS documented earlier (23–25, 27). Our experimental approach is focused on the capacity of the statin-induced, major HDL subfractions, HDL2 and HDL3, to attenuate formation of atherogenic secondary products of lipid oxidation in LDL by inactivation of redox-active PCOOH, with conversion to redox-inactive PCOH. We equally focus on the relevance of the content of both plasmalogens and polyunsaturated PLs in the pre- and poststatin HDL subfractions and in the LDL substrate to such HDL antioxidative activity. Our study design is distinguished by the combination of lipidomic and HPLC analyses with an in vitro experimental strategy providing a physiologically relevant estimation of the capacity of HDL subfractions to reduce LDL-derived PCOOH at baseline and following statin treatment.
MATERIALS AND METHODS
The CAPITAIN trial: criteria for recruitment and characteristics of the patient cohort
The CAPITAIN Study (An Open Label Study of the Chronic and Acute Effects of Pitavastatin on Monocyte Phenotype, Endothelial Dysfunction, and HDL Atheroprotective Function in Subjects with Metabolic Syndrome; ClinicalTrials.gov, #NCT01595828) was monocentric and recruited 12 dyslipidemic Caucasian male subjects with plasma LDL-C of 130–190 mg/dl (3.4–4.9 mM) and a MetS phenotype determined according to strict International Diabetes Federation (IDF) criteria (28). Inclusion criteria required participants to have central obesity (defined as a waist circumference ≥94 cm), plus any two of the following: i) elevated TG level ≥1.7 mM (>150 mg/dl); ii) subnormal HDL-C <1.03 mM (≤40 mg/dl) in males; iii) controlled hypertension [systolic blood pressure (SBP) ≥130 mm Hg or diastolic blood pressure (DBP) ≥85 mm Hg] or treatment for previously diagnosed hypertension with a calcium channel blocker that did not require treatment with a diuretic, beta-blocker, angiotensin converting enzyme inhibitor, or angiotensin II receptor blocker; or iv) fasting plasma glucose ≥5.6 mM (100 mg/dl). Exclusion criteria are detailed in Table 1.
TABLE 1.
Exclusion criteria
| All Subjects Included in the Study Did Not Meet Any of the Following Exclusion Criteria: |
| Women |
| Non-Caucasian |
| Excessive obesity defined as BMI above 35 kg/m2, rounded to the nearest whole number |
| LDL-C >190 mg/dl at screening |
| Fasting TGs >400 mg/dl at screening |
| Diabetes mellitus, defined as a fasting glucose >7 mM, or taking diabetic therapy at screening |
| History of symptomatic cardiovascular disease including angina pectoris, acute myocardial infarction, or peripheral arterial disease including intermittent claudication |
| History of symptomatic cerebrovascular disease, including cerebrovascular hemorrhage, transient ischemic attack, or carotid endarterectomy |
| A current smoker or have smoked in the preceding 12 months |
| Consume >10 g of alcohol (equivalent to one 100 ml glass of table wine) per day |
| Have received statins, fibric acid derivatives, bile acid sequestrants, cholesterol absorption inhibitors (including ezetimibe), or nicotinic acid >500 mg per day in the previous year |
| Have uncontrolled hypertension (SBP ≥140 mm Hg or DBP ≥90 mm Hg). Patients may have their hypertension controlled with a calcium channel blocker but must not receive treatment with a diuretic, beta-blocker, angiotensin converting enzyme inhibitor, or angiotensin II receptor blocker . If the patient has previously received treatment with these therapies, they must have been discontinued at least 2 months previously. |
| Any conditions that cause secondary dyslipidemia or increase the risk of statin therapy including alcoholism, autoimmune disease, nephrotic syndrome, uremia, any viral hepatitis clinically active within 12 months before study entry, obstructive hepatic or biliary disease, dysglobulinemia or macroglobulinemia, multiple myeloma, glycogen storage disease, porphyria, and uncontrolled hypothyroidism or hyperthyroidism. Controlled thyroid disease [normal serum thyroid stimulating hormone and stable therapy for at least 3 months] is permitted. |
| History of pancreatic injury or pancreatitis, or impaired pancreatic function/injury as indicated by abnormal lipase |
| Liver injury as indicated by serum transaminase levels (alanine aminotransferase/serum glutamic pyruvic transaminase, aspartate aminotransaminase/serum glutamic oxaloacetic transaminase) >3 × upper limit of the reference range (ULRR). |
| Impaired renal function as indicated by serum creatinine levels >1.5 × ULRR at screening or estimated glomerular filtration rate (eGFR) by Cockroft formula <60 ml/min. |
| History of any muscle disease or unexplained elevation (>3 × ULRR) of serum creatine kinase |
| Any surgical or medical condition that might significantly alter the absorption, distribution, metabolism, or excretion of the study drug, including the following: history of major gastrointestinal tract surgery (e.g., gastrectomy, gastroenterostomy or small bowel resection), gastritis or inflammatory bowel disease, current active ulcers, or gastrointestinal or rectal bleeding |
| Current obstruction of the urinary tract or difficulty in voiding likely to require intervention during the course of the study |
| Severe acute illness or severe trauma in the preceding 3 months |
| Evidence of symptomatic heart failure (New York Heart Association class III or IV): significant heart block or cardiac arrhythmia |
| History of uncontrolled complex ventricular arrhythmias, uncontrolled atrial fibrillation/flutter or uncontrolled supraventricular tachycardias with a ventricular response rate of >100 beats/min at rest. Patients whose electrophysiological instability is controlled with a pacemaker or implantable cardiac device are eligible. |
| History of drug abuse |
| History of allergy or intolerance to medication (including statins) |
| Current or recent (within 1 week) use of supplements or medications known to alter lipid metabolism including soluble fiber (including >2 teaspoons Metamucil or psyllium-containing supplement per day) or other dietary fiber supplements, fish oils containing omega-3 oils, “fat blockers” (e.g., orlistat), or other products at the discretion of the investigator |
| Any forbidden concomitant medication |
| Within the exclusion period defined in the National Register for Healthy Volunteers of the French Ministry of Health |
| Participation in any clinical trial with an investigational drug in the past 3 months preceding study entry |
| Forfeit their freedom by administrative or legal award or who are under guardianship |
Patient characteristics and anthropometric data were as follows: mean age, 50 ± 3 years; BMI, 31.7 ± 0.5 kg/m2; and waist circumference, 110 ± 3 cm. All subjects displayed atherogenic mixed dyslipidemia with plasma TGs >150 mg/dl; the complete plasma lipid profile of all participants is summarized in Table 2. Detailed baseline parameters of glucose homeostasis and insulin resistance in CAPITAIN subjects were reported earlier and revealed a prediabetic state with insulin resistance (39). In addition, all participants had been nonsmokers for at least 12 months prior to inclusion and had previously smoked <25 cigarettes/day on a regular basis. Study participants had no history of cardiovascular disease or type 2 diabetes; they acted as their own controls in order to limit confounding effects due to variation in genetic background and in baseline phenotype. The study was performed in accordance with the ethical principles set forth in the Declaration of Helsinki and received approval from the Ethics Committee of Pitié-Salpêtrière University Hospital. Written informed consent was given by each subject after the purpose and nature of the investigation had been explained.
TABLE 2.
Characteristics of dyslipidemic metabolic syndrome subjects at baseline (D0) and impact of pitavastatin (4 mg/day) treatment for 180 days
| Lipid and Apolipoprotein Parameters (mg/dl) | Baseline | D180 | % Change | P (D180 vs. Baseline) |
| Total cholesterol | 232.2 ± 17.6 | 161.7 ± 5.7 | −30% | <0.0005 |
| TGs | 215.9 ± 16.0 | 127.7 ± 8.1 | −41% | <0.0005 |
| LDL-C | 153.0 ± 6.2 | 96.1 ± 5.8 | −37% | <0.0001 |
| ApoB | 102.0 ± 4.2 | 72.8 ± 5.1 | −29% | <0.0001 |
| HDL-C | 46.3 ± 2.8 | 48.2 ± 3.6 | +4% | NS |
| ApoAI | 100.3 ± 4.8 | 106.6 ± 5.7 | +6% | NS |
| LDL-C/HDL-C ratio | 3.4 ± 0.2 | 2.1 ± 0.2 | −38% | <0.0001 |
| Lp(a)a | 8.8 (0.5–24.9) | 8.5 (0.9–32.2) | −3% | NS |
| Total LDL | 320.6 ± 20.0 | 225.5 ± 12.6 | −30% | <0.0001 |
| HDL2 | 103.7 ± 6.2 | 96.0 ± 8.9 | −7% | NS |
| HDL3 | 97.3 ± 4.3 | 104.6 ± 5.8 | +8% | NS |
Lp(a), lipoprotein (a). Values are expressed as means ± SEM (n = 12). Values for total native LDL, HDL2, and HDL3 are expressed as total mass (mg/dl).
Due to its asymmetric distribution, Lp(a) levels are expressed as median (minimum–maximum).
Blood sampling
Briefly, all participants in the CAPITAIN trial underwent screening within a 3-week period prior to inclusion and initiation of study drug administration. Subsequently, all subjects were treated with pitavastatin (4 mg/day) for a period of 180 days. The last meal consumed prior to clinical examination and blood sampling was a balanced mixed meal that contained 30–35% fat, 50–55% carbohydrate, and ∼15% protein as counseled by the study dietician (14). Blood samples were collected after overnight fasting before initiation of statin treatment (baseline, D0) and at 180 days (D180) after the final intake of drug. Blood samples were withdrawn in the Clinical Unit (14) by venipuncture from the cubital vein into precooled (4°C) EDTA-containing tubes (final concentration 1 mg/ml) at pretreatment (D0) and posttreatment (D180) time points. Plasma was separated from blood cells by low-speed centrifugation at 1,700 g for 20 min at 4°C; 0.6% sucrose was added to cryoprotect lipoproteins and plasma aliquoted within 2 h of blood collection (40). After freezing in liquid nitrogen, samples were stored at −80°C until analysis. Earlier studies have documented the absence of lipid- or protein-derived oxidation products in the component lipoproteins of such samples (32, 41).
Fractionation and preparative isolation of plasma lipoprotein fractions
Using our single-step, isopycnic nondenaturing density gradient procedure, plasma lipoprotein subfractions were preparatively isolated on the basis of their hydrated densities from plasma samples corresponding to the baseline D0 and D180 time points by ultracentrifugation in a Beckman SW41 Ti rotor at 40,000 rpm for 44 h in a Beckman Optima XPN-80 ultracentrifuge at 15°C (42). Upon completion of ultracentrifugation, each gradient was fractionated with a precision pipette into predefined volumes as previously described (42); in this way, 12 subfractions were obtained: VLDL + IDL (d <1.019 g/ml), LDL1 (d = 1.019–1.023 g/ml), LDL2 (d = 1.023–1.029 g/ml), LDL3 (d = 1.029–1.039 g/ml), LDL4 (d = 1.039–1.050 g/ml), LDL5 (d = 1.050–1.063 g/ml), HDL2b (d = 1.063–1.091 g/ml), HDL2a (d = 1.091–1.110 g/ml), HDL3a (d = 1.110–1.133 g/ml), HDL3b (d = 1.133–1.156 g/ml), HDL3c (d = 1.156–1.179 g/ml), and finally the bottom ultracentrifugal residue containing plasma proteins (d >1.179 g/ml).
Reconstitution of total LDL, dense LDL, HDL2, and HDL3
In order to study the susceptibility to oxidation of total native LDL, or of the native dense LDL subfraction alone, or the oxidative susceptibility of LDL in the presence of the HDL2 or HDL3 fractions, reconstitution of total LDL and HDL fractions was performed from their respective gradient subfractions isolated from either D0 plasma samples or from those obtained after statin treatment at D180, as follows: i) for total LDL (d = 1.019–1.063 g/ml), an equal volume of each of subfractions LDL1 to LDL5 was mixed; ii) for dense LDL (d = 1.039–1.063 g/ml), equal volumes of LDL4 and LDL5 were pooled; iii) for HDL2 (d = 1.063–1.110 g/ml), the whole volume of HDL2b was mixed with that of HDL2a; and finally, iv) the three component HDL3 subfractions were pooled in order to obtain HDL3 (d = 1.110–1.179 g/ml).
Desalting of lipoprotein fractions
LDL, HDL2, and HDL3 were dialyzed separately on PD-10 desalting columns (GE Healthcare) with the spin protocol described in the manufacturer’s instruction sheet. Briefly, each column was equilibrated with PBS 1× containing 1 g/l of Chelex (BioRad); each sample was then applied to the top of the column and elution performed by centrifugation at 1,000 g for 2 min: the eluate contained the desalted fraction.
Determination of the % weight chemical compositions of lipoprotein fractions
The weight % chemical composition of total LDL, dense LDL, HDL2, and HDL3 at D0 (baseline) and D180 (after treatment) was determined as previously described, and included PL, TG, free cholesterol (FC), CEs, and total protein (TP) (42). The total mass of each fraction corresponded to the sum of the mass of the individual lipid and protein components for each lipoprotein. Coefficients of intra- and interassay variation for the individual components ranged from 2% to 9%.
Lipoprotein oxidation with 2,2′-azobis(2-methylpropionamidine) dihydrochloride
2,2′-Azobis(2-methylpropionamidine) dihydrochloride (AAPH; Sigma-Aldrich), at a final concentration of 2 mM, was used to mediate oxidation of total or dense LDL (final concentration 10 mg TP/dl) in the absence or presence of HDL2 or HDL3 (final concentration 20 mg TP/dl) at 37°C for 6 h (32); mixtures of LDL ± HDL2 or HDL3 represented lipoprotein fractions isolated at D0 on the one hand and at D180 on the other. The LDL/HDL subfraction (2 or 3) mass ratio corresponds approximately to the mass ratio of these lipoprotein fractions in normolipidemic human plasma. We used AAPH, a well-characterized aqueous azo-initiator of oxidation to model free radical-induced LDL oxidation via formation of lipid hydroperoxide (LOOH) as a key step (43). Oxidation was terminated by addition of butylated hydroxytoluene and EDTA at final concentration of 10 µM for both (32).
