SUMMARY
Endometrial carcinomas (ECs) are heterogeneous at the genetic level. Whilst TP53 mutations are highly recurrent in serous endometrial carcinomas (SECs), these are also present in a subset of endometrioid endometrial carcinomas (EECs). Here we sought to define the frequency, pattern, distribution and type of TP53 somatic mutations in ECs by performing a re-analysis of the publicly available data from The Cancer Genome Atlas (TCGA). A total of 228 EECs (n=186) and SECs (n=42) from TCGA dataset, for which an integrated genomic characterization was performed, were interrogated for the presence and type of TP53 mutation, and for mutations in genes frequently mutated in ECs. TP53 mutations were found in 15% of EECs and 88% SECs, and in 91% of copy-number high and 35% of POLE integrative genomic subtypes. In addition to differences in prevalence, variation in the type and pattern of TP53 mutations were observed between histologic types and between the integrative genomic subtypes. TP53 hotspot mutations were significantly more frequently found in SECs (46%) than in EECs (15%). TP53-mutant EECs significantly more frequently harbored a co-occurring PTEN mutation than TP53-mutant SECs. Finally, a subset of TP53-mutant ECs (22%) was found to harbor frameshift or nonsense mutations. Given that nonsense and frameshift TP53 mutations result in distinct p53 immunohistochemical results that require careful interpretation, and that EECs and SECs display different patterns, types and distributions of TP53 mutations, the use of the TP53/p53 status alone for the differential diagnosis of EECs and SECs may not be sufficient.
Keywords: endometrial cancer, TP53 mutation, hotspot mutation, histologic subtype, genomic subtype
INTRODUCTION
Endometrial cancer (EC) is the most common malignant disease of the female genital system in North America, and comprises several histologic subtypes with distinct clinical behavior (1,2). Over the last decade it has become increasingly apparent that ECs are a heterogeneous group of tumors not only in terms of histology, biology, and clinical behavior, but also with respect to their genetic make-up (2-6). Endometrioid (EECs) and serous endometrial carcinomas (SECs), the two most common histologic EC subtypes, were found by candidate gene studies to be characterized by mutations affecting PTEN, ARID1A, PIK3CA, KRAS and CTNNB1 (EECs) and by TP53, PPP2R1A and FBXW7 mutations (SECs)(5,6). The Cancer Genome Atlas (TCGA) has recently reported a comprehensive genomic and transcriptomic analysis of EEC, SECs, and mixed endometrioid and serous carcinomas (4). On the basis of integration of mutation spectra defined by whole exome massively parallel sequencing analysis, copy number alterations and microsatellite instability, ECs were categorized into four genomic subtypes: POLE (ultramutated) tumors, microsatellite-instable (hypermutated) tumors (MSI), copy-number low (endometrioid) tumors (CN-low), and copy-number high (serous-like) tumors (CN-high). The latter genomic subtype of ECs, akin to serous carcinomas of the uterus, high-grade serous carcinomas of the ovary and basal-like breast cancers (4,7,8), displays recurrent mutations affecting TP53.
TP53 is the most commonly mutated gene in human cancers and alterations have been found in >50% of all human tumors (9,10). Whilst tumor suppressor genes are commonly inactivated by frameshift or nonsense mutations, the majority of mutations affecting TP53 in human tumors are missense, and primarily affect the DNA-binding domain of the protein (exons 5-8). In fact, a number of so-called mutation hotspots have been identified, and approximately a third of all missense mutations affect the amino acid residues R175, G245, R248, R249, R273 and R282 (10-12). Mutant p53 proteins mostly lose their tumor suppressive functions, and may exert dominant-negative activities, but may also gain new oncogenic properties (10,11,13).
With the availability of complete sequencing of the entire coding region of TP53 in multiple tumor types through large-scale massively parallel sequencing endeavors, it has become apparent that the frequency and spectrum of TP53 mutations may differ according to tumor type and to molecular subtypes. For instance, in breast cancer, TP53 mutations are found in approximately 85% of basal-like breast cancers, and in these tumors, a substantial proportion of the mutations are truncating or frameshift; on the other hand, luminal breast cancers harbor TP53 mutations in a minority of lesions and these are predominantly missense mutations (7). Differences in the type of TP53 mutations have been shown to have important consequences in the assessment of p53 by immunohistochemical analysis; whilst TP53 missense mutations largely result in detectable p53 overexpression by immunohistochemistry, truncating and frameshift mutations often result in a negative immunohistochemical result (14,15).
