Abstract
Background/Aim
Live attenuated vaccines confer partial protection in pigs before the appearance of neutralizing antibodies, suggesting the contribution of cell-mediated immunity (CMI). However, PRRSV-specific T-lymphocyte responses and protective mechanisms need to be further defined. To this end, the hypothesis was tested that PRRSV-specific T-lymphocytes induced by exposure to type-2 PRRSV can recognize diverse isolates.
Methods
An IFN-gamma ELISpot assay was used to enumerate PRRSV-specific T-lymphocytes from PRRSVSD23983-infected gilts and piglets born after in utero infection against 12 serologically and genetically distinct type-1 and -2 PRRSV isolates. The IFN-gamma ELISpot assay using synthetic peptides spanning all open reading frames of PRRSVSD23983 was utilized to localize epitopes recognized by T-lymphocytes. Virus neutralization tests were carried out using the challenge strain (type-2 PRRSVSD23983) and another strain (type-2 PRRSVVR2332) with high genetic similarity to evaluate cross-reactivity of neutralizing antibodies in gilts after PRRSVSD23983 infection.
Results
At 72 days post infection, T-lymphocytes from one of three PRRSVSD23983-infected gilts recognized all 12 diverse PRRSV isolates, while T-lymphocytes from the other two gilts recognized all but one isolate. Furthermore, five of nine 14-day-old piglets infected in utero with PRRSVSD23983 had broadly reactive T-lymphocytes, including one piglet that recognized all 12 isolates. Overlapping peptides encompassing all open reading frames of PRRSVSD23983 were used to identify ≥28 peptides with T-lymphocyte epitopes from 10 viral proteins. This included one peptide from the M protein that was recognized by T-lymphocytes from all three gilts representing two completely mismatched MHC haplotypes. In contrast to the broadly reactive T-lymphocytes, neutralizing antibody responses were specific to the infecting PRRSVSD23983 isolate.
Conclusion
These results demonstrated that T-lymphocytes recognizing antigenically and genetically diverse isolates were induced by infection with a type 2 PRRSV strain (SD23983). If these reponses have cytotoxic or other protective functions, they may help overcome the suboptimal heterologous protection conferred by conventional vaccines.
Introduction
Porcine reproductive and respiratory syndrome (PRRS) is one of the most economically important swine diseases worldwide. In the United States, direct economic loss due to PRRS is estimated to exceed $600 million per year [1,2]. PRRS virus (PRRSV) is an arterivirus in the Arteriviridae family with a single-stranded positive sense RNA genome of ~15 Kb encoding 10 open reading frames [3–5]. Rapidly and continuously increasing genetic diversity among PRRSV isolates [3,6,7] and emergence of highly pathogenic variants in different geographical regions [8–19] present a tremendous challenge to the control of PRRSV using conventional vaccines.
Both inactivated and live attenuated virus vaccines are used to aid PRRS control in swine herds, but the efficacy and/or safety of current licensed vaccines are not satisfactory [20–22]. Inactivated PRRSV vaccines induce weak neutralizing antibody responses even against homologous isolates and weaker to no response against heterologous isolates [21,23,24]. Live attenuated PRRSV vaccines contribute to clinical protection by unknown mechanisms without preventing infection, but a high probability of reversion to virulence is a major safety concern [9,25,26]. To better control PRRSV infections worldwide, it is crucial to develop a safer and more efficacious vaccine that confers protective immunity against diverse PRRSV isolates.
There have been numerous attempts to determine if neutralizing antibody provides protective immunity against PRRSV [27–33]. Passive transfer of hyperimmune serum containing high titer neutralizing antibody against PRRSV controlled viremia in pigs challenged with homologous PRRSV, but was not able to prevent viral replication in tissues [31,34]. In addition, neutralizing antibodies did not provide protection against heterologous challenge [32]. Several studies have found that immunization with live attenuated and killed PRRSV vaccines reduced clinical disease and/or viremia in pigs after PRRSV challenge, before the appearance of neutralizing antibody responses [29,30,33,35–37]. These observations, as well as the co-existence of infectious PRRSV and low titer neutralizing antibodies in the blood of infected pigs [27,28,38,39], lead to the conclusion that neutralizing antibodies alone are not potent enough to control PRRSV infection in pigs recovered from natural infections or vaccinated pigs.
Other mechanisms such as cell-mediated immunity (CMI) may contribute to control of PRRSV. Such correlates of protective immunity have not been clearly defined. Therefore, systematic approaches are needed to evaluate if CMI is more important than humoral immunity in the control of infections with diverse PRRSV isolates. A crucial step for defining the relative advantage of CMI versus humoral immunity in PRRSV-infected pigs is to determine if CMI has the potential to overcome the challenge of antigenic variation between diverse isolates. In this study, cross reactive T-lymphocyte responses against genetically and antigenically divergent PRRSV isolates were demonstrated in two pig groups of different ages using an interferon (IFN)-gamma ELISpot assay. Peptides containing T-lymphocyte epitopes were mapped to PRRSV proteins with several occurring in the M protein, one of which was recognized by T-lymphocytes from all three gilts used for mapping. These observations should help define both cytotoxic and helper T-lymphocyte epitopes that may be used for development of improved and novel PRRSV vaccines that provide protection against genetically and antigenically diverse isolates.
Methods
Sequencing and phylogenetic analysis of PRRSV open reading frame 5 (ORF5)
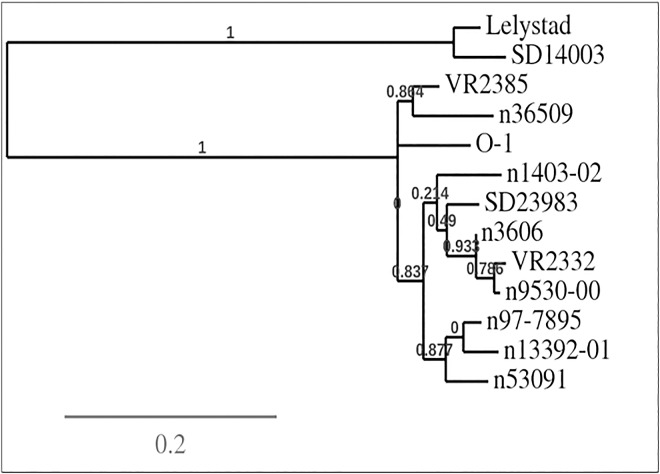
The extraction of viral RNA from three PRRSV isolates (O-1, 3606, 14003), reverse transcription (RT) and polymerase chain reaction (PCR) amplification of the gene encoded by ORF5, and DNA sequencing were carried out as previously described [40]. Briefly, total RNA was extracted from each of virus culture supernatants using the viral RNA isolation kit from Life Technologies (Grand Island, NY, USA). The RT reaction mixture consisted of 13 μL total RNA, 1 μL of 10 mM random primer (New England Biolabs, Ipswich, MA, USA), 1 μL (50 U) of reverse transcriptase (Promega, Madison, WI, USA), 4 μL of 5× buffer (Promega), and 1 μL of 10 mM dNTPs (Life Technologies). Reverse transcription was conducted at 37°C for 30 minutes using a thermal cycler (Applied Biosystems model 2720, Life Technologies) to produce full-length cDNA. ORF5 sequences of three PRRSV strains were then PCR amplified from cDNA using 1 μL of each of 100 μM concentration primers (VR-F: TTAGCCTGTCTTTTTGCCATTCT and VR-R: TGT GGAGCCGTGCTATCAT for type-2 PRRSVs [O-1, 3606]; LV-F: ATTGCTTGTTTGTTCGCCA, LV-R: AAGGCTAGCACGAGCTTTTGT for type-1 PRRSV [SD14003]) and 9 μL of PRRSV cDNAs in a PCR mixture which contained 0.4 μL of 5U/μL high fidelity DNA polymerase (Life Technologies), 5 μL of 10X buffer, 5 μL of 50 mM MgSO4 (Life Technologies), 5 μL of 10 mM dNTPs, and 23.6 μL of distilled water. PCR cycles were 94°C for 5 minutes, followed by 35 cycles of 94°C for 1 minute, 53°C for 1 minute, 72°C for 1 minute, and a final extension at 72°C for 10 minutes. The PCR products were purified using the QIAquick® Gel Extraction kit (QIAGEN, Hilden, Germany) and used for sequencing (Eurofins, Louisville, USA). Phylogenetic analyses were conducted of the three ORF5 sequences determined in this study coupled with 8 other sequences from GenBank (VR-2332: EF536003.1; SD23983: JX258843.1; 13392–01: EU556196.1; 53091: EU556181.1; 36509: EU556172.1; 97–7895: Ay545985.1; 1403–02: EU556164.1; 9530: EU556164.1; Lelystad: M96262.2; VR-2385: AFN88229.1) using PhyML 3.0 software (http://phylogeny.lirmm.fr) [41]. This program package consists of sequence alignment (MUSCLESetting), curation (GblocksSetting), phylogeny analysis (PhyMLSetting), and Tree Rendering (TreeDyn)Setting). These twelve PRRSV isolates were selected to stimulate T-lymphocytes based on significant genetic distances illustrated in Fig 1 and on previously reported serological distinctions [42].