Extraction of phosphatidylcholine, PCOOH, and plasmalogens in mixtures of native or oxidized lipoprotein fractions
Total phosphatidylcholine (PC) molecular species and total plasmalogen species were extracted from total native LDL, native HDL2, and native HDL3; PC and PCOOH were extracted from nonoxidized (total LDL, dense LDL, from mixtures of total LDL + HDL2, total LDL + HDL3, dense LDL + HDL2, and dense LDL + HDL3) and from mixtures of oxidized total LDL ± AAPH, oxidized dense LDL ± AAPH, oxidized total LDL + HDL2 ± AAPH, oxidized total LDL + HDL3 ± AAPH, oxidized dense LDL + HDL2 ± AAPH, and oxidized dense LDL + HDL3 ± AAPH. Samples corresponded to the D0 and to the D180 time points. Extraction of total molecular species of PC and PCOOH was performed on individual mixtures (500 µl aliquots) either in the absence of AAPH (PC), or in the presence of AAPH (PCOOH + PC), by adding methanol (2 ml) and hexane (5 ml) as previously described (32, 44). Extraction of total plasmalogen molecular species was performed on each nonoxidized mixture (100 µl aliquots) with equal volumes of methanol and hexane; each tube was vortexed for 1 min and centrifuged at 4,000 rpm for 10 min. The lower phase was collected (2.25 ml for PC and PCOOH; 1 ml for plasmalogen) and evaporated at 40°C under nitrogen. Acid hydrolysis, as previously described by Khaselev and Murphy (45) was performed on plasmalogens, but with the exception that methanol (200 µl) was used to dissolve the residue (as for PC and PCOOH); the residue was then dissolved by vortexing for 1 min followed by centrifugation at 4,000 rpm for 5 min. Extractions were performed for all mixtures at the D0 and D180 time points.
Quantitation of PC, PCOOH, plasmalogens, and ApoAI by HPLC
In the absence of validated MS methodology by which to identify and quantitate PCOOHs, total molecular species of PCOOH, PC, and plasmalogens [the latter including both PC and phosphatidylethanolamine (PE) species containing a vinyl ether bond] were identified and quantitated by reverse-phase HPLC (RP-HPLC) using a Kromasil 100-3.5-C18 (2.1 × 150 mm) column at 40°C with an isocratic mobile phase containing methanol (94%; v/v) and pH 5–10 mM ammonium acetate (6%; v/v) on an HPLC (Shimadzu) system; under these conditions, PC and PE plasmalogens coeluted. The duration of the elution was 40 min with a flow rate at 0.3 ml/min; 20 µl of sample was injected. PCOOH were detected by chemioluminescence (flow rate of 0.3 ml/min for 15 min/sample) in lipoprotein mixtures that had undergone oxidation, whereas PC and plasmalogens were detected by UV absorbance at 205 nm. The interindividual assay coefficient of variation for each PC and PCOOH molecular species was 3.5% and 9.5%, respectively; the limit of PCOOH detection was 30 pmol. The interindividual coefficients of variation for C18 (plasmalogen)-22:6 and C18 (plasmalogen)-20:4 were 1.6% and 2.9%, respectively. The limits of detection for C18(plasm)-22:6 and C18(plasm)-20:4 were 2 µg/ml and 4 µg/ml, respectively (48 pmol and 100 pmol, respectively).
Native and oxidized ApoAI were detected by UV absorbance at 214 nm after separation by RP-HPLC using an ACE 5 C18-300 (4.6 × 250 mm) column at 40°C with a gradient from 40% of acetonitrile containing 0.1% trifluoroacetic acid (TFA) (solution A) and 60% of water containing 0.1% TFA (solution B) to 65% of A and 35% of B in 50 min at a 1 ml/min flow rate (32); 50 µl of each sample was injected. Detection was performed on oxidized (following incubation with AAPH) and nonoxidized lipoprotein samples at D0 and D180: mixtures were as follows: total LDL + HDL2, total LDL + HDL3, and HDL2 and HDL3.
Purified standards for HPLC analyses
13S-Hydroperoxy-9Z,11E-octadecadienoic acid (Avanti Polar Lipids) was used as standard to quantify PCOOH 16:0/18:2 and PCOOH 18:0/18:2, while 15S-hydroperoxy-5Z,8Z,11Z,13E-eicosatetraenoic acid was used to quantify PCOOH 16:0/22:6 + 20:4 and 18:0/22:6 + 20:4. For identification and quantification of the major PC species, standard calibration curves for the following purified standards were determined: PC18:0/20:4, PC16:0/20:4, PC18:0/18:2, PC16:0/18:2, PC16:0/22:6, and PC18:0/22:6 (from Avanti Polar Lipids). Plasmalogen standards, C18 (plasmalogen)-20:4PC and C18(plasmalogen)-20:6PC (Avanti Polar lipids) were used following acid hydrolysis to identify lyso-PC species obtained after hydrolysis and to quantify plasmalogens (as the PC and PE species combined) in mixtures of lipoproteins before and after exposure to AAPH. Under these conditions, and as a consequence of the acid hydrolysis step, the lyso products of both the 16:0- and 18:0-containing species of PC and PE plasmalogens were quantitated together.
Lipidomic analysis
Lipidomic analysis was performed by LC/electrospray ionization-MS/MS using an Agilent 1200 LC system combined with an Applied Biosystems API 4000 Q/TRAP mass spectrometer with a turbo-ion spray source (350°C) and Analyst 1.5 data system. The methodology, instrumentation, and internal standards were identical to those used in earlier studies (14, 46); indeed, the conditions for MS/MS analysis of lipoprotein lipid classes and subclasses and the parameters of the assay performance were derived from a quality control plasma pool as detailed earlier (46). The following individual lipid classes and subclasses in LDL, HDL2, and HDL3 were analyzed and quantitated: PC, alkylphosphatidylcholine [PC(O)], alkenylphosphatidylcholine [plasmalogen, PC(P)], PE, alkylphosphatidylethanolamine [PE(O)], and alkenylphosphatidylethanolamine [plasmalogen, PE(P)]. The relative amounts of each molecular lipid species were calculated by expressing the peak area of each species relative to the peak area of the corresponding stable isotope or nonphysiological internal standard as described previously (46). A correction factor of 10 was applied to the PE(P) species to account for the lower signal response of these lipid species relative to the PE(17:0/17:0) internal standard. It is noteworthy that quantitation of plasmalogen species by HPLC was consistent with that by MS. Concentrations of total lipid classes were calculated from the sum of the individual lipid species within each class. Concentrations of lipoprotein lipids were expressed as nmol/mg of TP in each fraction.
Statistical analyses
The effect of statin treatment on each lipid parameter was determined by comparison of baseline D0 values with those obtained on samples at the D180 time point by the paired t-test. All results are expressed as means ± SEM for normally distributed variables and as median (minimum–maximum) for asymmetrically distributed parameters; distribution normality was assessed using the Kolmogorov-Smirnov test.
Using the data derived from the MS analysis of plasmalogens, we asked if there was a difference between the changes in plasmalogen content observed in the lipoprotein subclasses (LDL, HDL2, and HDL3) as a result of statin treatment. The change in each plasmalogen species or subclass was calculated and expressed as nmol/mg protein and as % change relative to baseline (D0); repeated measures (RM) ANOVA with post hoc paired Student’s t-test was then used to evaluate the significance of differences on treatment.
RESULTS
Baseline dyslipidemic profile and effect of statin treatment
Our male cohort (n = 12) displayed the MetS phenotype on the basis of three criteria, that is, central obesity (BMI = 31.7 ± 0.5 kg/m2), baseline hypertriglyceridemia (mean baseline TG level = 216 ± 16.0 mg/dl; 2.4 mM), and elevated SBP (131 ± 11 mm Hg) (14, 28, 29). As in our previous studies in MetS, in which HDL-C levels were subnormal (47 mg/dl) relative to the 50th percentile for the general population (23), male subjects in CAPITAIN displayed subnormal mean HDL-C and apoAI concentrations (46.3 mg/dl, 1.19 mM and 100.3 ± 4.8 mg/dl, 2.6 mM, respectively). It is, however, noteworthy that IDF proposed a lower cutoff for HDL-C of 40 mg/dl (1 mM) for Europid males with MetS (28). Significantly, our MetS group was equally hypercholesterolemic (LDL-C, 153 ± 6.2 mg/dl). Median Lp(a) concentrations were <10 mg/dl; from our earlier studies using density gradient fractionation of plasma lipoproteins, we estimate that contamination of LDL by Lp(a) represents <3% of LDL mass, and <10% mass of HDL2 and HDL3, respectively (47). After statin treatment for 180 days, plasma LDL-C levels decreased by 37% (P < 0.0001), TGs by 41% (P < 0.0005), and apoB by 29% (P < 0.0001); by contrast, despite small increments, there was no significant change in HDL-C, apoAI, or Lp(a) upon treatment (Table 2). Furthermore, there was no modification in total mass of HDL2 or HDL3, despite a net increment in HDL3 mass relative to that of HDL2 on treatment (Table 3).
TABLE 3.
Weight % chemical composition of total native LDL, HDL2, and HDL3 from dyslipidemic metabolic syndrome subjects at baseline (D0) and impact of pitavastatin (4 mg/day) treatment for 180 days (D180)
| Total LDL | HDL2 | HDL3 | ||||
| D0 | D180 | D0 | D180 | D0 | D180 | |
| Total mass (mg/dl) | 320.6 ± 20.0 | 225.5 ± 12.6a | 103.7 ± 6.2 | 96.1 ± 8.9 | 97.3 ± 4.3 | 104.6 ± 5.8 |
| % TG | 14.7 ± 1.3 | 13.6 ± 0.9 | 10.9 ± 1.7 | 6.3 ± 0.7b | 7.1 ± 0.9 | 4.1 ± 0.4b |
| % CE | 41.5 ± 1.3 | 41.3 ± 0.7 | 20.5 ± 0.7 | 23.1 ± 1.1c | 17.7 ± 0.8 | 19.5 ± 0.9c |
| % FC | 8.1 ± 0.3 | 8.5 ± 0.2 | 3.4 ± 0.1 | 3.7 ± 0.1b | 1.9 ± 0.1 | 1.9 ± 0.1 |
| % PL | 20.9 ± 0.8 | 21.3 ± 0.4 | 28.2 ± 0.8 | 29.4 ± 0.7 | 25.1 ± 0.6 | 26.4 ± 0.6b |
| % TP | 14.8 ± 1.1 | 15.3 ± 1.0 | 37.0 ± 1.9 | 37.5 ± 1.8 | 48.1 ± 1.6 | 48.1 ± 1.5 |
| CE/TG | 3.2 ± 0.4 | 3.2 ± 0.2 | 2.4 ± 0.3 | 4.1 ± 0.4b | 3.0 ± 0.4 | 5.3 ± 0.6b |
| (TP + FC + PL)/(CE + TG) | 0.8 ± 0.1 | 0.8 ± 0.03 | 2.3 ± 0.2 | 2.5 ± 0.2c | 3.3 ± 0.2 | 3.1 ± 0.2 |
Values are expressed as means ± SEM (n = 12). All components are expressed as % of total mass, except total mass (mg/dl).
P < 0.001.
0.001 < P < 0.01.
0.01 < P < 0.05 vs. D0.
% Weight chemical compositions of native total LDL, native HDL2, and native HDL3: effect of statin treatment
The % weight chemical compositions of both native HDL2 and HDL3 were modified after statin treatment (Table 3). Indeed, % weight TG content decreased by 42% (P < 0.01) in HDL2 and in HDL3 on treatment, consistent with marked statin-mediated reduction in TG-rich lipoprotein levels and in cholesteryl ester transfer protein (CETP) activity as a consequence of reduced numbers of apoB-containing particle acceptors for CETP-mediated heterotransfer of CEs and TGs (12). Concomitantly, % weight CE content increased by 12% and 10% (P < 0.05) in native HDL2 and native HDL3, respectively. Moreover, elevation in % FC content (+9%, P < 0.01) was documented in native HDL2 and in PL (+5%, P < 0.01) in native HDL3. The ratio CE/TG increased in HDL2 and in HDL3 by 74% (P < 0.01) and 77% (P < 0.01), respectively, primarily reflecting fall in TG content in the treatment phase. The ratio (TP + PL + FC)/(CE + TG), which corresponds essentially to the ratio of lipoprotein surface to core components, was elevated by statin treatment specifically in HDL2 (8%, P < 0.05).Changes in % weight chemical compositions trended toward those in normolipidemic subjects, with the exception of % PL content in poststatin HDL3, which increased by 25% (P < 0.05) relative to normolipidemic subjects (18–21, 23).
The molecular compositions of the PC and PE lipidomes in nonoxidized LDL, HDL2, and HDL3 and effect of statin treatment
Six molecular species of PC (16:0/22:6PC, 16:0/20:4PC, 16:0/18:2PC; 18:0/22:6PC, 18:0/20:4PC, and 18:0/18:2PC) were identified by HPLC and quantitated by UV absorption. These species were analyzed in nonoxidized total LDL (total native LDL), nonoxidized HDL2 (native HDL2), nonoxidized HDL3 (native HDL3), and in oxidized mixtures before (D0) and after a 180-day statin treatment (D180). The PC content in nonoxidized mixtures corresponded to the initial PC content before oxidation with AAPH. Statin treatment significantly reduced the content of PC 16:0/22:6 (−23%, P < 0.01), PC 16:0/18:2 (−21%, P < 0.001), PC 18:0/22:6 (−16%, P < 0.05) and PC 18:0/18:2 (−14%, P < 0.01) species in total native LDL (data not shown); comparable observations were made in dense LDL. These findings were entirely consistent with the corresponding analyses by MS (Table 4), although changes for the four PC species above did not attain significance on statin treatment, an observation that may be accounted for by differences in detection methodology and the relative sensitivity of detection.
