Abstract
Background/Aims
No clinical model exists to predict the occurrence of hepatocellular carcinoma in sustained virologic response-achieving (HCC after SVR) patients with chronic hepatitis C (CHC).
Methods
We performed a case-control study using a clinical database to research the risk factors for HCC after SVR. A predictive model based on risk factors was established, and the area under the receiver operating characteristic curve (AUC) was calculated.
Results
In the multivariate model, an initial diagnosis of compensated cirrhosis and post-SVR albumin reductions of 1 g/L were associated with 21.7-fold (95% CI, 4.2 to 112.3; p<0.001) and 1.3-fold (95% CI, 1.1 to 1.7; p=0.004) increases in the risk of HCC after SVR, respectively. A predictive model based on an initial diagnosis of compensated cirrhosis (yes, +1; no, 0) and post-SVR albumin ≤36.0 g/L (yes, +1; not, 0) predicted the occurrence of HCC after SVR with a cutoff value of >0, an AUC of 0.880, a sensitivity of 0.833, a specificity of 0.896, and a negative predictive value of 0.956.
Conclusions
An initial diagnosis of compensated cirrhosis combined with a post-SVR albumin value of ≤36.0 g/L predicts the occurrence of HCC after SVR in patients with CHC.
Keywords: Case-control studies, Chronic hepatitis C, He-patocellular carcinoma, Risk factors, Sustained virologic response
INTRODUCTION
Hepatitis C virus (HCV) infection affects more than 160 million people worldwide and is associated with viral persistence, which progresses to chronic hepatitis C (CHC) in 80% of all cases and is now recognized as one of the main causes of liver cirrhosis (LC), hepatocellular carcinoma (HCC) and death.1,2 Antiviral therapy with interferon-α plus ribavirin is the former standard of care for the clearance of HCV. A sustained virologic response (SVR), which is defined as an undetectable HCV RNA level at 24 weeks after the cessation of therapy, has proven to be durable and to equate to a cure.1,3 These data indicate that the diagnosis of HCC in SVR-achieving CHC patients (HCC after SVR) is rare.
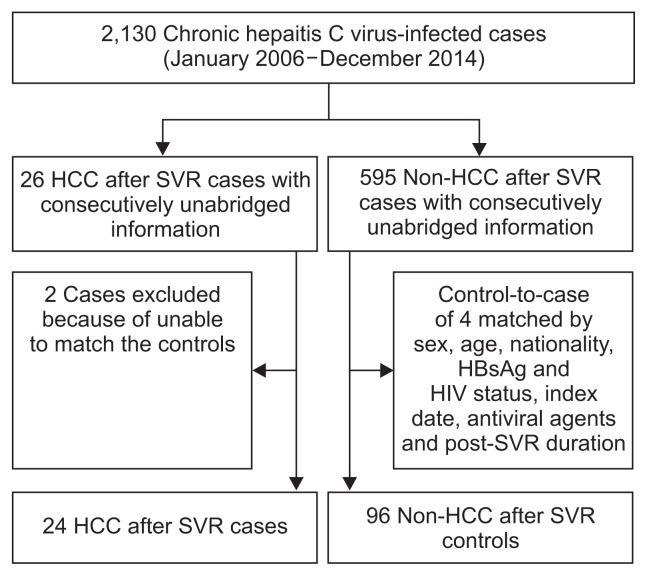
Indeed, we identified only 26 HCC after SVR cases in the clinical datasets of the First Affiliated Hospital of Zhengzhou University and the Beijing 302 Hospital (Fig. 1), which are largest general hospital and the largest liver disease treatment center in China, respectively. Nevertheless, the characteristics, particularly the risk factors for the occurrence of HCC after SVR are poorly understood.4 Prior prospective studies have suggested that SVR-achieving patients with advanced CHC exhibit significant reductions in liver transplantation and liver-related mortality, but they remain at risk for HCC.5–8 Moreover, patients with older ages at HCV eradication and heavy alcohol intake might be at an increased risk for the development of HCC within 5 years of HCV clearance.9 Recent studies have indicated that post-SVR albumin, alanine aminotransferase (ALT) and α-fetoprotein (AFP) levels are associated with the development of HCC in the future.4,10 This case control study was performed to investigate the risk factors for and form a predictive model for the occurrence of HCC after SVR CHC patients.