Given the existence of multiple histologic and molecular subtypes of EC and the fact that approximately 25% of all ECs were found to harbor TP53 mutations (4), we sought to define the frequency, pattern and type of TP53 somatic mutations in EC, and to determine whether the pattern of TP53 mutations would vary according to the histologic or genomic subtypes of the disease. We performed a re-analysis of the EC TCGA dataset, and observed that not only the TP53 mutation frequency (4), but also the TP53 mutation spectrum is histologic subtype and genomic subtype-specific. In addition, our analysis suggests that TP53 mutational or p53 immunohistochemical analyses may not be sufficient for the distinction between SECs and International Federation of Gynecology and Obstetrics (FIGO) grade 3 EECs, but should be combined with other immunohistochemical markers and/or a small set of genes frequently mutated in EECs and SECs.
MATERIALS AND METHODS
Patient selection
Clinico-pathologic data from endometrial cancers, including information on the four integrated genomic classes, were retrieved from the TCGA data portal (https://tcga-data.nci.nih.gov/docs/publications/ucec_2013/; file “Key Clinical Data”)(4). From the 232 ECs for which an integrated genomic characterization was performed, we selected tumors of endometrioid (n=186) and serous (n=42) histologic subtypes (n=228 total). Mixed endometrial cancers (n=4) were excluded from this study. These publicly available data were interrogated for the presence and type of TP53 mutations using cBioPortal (http://www.cbioportal.org; accessed January 2015)(16). All patients from the TCGA dataset had received no prior systemic treatment for their disease (4).
Histologic and genomic stratification
ECs were classified according to histologic type (i.e. endometrioid vs serous carcinomas), FIGO grade, and the four genomic subtypes as described by the TCGA (4), namely POLE (ultramutated), MSI (hypermutated), CN-low (endometrioid), and CN-high (serous-like). In addition to the presence and type of TP53 mutations, also the presence of ARID1A, FBXW7, PPP2R1A and PTEN mutations was assessed and visualized using cBioPortal (http://www.cbioportal.org; accessed January 2015)(16).
Classification of TP53 mutations
The TP53 mutations identified were classified according to the predicted effect on protein function using the IARC TP53 database (http://p53.iarc.fr; version R17, November 2013)(17). TP53 mutations were further stratified according to i) mutation type, including single nucleotide missense mutations and other mutations (i.e. splice-site, nonsense, in-frame and frameshift), ii) the protein domain targeted by mutations, including the DNA-binding motif and outside DNA-binding motif, and iii) the functional effect, including hotspot (i.e. R175, G245, R248, R249, R273 and R282) mutations, as previously described (9,10,17-19). Mutation diagrams (“lollipop plots”) were obtained from cBioPortal (www.cBioPortal.org)(16) and manually curated.
Statistical analysis
All statistical analyses were performed using the SPSS statistical software package (IBM SPSS, Version 21, IBM). Two-tailed Fisher’s exact or Chi-square tests were employed for comparisons between groups. Overall survival was expressed as the number of months from diagnosis to death (file “Key Clinical Data” (4)). Cumulative survival probabilities were calculated using the Kaplan–Meier method. Differences between survival rates were tested with the log-rank test (SPSS version 21; IBM). A p-value<0.05 was considered statistically significant.
RESULTS
TP53 mutational status in ECs
Of the 228 SECs and EECs included in this study, 64 (28%) harbored a mutation in TP53. Four EECs harbored multiple TP53 mutations; in these cases, we have observed a combination of multiple missense mutations (n=2), a missense and a frameshift mutation (n=1), or a missense and a nonsense mutation (n=1; Supplemental Table 1). For the subsequent analyses reported in this study, the two cases harboring a missense and a frameshift mutation or a missense and a nonsense mutation, were classified as TP53-mutant harboring a frameshift or a nonsense mutation, respectively, as these EECs were of ultramutated POLE genomic subtype (see below) and the TP53 missense mutations were rare non-hotpot mutations.
A comparative analysis of TP53 mutations according to histologic types revealed that SECs are significantly more frequently TP53-mutant than EECs (88% vs 15%, respectively; Fisher’s exact test p<0.0001; Table 1) (4-6,20). As expected, TP53-mutant ECs had a significantly worse overall survival than TP53 wild-type cancers (p=0.035; Supplemental Fig. 1A) (21,22). Within EECs, the frequency of TP53 mutations was significantly associated with tumor grade, with 3% (2/68), 11% (8/72) and 37% (17/46) of FIGO grade 1, grade 2, and grade 3 cancers harboring TP53 mutations, respectively (Chi-squared p<0.0001; Tables 1 and 2; Supplemental Table 2) (23). The presence of TP53 mutations in EECs, however, was not significantly associated with outcome in the series analyzed (p>0.1; Supplemental Fig. 1B).