TABLE 4.
The molecular compositions of the PC and PE lipidomes as determined by LC/MS in total native LDL, HDL2, and HDL3 from MetS subjects (D0) and % change from baseline (D0)a
| LDL | HDL2 | HDL3 | |||||||
| Lipid (nmol/mg TP) | D0 | % Change | P | D0 | % Change | P | D0 | % Change | P |
| Total PC | 1076 ± 47 | −8.7 | 1.26E-01 | 639 ± 27 | 0.6 | 8.72E-01 | 376 ± 12 | 6.3 | 9.09E-02 |
| Total PC(O) | 32 ± 1.5 | −2.6 | 6.04E-01 | 22 ± 1.31 | 5.8 | 3.05E-01 | 13 ± 0.66 | 13 | 6.61E-02 |
| Total PC(P) | 17 ± 1.1 | −1.2 | 6.04E-01 | 10 ± 0.77 | 4.4 | 5.82E-01 | 6.06 ± 0.31 | 12 | 1.52E-01 |
| Total PE | 11 ± 1.6 | −5.5 | 6.04E-01 | 11 ± 1.4 | 1.1 | 6.01E-01 | 6.83 ± 0.5 | 1.4 | 9.38E-01 |
| Total PE(O) | 1.5 ± 0.1 | 12 | 6.04E-01 | 1.01 ± 0.1 | 25 | 1.87E-01 | 0.6 ± 0.03 | 30 | 1.22E-01 |
| Total PE(P) | 17 ± 1.4 | 6.1 | 7.61E-01 | 12 ± 1.09 | 18 | 9.07E-02 | 7.36 ± 0.37 | 24 | 1.84E-02 |
| PC 28:0 | 0.2 ± 0 | 50 | 6.95E-01 | 0.06 ± 0.01 | 104 | 1.81E-01 | 0.03 ± 0 | 67 | 1.45E-01 |
| PC 29:0 | 0.1 ± 0 | 22 | 9.79E-01 | 0.02 ± 0 | 60 | 4.14E-01 | 0.01 ± 0 | 43 | 2.10E-01 |
| PC 30:0 | 3.5 ± 0.4 | 0.5 | 7.40E-01 | 1.19 ± 0.12 | 27 | 5.79E-01 | 0.63 ± 0.06 | 19 | 3.85E-01 |
| PC 31:0 | 1 ± 0.1 | 2 | 7.43E-01 | 0.36 ± 0.04 | 21 | 6.59E-01 | 0.2 ± 0.02 | 20 | 4.24E-01 |
| PC 31:1 | 1.3 ± 0.1 | 2.1 | 8.76E-01 | 0.38 ± 0.02 | 15 | 8.51E-02 | 0.19 ± 0.01 | 14 | 3.51E-02 |
| PC 32:0 | 11 ± 0.5 | −8.5 | 3.60E-01 | 4.45 ± 0.17 | 6.9 | 3.47E-01 | 2.37 ± 0.11 | 13 | 1.54E-03 |
| PC 32:1 | 19 ± 1.4 | −5.4 | 6.51E-01 | 9.13 ± 0.88 | 8.2 | 8.61E-01 | 5.16 ± 0.5 | 1.3 | 8.85E-01 |
| PC 32:2 | 5.1 ± 0.2 | −6 | 5.75E-01 | 2.08 ± 0.16 | 9.4 | 5.92E-01 | 1.12 ± 0.05 | 7.2 | 3.65E-01 |
| PC 32:3 | 0.2 ± 0 | −3.9 | 6.68E-01 | 0.1 ± 0.01 | 7.5 | 5.50E-01 | 0.05 ± 0 | 9.9 | 3.18E-01 |
| PC 33:0 | 1.2 ± 0.1 | −0.5 | 7.40E-01 | 0.51 ± 0.04 | 5.8 | 9.06E-01 | 0.28 ± 0.02 | 13 | 2.18E-01 |
| PC 33:1 | 3.7 ± 0.3 | −0.9 | 7.40E-01 | 1.86 ± 0.15 | 5.2 | 9.67E-01 | 1.08 ± 0.09 | 10 | 4.98E-01 |
| PC 33:2 | 2.4 ± 0.1 | −11.2 | 4.54E-01 | 1.37 ± 0.07 | 0.2 | 7.98E-01 | 0.8 ± 0.04 | −0.6 | 9.32E-01 |
| PC 33:3 | 0.1 ± 0 | 2.6 | 8.76E-01 | 0.03 ± 0 | 17 | 9.06E-01 | 0.02 ± 0 | 12 | 9.21E-01 |
| PC 34:0 | 2.9 ± 0.2 | −8.8 | 4.54E-01 | 1.09 ± 0.06 | 4.8 | 8.59E-01 | 0.59 ± 0.03 | 7 | 1.82E-01 |
| PC 34:1 | 132 ± 6.9 | −8.8 | 4.66E-01 | 79 ± 5.21 | −0.8 | 6.69E-01 | 45 ± 2.23 | 8.8 | 1.28E-01 |
| PC 34:2 | 213 ± 9.5 | −14.8 | 7.10E-02 | 133 ± 5.81 | −5.6 | 2.21E-01 | 78 ± 4.07 | 2.9 | 8.36E-01 |
| PC 34:3 | 9.1 ± 0.6 | −14.1 | 4.66E-01 | 6.14 ± 0.42 | 1.6 | 7.29E-01 | 3.6 ± 0.22 | −4.3 | 5.78E-01 |
| PC 34:4 | 1 ± 0.1 | 8.3 | 9.06E-01 | 0.59 ± 0.06 | 26 | 4.65E-01 | 0.36 ± 0.03 | 17 | 3.24E-01 |
| PC 34:5 | 0.1 ± 0 | 40 | 9.06E-01 | 0.04 ± 0.01 | 69 | 5.09E-01 | 0.02 ± 0 | 49 | 3.65E-01 |
| PC 35:0 | 0.2 ± 0 | 15 | 9.64E-01 | 0.04 ± 0.01 | 49 | 7.87E-01 | 0.02 ± 0 | 53 | 3.21E-01 |
| PC 35:1 | 5.8 ± 0.4 | 0.6 | 7.91E-01 | 2.82 ± 0.22 | 9.8 | 5.95E-01 | 1.54 ± 0.12 | 16 | 1.56E-01 |
| PC 35:2 | 6.8 ± 0.5 | −6 | 5.91E-01 | 4.44 ± 0.28 | 1.8 | 8.96E-01 | 2.6 ± 0.14 | 9.8 | 4.23E-01 |
| PC 35:3 | 1.2 ± 0.1 | −11.5 | 4.93E-01 | 0.8 ± 0.04 | −2.6 | 5.03E-01 | 0.49 ± 0.03 | −2.6 | 6.36E-01 |
| PC 35:4 | 0.8 ± 0.1 | 5 | 9.96E-01 | 0.56 ± 0.05 | 24 | 3.29E-02 | 0.34 ± 0.03 | 21 | 2.14E-02 |
| PC 35:5 | 0.1 ± 0 | 31 | 8.69E-01 | 0.04 ± 0.01 | 54 | 2.76E-01 | 0.03 ± 0 | 57 | 1.07E-01 |
| PC 36:0 | 0.3 ± 0 | −7 | 5.67E-01 | 0.07 ± 0.01 | 24 | 6.88E-01 | 0.04 ± 0 | 13 | 7.30E-01 |
| PC 36:1 | 39 ± 2.6 | 1.9 | 9.06E-01 | 16 ± 1.86 | 32 | 4.20E-01 | 9.34 ± 0.91 | 21 | 2.78E-01 |
| PC 36:2 | 137 ± 6.8 | −9.5 | 4.44E-01 | 79 ± 4.01 | −0.4 | 6.96E-01 | 45 ± 1.61 | 5.4 | 3.75E-01 |
| PC 36:3 | 91 ± 5.6 | −14.4 | 3.21E-01 | 67 ± 2.99 | −5.7 | 2.37E-01 | 40 ± 1.99 | −1.1 | 6.84E-01 |
| PC 36:5 | 11 ± 1.3 | 15 | 9.06E-01 | 8.34 ± 1.1 | 35 | 3.51E-01 | 4.9 ± 0.38 | 30 | 2.43E-01 |
| PC 36:6 | 0.5 ± 0.1 | −7.5 | 6.18E-01 | 0.33 ± 0.04 | 3.8 | 7.03E-01 | 0.19 ± 0.01 | −1.2 | 7.43E-01 |
| PC 37:4 | 3.7 ± 0.4 | 10 | 8.26E-01 | 3.34 ± 0.31 | 18 | 4.80E-02 | 2 ± 0.15 | 29 | 1.69E-02 |
| PC 37:5 | 0.6 ± 0.1 | 8.5 | 9.06E-01 | 0.47 ± 0.06 | 17 | 7.91E-01 | 0.29 ± 0.02 | 22 | 3.51E-01 |
| PC 37:6 | 0.5 ± 0.1 | −13.4 | 4.65E-01 | 0.3 ± 0.03 | −8.4 | 2.50E-01 | 0.18 ± 0.02 | −7.5 | 3.35E-01 |
| PC 38:2 | 7.4 ± 0.4 | −7 | 5.75E-01 | 1.57 ± 0.14 | 13 | 7.68E-01 | 0.91 ± 0.09 | −0.3 | 6.38E-01 |
| PC 38:3 | 35 ± 4.1 | −14.3 | 4.54E-01 | 24 ± 2.29 | −1.3 | 2.87E-01 | 14 ± 1.65 | −1.3 | 4.48E-01 |
| PC 38:4 | 125 ± 7.6 | 5.9 | 5.99E-01 | 44 ± 3.29 | 19 | 6.39E-03 | 27 ± 1.67 | 23 | 2.29E-03 |
| PC 38:5 | 34 ± 2.4 | −5.6 | 6.15E-01 | 28 ± 2.06 | 5.6 | 8.44E-01 | 17 ± 0.72 | 13 | 1.85E-01 |
| PC 38:7 | 0.8 ± 0.1 | −18.8 | 4.44E-01 | 0.61 ± 0.05 | −13.9 | 8.59E-02 | 0.38 ± 0.02 | −15.8 | 4.16E-02 |
| PC 39:5 | 0.6 ± 0.1 | −5.8 | 6.15E-01 | 0.41 ± 0.04 | −0.8 | 5.26E-01 | 0.25 ± 0.02 | 6.4 | 8.35E-01 |
| PC 39:6 | 1.2 ± 0.1 | −7.1 | 6.15E-01 | 0.96 ± 0.1 | −7.1 | 3.41E-01 | 0.59 ± 0.05 | 4.2 | 9.37E-01 |
| PC 39:7 | 0 ± 0 | −10.2 | 5.67E-01 | 0.03 ± 0 | −13.2 | 6.63E-02 | 0.02 ± 0 | −14.3 | 1.45E-01 |
| PC 40:4 | 2.5 ± 0.3 | −6.3 | 5.91E-01 | 1.66 ± 0.15 | 8.8 | 9.86E-01 | 1.04 ± 0.12 | 1.5 | 7.06E-01 |
| PC 40:5 | 9.7 ± 0.8 | −11.6 | 4.54E-01 | 6.33 ± 0.49 | −1.3 | 4.55E-01 | 3.81 ± 0.24 | −1 | 6.08E-01 |
| PC 40:6 | 18 ± 2 | −16.7 | 3.01E-01 | 10 ± 0.91 | −7.3 | 2.38E-01 | 6.09 ± 0.5 | −0.5 | 8.88E-01 |
| PC 40:7 | 2.6 ± 0.2 | −19.6 | 3.01E-01 | 2.36 ± 0.19 | −14.2 | 1.57E-02 | 1.42 ± 0.09 | −6.1 | 2.99E-01 |
| PC 40:8 | 0.8 ± 0.1 | −3.9 | 6.18E-01 | 0.5 ± 0.03 | −2.4 | 5.16E-01 | 0.32 ± 0.01 | −5.3 | 5.03E-01 |
| PC(16:0/20:4) | 83 ± 5.7 | 1.3 | 9.79E-01 | 57 ± 3.72 | 13 | 8.27E-02 | 35 ± 2.06 | 20 | 8.03E-03 |
| PC(16:0/22:6) | 38 ± 3.3 | −17.8 | 3.01E-01 | 29 ± 2.42 | −9.6 | 1.35E-01 | 17 ± 1.26 | −0.6 | 7.89E-01 |
| PC(18:1/18:3) | 7 ± 0.6 | −13 | 4.66E-01 | 4.69 ± 0.54 | 10 | 9.62E-01 | 2.7 ± 0.23 | 1.3 | 9.31E-01 |
| PC(18:2/20:4) | 2.6 ± 0.2 | −4.7 | 7.40E-01 | 2.22 ± 0.19 | 10 | 8.00E-01 | 1.34 ± 0.08 | −6.2 | 5.90E-01 |
| PC(O-32:0) | 1.8 ± 0.1 | −4.9 | 6.51E-01 | 0.58 ± 0.02 | 5.3 | 3.02E-01 | 0.29 ± 0.02 | 15 | 2.11E-02 |
| PC(O-32:1) | 0.4 ± 0 | −10.2 | 4.65E-01 | 0.2 ± 0.01 | −0.5 | 9.87E-01 | 0.11 ± 0.01 | 11 | 1.14E-01 |
| PC(O-32:2) | 0.1 ± 0 | −0.1 | 7.43E-01 | 0.03 ± 0 | 18 | 7.58E-01 | 0.01 ± 0 | 6.1 | 8.78E-01 |
| PC(O-34:1) | 2.6 ± 0.1 | −4.9 | 5.75E-01 | 1.26 ± 0.06 | 5.5 | 2.41E-01 | 0.71 ± 0.04 | 12 | 8.54E-02 |
| PC(O-34:2) | 2 ± 0.1 | 7 | 6.15E-01 | 1.2 ± 0.09 | 20 | 6.36E-02 | 0.7 ± 0.03 | 26 | 1.22E-02 |
| PC(O-34:3) | 0 ± 0 | 9.9 | 8.34E-01 | 0.02 ± 0 | 69 | 7.81E-02 | 0.01 ± 0 | 23 | 3.53E-01 |
| PC(O-34:4) | 0.1 ± 0 | 4.1 | 9.30E-01 | 0.07 ± 0.01 | 20 | 4.68E-01 | 0.04 ± 0 | 13 | 4.19E-01 |
| PC(O-35:4) | 0.1 ± 0 | 3.2 | 9.06E-01 | 0.03 ± 0 | 22 | 3.08E-01 | 0.02 ± 0 | 31 | 2.38E-01 |