Fig. 1.
Derivation and definitions of the study population.
SVR, sustained virologic response; HCC, hepatocellular carcinoma; HBsAg, hepatitis B surface antigen; HIV, human immunodeficiency virus.
MATERIALS AND METHODS
1. Data source and enrollment criteria
The clinical databases included the clinical history, related test results and prescription information for each patient. There were 2,130 inpatients with consecutively unabridged information between January 2006 and December 2014 in the databases (Fig. 1), which included cases with CHC, compensated LC, decompen-sated LC, HCC, and liver-related death. These datasets provided the opportunity to design a retrospective study to evaluate the associations of several potential risk factors with the occurrence of HCC after SVR. We enrolled all of the HCC after SVR inpatients from the clinical databases and identified four control inpatients per case.
2. Identification of the cases and controls
HCC after SVR was defined as a tumor occurrence at any time point after SVR, i.e., at least 6 months after the cessation of antiviral therapy. The control patients were matched to the cases in terms of sex, nationality, hepatitis B surface antigen (HBsAg) status, anti-human immunodeficiency virus antibody (anti-HIV) status, index date (the dates of hospital admission were within the same year), antiviral agents, equal or older age, and longer post-SVR durations than the interval between the SVR and the occurrence of HCC. Twenty-four of the overall 26 HCC after SVR cases had sufficient clinical histories and test information for further matching; thus, 96 matched controls were ultimately included (Fig. 1).
3. Potential factors
The factors that could potentially contribute to or could be related to disease progression were considered as risk factors in this study; these factors included the stage at which the liver disease was diagnosed (i.e., the initial diagnostic stage or the initial diagnosis), antivirals-administered disease stage (the disease stage at which the antiviral agents were administered, i.e., CHC or compensated LC), age at HCV clearance, diabetes, hypertension, alcohol consumption, anti-hepatitis B core (HBc) antibody status, history of transfusion before the diagnosis of CHC, smoking history, family history of viral hepatitis C, post-SVR albumin, post-SVR ALT and post-SVR AFP. The initial diagnostic stages and antivirals-administered disease stages were categorized into CHC and compensated LC. LC was diagnosed based on liver biopsies or the use of a combination of at least two imaging tools (i.e., abdominal ultrasonography, FibroScan® [Echosens, Paris, France], computed tomography or magnetic resonance imaging) plus clinical evidence of manifestations. HCC screening was performed at least every 2 months after the SVR. HCC was confirmed by liver histopathology, at least two imaging tools, or by one imaging diagnostic modality and a serum AFP level of 400 ng/mL or higher.11,12 Alcohol consumption was defined as alcohol consumption on at least 4 days per week for at least 5 years.11,12 Ever smokers were defined as patients who had smoked cigarettes at least 4 days per week for at least 5 years.11,12 The family history of viral hepatitis was defined according to direct relatives who were infected with HCV. The post-SVR albumin, ALT and AFP values were collected at 24 weeks immediately after the cessation of treatment.
4. Statistical analysis
For comparisons of proportions, chi-square statistics were used. For the comparisons of age between the case and control groups, Mann-Whitney U tests were performed. A conditional logistic regression (Cox regression) model was used to estimate the relative magnitudes in relation to the potential factors mentioned above. The odds ratios (ORs) and their 95% confidence intervals (CIs) were calculated using the patients with no exposure or with lower levels as the reference. We determined the cutoff values of the consecutive risk factor for the prediction of the development of HCC with receiver operating characteristic curves (ROCs). A simple risk score was derived from the variables that were significant in the multivariate analysis. This score (i.e., the predictive model for HCC after SVR) was taken as the weighted sum of the significant variables for which the weights were defined as the quotients (rounded to nearest integer) of the corresponding estimated coefficients from a Cox regression analysis divided by the smallest chi-square coefficient. The overall accuracy of the post-SVR score was estimated using an ROC and its 95% CI. The area under the ROC (AUC) was calculated. The analyses were performed using the SPSS version 16.0 (SPSS Inc., Chicago, IL, USA). All statistical tests were two sided. A p-value <0.05 was considered statistically significant.