Table 1.
TP53 mutational status according to clinico-pathologic characteristics and co-occurrence with somatic mutations in genes frequently altered in endometrial carcinomas.
| Total (n) |
TP53 gene status | p-value | |||
|---|---|---|---|---|---|
| Wild-type (n=164) |
Mutant (n=64) |
||||
| Histologic type | Endometrioid | 186 | 159 (85%) | 27 (15%) | <0.0001* |
| Serous | 42 | 5 (12%) | 37 (88%) | ||
| FIGO grade | Grade 1 | 68 | 66 (97%) | 2 (3%) | <0.0001** |
| Grade 2 | 72 | 64 (89%) | 8 (11%) | ||
| Grade 3 | 88 | 34 (39%) | 54 (61%) | ||
| Integrative genomic subtype |
CN-high | 57 | 5 (9%) | 52 (91%) | <0.0001** |
| CN-low | 89 | 88 (99%) | 1 (1%) | ||
| MSI | 65 | 60 (92%) | 5 (8%) | ||
| POLE | 17 | 11 (65%) | 6 (35%) | ||
| ARID1A gene status | Wild-type | 151 | 94 (62%) | 57 (38%) | <0.0001* |
| Mutant | 77 | 70 (91%) | 7 (9%) | ||
| FBXW7 gene status | Wild-type | 191 | 141 (74%) | 50 (26%) | 0.1641* |
| Mutant | 37 | 23 (62%) | 14 (38%) | ||
| PPP2R1A gene status | Wild-type | 204 | 152 (75%) | 52 (25%) | 0.0162* |
| Mutant | 24 | 12 (50%) | 12 (50%) | ||
| PTEN gene status | Wild-type | 81 | 35 (43%) | 46 (57%) | <0.0001* |
| Mutant | 147 | 129 (88%) | 18 (12%) | ||
Fisher’s exact test p-value;
Chi-square test p-value
CN-high, copy-number high (serous-like) integrative genomic subtype; CN-low, copy-number low (endometrioid) integrative genomic subtype; MSI, microsatellite instable (hypermutated) integrative genomic subtype; n, number of cases; POLE, POLE (ultramutated) integrative genomic subtype.
Table 2.
Clinico-pathologic features, distribution of TP53 mutations and mutations in genes recurrently altered in endometrial cancers in TP53-mutant endometrioid and serous endometrial carcinomas.
| Total (n) | Histologic type | p-value | |||
|---|---|---|---|---|---|
| Endometrioid (n=27) |
Serous (n=37) |
||||
| Tumor grade | Grade 1 | 2 | 2 | 0 | 0.0002** |
| Grade 2 | 8 | 8 | 0 | ||
| Grade 3 | 54 | 17 | 37 | ||
| Integrative genomic subtype | CN-high | 52 | 15 | 37 | 0.0002** |
| CN-low | 1 | 1 | 0 | ||
| MSI | 5 | 5 | 0 | ||
| POLE | 6 | 6 | 0 | ||
| Type of TP53 mutation | Frameshift | 7 | 3 | 4 | 0.7030** |
| Missense | 49 | 20 | 29 | ||
| Nonsense | 7 | 4 | 3 | ||
| Splice-site | 1 | 0 | 1 | ||
| Hotspot TP53 mutation | No | 43 | 23 | 20 | 0.0144* |
| Yes | 21 | 4 | 17 | ||
| ARID1A gene status | Wild-type | 57 | 23 | 34 | 0.4427* |
| Mutant | 7 | 4 | 3 | ||
| FBXW7 gene status | Wild-type | 50 | 24 | 26 | 0.1247* |
| Mutant | 14 | 3 | 11 | ||
| PPP2R1A gene status | Wild-type | 52 | 25 | 27 | 0.0578* |
| Mutant | 12 | 2 | 10 | ||
| PTEN gene status | Wild-type | 46 | 10 | 36 | <0.0001* |
| Mutant | 18 | 17 | 1 | ||
Fisher’s exact test p-value;
Chi-square test p-value.
CN-high, copy-number high (serous-like) integrative genomic subtype; CN-low, copy-number low (endometrioid) integrative genomic subtype; MSI, microsatellite instable (hypermutated) integrative genomic subtype; n, number of TP53-mutant cases; POLE, POLE (ultramutated) integrative genomic subtype. Hotspot TP53 mutations include R175, G245, R248, R249, R273 and R282.