Fig 1. Phylogenetic analysis using open reading frame 5 sequence of 12 serologically distinct PRRSV isolates.
Phylogenetic analyses were conducted using PhyML 3.0 software. Numbers in the figure represent Robinson and Foulds distance [69] measuring the topological difference between true and inferred trees.
Preparation of PRRS viruses
Twelve PRRSV isolates (VR-2332 [43], SD23983 [44], 1403–02, 13392–01, 97–7895 [45], 53091, O-1, 9530–00, 3606, 36509, Lelystad [46], SD14003) were used in this study. Lelystad and SD14003 were type-1 PRRSV and all others were type-2 PRRSV. All viruses were propagated in the MARC-145 cell line [47], known to be highly permissive to PRRSV. Confluent cell monolayers prepared in 150 cm2 tissue culture flasks were inoculated with a 0.01–0.1 multiplicity of infection. Virus-induced cytopathic effect (CPE) reached the maximum after incubation at 37°C in Eagle’s Modified Essential Medium (EMEM; Corning, Manassas, VA, USA) supplemented with 10% fetal bovine serum (FBS; Seradigm, Radnor, PA, USA) for five to seven days, at which point culture supernatants were combined and centrifuged at 4,000 × g for 10 minutes at 4°C to eliminate cell debris.
The titer of PRRSV in each cell culture supernatant was determined using a microtitration infectivity assay and the median tissue culture infective dose (TCID50) calculated according to the method of Reed and Muench [48]. Briefly, confluent monolayers of MARC-145 cells prepared in 96-well plates (Corning) were inoculated with 10-fold serial dilutions of PRRSV prepared in EMEM with 10% FBS. The development of CPE in the cells was monitored daily for seven days and the results were used to calculate TCID50/mL.
PRRSV-infected pigs
Three 12-month-old gilts (numbered 1–3) from the same dam were determined to be negative for porcine circovirus, porcine epidemic diarrhea virus, and PRRSV by realtime PCR and immunofluorescent antibody assay. They were then intranasally inoculated with 1.6 x 104 TCID50 of PRRSVSD23983 and subsequently maintained in isolation rooms at Washington State University for five months. All three gilts demonstrated clinical disease in the form of mild nasal discharge but recovered clinically within two weeks after PRRSV infection. Of three gilts, gilt-2 had positive viremia by realtime PCR at 2 (23.1 Ct), 4 (23.5 Ct), 6 (32.2 Ct), 8 (35.3 Ct) and 12 (35.5 Ct) dpi, and negative viremia (≥37.0 Ct) after 12 dpi. Gilt-3 had weak positive viremia (36.0 Ct) at 4 dpi only and negative viremia at later dpi. Gilt-1 had no detectable viremia (≥37.0 Ct) at all tested dpi although appearance of PRRSV-specific antibodies after intranasal inoculation of PRRSV SD23983 clearly demonstrates the infection. These gilts were the source of peripheral blood mononuclear cells (PBMC) collected at 30–33, 44, 58 and 72 days post-infection (dpi) for in vitro stimulation with PRRSV or peptides. Gilt 1 was artificially inseminated with semen of a PRRSV-negative boar and then infected with the virus at approximately 98 days of gestation. Nine piglets (numbered P13-P21) were born to gilt 1 at approximately 117 days of gestation and confirmed PRRSV-infected at birth prior to ingestion of colostrum, indicating in utero infection. PBMC were also collected from the nine piglets at 14 days of age (33 dpi). The animal use protocols for these animal studies were approved by the Institutional Animal Care and Use Committees at Washington State University. Proper sedation and anesthesia were used to minimize potential pain in sampling procedure from the gilts used in this study.
MHC class I and II typing of gilts and piglets
Gilts 1–3 and the nine piglets used in this study were genotyped for three swine leukocyte antigen (SLA) class I genes (SLA-1, -2, -3) and three class II genes (DRB1, DQB1, DQA) using the low resolution PCR-SSP (polymerase chain reaction with sequence-specific primers) typing panels as previously described [49,50]. Low resolution SLA class I and class II haplotypes were deduced based on published (15713212, 16305679, 18760302, 19317739) and unpublished (http://www.jimmunol.org/cgi/content/meetingabstract/186/1_MeetingAbstracts/170.1) haplotypes identified in outbred commercial pigs.
Preparation of peripheral blood mononuclear cells and storage
Swine PBMC from fresh venous blood collected in 5 mM EDTA (final concentration) were isolated by centrifugation on a discontinuous gradient using Lymphoprep™ (STEMCELL™ Technologies, Vancouver, Canada) as previously described [51]. Briefly, blood with 5 mM EDTA was diluted two-fold in 10mM phosphate-buffered saline (PBS, pH 7.4) containing 20% anti-coagulant citrate dextrose (ACD) and 2% FBS. Thirty-five milliliters of the diluted blood was added to a SepMate™-50 tube (STEMCELL Technologies) containing 15 mL of Lymphoprep™ and the tubes were centrifuged at 1,100 × g for 10 minutes. The buffy coat cells within the supernatant were collected and rinsed with PBS containing 20% ACD and 2% FBS until platelets were removed. Isolated PBMCs were counted, resuspended in a freezing medium containing 10% dimethyl sulfoxide (J.T. Baker, Center Valley, PA, USA) and 90% FBS, and stored in liquid nitrogen until use.
IFN-gamma enzyme-linked immunospot (ELISpot) assay
The IFN-gamma ELISpot assay used to define PRRSV-specific T-lymphocyte responses was performed using a commercial product (Mabtech, Cincinnati, OH, USA) as per manufacturer’s instructions. In brief, ELISpot plates were coated with anti-IFN-gamma antibody (1 ng/well) diluted in sterile 10 mM PBS (pH 7.4) by incubating at 4°C overnight. Wells were washed five times with PBS and blocked/equilibrated with RPMI-1640 media (Life Technology, Grand Island, NY, USA) containing 10% FBS for two hours at ambient temperature. PBMC (105 to 106 cells) in 100 μL of RPMI-1640 media supplemented with 10% FBS were added to each well of ELISpot plates. Each PRRSV antigen (e.g. live PRRSV at 105 TCID50, inactivated PRRSV or peptides at one to four μM) or mitogen (PHA 0.5 μg) suspended in 100 μL of RPMI-1640 media was then added to each well. The plates were incubated at 37°C for approximately 20 hours in a water-jacketed incubator conditioned with 5% CO2. To detect the spots associated with IFN-gamma secretion from antigen-specific T-lymphocytes, the plates were washed five times with PBS and biotinylated anti-IFN-gamma antibody (0.05 μg/100 μL/well) was added. Following two hours of incubation at ambient temperature, the plates were washed and then incubated with 100 μL of 1000-fold diluted streptoavidin-HRP (Mabtech) per well for one hour at ambient temperature. The plates were again washed five times with PBS and, following the addition of 100 μL of substrate solution, color spots were developed for approximately 90 seconds. The colorimetric reaction was stopped by rinsing with distilled water, and the plates were dried in the dark until spot forming units (SFU) were analyzed by an ELISpot reader (Autoimmun Diagnostika GmbH, Strasberg, Germany). The mean SFU/106 PBMC and standard error (SE) were calculated for the three non-stimulated negative control wells. For the test wells (two per test unless otherwise indicated), the mean was calculated and the negative control mean subtracted to determine mean antigen-specific SFU (representative data in S1 Fig). Those tests with a mean antigen-specific SFU higher than the mean SFU in non-stimulated wells plus five times the SE (≥99% confidence interval) were considered significant positive results.