5. Ethical evaluation
The study protocol was approved by the Research Ethics Committees of The First Affiliated Hospital of Zhengzhou University and the Beijing 302 Hospital. The committees waived the need for written informed consent from the participants because de-identified secondary data were analyzed in this study.
RESULTS
1. General characteristics of the study population
The records of 24 HCC after SVR cases and 96 matched controls were included for further analysis. Fig. 1 presents the derivation and definitions of the study population. Table 1 shows the general characteristics and selected medical conditions of the cases and controls. The earliest HCC case occurred at 11 months after SVR, i.e., 17 months after the cessation of antiviral therapy, and the largest tumor size was 3 cm among these 24 cases. There were no significant differences between cases and controls in terms of age, sex, nationality, HBsAg, anti-HIV or HCV RNA status. However, the post-SVR times, which denote the duration between the SVR and the occurrence of HCC for the case group and the post-SVR period for the control group, were significantly different (Table 1).
Table 1.
General Characteristics of Post-Sustained Virologic Response Hepatocellular Carcinoma Cases and Non-Hepatocellular Carcinoma Controls
| Variable | Case (n=24) | Control (n=96) | p-value |
|---|---|---|---|
| Age, yr | 58.5 (53.3–60.0) | 59.0 (56.0–63.0) | 0.385 |
| Sex | |||
| Male | 13 (54.2) | 52 (54.2) | NA |
| Female | 11 (45.8) | 44 (45.8) | NA |
| Han nationality | 24 (100.0) | 96 (100.0) | NA |
| HBsAg positive | 0 | 0 | NA |
| Anti-HIV antibody | 0 | 0 | NA |
| HCV genotype, 1b/2a/UD* | 20/2/2 | 85/5/6 | NA |
| HCV RNA positive | 0 | 0 | NA |
| HCV-diagnosed months† | 108.0 (53.0–138.0) | 84.0 (56.0–144.0) | 0.748 |
| Post-SVR months‡ | 17.5 (11.0–39.0) | 50.0 (33.0–62.8) | <0.001 |
| Tumor size, cm | 1.5 (1.5–2.0) | - | - |
Data are presented as median (range) or number (%).
NA, not applicable; HBsAg, hepatitis B surface antigen; HIV, human immunodeficiency virus; HCV, hepatitis C virus; UD, undermined; SVR, sustained virologic response.
Denotes the HCV genotype when diagnosed with chronic HCV infection;
Denotes the follow-up period after the diagnosis of an HCV infection;
Denotes the duration between the SVR and the occurrence of hepatocellular carcinoma in the case group and the post-SVR period for the control group.
2. Risk factors in the univariate model
The relationships between the potential risk factors and HCC after SVR as analyzed in the univariate model are shown in Table 2. Patients with initial diagnoses of a compensated cirrhosis stage exhibited a 25.6-fold (95% CI, 5.8 to 113.3; p<0.001) increase in the risk of the occurrence of HCC after SVR compared with those in the CHC stage. Moreover, antiviral therapy performed at the compensated cirrhotic stage was associated with 15.1-fold (95% CI, 4.3 to 52.2, p<0.001) increased in the HCC after SVR risk compared with antiviral agents administered in the CHC stage. Post-SVR albumin (every 1 g/L increase; OR, 0.7; 95% CI, 0.6 to 0.9; p<0.001) and post-SVR AFP (every 1 ng/mL increase; OR, 1.4; 95% CI, 1.1 to 1.8; p=0.034) were also risk factors for the occurrence of HCC after SVR. However, the other factors, such as HCV-diagnosed months, age at HCV clearance, diabetes, hypertension, alcohol consumption, anti-HBc status, transfusion history, smoking, family history of viral hepatitis, and post-SVR ALT level, were all excluded from the univariate model.
Table 2.