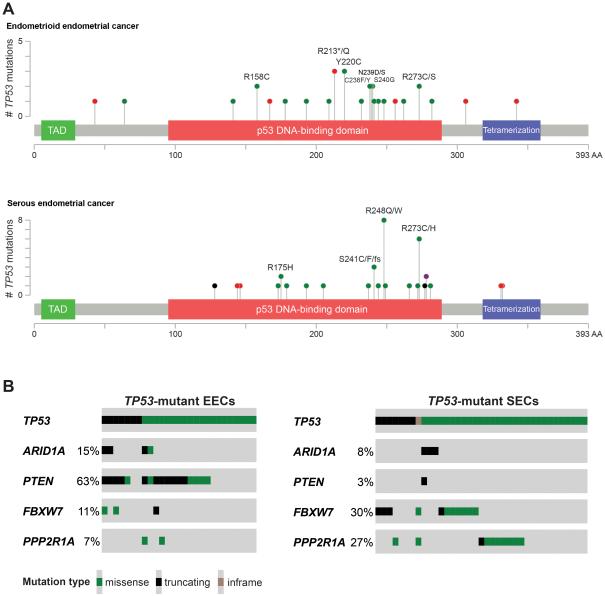
Given that TP53 mutations were relatively uncommon in EECs, and to understand the spectrum of TP53 mutations in ECs, we have focused only on TP53-mutant tumors for further analyses. Although no correlation between the type of TP53 mutation (i.e. missense, frameshift or nonsense) and tumor type was observed (Chi-squared p>0.1), we have found that SECs significantly more frequently harbored TP53 hotspot mutations than EECs (17/37, 46% vs 4/27, 15%, respectively, Fisher’s exact test p<0.05; Fig. 1A, Table 2).
Fig. 1. Distribution and spectrum of TP53 mutations in endometrial cancer.
(A) Distribution and spectrum of TP53 mutations in endometrioid endometrial cancer (top) and serous endometrial cancer (bottom). Diagrams represent the protein domains of p53 encoded by TP53. The presence of a mutation is shown on the x-axis (‘lollipop’), the frequency of mutations is shown on the y-axis. Missense mutations are presented as green circles, truncating mutations (i.e. nonsense, frameshift, splice-site) are depicted in red circles, in-frame insertions and deletions are shown in black circles, and circles colored in purple indicate residues affected by different types of mutation at the same proportion. Plots were generated using cBioPortal (www.cBioPortal.org) and manually curated. Note that mutations affecting the hotspots R175, R248 and R273 are more frequent in serous than in endometrioid endometrial cancers. (B) Prevalence of ARID1A, PTEN, FBXW7 and PPP2R1A mutations in TP53-mutant endometrioid endometrial cancers (EECs; left), and prevalence of ARID1A, PTEN, FBXW7 and PPP2R1A mutations in TP53-mutant serous endometrial cancers (SECs; right). Mutation types/ color-codes as depicted in the legend. Plots were generated using cBioPortal (www.cBioPortal.org) and manually curated.
Importantly, in the 64 TP53-mutant ECs identified in this dataset, TP53 frameshift or nonsense mutations were present in 14 cases (22%, Table 2), of which only two also displayed a missense mutation (Supplemental Table 1). Importantly, as somatic frameshift or nonsense mutations do not result in a p53 protein expression stabilization (15), in up to 22% of ECs (or in up to 19% if the two cases with both missense and truncating or frameshift mutations were excluded) these TP53 somatic mutations may theoretically not be detected as p53 overexpression by immunohistochemical analysis. Instead, frameshift and nonsense TP53 mutations are associated with a complete absence of p53 immunoreactivity, also referred to as ‘null pattern’, which requires careful interpretation of the immunohistochemical results (24-27).
Taken together, these observations demonstrate that TP53 mutations, although more frequently found in SECs and FIGO grade 3 EECs, are not restricted to these types of ECs, as a subset of FIGO grade 1 and 2 EECs may also harbor mutations affecting TP53. Furthermore, the spectrum of TP53 mutations differs between SECs and EECs, with the former displaying a significant enrichment for hotspot mutations.
TP53 mutation status according to integrative genomic subtypes
We next sought to define the spectrum of TP53 somatic mutations according to the integrative genomic subtypes of ECs. TP53 somatic mutations were found to be significantly more frequent in CN-high (91%) and POLE (35%) than in MSI (8%) and CN-low ECs (1%, Chi-squared p<0.0001; Table 1). Consistent with the ultra-high mutation rate of POLE ECs, all cases harboring more than one TP53 somatic mutation were of POLE subtype, and frameshift, nonsense and splice-site mutations were restricted to CN-high and POLE cancers (Table 3; Supplemental Table 1).
Table 3.