| PC(O-36:0) | 0 ± 0 | −2.4 | 7.40E-01 | 0.02 ± 0 | 14 | 4.75E-01 | 0.01 ± 0 | 10 | 5.04E-01 |
| PC(O-36:1) | 0.3 ± 0 | 5.8 | 9.06E-01 | 0.08 ± 0.01 | 22 | 2.03E-01 | 0.04 ± 0.01 | 8.2 | 5.07E-01 |
| PC(O-36:2) | 0.9 ± 0 | −4.2 | 7.40E-01 | 0.36 ± 0.03 | 15 | 5.83E-02 | 0.22 ± 0.02 | 6.6 | 5.84E-01 |
| PC(O-36:3) | 2.8 ± 0.1 | −3.7 | 7.40E-01 | 2.05 ± 0.1 | 4.3 | 4.73E-01 | 1.23 ± 0.05 | 11 | 1.15E-01 |
| PC(O-36:4) | 7.8 ± 0.5 | −0.3 | 8.99E-01 | 4.67 ± 0.36 | 7.6 | 3.75E-01 | 2.71 ± 0.18 | 23 | 7.44E-02 |
| PC(O-36:5) | 0.4 ± 0 | 18 | 9.06E-01 | 0.18 ± 0.03 | 54 | 3.87E-01 | 0.1 ± 0.01 | 32 | 3.75E-01 |
| PC(O-38:4) | 3.8 ± 0.2 | 2.1 | 9.79E-01 | 3.44 ± 0.26 | 11 | 1.40E-01 | 2.01 ± 0.17 | 13 | 3.47E-02 |
| PC(O-38:5) | 6.5 ± 0.4 | −5.7 | 6.15E-01 | 5.78 ± 0.44 | 1.7 | 8.84E-01 | 3.47 ± 0.2 | 8.6 | 3.38E-01 |
| PC(O-40:5) | 1.2 ± 0.1 | −6.1 | 5.75E-01 | 0.64 ± 0.05 | 4 | 3.91E-01 | 0.4 ± 0.04 | 2.4 | 5.64E-01 |
| PC(O-40:6) | 0.8 ± 0.1 | −4.1 | 6.15E-01 | 0.35 ± 0.04 | 4.7 | 9.40E-01 | 0.2 ± 0.02 | 9.3 | 5.62E-01 |
| PC(O-40:7) | 0.9 ± 0.1 | −8.2 | 6.15E-01 | 0.93 ± 0.09 | −5.9 | 3.76E-01 | 0.57 ± 0.05 | 3.6 | 9.62E-01 |
| PC(P-30:0) | 0.1 ± 0 | 5.5 | 9.06E-01 | 0.03 ± 0 | 22 | 9.82E-02 | 0.02 ± 0 | 17 | 1.06E-01 |
| PC(P-32:0) | 1.2 ± 0.1 | −5.5 | 5.75E-01 | 0.73 ± 0.03 | 5.3 | 2.85E-01 | 0.4 ± 0.02 | 11 | 6.52E-02 |
| PC(P-32:1) | 0.1 ± 0 | −8.4 | 4.54E-01 | 0.09 ± 0.01 | 3.9 | 4.14E-01 | 0.06 ± 0 | 8.9 | 1.25E-01 |
| PC(P-34:1) | 1.3 ± 0.1 | 4.6 | 9.06E-01 | 0.74 ± 0.05 | 15 | 3.58E-02 | 0.43 ± 0.02 | 21 | 3.30E-03 |
| PC(P-34:3) | 0.1 ± 0 | −8.2 | 4.44E-01 | 0.02 ± 0 | 5.8 | 7.16E-01 | 0.01 ± 0 | 9.5 | 1.82E-02 |
| PC(P-36:2) | 0.8 ± 0.1 | 7.5 | 6.95E-01 | 0.6 ± 0.05 | 16 | 2.03E-01 | 0.35 ± 0.03 | 20 | 4.87E-02 |
| PC(P-16:0/20:4) | 4.6 ± 0.4 | −3.9 | 6.15E-01 | 3.79 ± 0.33 | 1.9 | 9.50E-01 | 2.24 ± 0.13 | 13 | 1.86E-01 |
| PC(P-36:5) | 0.3 ± 0 | 16 | 9.06E-01 | 0.22 ± 0.03 | 24 | 5.77E-01 | 0.14 ± 0.01 | 24 | 5.01E-01 |
| PC(P-18:0/20:4) | 1.5 ± 0.1 | −5.6 | 5.67E-01 | 1.24 ± 0.12 | 13 | 2.26E-01 | 0.75 ± 0.06 | 8.7 | 2.91E-01 |
| PC(P-38:5) | 3.1 ± 0.3 | −6.3 | 6.15E-01 | 1.99 ± 0.18 | −1.5 | 7.48E-01 | 1.22 ± 0.09 | 8.3 | 5.04E-01 |
| PC(P-16:0/22:6) | 0.6 ± 0.1 | −9 | 5.75E-01 | 0.54 ± 0.06 | −3.1 | 5.28E-01 | 0.33 ± 0.03 | 2.3 | 9.37E-01 |
| PC(P-18:0/22:6) | 0.2 ± 0 | 3.4 | 9.64E-01 | 0.18 ± 0.02 | −3.9 | 5.04E-01 | 0.11 ± 0.01 | 6.1 | 5.02E-01 |
| PE 32:1 | 0 ± 0 | 28 | 9.06E-01 | 0.03 ± 0.01 | 38 | 9.00E-01 | 0.02 ± 0 | 15 | 7.80E-01 |
| PE 34:1 | 0.4 ± 0.1 | 1.6 | 7.43E-01 | 0.47 ± 0.06 | 2.3 | 6.77E-01 | 0.28 ± 0.02 | 2.1 | 8.36E-01 |
| PE 34:2 | 0.8 ± 0.1 | −20.7 | 4.54E-01 | 0.76 ± 0.08 | −14 | 1.87E-01 | 0.44 ± 0.03 | −13.8 | 7.72E-02 |
| PE 34:3 | 0.1 ± 0 | 2 | 7.43E-01 | 0.05 ± 0.01 | 22 | 9.87E-01 | 0.03 ± 0 | 16 | 8.46E-01 |
| PE 35:1 | 0 ± 0 | 9.9 | 9.64E-01 | 0.03 ± 0 | 11 | 7.65E-01 | 0.02 ± 0 | 29 | 2.50E-01 |
| PE 35:2 | 0.1 ± 0 | −8.5 | 6.15E-01 | 0.05 ± 0.01 | −16.8 | 8.22E-02 | 0.03 ± 0 | −2.5 | 6.73E-01 |
| PE 36:1 | 0.3 ± 0 | 18 | 9.06E-01 | 0.35 ± 0.04 | 30 | 4.99E-01 | 0.22 ± 0.02 | 19 | 3.26E-01 |
| PE 36:2 | 1.7 ± 0.2 | −12.5 | 5.35E-01 | 1.66 ± 0.17 | −7.8 | 2.00E-01 | 0.97 ± 0.06 | −8.5 | 1.81E-01 |
| PE 36:3 | 0.7 ± 0.1 | −13.8 | 4.93E-01 | 0.74 ± 0.08 | −5.1 | 2.88E-01 | 0.45 ± 0.03 | −3.3 | 5.20E-01 |
| PE 36:4 | 1.2 ± 0.2 | −7.2 | 5.75E-01 | 1.13 ± 0.16 | 1.7 | 5.88E-01 | 0.7 ± 0.06 | 3.1 | 8.94E-01 |
| PE 36:5 | 0.1 ± 0 | 22 | 7.43E-01 | 0.09 ± 0.02 | 38 | 9.91E-01 | 0.05 ± 0.01 | 22 | 8.42E-01 |
| PE 38:3 | 0.3 ± 0 | 5.6 | 6.18E-01 | 0.32 ± 0.04 | 7.9 | 5.76E-01 | 0.2 ± 0.03 | −1.8 | 3.10E-01 |
| PE 38:4 | 2 ± 0.3 | 5 | 8.63E-01 | 2.19 ± 0.27 | 10 | 2.99E-01 | 1.45 ± 0.12 | 7.7 | 4.83E-01 |
| PE 38:5 | 0.8 ± 0.1 | 4.1 | 7.40E-01 | 0.87 ± 0.14 | 11 | 9.87E-01 | 0.54 ± 0.05 | 13 | 2.32E-01 |
| PE 38:6 | 1.5 ± 0.3 | 3.5 | 5.67E-01 | 1.42 ± 0.26 | 4.7 | 5.79E-01 | 0.81 ± 0.1 | 10 | 6.82E-01 |
| PE 40:4 | 0 ± 0 | 25 | 9.06E-01 | 0.06 ± 0.01 | 19 | 8.15E-01 | 0.04 ± 0.01 | 7.7 | 8.22E-01 |
| PE 40:5 | 0.2 ± 0 | 9.9 | 6.85E-01 | 0.06 ± 0.01 | 19 | 9.86E-01 | 0.04 ± 0.01 | 25 | 2.43E-01 |
| PE 40:6 | 0.7 ± 0.1 | 5.9 | 6.18E-01 | 0.79 ± 0.14 | 4.7 | 6.53E-01 | 0.46 ± 0.05 | 8.9 | 8.01E-01 |
| PE 40:7 | 0.1 ± 0 | −1.8 | 6.15E-01 | 0.13 ± 0.02 | 14 | 6.12E-01 | 0.08 ± 0.01 | 21 | 4.44E-01 |
| PE(O-18:0/22:5) | 0.1 ± 0 | 17 | 6.15E-01 | 0.07 ± 0.01 | 28 | 5.78E-03 | 0.04 ± 0 | 29 | 3.59E-03 |
| PE(O-18:1/18:2) | 0.1 ± 0 | 14 | 6.78E-01 | 0.04 ± 0 | 27 | 3.39E-01 | 0.02 ± 0 | 45 | 1.70E-01 |
| PE(O-18:1/20:3) | 0.3 ± 0 | 13 | 7.43E-01 | 0.2 ± 0.02 | 33 | 1.83E-01 | 0.13 ± 0.01 | 24 | 2.07E-01 |
| PE(O-18:2/18:2) | 0.3 ± 0 | 12 | 7.96E-01 | 0.21 ± 0.02 | 27 | 2.51E-01 | 0.12 ± 0.01 | 43 | 1.69E-01 |
| PE(O-18:2/20:3) | 0.4 ± 0 | 14 | 8.34E-01 | 0.24 ± 0.03 | 22 | 2.80E-01 | 0.14 ± 0.01 | 30 | 1.58E-01 |
| PE(O-18:2/22:5) | 0.1 ± 0 | −0.4 | 7.67E-01 | 0.07 ± 0.01 | 4.4 | 8.85E-01 | 0.05 ± 0 | 9.6 | 7.88E-01 |
| PE(O-34:1) | 0.1 ± 0 | 30 | 3.12E-01 | 0.03 ± 0 | 36 | 5.83E-04 | 0.02 ± 0 | 46 | 7.46E-04 |
| PE(O-34:2) | 0 ± 0 | 14 | 6.66E-01 | 0.03 ± 0 | 14 | 2.67E-01 | 0.01 ± 0 | 33 | 7.50E-02 |
| PE(O-36:2) | 0.1 ± 0 | 21 | 4.93E-01 | 0.04 ± 0 | 37 | 5.71E-03 | 0.02 ± 0 | 34 | 8.27E-03 |
| PE(O-36:5) | 0 ± 0 | 25 | 8.99E-01 | 0.02 ± 0 | 159 | 1.36E-01 | 0.01 ± 0 | 54 | 2.79E-01 |
| PE(O-36:6) | 0 ± 0 | 23 | 9.94E-01 | 0.02 ± 0 | 30 | 9.45E-01 | 0.01 ± 0 | 74 | 7.57E-02 |
| PE(O-40:6) | 0.1 ± 0 | 4.5 | 8.65E-01 | 0.04 ± 0.01 | 17 | 8.72E-01 | 0.03 ± 0 | 8.5 | 8.11E-01 |
| PE(P-16:0/18:1) | 0.3 ± 0 | 20 | 4.89E-01 | 0.14 ± 0.02 | 60 | 1.24E-02 | 0.09 ± 0.01 | 45 | 3.72E-03 |
| PE(P-16:0/18:2) | 0.5 ± 0 | 13 | 6.85E-01 | 0.28 ± 0.03 | 32 | 8.74E-02 | 0.17 ± 0.01 | 29 | 3.59E-03 |
| PE(P-16:0/20:4) | 2.3 ± 0.3 | 10 | 9.06E-01 | 1.68 ± 0.2 | 24 | 7.66E-02 | 1.1 ± 0.07 | 35 | 2.56E-02 |
| PE(P-16:0/22:5) | 5.5 ± 0.5 | 3.8 | 9.79E-01 | 3.83 ± 0.37 | 12 | 2.50E-01 | 2.27 ± 0.14 | 24 | 5.79E-02 |
| PE(P-16:0/22:6) | 1.5 ± 0.2 | −0.2 | 8.76E-01 | 1.11 ± 0.13 | 15 | 3.06E-01 | 0.69 ± 0.06 | 21 | 8.72E-02 |
| PE(P-18:0/18:1) | 0.3 ± 0 | 38 | 3.01E-01 | 0.15 ± 0.01 | 44 | 3.79E-03 | 0.08 ± 0.01 | 62 | 1.54E-02 |
| PE(P-18:0/18:2) | 1 ± 0.1 | 12 | 7.43E-01 | 0.65 ± 0.06 | 18 | 1.61E-01 | 0.38 ± 0.02 | 31 | 8.49E-03 |
| PE(P-18:0/20:4) | 3 ± 0.3 | 12 | 7.43E-01 | 2.2 ± 0.19 | 26 | 7.95E-02 | 1.43 ± 0.09 | 21 | 7.68E-02 |
| PE(P-18:0/22:5) | 1.7 ± 0.1 | 5.6 | 9.06E-01 | 1.17 ± 0.09 | 18 | 1.46E-01 | 0.7 ± 0.04 | 22 | 2.64E-02 |
| PE(P-18:0/22:6) | 1.1 ± 0.1 | −2.5 | 8.04E-01 | 0.81 ± 0.09 | 12 | 3.39E-01 | 0.45 ± 0.03 | 20 | 6.26E-02 |
PC-P, phosphatidylcholine plasmalogen; PE-P, phosphatidylethanolamine plasmalogen. Values are expressed as means ± SEM (n = 12). P values are from paired t-test analysis and have been corrected for multiple comparisons by Benjamini-Hochberg. All values highlighted in bold are statistically significant at the level of ≤0.05.