Unadjusted Univariate Conditional Logistic Regression Analyses of the Potential Risk Factors for the Occurrence of Post-Sustained Virologic Response Hepatocellular Carcinoma
| Variable | Case (n=24) | Control (n=96) | UOR (95% CI) | B | p-value |
|---|---|---|---|---|---|
| Initial diagnostic stage | |||||
| Chronic hepatitis C | 10 (41.7) | 93 (96.9) | 1.0 (reference) | NA | NA |
| Compensated liver cirrhosis | 14 (58.3) | 3 (3.1) | 25.6 (5.8–113.3) | 3.2 | <0.001 |
| Antivirals-administered disease stage | |||||
| Chronic hepatitis C | 6 (25.0) | 82 (85.4) | 1.0 (reference) | NA | NA |
| Compensated liver cirrhosis | 18 (75.0) | 14 (14.6) | 15.1 (4.3–52.2) | 2.7 | <0.001 |
| At HCV clearance, yr | |||||
| Age ≥55 | 11 (45.8) | 49 (51.0) | 1.0 (reference) | NA | NA |
| Age <55 | 13 (54.2) | 47 (49.0) | NA | NA | 0.221 |
| Without diabetes | 15 (62.5) | 67 (69.8) | 1.0 (reference) | NA | NA |
| With diabetes | 9 (37.5) | 29 (30.2) | NA | NA | 0.505 |
| Without hypertension | 15 (62.5) | 71 (74.0) | 1.0 (reference) | NA | NA |
| With hypertension | 9 (37.5) | 25 (26.0) | NA | NA | 0.281 |
| Without alcohol consumption | 19 (78.6) | 70 (73.0) | 1.0 (reference) | NA | NA |
| With alcohol consumption | 5 (21.4) | 26 (27.0) | NA | NA | 0.460 |
| HBcAb negativity | 14 (58.3) | 48 (50.0) | 1.0 (reference) | NA | NA |
| HBcAb positivity | 10 (41.7) | 48 (50.0) | NA | NA | 0.450 |
| Without transfusion history | 11 (45.8) | 39 (40.6) | 1.0 (reference) | NA | NA |
| With transfusion history | 13 (54.2) | 57 (59.4) | NA | NA | 0.634 |
| Nonsmoker | 21 (87.5) | 81 (84.4) | 1.0 (reference) | NA | NA |
| Ever-smoker | 3 (12.5) | 15 (15.6) | NA | NA | 0.671 |
| Without family history of viral hepatitis | 24 (100.0) | 86 (89.6) | 1.0 (reference) | NA | NA |
| Family history of viral hepatitis | 0 | 10 (10.4) | NA | NA | 0.096 |
| Post-SVR albumin (by every 1 g/L increase) | 35.3±5.6 | 41.3±4.7 | 0.7 (0.6–0.9) | −0.3 | <0.001 |
| Post-SVR ALT (by every 1 IU/L increase) | 39.0±33.1 | 33.2±32.5 | NA | NA | 0.434 |
| Post-SVR AFP (by every 1 ng/mL increase) | 28.2±28.2 | 5.7±4.4 | 1.4 (1.1–1.8) | 0.4 | 0.004 |
Data are presented as number (%) or mean±SD.
UOR, unadjusted odds ratios; CI, confidence interval; B, regression coefficient; NA, not applicable; HCV, hepatitis C virus; HBcAb, anti-hepatitis B core antibody; SVR, sustained virologic response; ALT, alanine aminotransferase; AFP, α-fetoprotein.
3. Risk factors in the multivariate model
Initial diagnostic stage, antivirals-administered disease stage, post-SVR albumin and AFP levels were further included in the multivariate logistic regression analysis (Table 3). Finally, after adjustments for possible confounders, i.e., the initial diagnostic stage and the post-SVR AFP level, an initial diagnosis of compensated cirrhosis and each 1 g/L increase in the post-SVR albumin level were associated with 21.7-fold (95% CI, 4.2 to 112.3; p<0.001) and 0.8-fold (95% CI, 0.6 to 0.9; p=0.004) increases in the risk of HCC after SVR in the multivariate model.
Table 3.