Clinico-pathologic features, distribution of TP53 mutations and mutations in genes recurrently altered in endometrial cancers in TP53-mutant endometrial carcinomas classified according to integrative genomic subtypes.
| Total (n) |
Integrative genomic subtype | p-value | |||||
|---|---|---|---|---|---|---|---|
| CN-high (n) |
CN-low (n) |
MSI (n) |
POLE (n) |
||||
| Tumor grade | Grade 1 | 2 | 0 | 0 | 0 | 2 | <0.0001* |
| Grade 2 | 8 | 6 | 1 | 1 | 0 | ||
| Grade 3 | 54 | 46 | 0 | 4 | 4 | ||
| Histologic type | Endometrioid | 27 | 15 | 1 | 5 | 6 | <0.0001* |
| Serous | 37 | 37 | 0 | 0 | 0 | ||
| Type of TP53
mutation |
Frameshift | 7 | 6 | 0 | 0 | 1 | 0.1844** |
| Missense | 49 | 41 | 1 | 5 | 2 | ||
| Nonsense | 7 | 4 | 0 | 0 | 3 | ||
| Splice-site | 1 | 1 | 0 | 0 | 0 | ||
| Hotspot TP53
mutation |
No | 43 | 32 | 1 | 5 | 5 | 0.2545* |
| Yes | 21 | 20 | 0 | 0 | 1 | ||
| Cases with multiple TP53 mutations |
No | 60 | 52 | 1 | 5 | 2 | <0.0001* |
| Yes | 4 | 0 | 0 | 0 | 4 | ||
| ARID1A gene status | Wild-type | 57 | 49 | 1 | 4 | 3 | 0.0140* |
| Mutant | 7 | 3 | 0 | 1 | 3 | ||
| FBXW7 gene status | Wild-type | 50 | 41 | 1 | 5 | 3 | 0.2631* |
| Mutant | 14 | 11 | 0 | 0 | 3 | ||
|
PPP2R1A gene status |
Wild-type | 52 | 41 | 1 | 5 | 5 | 0.85549* |
| Mutant | 12 | 11 | 0 | 0 | 1 | ||
| PTEN gene status | Wild-type | 46 | 46 | 0 | 0 | 0 | <0.0001* |
| Mutant | 18 | 6 | 1 | 5 | 6 | ||
Fisher’s exact test p-value;
Chi-square test p-value.
CN-high, copy-number high (serous-like) integrative genomic subtype; CN-low, copy-number low (endometrioid) integrative genomic subtype; MSI, microsatellite instable (hypermutated) integrative genomic subtype; n, number of TP53-mutant cases; POLE, POLE (ultramutated) integrative genomic subtype. Hotspot TP53 mutations include R175, G245, R248, R249, R273 and R282.
The spectrum of TP53 somatic mutations in CN-high appears to be somewhat different from that of other subtypes of ECs, in that CN-high cancers harbored mutations affecting hotspot residues numerically more frequently (20/52, 38% TP53-mutant CN-high cancers vs 1/12, 8% TP53-mutant non-CN-high cancers), however this was not statistically significant (Fisher’s exact test p=0.084; Table 3). Given that the subset of CN-high ECs comprise both SECs and EECs, we next sought to define whether there would be differences in the frequency, type and pattern of TP53 mutations in CN-high ECs. Notably, SECs and EECs of CN-high genomic subtype had similar frequencies of TP53 somatic mutations, and also a similar distribution of missense and hotspot mutations (Table 3; Supplemental Table 3).
Taken together, our results demonstrate that the vast majority of CN-high ECs are TP53-mutant and that the spectrum of TP53 mutations is similar in SECs and EECs of CN-high subtype. Importantly, however, TP53 mutations cannot be employed as a defining feature of this subtype, given that up to a third of POLE and 8% of MSI (endometrioid) cancers are also TP53-mutant, and that 10% of CN-high SECs and 6% of CN-high EECs are TP53 wild-type (Supplemental Table 3).
Associations between highly recurrently mutated genes in ECs and TP53 mutational status
The TCGA study identified several mutated genes that were characteristic of the different genomic subtypes of ECs. Epistatic interactions between genes and mutations may not only determine the evolutionary properties of cancers but may also affect their fitness and response to therapies (28). Given that the frequency and type of TP53 mutations would not provide sufficient information to differentiate between FIGO grade 3 EECs and SECs, we sought to define whether mutations affecting genes preferentially mutated in EECs or SECs would provide additional information.