T-lymphocyte epitope mapping
Peptides consisting of 20 amino acids with 11 amino acid overlaps were synthesized for all structural and nonstructural proteins of PRRSVSD23983 by a commercial manufacturer (Mimotope Pty Ltd., Clayton, Australia). These synthetic peptides were used in ELISpot assay to define the location of epitopes recognized by T-lymphocytes from PRRSV-infected gilts.The initial round of screening was with pools containing 33–54 peptides per pool (~1 μM of each peptide) and those that were positive were tested in the second round with smaller pools containing two to four peptides (4 μM of each). The third round of screening tested each peptide individually from positive pools identified in the second round (4 μM of each). All individual peptides inducing a significant T-lymphocyte response in the third round were retested with four replicates of each peptide.
Fluorescent focusing neutralization (FFN) assay
The PRRSV neutralizing antibody response in serum was measured by FFN assay as previously described [52]. Briefly, two-fold serial dilutions (1:4 to 1:512) of heat-inactivated serum samples were prepared in 96-well plates using Minimum Essential Medium (MEM, GIBCO® brand; Life Technology, Grand Island, NY, USA) supplemented with 10% FBS. An equal volume (50 μL) of PRRSV (VR-2332 or SD23983 strain) at a concentration of 2 × 103 FFU50 per ml was added to each sample, incubated for one hour at 37°C, and transferred to a 96-well plate containing confluent MARC-145 cells. After 24 hours, the plates were washed, fixed in ice-cold 80% acetone (Fisherbrand®; Fisher Scientific, Hanover Park, IL, USA), and stained with fluorescein isothiocyanate-conjugated anti-PRRSV nucleocapsid monoclonal antibody SDOW17 (Rural Technologies, Brookings, SD, USA) diluted 1:100 in PBS. The neutralizing activity of each serum (i.e., FFN titer) was reported as the reciprocal of the highest dilution that caused a 90% or greater reduction in the number of fluorescent foci.
Results
T-lymphocytes from pigs infected with type-2 PRRSVSD23983 recognized highly diverse type-1 and -2 PRRSV isolates
To define T-lymphocyte responses reacting against diverse PRRSV isolates, PBMCs collected from three one-year-old gilts at 44 and 72 days following infection with type-2 PRRSVSD23983 were evaluated by IFN-gamma ELISpot assay using 12 diverse PRRSV isolates as antigen. Gilts 1 and 3 had identical MHC haplotypes (Class I = Lr-32.0/35.0, Class II = Lr-0.1/0.12) and had broadly cross-reactive PRRSV-specific T-lymphocyte responses at 44 dpi, recognizing 11 of 12 heterologous PRRSV isolates significantly high (>5 SE) (Table 1). Notably, one of the recognized isolates included the type-1 PRRSVLelystad, which has more than 40% difference in GP5 and complete ORF sequences from major type-2 PRRSV isolates, including the type-2 PRRSVSD2398 inoculum virus as well as PRRSVVR2332 (Fig 1). Gilt 2 had a different MHC haplotype (Class I = Lr-4.0/39.0, Class II = Lr-0.2/0.23) and demostrated significantly high (>5 SE) T-lymphocyte responses against 10 of 12 strains at 44 dpi. By 72 dpi T-lymphocytes from gilt 3 had significantly high (>5 SE) recognition of all 12 PRRSV isolates and those from gilts 1 and 2 recognized 11 of 12 isolates (Table 1).
Table 1. T-lymphocyte responses from one-year-old gilts infected with PRRSVSD23983 at 44 and 72 dpi in an IFN-gamma ELISpot assay against various type-1 and type-2 PRRSV isolates.
| PRRSV type: strain | 44 dpi PBMC | 72 dpi PBMC | ||||
|---|---|---|---|---|---|---|
| Gilt 1 | Gilt 2 | Gilt 3 | Gilt 1 | Gilt 2 | Gilt 3 | |
| Unstimulated: mean SFU (5 x SE) | 2 (5) | 6.5 (2.5) | 15 (30) | 4 (0) | 28 (5) | 30 (10) |
| 1: PRRSVLV | 43+* | 11.5+ | 97+ | 73+ | 46+ | >262+ |
| 1: PRRSV14003 | 6 | 6 | 7 | 4 | 24 | 89+ |
| 2: PRRSVVR2332 | 148+ | 61+ | 242+ | >262+ | >262+ | >262+ |
| 2: PRRSV13392 | 199+ | 71+ | 262+ | >262+ | >262+ | >262+ |
| 2: PRRSV53091 | 136+ | 72+ | 218+ | >262+ | >262+ | >262+ |
| 2: PRRSV36509 | >262+ | 69+ | 218+ | >262+ | >262+ | >262+ |
| 2: PRRSV97-7895 | >262+ | 61+ | 230+ | >262+ | >262+ | >262+ |
| 2: PRRSVO-1 | >262+ | 59+ | >262+ | >262+ | >262+ | >262+ |
| 2:PRRSV1403-02 | >262+ | 61+ | 238+ | >262+ | >262+ | >262+ |
| 2: PRRSV9530 | >262+ | 58+ | 210+ | >262+ | >262+ | >262+ |
| 2: PRRSV3606-98 | 165+ | 4 | 139+ | >262+ | >262+ | >262+ |
| 2: PRRSVSD23983 | 259+ | 52+ | 226+ | >262+ | >262+ | >262+ |
*Numbers are mean SFU/106 PBMC minus the mean of the appropriate unstimulated control; those adjusted SFU means that were greater than the unstimulated control SFU mean plus 5 times the SE (standard error) were scored as positive and marked with a + in the table (for gilt 1 at 44 dpi those test SFU means >7 were positive; for gilt 2, >9; for gilt 3, >45 and so on). SFU means with >262+ indicate that the SFU was higher than the upper count limit for ELISpot reader.
T-lymphocytes from nine 14-day old piglets born to gilt 1 after in utero infection with PRRSVSD23983 were evaluated by IFN-gamma ELISpot assay using the 12 described PRRSV isolates. The infection period totaled 33 days (including 19 days in utero and 14 days after birth) to allow stimulation of PRRSVSD23983-specifc T-lymphocytes in all piglets. All nine had significant (>5 SE) T-lymphocyte responses against two or more of the heterologous PRRSV isolates tested (Table 2). In particular, piglet 13 had T-lymphocytes recognizing all 12 PRRSV isolates including the two type-1 PRRSV isolates.
Table 2. T-lymphocyte responses from 14-day-old piglets infected in utero with PRRSVSD23983 in an IFN-gamma ELISpot assay against phytohemagglutin (PHA) and various type-1 and type-2 PRRSV isolates.
| Pig number and SLA genotype | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| PHA or PRRSV type: isolate | P13 | P14 | P15 | P16 | P17 | P18 | P19 | P20 | P21 |
| Lr49.29+Lr35.12 | Lr49.29+Lr32.1 | Lr4.2+Lr32.1 | Lr4.2+Lr32.1 | Lr4.2+Lr35.12 | Lr4.2+Lr32.1 | Lr49.2+Lr32.1 | Lr4.2+Lr32.1 | Lr4.2+Lr35.12 | |
| PHA | <353 | 337 (29) | <353 | 149 (6) | <353 | 220 (26) | <353 | 205 (87) | <353 |
| Unstimulated: mean SFU (5 x SE) | 6.5 (7.5) | 11 (20) | 6.5 (2.5) | 10.5 (12.5) | 21 (45) | 1 (5) | 9.5 (17.5) | 2 (5) | 7 (0) |
| 1: PRRSVLV | 63+* | 4 | 24+ | 0 | 3 | 3 | 3 | 0 | 13+ |
| 1: PRRSV14003 | 40+ | 7 | 3 | 0 | 0 | 1 | 15 | 0 | 1 |
| 2: PRRSVVR2332 | 320+ | 45+ | 3 | 0 | 30 | 15+ | 38+ | 23+ | 60+ |
| 2: PRRSV13392 | 181+ | 28 | 20+ | 15 | 32 | 7+ | 86+ | 28+ | 30+ |
| 2: PRRSV53091 | 231+ | 30 | 17+ | 13 | 71+ | 9+ | 37+ | 18+ | 30+ |
| 2: PRRSV36509 | 235+ | 30 | 23+ | 4 | 27 | 8+ | 25 | 0 | 69+ |
| 2: PRRSV97-7895 | 353+ | 27 | 27+ | 34+ | 69+ | 21+ | 51+ | 0 | 71+ |
| 2: PRRSVO-1 | 285+ | 25 | 44+ | 11 | 47 | 18+ | 46+ | 0 | 104+ |
| 2:PRRSV1403-02 | 235+ | 23 | 50+ | 29+ | 38 | 7+ | 67+ | 76+ | 68+ |
| 2: PRRSV9530 | 276+ | 41+ | 27+ | 6 | 51 | 6 | 43+ | 0 | 66+ |
| 2: PRRSV3606-98 | 214+ | 25 | 27+ | 2 | 30 | 11+ | 43+ | 0 | 76+ |
| 2: PRRSVSD23983 | 329+ | 51+ | 30+ | 9 | 60 | 17+ | 132+ | 5 | 73+ |
* Numbers are mean SFU/106 PBMC minus the mean of the appropriate unstimulated control; those adjusted SFU means that were greater than the unstimulated control SFU mean plus 5 times the SE (standard error) were scored as positive and marked with a + in the table (for P13 those test SFU means >14 were positive; for P14, >31; for P15, >9 and so on).
