Abstract
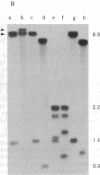
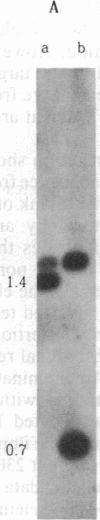
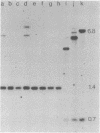
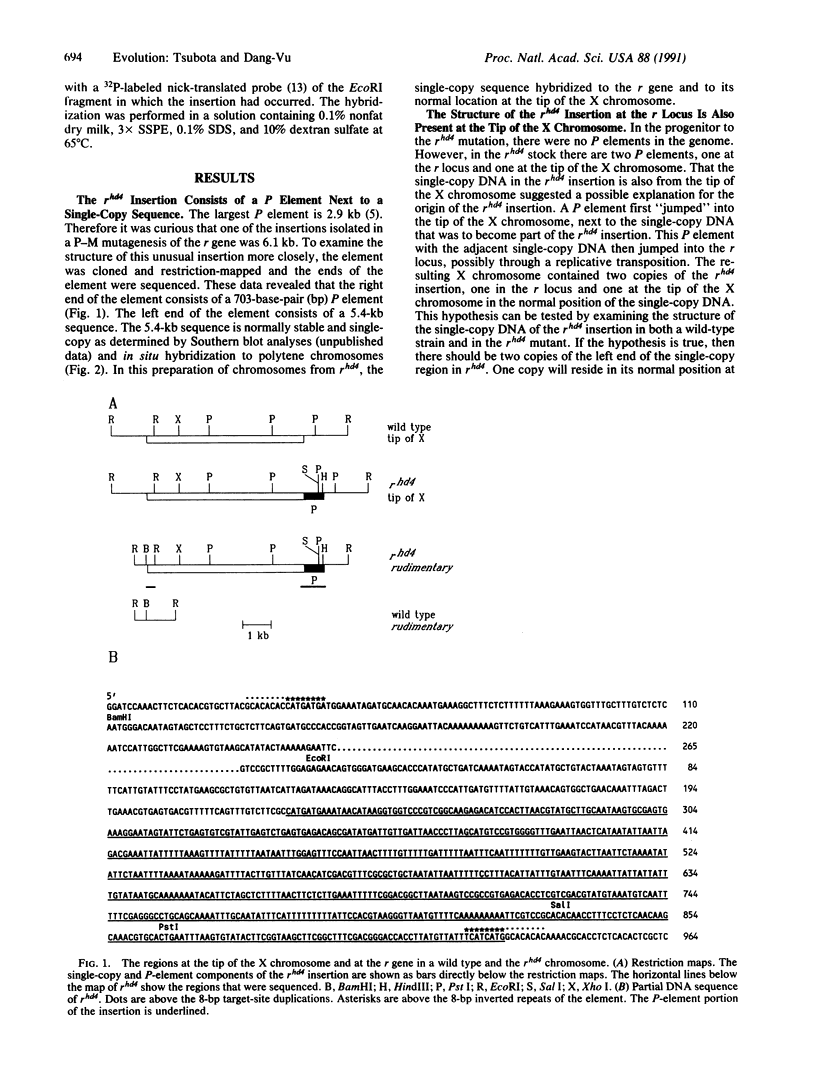
A 6.1-kilobase insertion into the rudimentary (r) gene was cloned and partially sequenced. The insertion consists of a 703-base-pair (bp) P element next to a 5.4-kilobase single-copy sequence. The normal position of the single-copy sequence is near the tip of the X chromosome. Upon insertion into the r gene, this chimeric element generated an 8-bp target-site duplication, characteristic of P elements. At the non-P-element end of the insertion, the first 8 bp are identical to the first 8 bp of the inverted terminal repeats of the P element. Thus, this element has inverted terminal repeats of 8 bp. This large element can excise from the r gene under conditions of hybrid dysgenesis, which indicates that it behaves like a normal P element. These data support the conclusion that a normally stable single-copy sequence has now become unstable and duplicated within the genome.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bingham P. M., Kidwell M. G., Rubin G. M. The molecular basis of P-M hybrid dysgenesis: the role of the P element, a P-strain-specific transposon family. Cell. 1982 Jul;29(3):995–1004. doi: 10.1016/0092-8674(82)90463-9. [DOI] [PubMed] [Google Scholar]
- Bingham P. M., Levis R., Rubin G. M. Cloning of DNA sequences from the white locus of D. melanogaster by a novel and general method. Cell. 1981 Sep;25(3):693–704. doi: 10.1016/0092-8674(81)90176-8. [DOI] [PubMed] [Google Scholar]
- Karess R. E., Rubin G. M. Analysis of P transposable element functions in Drosophila. Cell. 1984 Aug;38(1):135–146. doi: 10.1016/0092-8674(84)90534-8. [DOI] [PubMed] [Google Scholar]
- Kidwell M. G., Kidwell J. F., Sved J. A. Hybrid Dysgenesis in DROSOPHILA MELANOGASTER: A Syndrome of Aberrant Traits Including Mutation, Sterility and Male Recombination. Genetics. 1977 Aug;86(4):813–833. doi: 10.1093/genetics/86.4.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins M. C., Rio D. C., Rubin G. M. cis-acting DNA sequence requirements for P-element transposition. Genes Dev. 1989 May;3(5):729–738. doi: 10.1101/gad.3.5.729. [DOI] [PubMed] [Google Scholar]
- O'Hare K., Rubin G. M. Structures of P transposable elements and their sites of insertion and excision in the Drosophila melanogaster genome. Cell. 1983 Aug;34(1):25–35. doi: 10.1016/0092-8674(83)90133-2. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Rubin G. M., Kidwell M. G., Bingham P. M. The molecular basis of P-M hybrid dysgenesis: the nature of induced mutations. Cell. 1982 Jul;29(3):987–994. doi: 10.1016/0092-8674(82)90462-7. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Tsubota S., Ashburner M., Schedl P. P-element-induced control mutations at the r gene of Drosophila melanogaster. Mol Cell Biol. 1985 Oct;5(10):2567–2574. doi: 10.1128/mcb.5.10.2567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsubota S., Schedl P. Hybrid dysgenesis-induced revertants of insertions at the 5' end of the rudimentary gene in Drosophila melanogaster: transposon-induced control mutations. Genetics. 1986 Sep;114(1):165–182. doi: 10.1093/genetics/114.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voelker R. A., Greenleaf A. L., Gyurkovics H., Wisely G. B., Huang S. M., Searles L. L. Frequent Imprecise Excision among Reversions of a P Element-Caused Lethal Mutation in Drosophila. Genetics. 1984 Jun;107(2):279–294. doi: 10.1093/genetics/107.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]