Mutations affecting ARID1A and PTEN have been reported to be characteristic of EECs, whereas SECs show an enrichment for mutations affecting FBXW7 and PPP2R1A (4,6,20,23,29-32). As expected, in this dataset of 228 EECs and SECs, ARID1A and PTEN were significantly more frequently mutated in EECs (ARID1A, 39% EEC vs 10% SECs; PTEN, 78% EECs vs 2% SECs; Fisher’s exact test p<0.001), whereas FBXW7 and PPP2R1A were significantly more frequently mutated in SECs (FBXW7, 12% EECs vs 33% SECs; PPP2R1A, 7% EECs vs 26% SECs; Fisher’s exact test p<0.005; Supplemental Table 4).
The associations between TP53 somatic mutations and mutations affecting ARID1A, FBXW7, PPP2R1A and PTEN varied according to histologic type. In EECs, although no differences in the prevalence of FBXW7 and PPP2R1A mutations were identified, a significant inverse association between TP53 somatic mutations and ARID1A somatic mutations was observed, where ARID1A somatic mutations were found in 15% and 43% of the TP53-mutant and wild-type EECs, respectively (Fisher’s exact test p<0.001; Table 1, Fig. 1B, Supplemental Table 4). We found that ARID1A mutations were particularly frequent in EECs of MSI (endometrioid) integrative subtype (40%), a subtype that generally harbored few TP53 mutations (Table 1). PTEN somatic mutations were significantly less frequently found in TP53-mutant (63%) than in TP53 wild-type EECs (81%; Fisher’s exact test p<0.05; Fig. 1B; Supplemental Table 4). Given that 88% of SECs harbored a TP53 somatic mutation, no associations between TP53 mutations and mutations affecting ARID1A, FBXW7, PPP2R1A and PTEN were identified.
Within the subset of TP53-mutant ECs, a significant association between histologic type and PTEN mutations was identified (Table 1). Whilst 63% of all TP53-mutant EECs displayed a PTEN somatic mutation, only 3% of TP53-mutant SECs harbored somatic mutations affecting this gene (Fisher’s exact test p<0.0001; Supplemental Table 4). When focusing on high-grade ECs of CN-high subtype only, however, PTEN somatic mutations were observed at similar frequencies in CN-high TP53-mutant FIGO grade 3 EECs (2/9, 22%) and CN-high TP53-mutant SECs (3/37, 8%; Fisher’s exact test p=0.2484; Supplemental Table 3). Conversely, FIGO grade 3 TP53 wild-type EECs were statistically significantly more likely to harbor a PTEN mutation (22/29, 76%) than TP53 wild-type SECs (0/5, 0%; Fisher’s exact test, p=0.0028), providing evidence to suggest that the analysis of PTEN in high-grade ECs may help in discriminating FIGO grade 3 TP53 wild-type EECs from TP53 wild-type SECs (33-36). It should be noted, however, that the distinction between SEC and FIGO grade 3 EEC is challenging, even among expert gynecologic pathologists (33,37).
We next sought to define whether the information provided by the mutational status of FBXW7 or PPP2R1A genes, which are significantly more frequently targeted by mutations in SECs than in EECs (4,29) (Supplemental Table 4), would be useful in the discrimination of FIGO grade 3 EECs and SECs. We found that 20/37 (54%) of TP53-mutant SECs harbored a mutation affecting FBXW7 and/or PPP2R1A, as compared to 2/17 (12%) of FIGO grade 3 TP53-mutant EECs (Fisher’s exact test p=0.0062; Fig. 1B; Supplemental Table 4). In the remaining cases, TP53-mutant FBXW7/PPP2R1A wild-type SECs were significantly less frequently affected by PTEN mutations (1/17, 6%) as compared to FIGO grade 3 TP53-mutant FBXW7/PPP2R1A wild-type EECs (8/15, 53%, Fisher’s exact test p=0.0049).
Spectrum of TP53 mutations in ECs
Given that POLE cancers often harbored more than one TP53 somatic mutation, we next investigated the distribution of the TP53 mutations according to exons of the TP53 gene. In this dataset, 69 TP53 mutations were found in SECs and EECs (Table 4).
Table 4.