T-lymphocytes from three gilts infected with PRRSVSD23983 at one year of age recognized several epitopes in various viral proteins
PRRSV epitopes were initially mapped with high stringency IFN-gamma ELISpot assays using 20 peptide pools (33 to 54 overlapping peptides per pool) spanning all PRRSVSD23983 ORFs. In the first round of epitope mapping, PBMC collected at 30 or 33 dpi had significant T-lymphocyte responses against four to 14 of the 20 peptide pools, depending on the source animal (Table 3). This suggested that T-lymphocyte responses to multiple epitopes in several proteins contributed to recognition of diverse PRRSV isolates. In general, the number of peptide pools recognized by T-lymphocytes from the three gilts at 44 or 58 dpi was similar to the findings at 30–33 dpi (Table 3).
Table 3. Mapping of T-lymphocyte epitopes on PRRSV proteins using overlapping peptide pools encompassing all open reading frames of PRRSVSD23983.
| Peptide pool | Gilt 1 PBMC | Gilt 2 PBMC | Gilt 3 PBMC | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 30dpi | 44dpi | 58dpi | 33dpi | 44dpi | 58dpi | 33dpi | 44dpi | 58dpi | |
| Unstimulated: mean SFU (5 x SE) | 19 (15) | 60.5 (52.5) | 24.5 (2.5) | 37 (10) | 3 (10) | 4 (5) | 26.5 (22.5) | 6 (5) | 5.5 (7.5) |
| nsp1 | 129+* | 183+ | 141+ | 26 | 15 | 24+ | 29 | 18+ | 9 |
| nsp2-1 | 49+ | 92 | 56+ | 2 | 8 | 13+ | 13 | 3 | 1 |
| nsp2-2 | 133+ | 245+ | 179+ | 18 | 0 | 8 | 34 | 13+ | 9 |
| nsp2-3 | 31 | 45 | 30+ | 19 | 0.5 | 0 | 9 | 0 | 0 |
| nsp3,4 | 49+ | 72 | 47+ | 115+ | 3 | 3 | 20 | 1 | 0 |
| nsp5,6,7 | 76+ | 75 | 73+ | 26 | 6 | 11+ | 16 | 6 | 2 |
| nsp8,9 | 29 | 22 | 27 | 55+ | 4 | 8 | 11 | 5 | 3 |
| nsp9,10 | 35+ | 48 | 30+ | 10 | 1 | 4 | 14 | 1 | 0 |
| nsp10,11 | 20 | 44 | 21 | 9 | 1 | 3 | 23 | 0 | 0 |
| nsp12, 2TF | 20 | 18 | 20 | 4 | 0 | 2 | 11 | 9 | 0 |
| GP2a and b, ORF5a | 143+ | 141+ | 74+ | 17 | 7 | 27+ | 28 | 46+ | 11 |
| GP3, GP4 | 212+ | 226+ | 180+ | 59+ | 25+ | 47+ | 60+ | 74+ | 19+ |
| GP5, M, N | 333+ | >345+ | 308+ | 56+ | 30+ | 65+ | 88+ | 155+ | 80+ |
| M | 218+ | 180+ | 134+ | 26 | 35+ | 93+ | 61+ | 129+ | 55+ |
| E (GP2b) | 14 | 13 | 16 | 6 | 1 | 5 | 4 | 7 | 4 |
| GP3 | 42+ | 29 | 28+ | 27 | 17+ | 46+ | 17 | 8 | 3 |
| GP4 | 194+ | 163+ | 139+ | 38 | 24+ | 42+ | 33 | 65+ | 26+ |
| GP5 | 200+ | 263+ | 214+ | 23 | 13 | 25+ | 65+ | 75+ | 34+ |
| ORF5a | 11 | 19 | 14 | 25 | 1 | 0 | 22 | 6 | 2 |
| N | 22 | NA | NA | 3 | 4 | 7 | 7.5 | 14+ | 6 |
| Inactivated PRRSVSD23983 | 182+ | 204+ | 177+ | 27 | 0 | 3 | 51+ | 1 | 0 |
* Numbers are mean IFN-gamma ELISpot assay SFU/106 PBMC minus the mean of the appropriate unstimulated control; those adjusted SFU means that were greater than the unstimulated control SFU mean plus 5 times the SE were scored as positive and marked with a + in the table (for gilt 1 at 30 dpi those test SFU means >34 were positive; for gilt 1 at 44 dpi, >113; for gilt 1 at 58 dpi, >27 and so on). SFU means with > indicate that the SFU was higher than the upper count limit for ELISpot reader.
Second and third round T-lymphocyte epitope mapping identified a total of 28 peptides recognized by T-lymphocytes from gilt 1 which included four overlapping regions, resulting in mapping of epitopes on ≥24 peptides (Table 4). The viral proteins containing these peptides with epitopes were nsp1 alpha (five peptides, five epitopes), nsp2 (five peptides, ≥five epitopes), GP2a (two peptides, ≥two epitopes), GP2b (one peptide, ≥one epitope), GP4 (six peptides, ≥five epitopes), GP5 (three peptides, ≥three epitopes) and M (six peptides, ≥three epitopes). Gilt 2 T-lymphocytes recognized six peptides including two overlapping regions (≥four epitopes) in viral proteins nsp4 (two peptides, ≥one epitope), GP3 (one peptide, ≥one epitope), GP4 (one peptide, ≥one epitope) and M (two peptides, ≥one epitopes). Gilt 3 T-lymphocytes recognized seven peptides including two overlapping regions (≥five epitopes) in viral proteins GP5 (one peptide, ≥one epitope), M (five peptides, ≥three epitopes) and N (one peptide, ≥one epitope). Of these seven peptides, six were also recognized by T-lymphocytes from gilt 1 with the same MHC class I and II haplotypes. It is noteworthy that one peptide (P579 TWKFITSRCRLCLLGRKYIL) in the M protein was recognized by T-lymphocytes from all three gilts representing two distinct MHC class I and II haplotypes.
Table 4. Peptides containing epitopes recognized by T-lymphocytes from three gilts infected with PRRSVSD23983.