Percent change from baseline (D0) subsequent to pitavastatin (4 mg/day) treatment for 180 days (D180).
In contrast to LDL, the content of major PC species in native HDL2 was not significantly modified after statin treatment as determined by HPLC, whereas minor statin-induced changes in the contents of the PC 35:4, 37:4, 38:4, and 40:7 species (+24, +18, +19, and −14%, respectively) attained significance by MS measurement (Table 4). Equally, MS analysis did not show any change in total PC content in HDL2 upon statin treatment, nor in HDL3. In HPLC analysis of native HDL3, the contents of the PC 16:0/20:4 and the PC 18:0/20:4 species were, however, increased to a minor degree upon treatment (12%, P < 0.05; and 22%, P < 0.01), respectively. Overall, poststatin changes in the PC lipidome of HDL2 paralleled those in HDL3 when analyzed by MS, but in addition, HDL3 was distinguished by changes in PC 31:1, PC 32:0, PC 35:4, and PC 38:7 (+14, +13, −21, and −16%, respectively). Importantly, HPLC and MS measurements of the highly oxidizable PC 16:0/20:4 species confirmed an increment of +20% on treatment.
Total contents of PC(O), PC(P), PE, and PE(O) were similar in HDL2 and HDL3 at baseline and on treatment when expressed as nmol/mg TP (Table 4). By contrast, statin-associated changes in total PE(P) species differed between HDL subfractions (see below).
PCOOH and PCOH formation: effect of statin treatment
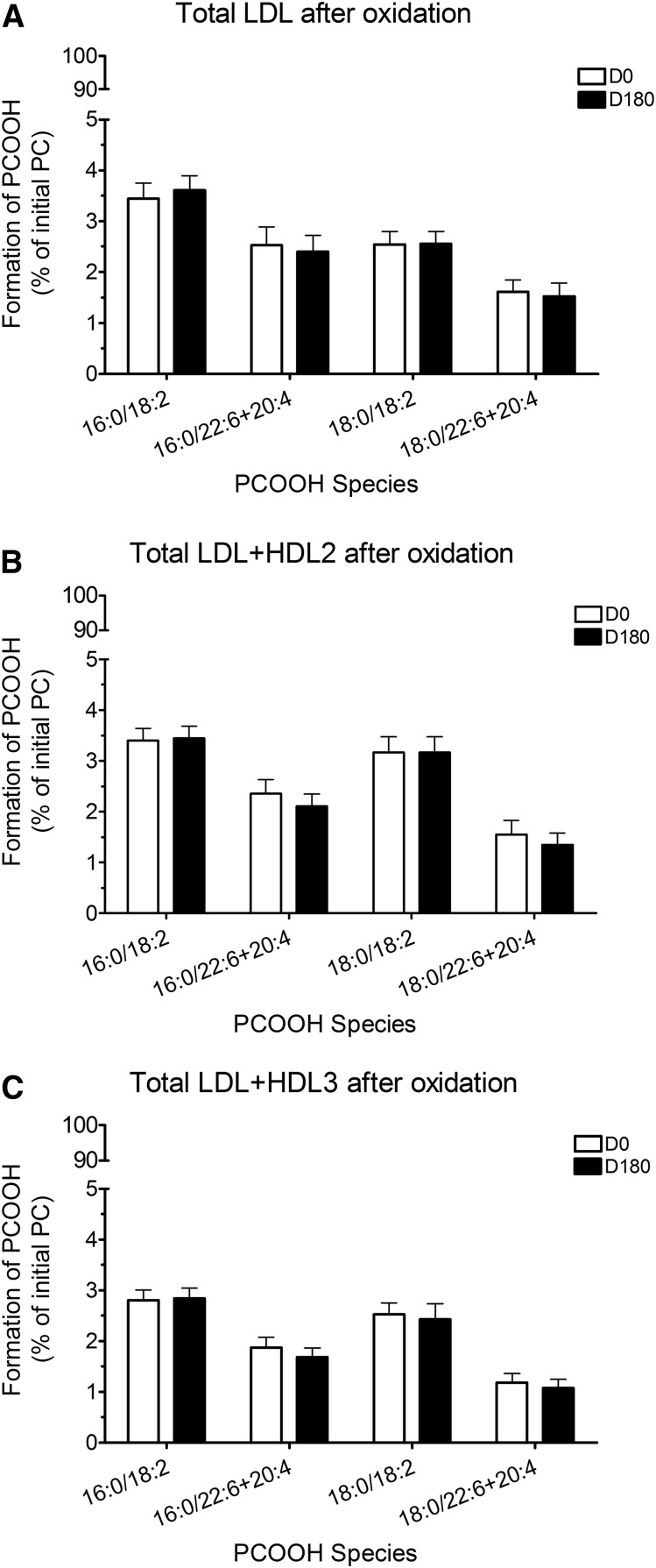
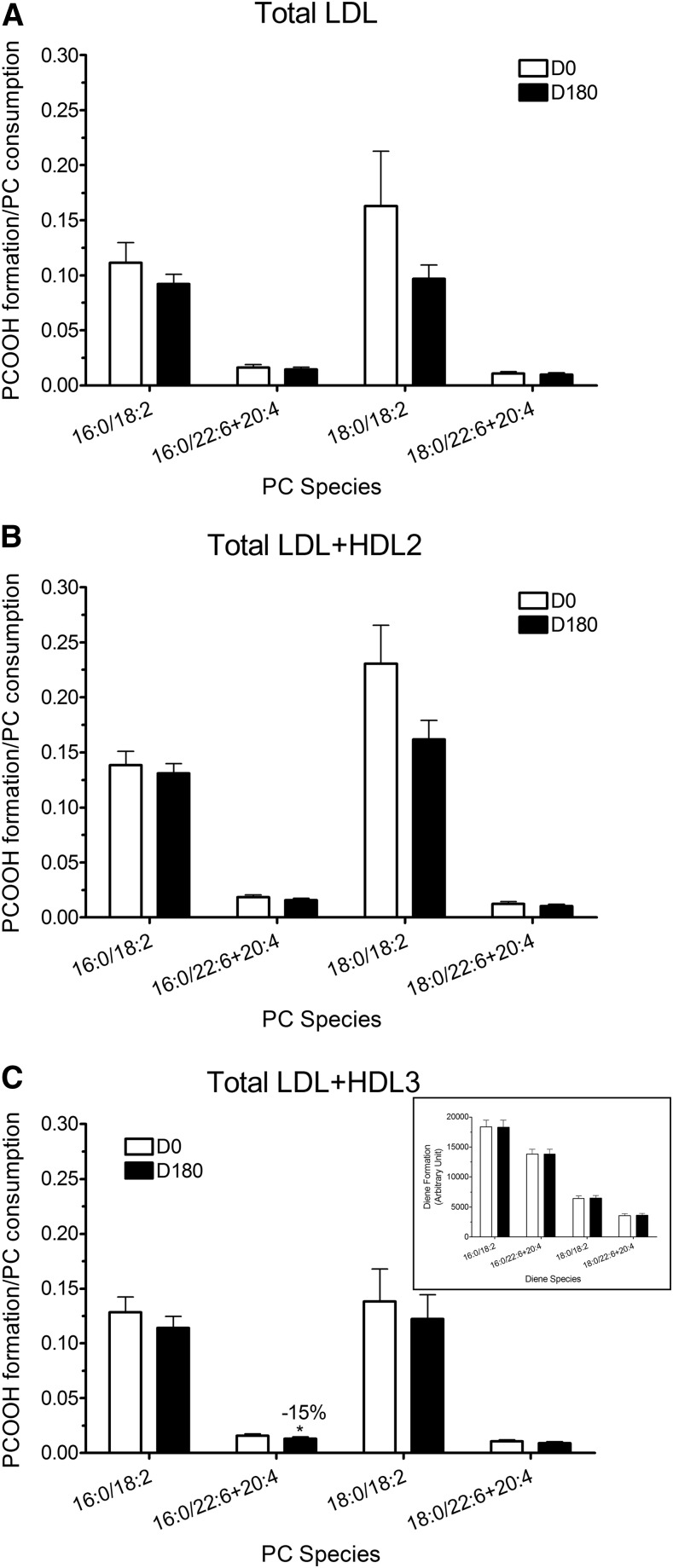
Formation of PCOOH was expressed as % of initial PC present, that is, as % PC content in nonoxidized lipoprotein mixtures (Fig. 1). Statin treatment did not, however, modify the formation of individual PCOOH species detected in samples of mixtures of oxidized total LDL (Fig. 1A), total LDL + HDL2 (Fig. 1B), and total LDL + HDL3 (Fig. 1C) isolated from plasmas at D180. Nonetheless, the ratio of PCOOH formed to PC consumed was significantly decreased in the PC 16:0/22:6 and PC 16:0/20:4 species when total LDL were oxidized in the presence of statin-induced HDL3 (−15%, P < 0.05; Fig. 2C). Diene formation (insert, Fig. 2C), which is the sum of PCOOH and their corresponding hydroxides, was unchanged in the presence of pitavastatin-induced HDL3, indicating that PCOOH formed were more efficiently reduced to inactive PCOH in the presence of statin-induced HDL3 as compared with the corresponding HDL2 subfraction.
Fig. 1.
Formation of PCOOH expressed as % initial PC in total LDL (A), total LDL + HDL2 mixture (B), and total LDL + HDL3 mixture (C) before (D0) and after pitavastatin treatment for 180 days (D180).
Fig. 2.
Ratio of PCOOH formation to PC consumption after oxidation in total LDL (A), total LDL + HDL2 mixtures (B), and total LDL + HDL3 mixtures (C) before (D0) and after pitavastatin treatment for 180 days (D180). C (insert): Diene formation expressed in arbitrary units in total LDL + HDL3 mixtures before (D0) and after pitavastatin treatment for 180 days (D180). * P < 0.05 vs. D0.
Plasmalogen content in native and oxidized total LDL, HDL2, and HDL3: effect of statin treatment
Two plasmalogen molecular species(plasm)-22:6 and (plasm)-20:4 containing DHA and arachidonic acid (AA), respectively, at the sn-2 position, were identified in native total LDL, native HDL2, and native HDL3 by HPLC, which did not differentiate the PC(P) and PE(P) species. The (plasm)-20:4 species (expressed as nmol of plasmalogen/mg TP) were the most abundant molecular species in total native LDL, native HDL2, and native HDL3 (data not shown). While statin treatment did not significantly modify total plasmalogen content in native LDL and in native HDL2, nonetheless, there was a distinct trend for plasmalogen 22:6 to decrease in LDL on treatment. HDL3 was distinct in that total plasmalogens increased (+18%; Table 5), and in addition, the 20:4 species trended to increase (P < 0.055). Upon oxidation of lipoprotein fractions at baseline and after statin treatment, <20% of total native plasmalogen remained in total oxidized LDL (range 10–18%), <25% in oxidized HDL2 (range 16–24%), and <40% in oxidized HDL3 (range 15–37%). Absolute amounts of total PC(P) and PE(P) species after oxidation in LDL were 7.0 ± 4.0 and 8.3 ± 1.7 nmol/mg TP at baseline and after statin treatment, respectively; for HDL2, 12.9 ± 1.7 and 9.4 ± 1.3 nmol/mg TP, respectively; and for HDL3, 10.3 ± 3.3 and 10.4 ± 4.4 nmol/ mg TP, respectively. Clearly, major plasmalogen species in LDL and HDL subfractions were oxidatively degraded to a major degree under oxidative conditions.
TABLE 5.