Spectrum of TP53 mutations according to histologic types and integrative genomic subtypes.
| Total (n) |
Histologic type | p-value | Integrative genomic subtype | p-value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Endometrioid (n) |
Serous (n) |
CN-high (n) |
CN-low (n) |
MSI (n) |
POLE (n) |
|||||
| Type of TP53
mutation |
Frameshift | 8 | 4 | 4 | 0.6063** | 6 | 0 | 0 | 2 | 0.3111** |
| Missense | 52 | 23 | 29 | 41 | 1 | 5 | 5 | |||
| Nonsense | 8 | 5 | 3 | 4 | 0 | 0 | 4 | |||
| Splice-site | 1 | 0 | 1 | 1 | 0 | 0 | 0 | |||
| Exon | Exon 4 | 2 | 2 | 0 | 0.0366*** | 1 | 0 | 0 | 1 | 0.1311*** |
| Exon 5 | 12 | 5 | 7 | 10 | 0 | 1 | 1 | |||
| Exon 6 | 10 | 8 | 2 | 6 | 0 | 0 | 4 | |||
| Exon 7 | 25 | 11 | 14 | 17 | 1 | 4 | 3 | |||
| Exon 8 | 17 | 5 | 12 | 16 | 0 | 0 | 1 | |||
| Exon 9 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | |||
| Exon 10 | 2 | 1 | 1 | 1 | 0 | 0 | 1 | |||
| Hotspot | No | 48 | 28 | 20 | 0.0036* | 32 | 1 | 5 | 10 | 0.08678* |
| Yes | 21 | 4 | 17 | 20 | 0 | 0 | 1 | |||
| Multiple TP53
mutations |
No | 60 | 23 | 37 | 0.0276* | 52 | 1 | 5 | 2 | <0.0001* |
| Yes | 4 | 4 | 0 | 0 | 0 | 0 | 4 | |||
Fisher’s exact test p-value;
Chi-square test p-value;
Fisher’s exact test p-value comparing exon 6 vs mutations affecting other exons.
CN-high, copy-number high (serous-like) integrative genomic subtype; CN-low, copy-number low (endometrioid) integrative genomic subtype; MSI, microsatellite instable (hypermutated) integrative genomic subtype; n, number; POLE, POLE (ultramutated) integrative genomic subtype.
When we assessed the single base substitutions, we observed that in EECs, these were predominantly T>C (8/28, 28%) and G>A (8/28, 28%) transitions, followed by C>T (5/28, 17%) transitions and C>A transversions (3/28, 10%). By contrast, in SECs, the most common single base substitutions were C>T transitions (12/35, 34%), followed by G>A transitions (11/35, 31.5%) and C>A transversions (3/35, 8.5%). EECs significantly more often harbored T>C transitions than SECs (EECs 8/28, SECs 2/35; Fisher’s exact test p=0.0179; data not shown).
Consistent with the notion that TP53 mutations preferentially affect exons 5-8 (10-12), in this dataset of ECs, TP53 mutations were found to primarily affect exon 7 (25/69, 36%) and exon 8 (17/69, 25%) of the TP53 gene, whereas exons 4, 9 and 10 harbored the lowest number of mutations (n=2, n=1 and n=2, respectively; Table 4). Interestingly, whilst the number of TP53 mutations affecting exons 7 and 8 were similar between EECs and SECs (exon 7: 11/32, 34% EECs; 14/37, 38%, SECs, Fisher’s exact test p=0.806; exon 8: 5/32, 5% EECs; 12/37, 32%, SECs, Fisher’s exact test p=0.161), we observed that EECs statistically significantly more frequently harbored TP53 exon 6 mutations than SECs (exon 6: 8/32, 25% EECs; 2/37, 5% SECs; Fisher’s exact test, p=0.037; Table 4). In fact, mutations affecting exon 6 of TP53 were significantly more frequently found in POLE cancers than in other genomic subtypes (36% of the TP53 mutations affected exon 6 in POLE cancers, whereas only 12% of TP53 mutations affected exon 6 in the other integrative genomic subtypes, Fisher’s exact test p-value<0.05).
DISCUSSION
Here we demonstrate that TP53 mutations are present in 28% of the 228 ECs analyzed, and that although the vast majority of SECs harbor TP53 mutations, up to 15% of all EECs are also TP53-mutant. Within EECs, we have observed that TP53 mutations are more frequently found in tumors of FIGO grade 3, and of CN-high and POLE integrative genomic subtypes (4). Given that up to a third of POLE and 8% of MSI (endometrioid) cancers are TP53-mutant, and that 10% of CN-high SECs and 6% of CN-high EECs are TP53 wild-type, TP53 mutations cannot be employed as a defining feature of ECs of CN-high (serous-like) integrative subtype or the discrimination between EECs and SECs of this genomic subgroup. The 12% of SECs in this series not harboring TP53 mutations were of CN-high (4/5) or CN-low (1/5) integrative genomic subtypes, and four cases harbored a median of 690 (range 238-1133) gene copy number alterations and 41 (range 30-43) non-synonymous somatic mutations (Supplemental Table 5). One TP53 wild-type SEC, however, harbored 1,324 non-synonymous mutations and few copy number altered genes (n=5), and mutations characteristic for both SECs and EECs (Supplemental Table 5).