| Pig ID (dpi) | Peptide number and sequence | Protein | Mean SFU (5 x SE) |
|---|---|---|---|
| Gilt 1 (30) | Unstimulated | 2.2 (6.5) | |
| P2 CTPNARVFMAEGQVYCTRCL | nsp1alpha | 12+* | |
| P10 FPIARMTSGNLNFQQRMVRV | nsp1alpha | 12+ | |
| P13 GQLTPAVLKALQVYERGCRW | nsp1alpha | 21+ | |
| P15 RWYPIVGPVPGVAVYANSLH | nsp1alpha | 148+ | |
| P18 GATHVLTNLPLPQRPKPEDF | nsp1alpha | 25+ | |
| P53 ATDEDLVNAIQILRLPAALD | nsp2 | 14+ | |
| P94 APRRKVGTNCGSPISLGDNI | nsp2 | 48+ | |
| P121 SAYQAFRTLDGRLKFLPKMI | nsp2 | 16+ | |
| P132 PDESTSAPPTGTGGAGSFTD | nsp2 | 22+ | |
| P136 TIKRKAEGLFDRLSRQVFNL | nsp2 | 25+ | |
| P470 TKHPLGMFWHHKVSTLIDEM | GP2a | 18+ | |
| P478 ASRLPMLHNLRMTGSNVTIV | GP2a | 39+ | |
| P493 VVFCIRLVCSAILRTRPAIH | E (GP2b) | 12+ | |
| P528 VLQDISCLRHRNSAPEALRK | GP4 | 42+ | |
| P530 RKIPQCRTAIGTPVYITITA | GP4 | 23+ | |
| P532 TANVTDENYLHSSDLLMLSS | GP4 | 30+ | |
| P533 LHSSDLLMLSSCLFYASEMS | GP4 | 30+ | |
| P538 QHVREFTQRSLMVDHVRLLH | GP4 | 16+ | |
| P540 LHFMTPETMRWATVLACLFA | GP4 | 22+ | |
| P550 LTHIVSYGALTTSHFLDTIA | GP5 | 25+ | |
| P555 AALTCFVIRFVKNCMSWRYS | GP5 | 17+ | |
| P558 LLDTKGRLYRWRSPVIIEKR | GP5 | 96+ | |
| P571 LLAFSITYTPVMIYALKVSR | M | 24+ | |
| P572 PVMIYALKVSRGRLLGLLHL | M | 65+ | |
| P573 SRGRLLGLLHLLIFLNCAFT | M | 21+ | |
| P578 LWGVYSAIETWKFITSRCRL | M | 23+ | |
| P579 TWKFITSRCRLCLLGRKYIL | M | 24+ | |
| P586 LVLGGRKAVKQGVVNLVKYA | M | 65+ | |
| Total 28 peptides: ≤24 epitopes | |||
| Gilt 2 (33) | Unstimulated | 5.3 (8.5) | |
| P221 PLGDVKVGSHIIKDIGEVPS | nsp4 | 34+ | |
| P222 HIIKDIGEVPSDLCALLAAK | nsp4 | 24+ | |
| P521 LGIATRPLRRFAKSLSAVRR | GP3 | 15+ | |
| P538 QHVREFTQRSLMVDHVRLLH | GP4 | 24+ | |
| P579 TWKFITSRCRLCLLGRKYIL | M | 35+ | |
| P580 RLCLLGRKYILAPAHHVESA | M | 20+ | |
| Total 6 peptides: ≤4 epitopes | |||
| Gilt 3 (33) | Unstimulated | 3.5 (7.5) | |
| P558 LLDTKGRLYRWRSPVIIEKR | GP5 | 25+ | |
| P571 LLAFSITYTPVMIYALKVSR | M | 22+ | |
| P572 PVMIYALKVSRGRLLGLLHL | M | 23+ | |
| P578 LWGVYSAIETWKFITSRCRL | M | 17+ | |
| P579 TWKFITSRCRLCLLGRKYIL | M | 10+ | |
| P586 LVLGGRKAVKQGVVNLVKYA | M | 37+ | |
| P589 KRKKGDGQPVNQLCQMLGKI | N | 15+ | |
| Total 7 peptides: ≤5 epitopes | |||
* Numbers are mean IFN-gamma ELISpot assay SFU/106 PBMC minus the mean of the appropriate unstimulated control; those adjusted SFU means that were greater than the unstimulated control SFU mean plus 5 times the SE were scored as positive and marked with a + in the table: for gilts 1, 2 and 3 were >8.7, >13.5 and >11, respectively.
Neutralizing antibody responses were weak and isolate-specific while T-lymphocytes recognized diverse isolates
To define neutralizing antibody responses, virus neutralizing antibody titers were determined by FFN assay using two type-2 PRRSV, the inoculum PRRSVSD23983 as well as PRRSVVR2332, which are closely related according to phylogenetic analysis (Fig 1). All three gilts had weak to moderate neutralizing antibody titers against the homologous PRRSVSD23983, ranging between 1:4 and 1:32 at 30–72 dpi (Table 5). However, there was no detectable level of neutralizing antibodies against the heterologous but closely related PRRSVVR2332 in any of the serum samples from various dpi time points tested (Table 5).
Table 5. Neutralizing antibody responses in three gilts after intranasal challenge with PRRSVSD23983 and in piglets at 14-days of birth to gilt 1 exposed to PRRSVSD23983 at 98 days of gestation.
| Pig identification | dpi | Neutralizing antibody titer against homologous isolate | Neutralizing antibody titer against VR2332 isolate |
|---|---|---|---|
| Gilt 1 | 30 | 1:4* | <1:4 |
| Gilt 1 | 58 | 1:16 | <1:4 |
| Gilt 1 | 72 | 1:8 | <1:4 |
| Piglet 13 | 32** | 1:16 | <1:4 |
| Piglet 14 | 32 | 1:16 | <1:4 |
| Piglet 15 | 32 | 1:32 | <1:4 |
| Piglet 16 | 32 | 1:16 | <1:4 |
| Piglet 17 | 32 | 1:32 | <1:4 |
| Piglet 18 | 32 | 1:8 | <1:4 |
| Piglet 19 | 32 | 1:16 | <1:4 |
| Piglet 20 | 32 | 1:16 | <1:4 |
| Piglet 21 | 32 | 1:16 | <1:4 |
| Gilt 2 | 30 | 1:32 | <1:4 |
| Gilt 2 | 58 | 1:32 | <1:4 |
| Gilt 2 | 72 | 1:32 | <1:4 |
| Gilt 3 | 30 | 1:16 | <1:4 |
| Gilt 3 | 58 | 1:32 | <1:4 |
| Gilt 3 | 72 | 1:32 | <1:4 |
*Neutralizing antibody titer is the last serum dilution with detectable neutralizing activity.
**32 dpi for piglets born to gilt 1 includes 18 days gestation following infection of gilt plus 14 days after birth, at which point the serum sample was obtained.
Five of nine piglets had PRRSV-specific T-lymphocyte responses reacting against diverse type 2 PRRSV isolates (≥9 of 12 isolates). Of two type-1 isolates, piglet 13 significantly (>5SE) recognized both, while piglets 15 and 21 each recognized one. In contrast, neutralizing antibody responses in all nine piglets were weak against the homologous PRRSVSD23983 isolate with titers ranging between 1:8 and 1:32, and there was no neutralization of slightly heterologous PRRSVVR2332 (Table 5). Neutralization results were similar to serum from the dam (gilt 1), with isolate-specific neutralization (Table 5).
Discussion
PRRSV infects porcine macrophages using CD163 and CD169 as receptors [53–56]. This virus then persists in tissue macrophages and modulates the immune system, resulting in weak and/or delayed adaptive and innate immune responses [51,57,58]. Neutralizing antibody responses are weak and inefficient in preventing PRRSV infection of target cells in tissues even in a homologous challenge model [31]. Therefore, CMI has been frequently discussed as a protective mechanism against PRRSV infection [58–64] [65] [66]. Studies to date have demonstrated the existence of T-lymphocyte epitopes in several PRRSV proteins including both structural (GP2, GP3, GP5, M, N) and non-structural proteins (nsp1, nsp2, nsp5, nsp7, nsp9 and nsp10) [58–64]. PRRSV-specific T-lymphocytes in PBMC from infected pigs appeared at 14 dpi and remained longer than 50 days when evaluated in IFN-gamma ELISpot assay using the mixture of PRRSVMN30100 and recombinant N protein as the stimulation antigen [65]. Furthermore, PRRSV-specific T-lymphocyte responses were highly variable between different age groups, potentially related to infection strains and virus titer [66]. To date, no systematic evaluation of the cross-recognition potential of PRRSV-specific T-lymphocyte responses to heterologous PRRSV isolates has been performed. If T-lymphocyte responses recognize multiple epitopes in diverse PRRSV isolates and have a protective function, they may overcome the challenges presented by antigenic variation among PRRSV isolates and weak, isolate-specific neutralizing antibody responses.
The objective of this study was to define broadly cross-reactive PRRSV-specific T-lymphocyte responses induced in gilts and piglets after infection with a type-2 PRRSV and map epitope-containing sequence within T-lymphocyte-recognized viral proteins. T-lymphocytes from both age groups recognized multiple diverse PRRSV isolates. In the three 12-month-old gilts, intranasal infection with PRRSVSD23983 induced T-lymphocyte responses that reacted against 10 type-2 isolates and one or both of the two highly divergent type-1 isolates when evaluated by 72 dpi. The recognition of all 12 PRRSV isolates including two type-1 isolates by gilt 3 T-lymphocytes was unexpected considering the antigenic and genetic differences between inoculum PRRSVSD23983 and tested divergent isolates. T-lymphocytes from all nine 14-day-old piglets infected in utero recognized at least two of the 12 PRRSV isolates tested. Importantly, T-lymphocytes from one of the nine piglets recognized all 12 PRRSV isolates and another recognized 11 isolates. This finding could be reinforced through further studies with more diverse PRRSV isolates including high pathogenic strains as well as PBMC at various dpi time points.