Plasmalogen content determined by LC/MS in total native LDL, HDL2, and HDL3 from dyslipidemic MetS subjects (D0) and % change from baseline (D0)a
| Plasmalogen Content (nmol/mg TP) | LDL | HDL2 | HDL3 | LDL vs HDL3 | |||
| D0 | % Change | D0 | % Change | D0 | % Change | RM-ANOVA % Change | |
| PC-P(16:0/20:4) | 4.6 ± 0.4 | −3.9% | 3.8 ± 0.3 | +1.9% | 2.2 ± 0.1 | +13.3% | 0.0091c |
| PC-P(18:0/20:4) | 1.5 ± 0.1 | −5.6% | 1.2 ± 0.1 | +12.5% | 0.8 ± 0.1 | +8.7% | 0.0616 |
| Total PC-P(20:4) | 1.5 ± 0.1 | -4.4% | 1.2 ± 0.1 | +4.2% | 0.8 ± 0.1 | +11.9% | 0.0141b |
| PC-P(16:0/22:6) | 0.6 ± 0.1 | −9.0% | 0.5 ± 0.1 | −3.1% | 0.3 ± 0 | +2.3% | 0.0626 |
| PC-P(18:0/22:6) | 0.2 ± 0 | +3.4% | 0.2 ± 0 | −3.9% | 0.1 ± 0 | +6.1% | 0.672 |
| Total PC-P(22:6) | 1.5 ± 0.1 | -6.4% | 1.2 ± 0.1 | -3.5% | 0.8 ± 0.1 | +3.1% | 0.0998 |
| Total PC-P | 17 ± 1.1 | -1.2% | 10 ± 0.8 | +4.4% | 6.1 ± 0.3 | +11.6% | 0.059 |
| PE-P(16:0/20:4) | 2.3 ± 0.3 | +10.4% | 1.7 ± 0.2 | +23.8% | 1.1 ± 0.1 | +34.8%b | 0.065 |
| PE-P(18:0/20:4) | 3 ± 0.3 | +12.0% | 2.2 ± 0.2 | +25.7% | 1.4 ± 0.1 | +20.7% | 0.36 |
| Total PE-P(20:4) | 5.3 ± 0.5 | +10.2% | 3.9 ± 0.4 | +23.6% | 2.5 ± 0.1 | +26.0%b | 0.103 |
| PE-P(16:0/22:6) | 1.5 ± 0.2 | −0.2% | 1.1 ± 0.1 | +14.6% | 0.7 ± 0.1 | +20.5% | 0.009c |
| PE-P(18:0/22:6) | 1.1 ± 0.1 | −2.5% | 0.8 ± 0.1 | +11.7% | 0.5 ± 0 | +19.7% | 0.019b |
| Total PE-P(22:6) | 2.6 ± 0.3 | -1.7% | 1.9 ± 0.2 | +12.9% | 1.1 ± 0.1 | +19.4% | 0.004c |
| Total PE-P | 17 ± 1.4 | +6.1% | 12 ± 1.1 | +18.0% | 7.4 ± 0.4 | +24.1%b | 0.028b |
| Total PC-P + PE-P(20:4) | 11 ± 1 | +1.6% | 8.9 ± 0.8 | +12.1% | 5.5 ± 0.3 | +18.2% | 0.025b |
| Total PC-P + PE-P(22:6) | 3.4 ± 0.3 | -3.0% | 2.6 ± 0.3 | +8.2% | 1.6 ± 0.1 | +14.7% | 0.007c |
| Total PC-P + PE-P | 34 ± 2.5 | +2.1% | 22 ± 1.8 | +11.5% | 13 ± 0.7 | +18.3%b | 0.027b |
Values are expressed as means ± SEM (n = 12). Data for baseline concentrations of individual PC(P) and PE(P) molecular species are presented in Table 4, together with % change upon statin treatment.
Percent change from baseline (D0) subsequent to pitavastatin (4 mg/day) treatment for 180 days (D180).
0.01 < P < 0.05 vs. D0.
0.001 < P < 0.01.
Overall, these HPLC-based findings are consistent with those obtained in our MS analyses (Table 5), with the exception that individual PC-P and PE-P species were identified in all lipoprotein subfractions at D0 and at D180. Indeed, both absolute levels and profiles of total PC-P + PE-P(20:4) and PC-P + PE-P(22:6) at D0 and at D180 in native LDL, HDL2, and HDL3 obtained by LC/MS closely resembled those quantitated as total plasmalogen by HPLC above. When PC-P(20:4), PE-P(20:4), PC-P(22:6), and PE-P(22:6) determined by MS were considered together, total PC-P + PE-P was significantly increased poststatin in HDL3 (+18.3%, P < 0.05), whereas a lesser increment occurred in HDL2 (+11.5%), and no net change in LDL. The significant increment in PC-P + PE-P in HDL3 primarily reflected an increase in total PE-P (+24.1%, P < 0.05) with lesser elevation in total PC-P (+11.9%, NS). Similar but lesser, nonsignificant trends were observed in HDL2 (PE-P, +18.0% and PC-P, +4.4%, respectively). The significant statin-induced elevation in total PE-P in HDL3 arose from increment in PE-P(20:4) (+26%, P < 0.05) and more specifically from that of the PE-P(16:0-20:4) molecular species (34.8%, P < 0.05). It is interesting to note that there was a trend to similar but nonsignificant observations in HDL2. When plasmalogen changes in LDL on treatment were compared with those detected in HDL3, the differences were significant for several species, that is, PC-P(16:0/20:4) (P < 0.01), total PC-P(20:4) (P < 0.05), PE-P(16:0/22:6) (P < 0.01), PE-P(18:0/22:6) (P < 0.05), total PE-P(22:6) (P < 0.01), total PE-P (P < 0.05), PC-P + PE-P(20:4) (P < 0.05), PC-P + PE-P(22:6) (P < 0.01), and total plasmalogens (P < 0.05) (Table 5). These findings substantiate the contention that increments in PE-P and PC-P occurred preferentially in HDL3 upon statin treatment.
As previously shown (32), we confirmed that the HDL content of native apoAI and apoAII decreased and that of oxidized forms of apoAI (apoAI + 16, apoAI + 32, apoAII + 16) increased in parallel with progression of AAPH-induced oxidation of HDL2 + LDL and HDL3 + LDL mixtures. These findings suggest that the formation of specifically oxidized forms of apoAI and apoAII in HDL2 and HDL3 is directly related to reduction of PCOOH as observed previously (32).
DISCUSSION
In the context of the CAPITAIN trial (14, 39), we presently evaluated the potential impact of statin treatment on the capacity of HDL to inactivate proinflammatory PCOOHs in mixed dyslipidemic, insulin-resistant subjects in a manner relevant to the pathophysiology of atherosclerotic vascular disease. Specifically, and for the first time to our knowledge, we observed that statin treatment i) reduced the content of oxidizable, PUPC molecular species containing DHA and linoleic acid (LA) in LDL; ii) preferentially increased the content of PC molecular species containing AA in small, dense HDL3 relative to HDL2; iii) induced significant elevation in the content of antioxidant PC and PE plasmalogens containing AA and DHA preferentially in HDL3; and finally, iv) induced formation of HDL3 particles with increased capacity to reduce PCOOH to redox-inactive PCOH, thereby attenuating propagation of lipid peroxidation and formation of potentially atherogenic secondary oxidation products. Interestingly, minor changes occurred in the absolute concentrations of HDL2 and HDL3 on treatment, but did not attain significance; these findings contrast with those of Asztalos et al. (9) who documented preferential increase in large CE-rich α-migrating HDL particles by 2D electrophoresis in patients treated with either atorvastatin, simvastatin, pravastatin, lovastatin, or fluvastatin. The mechanistic basis for such differences in HDL particle profile are indeterminate but may derive from differences between statins on their impact on direct pathways for secretion of HDL particles from the intestine and liver (48). Interestingly, CE/TG ratios in both HDL subfractions increased significantly on pitavastatin treatment, consistent with marked reduction in VLDL-TG levels and in plasma CETP activity (unpublished observations). Critically, our experimental design involved use of the same relative mass concentrations of LDL and HDL subfractions (HDL2 and HDL3) in vitro as those in native plasmas of the MetS subjects pre- and poststatin treatment.
The susceptibility of LDL particles to oxidative modification has multiple, mutually interactive lipid and protein determinants, including content of esterified PUFAs, vitamin E, other small molecules with antioxidant activity, sphingophospholipidome composition, and neutral core lipid composition (29–32, 36, 42–44). Importantly, both 1-electron and 2-electron oxidants (lipophilic and hydrophilic free radicals on the one hand and hypochlorite and peroxynitrite on the other) contribute to LDL oxidation in vivo (49). Thus, oxidized LDL contains multiple products of free-radical-induced lipid peroxidation, and notably, LOOHs, short-chain oxidized PLs and oxidized sterols (49). HDL particles efficaciously inhibit the formation of such primary and secondary peroxidation products in LDL (50). Thus, while there is ample evidence for the presence of oxidatively modified LDL in atherosclerotic plaques in humans and in animal species (38), and while both enzymatic and nonenzymatic mechanisms are implicated in the underlying oxidative processes, HDL protects against the action of a wide spectrum of reactive oxygen species (ROS) and attenuates LDL oxidation in part by removal of seeding LOOH species (21, 22, 24, 26, 27, 32, 34, 51). Central to HDL-mediated antioxidative activity is the direct reduction of LOOHs by methionine residues of apoAI and apoAII, with conversion to residue-specific methionine sulfoxides (apoAI + 16, apoAI + 32, and apoAII + 16) (21, 30–32). Such LOOHs have their origin in LDL; transfer to HDL occurs by aqueous diffusion in the absence of CETP and is limited to PL-derived LOOH (32).
Our experimental approach involved an in vitro model of mild LDL oxidation resembling oxidative processes as they occur in the arterial intima (38, 51). In this way, the rate of formation of free radicals is constant and apolipoprotein oxidation is a secondary process mediated by LOOH (32, 51). Furthermore purified HDL subfractions (HDL2 or HDL3) were added to LDL in the in vitro oxidation system prior to initiation of oxidation itself. Indeed, we established earlier that HDL3 particles protect LDL from oxidative free radical damage via apoAI-mediated reduction of PCOOH to the corresponding redox-inactive PCOH (32). For this reason, we chose to measure the maximum formation of PCOOH and of CDs at the end of the propagation phase in LDL + HDL mixtures at 6 h; the pitavastatin molecule was absent from these oxidation systems in order to exclude any possible endogenous antioxidative activity. It is noteworthy that pitavastatin is an efficacious LDL lowering agent and was used here as a model for the statin class (Table 2).
Multiple molecular mechanisms are implicated in the observed enhancement of the PCOOH-inactivating capacity of statin-induced HDL3, which were concomitant with reduced content of oxidizable PUPC in statin-induced LDL. Indeed, statin treatment favored a reduction (−10% to −18%) in LDL content of four diacyl species of PUPCs (PC 16:0/18:2, PC 16:0/22:6, PC 18:0/18:2, and PC 18:0/22:6); this modification occurred, however, in the absence of change in % weight composition of LDL lipids and protein (Table 2). This finding is not inconsistent with our recent report that plasma enrichment of PC diacylglycerol species containing AA is observed when lipid levels are normalized to nonHDL-C in patients with mixed dyslipidemia and MetS (14).
In contrast to poststatin LDL, the preferential elevation in PUPC (i.e., diacyl PCs 16:0/20:4 and 18:0/20:4) seen in HDL3 can result from several mechanisms. One involves reduction in oxidative PUPC degradation as a result of statin-induced decrement in the production of ROS (52). A second may arise from enhanced protection of HDL3-PUPC against oxidative degradation due to elevated poststatin content of plasmalogens in HDL3 (see below); these lipids are highly effective antioxidants (33–37). Third, pitavastatin has been shown to reduce endothelial lipase (EL) activity by up to 15% (53); this enzyme exerts phospholipase activity on HDL (54), and therefore, we cannot exclude the possibility that statin-mediated inhibition of EL might contribute to sparing of PUPC.
We recently reported a significant trend to normalization of the abnormal plasma lipidome in insulin-resistant, MetS subjects with a high TG/low HDL-C phenotype in the CAPITAIN study and demonstrated a relative enrichment of both the alkyl (ether-linked) PC and PE species as well as of the alkenyl (vinyl ether-linked) species of PE and PC (the latter corresponding specifically to plasmalogens) after 4 mg/day pitavastatin during 26 weeks (14). The present investigations, which focus on vinyl ether-linked plasmalogen species, confirm these data and reveal that statin-induced, small, dense HDL3 are preferentially and significantly enriched in PC-P and PE-P containing AA and DHA at the sn-2 position respectively (PC-P, +12%; PE-P, +24%; PC-P + PE-P, +18%) as compared with LDL and HDL2 in which lesser, nonsignificant changes were observed; values were normalized to nonHDL-C in order to assess these findings independently of change in baseline plasma cholesterol levels (Table 5). Plasmalogens are mainly classified into vinyl-ether choline plasmalogens (PC-P) or ethanolamine plasmalogens (PE-P). The predominant plasmalogen species in plasma LDL, HDL2, and HDL3 were previously shown to be PE-P containing AA (20:4) and DHA (22:6) in the sn-2 position (14, 34, 35); in this context, it is relevant that statin action may upregulate the hepatic biosynthetic pathway from LA to AA, as suggested earlier (14). Interestingly, total concentrations of PC and PE alkyl ethers [PC(O) and PE(O)] resembled those of the total plasmalogens across the three lipoprotein classes (Table 5). It is established that HDL contained the majority (60%) of plasma PE-P, PE-P species containing 20:4 at the sn-2 position predominating as seen earlier (34, 35). Their vinyl-ether bond, coupled with enrichment in AA and DHA at the sn-2 position, endow plasmalogens with unique biological functions in an environment of oxidative stress, such as a reservoir for second messengers and the ability to protect membrane lipids from oxidation by scavenging ROS via the vinyl-ether moiety (33–36). Plasmalogens are consumed during this reaction, leading to the conclusion that they spare the oxidation of unsaturated esterified fatty acids in diacylglycerophospholipids such as PC and PE (33, 35, 36, 55); our present experimental findings confirm major loss of plasmalogens over the oxidation time course. It was equally established that the oxidation products of plasmalogens are unable to further propagate lipid peroxidation (33, 35, 36).