Adjusted Multivariate Conditional Logistic Regression Analysis of the Potential Risk Factors for Post-Sustained Virologic Response Hepatocellular Carcinoma
| Variable | AOR (95% CI) | B | p-value |
|---|---|---|---|
| Initial diagnostic stage | |||
| Chronic hepatitis C | 1.0 (reference) | NA | NA |
| Compensated liver cirrhosis | 21.7 (4.2–112.3) | 3.1 | <0.001 |
| Post-SVR albumin (by every 1 g/L increase) | 0.8 (0.6–0.9) | −0.3 | 0.004 |
| Antivirals-administered disease stage | |||
| Chronic hepatitis C | 1.0 (reference) | NA | NA |
| Compensated liver cirrhosis | NA | NA | 0.113 |
| Post-SVR AFP (by every 1 ng/mL increase) | NA | NA | 0.196 |
AOR, adjusted odds ratios; CI, confidence interval; B, regression coefficient; NA, not applicable; SVR, sustained virologic response; AFP, α-fetoprotein.
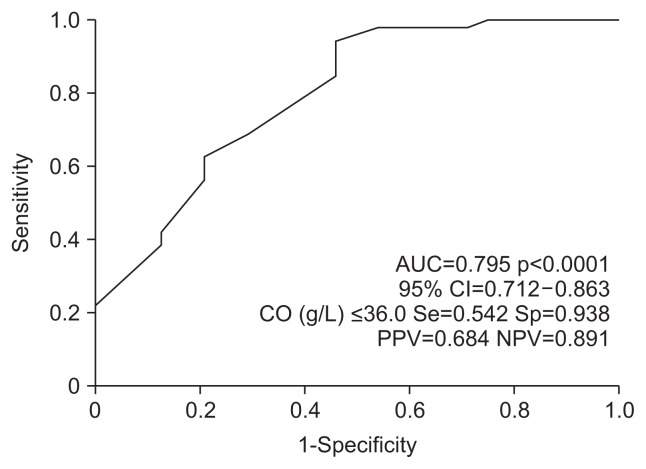
4. Receiver operator characteristics of the post-SVR albumin
We further determined the cutoff value for the post-SVR albumin level by predicting the development of HCC using ROC analysis. As shown in Fig. 2, ≤36.0 g/L was identified as the cutoff value for post-SVR albumin and resulted in an area under the ROC of 0.795.
Fig. 2.
Receiver operating characteristic curve for the post-sustained virologic response (SVR) albumin level in the prediction of hepatocellular carcinoma after SVR.
AUC, area under the receiver operating characteristic curve; CI, confidence interval; CO, cutoff value; Se, sensitivity; Sp, specificity; PPV, positive predictive value; NPV, negative predictive value.
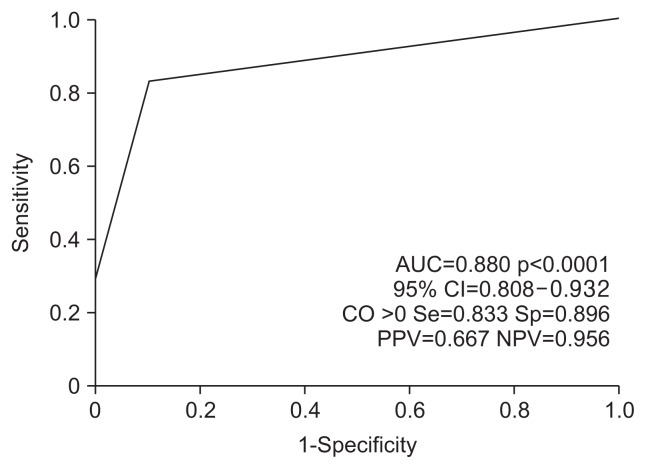
5. Predictive score for the HCC after SVR
A prediction score was attributed to each parameter according to its relative contribution to the Cox proportional hazards model as determined by the chi-square score (Table 4). This prediction score ranged from 0 to 2, and the cutoff value was >0 (Fig. 3). The AUC was 0.880 (95% CI, 0.808 to 0.932; p<0.001), the sensitivity was 0.833, the specificity was 0.896, the positive predictive value was 0.667, and the negative predictive value was 0.956.
Table 4.