The broadly cross-reactive T-lymphocyte responses observed in this study were in direct contrast to the weak and isolate-specific neutralizing antibody responses to the homologous PRRSVSD23983 in gilts when tested at various dpi time points. This strongly supports the effort to define T-lymphocyte responses further with regard to protective mechanisms. Porcine T-lymphocytes that could contribute to protective responses include CD4+ T-lymphocytes, CD8+ T-lymphocytes and memory T-lymphocytes with the double positive CD4+CD8+ phenotype. To this end, a companion study using T-lymphocytes from the three gilts in this study demonstrated cytotoxic responses by both CD8+ and CD4+ T-lymphocytes [67]. Additional large scale studies using pigs with systematically defined MHC haplotypes are also indicated to investigate the association between MHC haplotypes and PRRSV-specific IFN-gamma-secreting T-lymphocyte responses, as this limited analysis of gilts and congenitally infected piglets is not definitive.
The epitope mapping results in this study identified peptides containing T-lymphocyte epitopes in multiple proteins including nsp4, in which no other T-lymphocyte epitopes have been reported to date. T-lymphocyte epitopes have been previously described in PRRSV nsp1, nsp2, GP2, GP4, GP5, M and N proteins [58–64], however in this study the exact locations for 23 of 33 epitope-containing peptides identified in these proteins were novel. Locations of ten peptides including seven M peptides, two GP5 peptides, and one nsp2 peptide were similar to those previously reported. M protein had several epitopes recognized by two of three gilts tested and, in particular, one epitope-containing peptide (P579) that was recognized by T-lymphocytes from all three. It will be interesting to determine if either cytotoxic T-lymphocytes (CTL) and/or helper T-lymphocytes recognize this peptide and whether they potentially recognize the same epitope. In addition, the same repertoire of epitope-containing peptides from the M protein were recognized by two gilts (gilt 1 and gilt 3) with identical MHC class-I and -II haplotypes. Despite differing MHC class-I and -II haplotypes, T-lymphocytes in gilt 1 and 2 recognized a common epitope-containing peptide (P538) in GP4. Identification of M protein sequences recognized by T-lymphocytes in several previous reports [58,59,62,68] as well as in the current study suggest that M protein is epitope-rich region suitable for inducing T-lymphocytes recognizing diverse PRRSV isolates, particularly since PBMCs have originated from genetically diverse pigs infected with various PRRSV genotypes.
Four PRRSV non-structural proteins (nsp5, nsp7, nsp9 and nsp10) reported to contain T-lymphocyte epitopes in previous studies [58,63,64] had no epitopes identified in this study. This may have been due to the use of only three gilts for epitope mapping and/or the use of limited dpi time points. Additional studies are planned to undertake epitope mapping and functional analysis of T-lymphocytes through virus suppression and cytotoxic assays using PBMC collected at various dpi from pigs infected with type-1 and type-2 PRRSV strains in order to extend the current findings. In addition, inclusion of high pathogenic PRRSV strains in diverse PRRSV panel as T-lymphocyte stimulation antigen in ELISpot assay may be valuable. Thorough epitope mapping will be crucial in the development of the next generation of PRRSV vaccines, particularly those epitopes that induce CTL and helper T-lymphocytes for both CTL and B-lymphocyte responses. Epitopes recognized by helper T-lymphocytes can be used as an adjuvant for either conventional or novel PRRSV vaccines to boost PRRSV-specific T- or B-lymphocyte responses, and PRRSV-specific CTL epitopes could be included to improve protective vaccine efficacy.
Supporting Information
(DOCX)
Acknowledgments
This work was supported by funding from Animal and Plant Quarantine Agency, Republic of Korea (QIA I-1541780-2012-14-03). The authors are grateful to Drs. Robert H. Mealey and Siddra Hines for critical review of manuscript, as well as Mr. TJ Heiniger and Ms. Jan Luft for providing assistance in animal care.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by funding from Animal and Plant Quarantine Agency, Republic of Korea (QIA I-1541780-2012-14-03; http://qia.go.kr) given to CJC.
References
- 1.Holtkamp DJ: 2011, Assessment of the economic impact of porcine reproductive and respira-tory syndrome virus on U.S. pork producers. In: International PRRS symposium 86.
- 2.Neumann EJ, Kliebenstein JB, Johnson CD, Mabry JW, Bush EJ, Seitzinger AH, et al. : 2005, Assessment of the economic impact of porcine reproductive and respiratory syndrome on swine production in the United States. J Am Vet Med Assoc 227: 385–392. [DOI] [PubMed] [Google Scholar]
- 3.Brar MS, Shi M, Hui RK, Leung FC: 2014, Genomic evolution of porcine reproductive and respiratory syndrome virus (PRRSV) isolates revealed by deep sequencing. PLoS One 9: e88807 10.1371/journal.pone.0088807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li Y, Treffers EE, Napthine S, Tas A, Zhu L, Sun Z, at al.: 2014, Transactivation of programmed ribosomal frameshifting by a viral protein. Proc Natl Acad Sci U S A 111: E2172–E2181. 10.1073/pnas.1321930111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Snijder EJ, Kikkert M, Fang Y: 2013, Arterivirus molecular biology and pathogenesis. J Gen Virol 94: 2141–2163. 10.1099/vir.0.056341-0 [DOI] [PubMed] [Google Scholar]
- 6.Brar MS, Shi M, Murtaugh MP, Leung FC: 2015, Evolutionary Diversification of Type 2 Porcine Reproductive and Respiratory Syndrome Virus. J Gen Virol. vir.0.000104 [DOI] [PubMed] [Google Scholar]
- 7.Shi M, Lam TT, Hon CC, Hui RK, Faaberg KS, Wennblom T, et al. : 2010, Molecular epidemiology of PRRSV: a phylogenetic perspective. Virus Res 154: 7–17. 10.1016/j.virusres.2010.08.014 [DOI] [PubMed] [Google Scholar]
- 8.Shi M, Holmes EC, Brar MS, Leung FC: 2013, Recombination is associated with an outbreak of novel highly pathogenic porcine reproductive and respiratory syndrome viruses in China. J Virol 87: 10904–10907. 10.1128/JVI.01270-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Opriessnig T, Halbur PG, Yoon KJ, Pogranichniy RM, Harmon KM, Evans R, et al. : 2002, Comparison of molecular and biological characteristics of a modified live porcine reproductive and respiratory syndrome virus (PRRSV) vaccine (ingelvac PRRS MLV), the parent strain of the vaccine (ATCC VR2332), ATCC VR2385, and two recent field isolates of PRRSV. J Virol 76: 11837–11844. 10.1128/JVI.76.23.11837-11844.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tian K, Yu X, Zhao T, Feng Y, Cao Z, Wang C,et al. : 2007, Emergence of fatal PRRSV variants: unparalleled outbreaks of atypical PRRS in China and molecular dissection of the unique hallmark. PLoS One 2: e526 10.1371/journal.pone.0000526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Metwally S, Mohamed F, Faaberg K, Burrage T, Prarat M, Moran K, et al. : 2010, Pathogenicity and molecular characterization of emerging porcine reproductive and respiratory syndrome virus in Vietnam in 2007. Transbound Emerg Dis 57: 315–329. 10.1111/j.1865-1682.2010.01152.x [DOI] [PubMed] [Google Scholar]
- 12.Karniychuk UU, Geldhof M, Vanhee M, Van DJ, Saveleva TA, Nauwynck HJ: 2010, Pathogenesis and antigenic characterization of a new East European subtype 3 porcine reproductive and respiratory syndrome virus isolate. BMC Vet Res 6: 30 10.1186/1746-6148-6-30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weesendorp E, Morgan S, Stockhofe-Zurwieden N, Popma-De Graaf DJ, Graham SP, Rebel JM: 2013. Comparative analysis of immune responses following experimental infection of pigs with European porcine reproductive and respiratory syndrome virus strains of differing virulence. Vet Microbiol 163: 1–12. 10.1016/j.vetmic.2012.09.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morgan SB, Graham SP, Salguero FJ, Sanchez Cordon PJ, Mokhtar H, Rebel JM, et al. : 2013, Increased pathogenicity of European porcine reproductive and respiratory syndrome virus is associated with enhanced adaptive responses and viral clearance. Vet Microbiol 163: 13–22. 10.1016/j.vetmic.2012.11.024 [DOI] [PubMed] [Google Scholar]