Interestingly, evidence is emerging to link plasma plasmalogen levels on the one hand, and plasma LOOH concentrations on the other, to cardiovascular risk. Thus HDL-PC plasmalogen levels are inversely associated with both stable and acute coronary artery disease; concomitantly, HDL antiapoptotic activity was defective (34). On the other hand, plasma PCOOH levels are 2-fold elevated in hyperlipidemic subjects relative to controls [330 nM vs. 160 nM (56)]; interestingly, under our experimental conditions, we observed a reduction of PCOOH formed after oxidation in the LDL + HDL3 mixture of 58 nM, a value suggestive of potential physiological/pathophysiological relevance. Furthermore, elevated circulating LOOH levels were predictive of nonfatal vascular events and procedures in patients with stable CAD, independent of traditional risk factors and inflammatory markers (57). Indeed, in humans, the ratio of oxidized PL/apoB has been shown to be both a prognostic indicator and a separate risk factor for coronary events (58). Abundant evidence equally attests to the proinflammatory action of oxidized PLs at the arterial wall, and indeed in vivo murine studies suggest that they can be considered as triggers of the inflammatory dimension of atherosclerosis (59).
The statin-mediated mechanisms driving the elevation of plasmalogens in both HDL2 and HDL3, but preferentially in HDL3, are open to speculation. A simple explanation may derive from statin-mediated reduction in systemic oxidative stress, prevalent in MetS (3, 23, 52). However, as the major source of circulating plasmalogens in humans is the liver, and as their synthesis is independent of that of cholesterol, several additional mechanisms may contribute. The possibility that statins may upregulate the early, rate-limiting stages of plasmalogen synthesis in hepatic peroxisomes via activation of PPARs cannot be excluded at this time. Indeed, PPAR activation has been suggested to be an integral component of the pleiotropic effects of statins (60). Increased amounts of plasmalogens so formed could then be secreted in hepatic VLDL particles, with sequestration to the HDL pool as components of surface fragments released upon VLDL hydrolysis; this hypothesis is consistent with the major reduction seen in plasma levels of TGs as well as VLDL and apoCIII (unpublished observations) in the CAPITAIN study, which is indicative in part of enhanced lipolytic degradation of VLDL upon statin treatment. Direct hepatic and/or intestinal secretion of plasmalogen-enriched small HDL may equally contribute to our finding.
It is relevant that the impact of a lower SM/PC ratio observed in statin-induced HDL3 as compared with HDL2 (unpublished observations) may favor enhanced activity of HDL3 as an acceptor of redox-active PCOOH from LDL, given that SM is known to rigidify the HDL surface lipid monolayer (18, 20, 21, 27, 32). Indeed, an elevated SM/PC ratio and a decreased PUFA content can augment HDL surface rigidity, thereby decreasing HDL antioxidative functionality (18, 20, 21, 27, 32). Diacyl PC containing 16:0/20:4 and 18:0/20:4 were increased in HDL3 compared with HDL2, consistent with a higher fluidity of HDL3. Furthermore, we previously reported that HDL3 are enriched in phosphatidylserine (PS) compared with HDL2, thereby improving the interaction of apoAI with oxidized PC (18). Modification of surface lipid architecture involving a lower SM/PC ratio and PS enrichment in HDL3 may confer a conformational change on apoAI in HDL3, leading potentially to an elevated capacity of statin-induced HDL3 to accept and inactivate redox-active PCOOH (18, 32). The superior degree of normalization of the CE/TG core ratio in HDL3 vs HDL2 on statin treatment, reflecting attenuated CETP activity due to concomitant reduction in apoB-containing particle acceptors for HDL CEs, may enhance this effect (Table 2); our suggestion is consistent with earlier studies indicating that the antioxidative function of HDL3 particles is systematically superior to that of HDL2 on a per particle basis (18, 19, 32).
Several conclusions are in order. Thus, pitavastatin treatment of MetS subjects displaying an atherogenic mixed dyslipidemia resulted in i) formation of LDL with attenuated oxidability due to reduction in PUPC content, ii) preferential elevation in the capacity of HDL3 relative to HDL2 to inactivate LDL-derived PCOOH with production of PCOH and termination of the propagation of lipid oxidation, and iii) an elevation in antioxidant plasmalogen content. Overall then, the statin-mediated increment in HDL3 lipidome, and specifically its plasmalogen content, can be considered to be intimately linked to its enhanced capacity to reduce proinflammatory and potentially proatherogenic PCOOH to inactive PCOHs. These findings subscribe then to a potential anti-inflammatory effect of statin treatment, which by virtue of a reduction in circulating levels of oxidized PLs may be atheroprotective.
LIMITATIONS AND FUTURE RESEARCH
Limitations in these studies concern the lack of a statin comparator (14, 39). To what degree the effects observed on the structure, composition, and antioxidative activity of HDL2 and HDL3 might be distinct for pitavastatin as compared with other members of the class of elevated efficacy is indeterminate. Nonetheless, it is relevant that pitavastatin has been reported to enhance the biological activities of HDL in dyslipidemic subjects, as indicated by increase in cellular cholesterol efflux activity and antioxidant activity (as paraoxonase) of HDL (approximately +10% vs. baseline) (61).
Our experimental findings provoke new working hypotheses surrounding the atheroprotective action of statins, which reach well beyond reduction of plasma cholesterol and triacylglycerol levels. Indeed it is plausible that statin-induced, plasmalogen-enriched HDL contribute to attenuation of atherosclerotic vascular disease via enhancement of their capacity to reduce PCOOH, with reduction of the formation of proatherogenic secondary oxidation products. Evidence is equally available for a role of plasmalogens in the antiapoptotic activity of HDL (34). Could upregulation of plasmalogen synthesis therefore represent a therapeutic target to attenuate PCOOH-induced inflammation of the vasculature in subjects presenting with dyslipidemia, premature atherosclerosis, and concomitant oxidative stress? The interface of statin action with plasmalogen and plasma lipoprotein metabolism clearly merits future research focus.
Finally, the clinical significance of our findings in this exploratory study now requires further evaluation in a long term, placebo-controlled intervention study designed to evaluate the potential contribution of HDL biological activities to reduction in cardiovascular risk.
Acknowledgments
The authors gratefully acknowledge the contributions of Dr. P. Betting and colleagues at the Clinical Unit of SGS Aster, Paris, for patient recruitment and follow-up, and of Dr. A. Carrie for apoE genotyping. The authors are indebted to Dr. N. Hounslow for critical discussion of the clinical protocol, Mr. M. Suzuki for critical logistical support in clinical studies, and Ms. N. Mellett for critical support in database analyses.
Footnotes
Abbreviations:
- AA
- arachidonic acid
- AAPH
- 2,2′-azobis(2-methylpropionamidine) dihydrochloride
- CE
- cholesteryl ester
- CETP
- cholesteryl ester transfer protein
- FC
- free cholesterol
- HDL-C
- HDL-cholesterol
- LDL-C
- LDL-cholesterol
- LOOH
- lipid hydroperoxide
- Lp(a)
- lipoprotein (a)
- MetS
- metabolic syndrome
- PC
- phosphatidylcholine
- PC(O)
- alkylphosphatidylcholine
- PCOH
- phospholipid hydroxide
- PCOOH
- phospholipid hydroperoxide
- PC-P
- phosphatidylcholine plasmalogen
- PC(P)
- alkenylphosphatidylcholine
- PE
- phosphatidylethanolamine
- PE(O)
- alkylphosphatidylethanolamine
- PE-P
- phosphatidylethanolamine plasmalogen
- PE(P)
- alkenylphosphatidylethanolamine
- PL
- phospholipid
- PUPC
- polyunsaturated phosphatidylcholine
- ROS
- reactive oxygen species
- SBP
- systolic blood pressure
- TP
- total protein content
The authors are indebted to Kowa Research Europe for the award of a Clinical Research Grant to support all aspects of the CAPITAIN study and lipidomic analyses (ClinicalTrials.gov, #NCT01595828), and to Institut National de la Santé et de la Recherche Médicale, the Nouvelle Société Française d’Athérosclérose, and the Association for Research on Lipoproteins and Atherogenesis (ARLA) for additional support. A. Orsoni gratefully acknowledges the award of a postdoctoral fellowship from ARLA. This work was equally supported by funding from the National Health and Medical Research Council of Australia (NHMRC), the Operational Infrastructure Support (OIS) Program of the State Government of Victoria, Australia. P. J. Meikle is supported by an NHMRC Senior Research Fellowship. The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the aforementioned funding bodies.
REFERENCES
- 1.Baigent C., Blackwell L., Emberson J., Holland L. E., Reith C., Bhala N., Peto R., Barnes E. H., Keech A., Simes J., et al. 2010. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 376: 1670–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nicholls S. J., Ballantyne C. M., Barter P. J., Chapman M. J., Erbel R. M., Libby P., Raichlen J. S., Uno K., Borgman M., Wolski K., et al. 2011. Effect of two intensive statin regimens on progression of coronary disease. N. Engl. J. Med. 365: 2078–2087. [DOI] [PubMed] [Google Scholar]
- 3.Sposito A. C., and Chapman M. J.. 2002. Statin therapy in acute coronary syndromes: mechanistic insight into clinical benefit. Arterioscler. Thromb. Vasc. Biol. 22: 1524–1534. [DOI] [PubMed] [Google Scholar]
- 4.Goldstein J. L., and Brown M. S.. 2015. A century of cholesterol and coronaries: from plaques to genes to statins. Cell. 161: 161–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barter P. J., Brandrup-Wognsen G., Palmer M. K., and Nicholls S. J.. 2010. Effect of statins on HDL-C: a complex process unrelated to changes in LDL-C: analysis of the VOYAGER Database. J. Lipid Res. 51: 1546–1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hegele R. A., Ginsberg H. N., Chapman M. J., Nordestgaard B. G., Kuivenhoven J. A., Averna M., Boren J., Bruckert E., Catapano A. L., Descamps O. S., et al. 2014. The polygenic nature of hypertriglyceridaemia: implications for definition, diagnosis, and management. Lancet Diabetes Endocrinol. 2: 655–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jones P. H., Davidson M. H., Stein E. A., Bays H. E., McKenney J. M., Miller E., Cain V. A., and Blasetto J. W.. 2003. Comparison of the efficacy and safety of rosuvastatin versus atorvastatin, simvastatin, and pravastatin across doses (STELLAR* Trial). Am. J. Cardiol. 92: 152–160. [DOI] [PubMed] [Google Scholar]
- 8.Nordestgaard B. G., and Varbo A.. 2014. Triglycerides and cardiovascular disease. Lancet. 384: 626–635. [DOI] [PubMed] [Google Scholar]
- 9.Asztalos B. F., Horvath K. V., McNamara J. R., Roheim P. S., Rubinstein J. J., and Schaefer E. J.. 2002. Comparing the effects of five different statins on the HDL subpopulation profiles of coronary heart disease patients. Atherosclerosis. 164: 361–369. [DOI] [PubMed] [Google Scholar]
- 10.Nicholls S. J., Tuzcu E. M., Sipahi I., Grasso A. W., Schoenhagen P., Hu T., Wolski K., Crowe T., Desai M. Y., Hazen S. L., et al. 2007. Statins, high-density lipoprotein cholesterol, and regression of coronary atherosclerosis. JAMA. 297: 499–508. [DOI] [PubMed] [Google Scholar]
- 11.Rye K. A., and Barter P. J.. 2014. Regulation of high-density lipoprotein metabolism. Circ. Res. 114: 143–156. [DOI] [PubMed] [Google Scholar]
- 12.Chapman M. J., Le Goff W., Guerin M., and Kontush A.. 2010. Cholesteryl ester transfer protein: at the heart of the action of lipid-modulating therapy with statins, fibrates, niacin, and cholesteryl ester transfer protein inhibitors. Eur. Heart J. 31: 149–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liao J. K. 2002. Isoprenoids as mediators of the biological effects of statins. J. Clin. Invest. 110: 285–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meikle P. J., Wong G., Tan R., Giral P., Robillard P., Orsoni A., Hounslow N., Magliano D. J., Shaw J. E., Curran J. E., et al. 2015. Statin action favors normalization of the plasma lipidome in the atherogenic mixed dyslipidemia of MetS: potential relevance to statin-associated dysglycemia. J. Lipid Res. 56: 2381–2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ng T. W., Ooi E. M., Watts G. F., Chan D. C., Weir J. M., Meikle P. J., and Barrett P. H.. 2014. Dose-dependent effects of rosuvastatin on the plasma sphingolipidome and phospholipidome in the metabolic syndrome. J. Clin. Endocrinol. Metab. 99: E2335–E2340. [DOI] [PubMed] [Google Scholar]