Components of the Post-Sustained Virologic Response Hepatocellular Carcinoma Prediction Score
| Factors | AOR (95% CI) | B | Chi-square score | p-value | Score |
|---|---|---|---|---|---|
| Initial diagnostic stage | |||||
| Chronic hepatitis C | 1.0 (reference) | NA | NA | NA | 0 |
| Compensated liver cirrhosis | 37.6 (6.0–237.1) | 3.6 | 14.9 | <0.001 | +1 |
| Post-SVR albumin, g/L | |||||
| >36.0 | 1.0 (reference) | NA | NA | NA | 0 |
| ≤36.0 | 18.0 (3.6–90.2) | 2.9 | 12.4 | <0.001 | +1 |
AOR, adjusted odds ratios; CI, confidence interval; B, regression coefficient; NA, not applicable; SVR, sustained virologic response.
Fig. 3.
Receiver operating characteristic curve for the score predicting post-sustained virologic response hepatocellular carcinoma.
AUC, area under the receiver operating characteristic curve; CI, confidence interval; CO, cutoff value; Se, sensitivity; Sp, specificity; PPV, positive predictive value; NPV, negative predictive value.
DISCUSSION
In the present study, we aimed to investigate the risk factors and a predictive system for HCC after SVR using a strict case control study that involved matching the cases according to gender, nationality, HBsAg status, anti-HIV status, index date of admission, antiviral agents, equal or older age, and a median of a 50-month (range, 33.0 to 62.8 months) post-SVR period (Table 1). We then examined 13 potential risk factors, and four factors emerged in the unadjusted univariate model (Table 2). An initial diagnosis of compensated cirrhosis and each 1 g/L increase in post-SVR albumin were associated with 21.7- and 0.8-fold increases in the risk of HCC after SVR in the multivariate model (Table 3). Furthermore, ROC analysis indicated that a post-SVR albumin level ≤36.0 g/L was the cutoff value (Fig. 2). Finally, a prediction score for HCC after SVR was established with a cutoff value of >0, which means that patients with either an initial diagnosis of compensated cirrhosis or a post-SVR albumin level ≤36.0 g/L have high probability of experiencing HCC after SVR.
A previous study suggested that an age greater than 55 years at the time of HCV eradication is an independent risk factor for the development of HCC.9 Some comorbidities, such as alcohol consumption in presence of viral hepatitis and obesity, have been found to synergistically increase the risks of HCC and death,11,13 and diabetes has been found to be relevant to poor outcomes in CHC patients.14,15 Anti-HBc positivity has been proven to be unrelated to the prognoses of HCV infections,16 and whether smoking is a risk factor for disease progression and poor prognosis remains controversial.17,18 A recent study reported that the post-SVR ALT level is relevant to HCC.10 All of these above-mentioned factors inspired us to include them as factors in the evaluations performing in this study. However, to our knowledge, there are no data concerning the associations of hypertension, the route of transmission, and the family history of viral hepatitis with the risk of HCC after SVR. We observed that these variables were not associated with increased risks of HCC after SVR in either the univariate or multivariate models.
Prior studies have reported that SVR-achieving CHC patients with advanced disease remain at risk for HCC,5–7 although the rate of liver-related death in this population is markedly reduced.8 However, relative magnitude of the influence of this potential factor on the occurrence of HCC remains unknown. The post-SVR ALT and AFP levels have been observed to be predictive factors for HCC,10 although some critical opinions regarding these findings exist.19,20 Furthermore, the post-SVR albumin level has also been reported to be related to the occurrence of HCC.4 All of these factors were analyzed as potential risk factors in this strictly matched case control study. However, the post-SVR AFP was significant in only the univariate model, and an initial diagnosis of compensated cirrhosis and reductions in post-SVR albumin level were finally observed to be the independent risk factors for HCC after SVR (Table 3).