- 15.An TQ, Tian ZJ, Leng CL, Peng JM, Tong GZ: 2011, Highly pathogenic porcine reproductive and respiratory syndrome virus, Asia. Emerg Infect Dis 17: 1782–1784. 10.3201/eid1709.110411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.An TQ, Tian ZJ, Xiao Y, Li R, Peng JM, Wei TC,et al. : 2010, Origin of highly pathogenic porcine reproductive and respiratory syndrome virus, China. Emerg Infect Dis 16: 365–367. 10.3201/eid1602.090005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tong GZ, Zhou YJ, Hao XF, Tian ZJ, An TQ, Qiu HJ: 2007, Highly pathogenic porcine reproductive and respiratory syndrome, China. Emerg Infect Dis 13: 1434–1436. 10.3201/eid1309.070399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ni J, Yang S, Bounlom D, Yu X, Zhou Z, Song J, et al. : 2012, Emergence and pathogenicity of highly pathogenic Porcine reproductive and respiratory syndrome virus in Vientiane, Lao People's Democratic Republic. J Vet Diagn Invest 24: 349–354. 10.1177/1040638711434111 [DOI] [PubMed] [Google Scholar]
- 19.Wei Y, Li S, Huang L, Tang Q, Liu J, Liu D, et al. : 2013, Experimental infection and comparative genomic analysis of a highly pathogenic PRRSV-HBR strain at different passage levels. Vet Microbiol 166: 337–346. 10.1016/j.vetmic.2013.05.014 [DOI] [PubMed] [Google Scholar]
- 20.Charerntantanakul W: 2012, Porcine reproductive and respiratory syndrome virus vaccines: Immunogenicity, efficacy and safety aspects. World J Virol 1: 23–30. 10.5501/wjv.v1.i1.23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Geldhof MF, Vanhee M, Van BW, Van DJ, Karniychuk UU, Nauwynck HJ: 2012, Comparison of the efficacy of autogenous inactivated Porcine Reproductive and Respiratory Syndrome Virus (PRRSV) vaccines with that of commercial vaccines against homologous and heterologous challenges. BMC Vet Res 8: 182 10.1186/1746-6148-8-182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park C, Seo HW, Han K, Kang I, Chae C: 2014, Evaluation of the efficacy of a new modified live porcine reproductive and respiratory syndrome virus (PRRSV) vaccine (Fostera PRRS) against heterologous PRRSV challenge. Vet Microbiol 172: 432–442. 10.1016/j.vetmic.2014.05.030 [DOI] [PubMed] [Google Scholar]
- 23.Kim WI, Yoon KJ: 2008, Molecular assessment of the role of envelope-associated structural proteins in cross neutralization among different PRRS viruses. Virus Genes 37: 380–391. 10.1007/s11262-008-0278-1 [DOI] [PubMed] [Google Scholar]
- 24.Okuda Y, Kuroda M, Ono M, Chikata S, Shibata I: 2008, Efficacy of vaccination with porcine reproductive and respiratory syndrome virus following challenges with field isolates in Japan. J Vet Med Sci 70: 1017–1025. [DOI] [PubMed] [Google Scholar]
- 25.Li B, Fang L, Xu Z, Liu S, Gao J, Jiang Y, et al. : 2009, Recombination in vaccine and circulating strains of porcine reproductive and respiratory syndrome viruses. Emerg Infect Dis 15: 2032–2035. 10.3201/eid1512.090390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nielsen HS, Oleksiewicz MB, Forsberg R, Stadejek T, Botner A, Storgaard T: 2001, Reversion of a live porcine reproductive and respiratory syndrome virus vaccine investigated by parallel mutations. J Gen Virol 82: 1263–1272. 10.1099/0022-1317-82-6-1263 [DOI] [PubMed] [Google Scholar]
- 27.Allende R, Laegreid WW, Kutish GF, Galeota JA, Wills RW, Osorio FA: 2000, Porcine reproductive and respiratory syndrome virus: description of persistence in individual pigs upon experimental infection. J Virol 74: 10834–10837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Batista L, Pijoan C, Dee S, Olin M, Molitor T, Joo HS, et al. : 2004, Virological and immunological responses to porcine reproductive and respiratory syndrome virus in a large population of gilts. Can J Vet Res 68: 267–273. [PMC free article] [PubMed] [Google Scholar]
- 29.Leng X, Li Z, Xia M, He Y, Wu H: 2012, Evaluation of the efficacy of an attenuated live vaccine against highly pathogenic porcine reproductive and respiratory syndrome virus in young pigs. Clin Vaccine Immunol 19: 1199–1206. 10.1128/CVI.05646-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li J, Murtaugh MP: 2012, Dissociation of porcine reproductive and respiratory syndrome virus neutralization from antibodies specific to major envelope protein surface epitopes. Virology 433: 367–376. 10.1016/j.virol.2012.08.026 [DOI] [PubMed] [Google Scholar]
- 31.Lopez OJ, Oliveira MF, Garcia EA, Kwon BJ, Doster A, Osorio FA: 2007, Protection against porcine reproductive and respiratory syndrome virus (PRRSV) infection through passive transfer of PRRSV-neutralizing antibodies is dose dependent. Clin Vaccine Immunol 14: 269–275. 10.1128/CVI.00304-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meng XJ: 2000, Heterogeneity of porcine reproductive and respiratory syndrome virus: implications for current vaccine efficacy and future vaccine development. Vet Microbiol 74: 309–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zuckermann FA, Garcia EA, Luque ID, Christopher-Hennings J, Doster A, Brito M, et al. : 2007, Assessment of the efficacy of commercial porcine reproductive and respiratory syndrome virus (PRRSV) vaccines based on measurement of serologic response, frequency of gamma-IFN-producing cells and virological parameters of protection upon challenge. Vet Microbiol 123: 69–85. 10.1016/j.vetmic.2007.02.009 [DOI] [PubMed] [Google Scholar]
- 34.Osorio FA, Galeota JA, Nelson E, Brodersen B, Doster A, Wills R, et al. 2002, Passive transfer of virus-specific antibodies confers protection against reproductive failure induced by a virulent strain of porcine reproductive and respiratory syndrome virus and establishes sterilizing immunity. Virology 302: 9–20. [DOI] [PubMed] [Google Scholar]
- 35.Costers S, Lefebvre DJ, Goddeeris B, Delputte PL, Nauwynck HJ: 2009, Functional impairment of PRRSV-specific peripheral CD3+CD8high cells. Vet Res 40: 46 10.1051/vetres/2009029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yoon KJ, Zimmerman JJ, Swenson SL, McGinley MJ, Eernisse KA, Brevik A, et al. : 1995, Characterization of the humoral immune response to porcine reproductive and respiratory syndrome (PRRS) virus infection. J Vet Diagn Invest 7: 305–312. [DOI] [PubMed] [Google Scholar]
- 37.Labarque GG, Nauwynck HJ, van RK, Pensaert MB: 2000, Effect of cellular changes and onset of humoral immunity on the replication of porcine reproductive and respiratory syndrome virus in the lungs of pigs. J Gen Virol 81: 1327–1334. 10.1099/0022-1317-81-5-1327 [DOI] [PubMed] [Google Scholar]
- 38.Christianson WT, Collins JE, Benfield DA, Harris L, Gorcyca DE, Chladek DW, et al. : 1992, Experimental reproduction of swine infertility and respiratory syndrome in pregnant sows. Am J Vet Res 53: 485–488. [PubMed] [Google Scholar]
- 39.Shimizu M, Yamada S, Kawashima K, Ohashi S, Shimizu S, Ogawa T: 1996, Changes of lymphocyte subpopulations in pigs infected with porcine reproductive and respiratory syndrome (PRRS) virus. Vet Immunol Immunopathol 50: 19–27. [DOI] [PubMed] [Google Scholar]
- 40.Mateu E, Martin M, Vidal D: 2003, Genetic diversity and phylogenetic analysis of glycoprotein 5 of European-type porcine reproductive and respiratory virus strains in Spain. J Gen Virol 84: 529–534. 10.1099/vir.0.18478-0 [DOI] [PubMed] [Google Scholar]
- 41.Guindon S, Dufayard JF, Lefort V, Anisimova M, Hordijk W, Gascuel O: 2010, New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst Biol 59: 307–321. 10.1093/sysbio/syq010 [DOI] [PubMed] [Google Scholar]
- 42.Vu HLX, Brito M, Kim WI, Yoon KJ, Laegreid W, Osorio FA: 2008, Sub-typing PRRSV isolates by means of measurement of cross neutralization reactions. Proceeding of CRWAD meeting, Chicago, IL 119.