- 16.Caslake M. J., Stewart G., Day S. P., Daly E., McTaggart F., Chapman M. J., Durrington P., Laggner P., Mackness M., Pears J., et al. 2003. Phenotype-dependent and -independent actions of rosuvastatin on atherogenic lipoprotein subfractions in hyperlipidaemia. Atherosclerosis. 171: 245–253. [DOI] [PubMed] [Google Scholar]
- 17.Guerin M., Lassel T. S., Le Goff W., Farnier M., and Chapman M. J.. 2000. Action of atorvastatin in combined hyperlipidemia: preferential reduction of cholesteryl ester transfer from HDL to VLDL1 particles. Arterioscler. Thromb. Vasc. Biol. 20: 189–197. [DOI] [PubMed] [Google Scholar]
- 18.Camont L., Lhomme M., Rached F., Le Goff W., Negre-Salvayre A., Salvayre R., Calzada C., Lagarde M., Chapman M. J., and Kontush A.. 2013. Small, dense high-density lipoprotein-3 particles are enriched in negatively charged phospholipids: relevance to cellular cholesterol efflux, antioxidative, antithrombotic, anti-inflammatory, and antiapoptotic functionalities. Arterioscler. Thromb. Vasc. Biol. 33: 2715–2723. [DOI] [PubMed] [Google Scholar]
- 19.Kontush A., Lhomme M., and Chapman M. J.. 2013. Unraveling the complexities of the HDL lipidome. J. Lipid Res. 54: 2950–2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kontush A., Lindahl M., Lhomme M., Calabresi L., Chapman M. J., and Davidson W. S.. 2015. Structure of HDL: particle subclasses and molecular components. Handb. Exp. Pharmacol. 224: 3–51. [DOI] [PubMed] [Google Scholar]
- 21.Camont L., Chapman M. J., and Kontush A.. 2011. Biological activities of HDL subpopulations and their relevance to cardiovascular disease. Trends Mol. Med. 17: 594–603. [DOI] [PubMed] [Google Scholar]
- 22.de Souza J. A., Vindis C., Hansel B., Nègre-Salvayre A., Therond P., Serrano C. V. Jr., Chantepie S., Salvayre R., Bruckert E., Chapman M. J., et al. 2008. Metabolic syndrome features small, apolipoprotein A-I-poor, triglyceride-rich HDL3 particles with defective anti-apoptotic activity. Atherosclerosis. 197: 84–94. [DOI] [PubMed] [Google Scholar]
- 23.Hansel B., Giral P., Nobecourt E., Chantepie S., Bruckert E., Chapman M. J., and Kontush A.. 2004. Metabolic syndrome is associated with elevated oxidative stress and dysfunctional dense high-density lipoprotein particles displaying impaired antioxidative activity. J. Clin. Endocrinol. Metab. 89: 4963–4971. [DOI] [PubMed] [Google Scholar]
- 24.Kontush A., and Chapman M. J.. 2006. Functionally defective high-density lipoprotein: a new therapeutic target at the crossroads of dyslipidemia, inflammation, and atherosclerosis. Pharmacol. Rev. 58: 342–374. [DOI] [PubMed] [Google Scholar]
- 25.Nobécourt E., Jacqueminet S., Hansel B., Chantepie S., Grimaldi A., Chapman M. J., and Kontush A.. 2005. Defective antioxidative activity of small dense HDL3 particles in type 2 diabetes: relationship to elevated oxidative stress and hyperglycaemia. Diabetologia. 48: 529–538. [DOI] [PubMed] [Google Scholar]
- 26.Riwanto M., Rohrer L., von Eckardstein A., and Landmesser U.. 2015. Dysfunctional HDL: from structure-function-relationships to biomarkers. Handb. Exp. Pharmacol. 224: 337–366. [DOI] [PubMed] [Google Scholar]
- 27.Rosenson R. S., Brewer H. B. Jr., Ansell B. J., Barter P., Chapman M. J., Heinecke J. W., Kontush A., Tall A. R., and Webb N. R.. 2016. Dysfunctional HDL and atherosclerotic cardiovascular disease. Nat. Rev. Cardiol. 13: 48–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alberti G., Zimmet P., Shaw J., and Grundy S. M.. 2006. The IDF Consensus Worldwide Definition of the Metabolic Syndrome. International Diabetes Federation, Brussels, Belgium. [Google Scholar]
- 29.Chapman M. J., Ginsberg H. N., Amarenco P., Andreotti F., Boren J., Catapano A. L., Descamps O. S., Fisher E., Kovanen P. T., Kuivenhoven J. A., et al. 2011. Triglyceride-rich lipoproteins and high-density lipoprotein cholesterol in patients at high risk of cardiovascular disease: evidence and guidance for management. Eur. Heart J. 32: 1345–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Garner B., Waldeck A. R., Witting P. K., Rye K. A., and Stocker R.. 1998. Oxidation of high density lipoproteins. II. Evidence for direct reduction of lipid hydroperoxides by methionine residues of apolipoproteins AI and AII. J. Biol. Chem. 273: 6088–6095. [DOI] [PubMed] [Google Scholar]
- 31.Garner B., Witting P. K., Waldeck A. R., Christison J. K., Raftery M., and Stocker R.. 1998. Oxidation of high density lipoproteins. I. Formation of methionine sulfoxide in apolipoproteins AI and AII is an early event that accompanies lipid peroxidation and can be enhanced by alpha-tocopherol. J. Biol. Chem. 273: 6080–6087. [DOI] [PubMed] [Google Scholar]
- 32.Zerrad-Saadi A., Therond P., Chantepie S., Couturier M., Rye K-A., Chapman M. J., and Kontush A.. 2009. HDL3-mediated inactivation of LDL-associated phospholipid hydroperoxides is determined by the redox status of apolipoprotein A-I and HDL particle surface lipid rigidity: relevance to inflammation and atherogenesis. Arterioscler. Thromb. Vasc. Biol. 29: 2169–2175. [DOI] [PubMed] [Google Scholar]
- 33.Reiss D., Beyer K., and Engelmann B.. 1997. Delayed oxidative degradation of polyunsaturated diacyl phospholipids in the presence of plasmalogen phospholipids in vitro. Biochem. J. 323: 807–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sutter I., Velagapudi S., Othman A., Riwanto M., Manz J., Rohrer L., Rentsch K., Hornemann T., Landmesser U., and von Eckardstein A.. 2015. Plasmalogens of high-density lipoproteins (HDL) are associated with coronary artery disease and anti-apoptotic activity of HDL. Atherosclerosis. 241: 539–546. [DOI] [PubMed] [Google Scholar]
- 35.Wallner S., and Schmitz G.. 2011. Plasmalogens the neglected regulatory and scavenging lipid species. Chem. Phys. Lipids. 164: 573–589. [DOI] [PubMed] [Google Scholar]
- 36.Engelmann B. 2004. Plasmalogens: targets for oxidants and major lipophilic antioxidants. Biochem. Soc. Trans. 32: 147–150. [DOI] [PubMed] [Google Scholar]
- 37.Hahnel D., Thiery J., Brosche T., and Engelmann B.. 1999. Role of plasmalogens in the enhanced resistance of LDL to copper-induced oxidation after LDL apheresis. Arterioscler. Thromb. Vasc. Biol. 19: 2431–2438. [DOI] [PubMed] [Google Scholar]
- 38.Ylä-Herttuala S., Palinski W., Rosenfeld M. E., Parthasarathy S., Carew T. E., Butler S., Witztum J. L., and Steinberg D.. 1989. Evidence for the presence of oxidatively modified low density lipoprotein in atherosclerotic lesions of rabbit and man. J. Clin. Invest. 84: 1086–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chapman M. J., Orsoni A., Robillard P., Hounslow N., Sponseller C. A., and Giral P.. 2014. Effect of high-dose pitavastatin on glucose homeostasis in patients at elevated risk of new-onset diabetes: insights from the CAPITAIN and PREVAIL-US studies. Curr. Med. Res. Opin. 30: 775–784. [DOI] [PubMed] [Google Scholar]
- 40.Rumsey S. C., Stucchi A. F., Nicolosi R. J., Ginsberg H. N., Ramakrishnan R., and Deckelbaum R. J.. 1994. Human plasma LDL cryopreserved with sucrose maintains in vivo kinetics indistinguishable from freshly isolated human LDL in cynomolgus monkeys. J. Lipid Res. 35: 1592–1598. [PubMed] [Google Scholar]
- 41.Kontush A., Chantepie S., and Chapman M. J.. 2003. Small, dense HDL particles exert potent protection of atherogenic LDL against oxidative stress. Arterioscler. Thromb. Vasc. Biol. 23: 1881–1888. [DOI] [PubMed] [Google Scholar]
- 42.Orsoni A., Saheb S., Levels J. H. M., Dallinga-Thie G., Atassi M., Bittar R., Robillard P., Bruckert E., Kontush A., Carrié A., et al. 2011. LDL-apheresis depletes apoE-HDL and pre-β1-HDL in familial hypercholesterolemia: relevance to atheroprotection. J. Lipid Res. 52: 2304–2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bowry V. W., and Stocker R.. 1993. Tocopherol-mediated peroxidation. The prooxidant effect of vitamin E on the radical-initiated oxidation of human low-density lipoprotein. J. Am. Chem. Soc. 115: 6029–6044. [Google Scholar]
- 44.Chancharme L., Therond P., Nigon F., Lepage S., Couturier M., and Chapman M. J.. 1999. Cholesteryl ester hydroperoxide lability is a key feature of the oxidative susceptibility of small, dense LDL. Arterioscler. Thromb. Vasc. Biol. 19: 810–820. [DOI] [PubMed] [Google Scholar]
- 45.Khaselev N., and Murphy R. C.. 1999. Susceptibility of plasmenyl glycerophosphoethanolamine lipids containing arachidonate to oxidative degradation. Free Radic. Biol. Med. 26: 275–284. [DOI] [PubMed] [Google Scholar]
- 46.Weir J. M., Wong G., Barlow C. K., Greeve M. A., Kowalczyk A., Almasy L., Comuzzie A. G., Mahaney M. C., Jowett J. B. M., Shaw J., et al. 2013. Plasma lipid profiling in a large population-based cohort. J. Lipid Res. 54: 2898–2908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bergmark C., Dewan A., Orsoni A., Merki E., Miller E. R., Shin M-J., Binder C. J., Hörkkö S., Krauss R. M., Chapman M. J., et al. 2008. A novel function of lipoprotein [a] as a preferential carrier of oxidized phospholipids in human plasma. J. Lipid Res. 49: 2230–2239. [DOI] [PubMed] [Google Scholar]
- 48.Mendivil C. O., Furtado J., Morton A. M., Wang L., and Sacks F. M.. 2016. Novel pathways of apolipoprotein A-I metabolism in high-density lipoprotein of different sizes in humans. Arterioscler. Thromb. Vasc. Biol. 36: 156–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stocker R., and Keaney J. F.. 2004. Role of oxidative modifications in atherosclerosis. Physiol. Rev. 84: 1381–1478. [DOI] [PubMed] [Google Scholar]
- 50.Navab M., Ananthramaiah G. M., Reddy S. T., Van Lenten B. J., Ansell B. J., Fonarow G. C., Vahabzadeh K., Hama S., Hough G., Kamranpour N., et al. 2004. The oxidation hypothesis of atherogenesis: the role of oxidized phospholipids and HDL. J. Lipid Res. 45: 993–1007. [DOI] [PubMed] [Google Scholar]
- 51.Navab M., Hama S. Y., Cooke C. J., Anantharamaiah G. M., Chaddha M., Jin L., Subbanagounder G., Faull K. F., Reddy S. T., Miller N. E., et al. 2000. Normal high density lipoprotein inhibits three steps in the formation of mildly oxidized low density lipoprotein: step 1. J. Lipid Res. 41: 1481–1494. [PubMed] [Google Scholar]
- 52.Lim S., and Barter P.. 2014. Antioxidant effects of statins in the management of cardiometabolic disorders. J. Atheroscler. Thromb. 21: 997–1010. [DOI] [PubMed] [Google Scholar]
- 53.Kojima Y., Ishida T., Sun L., Yasuda T., Toh R., Rikitake Y., Fukuda A., Kume N., Koshiyama H., Taniguchi A., et al. 2010. Pitavastatin decreases the expression of endothelial lipase both in vitro and in vivo. Cardiovasc. Res. 87: 385–393. [DOI] [PubMed] [Google Scholar]
- 54.Broedl U. C., Maugeais C., Millar J. S., Jin W., Moore R. E., Fuki I. V., Marchadier D., Glick J. M., and Rader D. J.. 2004. Endothelial lipase promotes the catabolism of ApoB-containing lipoproteins. Circ. Res. 94: 1554–1561. [DOI] [PubMed] [Google Scholar]
- 55.Sindelar P. J., Guan Z., Dallner G., and Ernster L.. 1999. The protective role of plasmalogens in iron-induced lipid peroxidation. Free Radic. Biol. Med. 26: 318–324. [DOI] [PubMed] [Google Scholar]
- 56.Kinoshita M., Oikawa S., Hayasaka K., Sekikawa A., Nagashima T., Toyota T., and Miyazawa T.. 2000. Age-related increases in plasma phosphatidylcholine hydroperoxide concentrations in control subjects and patients with hyperlipidemia. Clin. Chem. 46: 822–828. [PubMed] [Google Scholar]
- 57.Walter M. F., Jacob R. F., Bjork R. E., Jeffers B., Buch J., Mizuno Y., Mason R. P. ; and PREVENT Investigators. 2008. Circulating lipid hydroperoxides predict cardiovascular events in patients with stable coronary artery disease: the PREVENT study. J. Am. Coll. Cardiol. 51: 1196–1202. [DOI] [PubMed] [Google Scholar]
- 58.Berliner J. A., Leitinger N., and Tsimikas S.. 2009. The role of oxidized phospholipids in atherosclerosis. J. Lipid Res. 50 (Suppl.): S207–S212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Furnkranz A., Schober A., Bochkov V. N., Bashtrykov P., Kronke G., Kadl A., Binder B. R., Weber C., and Leitinger N.. 2005. Oxidized phospholipids trigger atherogenic inflammation in murine arteries. Arterioscler. Thromb. Vasc. Biol. 25: 633–638. [DOI] [PubMed] [Google Scholar]
- 60.Paumelle R., and Staels B.. 2007. Peroxisome proliferator-activated receptors mediate pleiotropic actions of statins. Circ. Res. 100: 1394–1395. [DOI] [PubMed] [Google Scholar]
- 61.Miyamoto-Sasaki M., Yasuda T., Monguchi T., Nakajima H., Mori K., Toh R., Ishida T., and Hirata K.. 2013. Pitavastatin increases HDL particles functionally preserved with cholesterol efflux capacity and antioxidative actions in dyslipidemic patients. J. Atheroscler. Thromb. 20: 708–716. [DOI] [PubMed] [Google Scholar]