Our study has the strength of the strict matching conditions and the significant longer post-SVR durations in the controls. However, the limitations of the present study should also be noted. Although 2,130 inpatients were involved, only 24 cases of the 26 HCC after SVR cases were able to be further matched with controls due to the rarity of this clinical entity and the strict matching criterion, which might have influenced the statistical power of this study. Nevertheless, this issue might also have greatly minimized the confounding factors. Moreover, some predictive factors, such as the HCV genotype and the IL-28B polymorphism, were not included in the evaluation because these factors were either applied in the prediction of antiviral efficacy or, in the case of IL-28B, because we lacked the relevant data. Notably, IL-28B was identified as a predictive factor in 2009.21,22 More importantly, the cases and controls all achieved SVR in the present study. Additionally, although some noninvasive tests, such as the FibroScan®, that were applied in our two hospitals in 2010, potentially could play roles in the prediction of the occurrence of HCC,23 we were unable to use the FibroScan® for the evaluation for all cases because we did not have the relevant data before 2010. More importantly, all of the cirrhosis were clearly diagnosed without the FibroScan® in present study. Furthermore, as with any observational study, residual confounds due to unmeasured factors that varied between the cases and controls are also possible; however, the confounding effect of medical attention might be corrected for by hospitalization because all of the subjects in this study were consecutive inpatients.
In summary, to our knowledge, this study is the first to investigate the risk factors for HCC after SVR using a case control study design. Our results indicated that an initial diagnosis of compensated cirrhosis and a post-SVR albumin level ≤36.0 g/L were independent risk factors for the occurrence of HCC after SVR. Moreover, a prediction score for HCC after SVR was established based on the above-mentioned risk factors, and the cutoff value for this score was >0 score. Physicians should consider the probability of HCC after SVR when cirrhotic hepatitis C patients are faced with the option of antiviral therapy. Additionally, the measurement of the post-SVR albumin level is beneficial for the prediction of the future HCC risk. Further validation of this risk score based on data from a prospective study is desirable. Additionally, further study is needed to investigate whether HCC after SVR also occurs in the era of direct-acting antiviral agents.
ACKNOWLEDGEMENTS
This study was supported by the National Natural Science Foundation of China (number: 81302593) and the Key Scientific Research Project of Henan Higher Education Institutions of China (number: 15A320083).
Footnotes
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
REFERENCES
- 1.European Association for the Study of the Liver. EASL Clinical Practice Guidelines: management of hepatitis C virus infection. J Hepatol. 2011;55:245–264. doi: 10.1016/j.jhep.2011.02.023. [DOI] [PubMed] [Google Scholar]
- 2.Lavanchy D. Evolving epidemiology of hepatitis C virus. Clin Microbiol Infect. 2011;17:107–115. doi: 10.1111/j.1469-0691.2010.03432.x. [DOI] [PubMed] [Google Scholar]
- 3.Swain MG, Lai MY, Shiffman ML, et al. A sustained virologic response is durable in patients with chronic hepatitis C treated with peginterferon alfa-2a and ribavirin. Gastroenterology. 2010;139:1593–1601. doi: 10.1053/j.gastro.2010.07.009. [DOI] [PubMed] [Google Scholar]
- 4.Sato A, Sata M, Ikeda K, et al. Clinical characteristics of patients who developed hepatocellular carcinoma after hepatitis C virus eradication with interferon therapy: current status in Japan. Intern Med. 2013;52:2701–2706. doi: 10.2169/internalmedicine.52.1180. [DOI] [PubMed] [Google Scholar]
- 5.Morgan TR, Ghany MG, Kim HY, et al. Outcome of sustained virological responders with histologically advanced chronic hepatitis C. Hepatology. 2010;52:833–844. doi: 10.1002/hep.23744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shiffman ML. Impact of peginterferon maintenance therapy on the risk of developing hepatocellular carcinoma in patients with chronic hepatitis C virus. Oncology. 2010;78( Suppl 1):11–16. doi: 10.1159/000315224. [DOI] [PubMed] [Google Scholar]