- 43.Collins JE, Benfield DA, Christianson WT, Harris L, Hennings JC, Shaw DP, et al. : 1992, Isolation of swine infertility and respiratory syndrome virus (isolate ATCC VR-2332) in North America and experimental reproduction of the disease in gnotobiotic pigs. J Vet Diagn Invest 4: 117–126. [DOI] [PubMed] [Google Scholar]
- 44.Feng W, Laster SM, Tompkins M, Brown T, Xu JS, Altier C, et al. 2001, In utero infection by porcine reproductive and respiratory syndrome virus is sufficient to increase susceptibility of piglets to challenge by Streptococcus suis type II. J Virol 75: 4889–4895. 10.1128/JVI.75.10.4889-4895.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Petry DB, Holl JW, Weber JS, Doster AR, Osorio FA, Johnson RK: 2005, Biological responses to porcine respiratory and reproductive syndrome virus in pigs of two genetic populations. J Anim Sci 83: 1494–1502. [DOI] [PubMed] [Google Scholar]
- 46.Wensvoort G, Terpstra C, Pol JM, ter Laak EA, Bloemraad M, de Kluyver EP, et al. : 1991, Mystery swine disease in The Netherlands: the isolation of Lelystad virus. Vet Q 13: 121–130. 10.1080/01652176.1991.9694296 [DOI] [PubMed] [Google Scholar]
- 47.Han J, Liu G, Wang Y, Faaberg KS: 2007, Identification of nonessential regions of the nsp2 replicase protein of porcine reproductive and respiratory syndrome virus strain VR-2332 for replication in cell culture. J Virol 81: 9878–9890. 10.1128/JVI.00562-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Reed LJ: 1938, A simple method of estimating fifty percent endpoints. Am J Hygiene 27: 493–497. [Google Scholar]
- 49.Ho CS, Lunney JK, Lee JH, Franzo-Romain MH, Martens GW, Rowland RR, et al. :2010, Molecular characterization of swine leucocyte antigen class II genes in outbred pig populations. Anim Genet 41: 428–432. 10.1111/j.1365-2052.2010.02019.x [DOI] [PubMed] [Google Scholar]
- 50.Ho CS, Lunney JK, Franzo-Romain MH, Martens GW, Lee YJ, Lee JH, et al. : 2009, Molecular characterization of swine leucocyte antigen class I genes in outbred pig populations. Anim Genet 40: 468–478. 10.1111/j.1365-2052.2009.01860.x [DOI] [PubMed] [Google Scholar]
- 51.Lopez FL, Domenech N, Alvarez B, Ezquerra A, Dominguez J, Castro JM, et al. : 1999, Analysis of cellular immune response in pigs recovered from porcine respiratory and reproductive syndrome infection. Virus Res 64: 33–42. [DOI] [PubMed] [Google Scholar]
- 52.Wu WH, Fang Y, Farwell R, Steffen-Bien M, Rowland RR, Christopher-Hennings J, et al. : 2001, A 10-kDa structural protein of porcine reproductive and respiratory syndrome virus encoded by ORF2b. Virology 287: 183–191. 10.1006/viro.2001.1034 [DOI] [PubMed] [Google Scholar]
- 53.Duan X, Nauwynck HJ, Pensaert MB: 1997, Virus quantification and identification of cellular targets in the lungs and lymphoid tissues of pigs at different time intervals after inoculation with porcine reproductive and respiratory syndrome virus (PRRSV). Vet Microbiol 56: 9–19. 10.1016/S0378-1135(96)01347-8 [DOI] [PubMed] [Google Scholar]
- 54.Van BW, Delputte PL, Van GH, Misinzo G, Vanderheijden N, Duan X, et al. 2010, Porcine reproductive and respiratory syndrome virus entry into the porcine macrophage. J Gen Virol 91: 1659–1667. 10.1099/vir.0.020503-0 [DOI] [PubMed] [Google Scholar]
- 55.Van GH, Van BW, Delputte PL, Nauwynck HJ: 2008, Sialoadhesin and CD163 join forces during entry of the porcine reproductive and respiratory syndrome virus. J Gen Virol 89: 2943–2953. 10.1099/vir.0.2008/005009-0 [DOI] [PubMed] [Google Scholar]
- 56.Welch SK, Calvert JG: 2010, A brief review of CD163 and its role in PRRSV infection. Virus Res 154: 98–103. 10.1016/j.virusres.2010.07.018 [DOI] [PubMed] [Google Scholar]
- 57.Meier WA, Galeota J, Osorio FA, Husmann RJ, Schnitzlein WM, Zuckermann FA: 2003, Gradual development of the interferon-gamma response of swine to porcine reproductive and respiratory syndrome virus infection or vaccination. Virology 309: 18–31. [DOI] [PubMed] [Google Scholar]
- 58.Mokhtar H, Eck M, Morgan SB, Essler SE, Frossard JP, Ruggli N, et al. : 2014, Proteome-wide screening of the European porcine reproductive and respiratory syndrome virus reveals a broad range of T cell antigen reactivity. Vaccine 32: 6828–6837. 10.1016/j.vaccine.2014.04.054 [DOI] [PubMed] [Google Scholar]
- 59.Wang YX, Zhou YJ, Li GX, Zhang SR, Jiang YF, Xu AT,et al. : 2011, Identification of immunodominant T-cell epitopes in membrane protein of highly pathogenic porcine reproductive and respiratory syndrome virus. Virus Res 158: 108–115. 10.1016/j.virusres.2011.03.018 [DOI] [PubMed] [Google Scholar]
- 60.Diaz I, Pujols J, Ganges L, Gimeno M, Darwich L, Domingo M, et al. 2009, In silico prediction and ex vivo evaluation of potential T-cell epitopes in glycoproteins 4 and 5 and nucleocapsid protein of genotype-I (European) of porcine reproductive and respiratory syndrome virus. Vaccine 27: 5603–5611. 10.1016/j.vaccine.2009.07.029 [DOI] [PubMed] [Google Scholar]
- 61.Vashisht K, Goldberg TL, Husmann RJ, Schnitzlein W, Zuckermann FA: 2008, Identification of immunodominant T-cell epitopes present in glycoprotein 5 of the North American genotype of porcine reproductive and respiratory syndrome virus. Vaccine 26: 4747–4753. 10.1016/j.vaccine.2008.06.047 [DOI] [PubMed] [Google Scholar]
- 62.Bautista EM, Suarez P, Molitor TW, 1999, T cell responses to the structural polypeptides of porcine reproductive and respiratory syndrome virus. Arch Virol 144: 117–134. [DOI] [PubMed] [Google Scholar]
- 63.Parida R, Choi IS, Peterson DA, Pattnaik AK, Laegreid W, Zuckermann FA, et al. : 2012, Location of T-cell epitopes in nonstructural proteins 9 and 10 of type-II porcine reproductive and respiratory syndrome virus. Virus Res 169: 13–21. 10.1016/j.virusres.2012.06.024 [DOI] [PubMed] [Google Scholar]
- 64.Burgara-Estrella A, Diaz I, Rodriguez-Gomez IM, Essler SE, Hernandez J, Mateu E: 2013, Predicted peptides from non-structural proteins of porcine reproductive and respiratory syndrome virus are able to induce IFN-gamma and IL-10. Viruses 5: 663–677. 10.3390/v5020663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Xiao Z, Batista L, Dee S, Halbur P, Murtaugh MP: 2004, The level of virus-specific T-cell and macrophage recruitment in porcine reproductive and respiratory syndrome virus infection in pigs is independent of virus load. J Virol 78: 5923–5933. 10.1128/JVI.78.11.5923-5933.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Klinge KL, Vaughn EM, Roof MB, Bautista EM, Murtaugh MP: 2009, Age-dependent resistance to Porcine reproductive and respiratory syndrome virus replication in swine. Virol J 6: 177 10.1186/1743-422X-6-177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chung C, Cha SH, Grimm AL, Rzepka J, Ajithdoss D, Chung G, et al.: 2015, Proceeding of the 7th international symposium on emerging and re-emerging pig diseases 66.
- 68.Jeong HJ, Song YJ, Lee SW, Lee JB, Park SY, Song CS, et al. : 2010, Comparative measurement of cell-mediated immune responses of swine to the M and N proteins of porcine reproductive and respiratory syndrome virus. Clin Vaccine Immunol 17: 503–512. 10.1128/CVI.00365-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Robinson DF, Foulds LR: 1981, Comparison of phylogenetic trees. Mathematical Biosciences 53: 131–147. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.