- 7.Bruix J, Poynard T, Colombo M, et al. Maintenance therapy with peginterferon alfa-2b does not prevent hepatocellular carcinoma in cirrhotic patients with chronic hepatitis C. Gastroenterology. 2011;140:1990–1999. doi: 10.1053/j.gastro.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 8.Dieperink E, Pocha C, Thuras P, Knott A, Colton S, Ho SB. All-cause mortality and liver-related outcomes following successful antiviral treatment for chronic hepatitis C. Dig Dis Sci. 2014;59:872–880. doi: 10.1007/s10620-014-3050-5. [DOI] [PubMed] [Google Scholar]
- 9.Nagaoki Y, Aikata H, Miyaki D, et al. Clinical features and prognosis in patients with hepatocellular carcinoma that developed after hepatitis C virus eradication with interferon therapy. J Gastroenterol. 2011;46:799–808. doi: 10.1007/s00535-011-0384-z. [DOI] [PubMed] [Google Scholar]
- 10.Asahina Y, Tsuchiya K, Nishimura T, et al. Alpha-fetoprotein levels after interferon therapy and risk of hepatocarcinogenesis in chronic hepatitis C. Hepatology. 2013;58:1253–1262. doi: 10.1002/hep.26442. [DOI] [PubMed] [Google Scholar]
- 11.Loomba R, Yang HI, Su J, et al. Synergism between obesity and alcohol in increasing the risk of hepatocellular carcinoma: a prospective cohort study. Am J Epidemiol. 2013;177:333–342. doi: 10.1093/aje/kws252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zeng QL, Feng GH, Zhang JY, et al. Risk factors for liver-related mortality in chronic hepatitis C patients: a deceased case-living control study. World J Gastroenterol. 2014;20:5519–5526. doi: 10.3748/wjg.v20.i18.5519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zakhari S. Bermuda Triangle for the liver: alcohol, obesity, and viral hepatitis. J Gastroenterol Hepatol. 2013;28( Suppl 1):18–25. doi: 10.1111/jgh.12207. [DOI] [PubMed] [Google Scholar]
- 14.Kita Y, Mizukoshi E, Takamura T, et al. Impact of diabetes mellitus on prognosis of patients infected with hepatitis C virus. Metabolism. 2007;56:1682–1688. doi: 10.1016/j.metabol.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 15.Giordanino C, Ceretto S, Bo S, et al. Type 2 diabetes mellitus and chronic hepatitis C: which is worse? Results of a long-term retrospective cohort study. Dig Liver Dis. 2012;44:406–412. doi: 10.1016/j.dld.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 16.Tsubouchi N, Uto H, Kumagai K, et al. Impact of antibody to hepatitis B core antigen on the clinical course of hepatitis C virus carriers in a hyperendemic area in Japan: a community-based cohort study. Hepatol Res. 2013;43:1130–1138. doi: 10.1111/hepr.12075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fujita Y, Shibata A, Ogimoto I, et al. The effect of interaction between hepatitis C virus and cigarette smoking on the risk of hepatocellular carcinoma. Br J Cancer. 2006;94:737–739. doi: 10.1038/sj.bjc.6602981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pyong SJ, Tsukuma H, Hiyama T. Case-control study of hepatocellular carcinoma among Koreans living in Osaka, Japan. Jpn J Cancer Res. 1994;85:674–679. doi: 10.1111/j.1349-7006.1994.tb02413.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Asahina Y, Tsuchiya K, Izumi N. Reply: to PMID 23564522. Hepatology. 2014;60:764. doi: 10.1002/hep.27066. [DOI] [PubMed] [Google Scholar]
- 20.Toyoda H, Kumada T, Tada T. Postinterferon alpha-fetoprotein elevation and risk of hepatocellular carcinoma development after sustained virological response: cause or results? Hepatology. 2014;60:762–763. doi: 10.1002/hep.27064. [DOI] [PubMed] [Google Scholar]
- 21.Ge D, Fellay J, Thompson AJ, et al. Genetic variation in IL28B predicts hepatitis C treatment-induced viral clearance. Nature. 2009;461:399–401. doi: 10.1038/nature08309. [DOI] [PubMed] [Google Scholar]
- 22.Thomas DL, Thio CL, Martin MP, et al. Genetic variation in IL28B and spontaneous clearance of hepatitis C virus. Nature. 2009;461:798–801. doi: 10.1038/nature08463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tamaki N, Kurosaki M, Matsuda S, et al. Non-invasive prediction of hepatocellular carcinoma development using serum fibrosis marker in chronic hepatitis C patients. J Gastroenterol. 2014;49:1495–1503. doi: 10.1007/s00535-013-0914-y. [DOI] [PubMed] [Google Scholar]