The type of TP53 mutations present in ECs varied according to histologic and molecular subtypes. TP53 hotspot mutations were significantly more frequently found in SECs than in EECs. These hotspot mutations affect residues that are either involved in DNA binding (e.g., R248 and R273) or in supporting the structure/conformation of the DNA-binding surface (e.g., R175, G245, R249, R282)(12,38). There is burgeoning evidence to demonstrate that all TP53 hotspot mutations are in fact pathogenic and result in loss of p53 protein activity, whereas this is less clear for missense mutations that affect codons other than the non-hotspot residues (38,39). In this study, 46% of the TP53 mutations in SECs affected hotspot residues, whereas in EECs hotspot mutations were significantly less frequent (15%) and numerically less frequent in CN-high EECs than in CN-high SECs (20% vs 46%, respectively, Fisher’s exact test p-value=0.084). These observations suggest that even when present in EECs, the biological impact of TP53 mutations may differ between EECs and SECs.
Data from breast cancer studies have demonstrated that TP53 mutations are found in all molecular subtypes, but differ in their frequency, type and pattern according to the molecular subtypes (7,18). Our results demonstrate that akin to breast cancers, the frequency and type of TP53 mutations also varies according to the EC integrative genomic subtypes. Unlike breast cancers, however, where the basal-like group is the most frequently mutated and is enriched for frameshift and nonsense mutations (7,18), in ECs, the CN-high genomic subtype is the most frequently mutated but displays an enrichment for missense mutations affecting hotspot residues. In fact, a re-analysis of the TCGA ovarian cancer study revealed that the repertoire of TP53 mutations in SECs is more similar to that of high-grade serous ovarian carcinomas than to that of basal-like breast cancers (4,7,8), further supporting that although there are similarities between these tumors, important differences are also observed.
Although the protein product of TP53 harboring missense mutations has been shown to have a longer half-life and, therefore, amenable to immunohistochemical detection, cases harboring TP53 frameshift and nonsense mutations have been shown to frequently yield negative immunohistochemical results (i.e. p53 overexpression cannot be detected)(15,40). Immunohistochemical analysis of p53, however, has been suggested to provide ancillary information for accurate diagnosis of SECs (6). Our results demonstrate that approximately one fifth of all TP53-mutant ECs harbor frameshift or nonsense mutations. In these cases, careful interpretation of the p53 immunohistochemical results is essential as frameshift and nonsense TP53 mutations lead to a complete lack of p53 protein expression (‘null pattern’)(24-27), which may be misinterpreted as wild-type p53 expression pattern. Further studies investigating the type of TP53 mutation and the respective p53 protein expression patterns by immunohistochemistry using distinct p53 antibody clones are warranted.
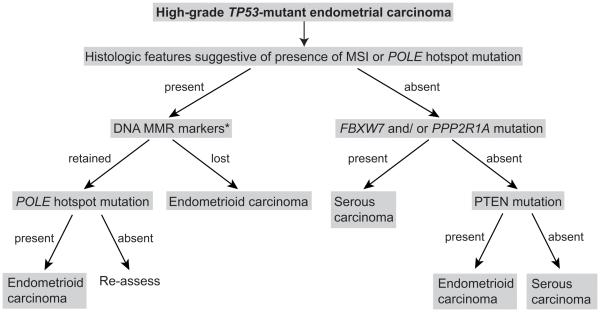
Finally, we sought to define whether sequencing analysis of additional genes highly mutated in ECs may assist in the differentiation between SECs and high-grade EECs. In fact, we have observed that within the group of TP53-mutant ECs, somatic mutations affecting PTEN are significantly more frequently found in EECs than in SECs. Importantly, however, not all high-grade EECs, and in particular only a small subset of FIGO grade 3 EECs of CN-high integrative subtype, harbor PTEN somatic mutations and, therefore, would have retained PTEN expression by immunohistochemical analysis, given the strong correlation between PTEN somatic mutations and lack of protein expression in ECs (41). We further demonstrated that the mutational analysis of FBXW7, PPP2R1A and PTEN together with the assessment of histologic features associated with microsatellite instable (MSI) ECs and ECs harboring POLE hotspot mutations (i.e. enrichment in tumor-infiltrating lymphocytes and/or peri-tumoral lymphocytes (42,43)) and the immunohistochemical analysis of the DNA mismatch repair markers (MMR) (4,43), may aid in the distinction of between SECs and FIGO grade 3 EECs (Fig. 2). In the sporadic ECs analyzed by TCGA (4), high-level MSI was associated with MLH1 hypermethylation and endometrioid histology in all cases studied, thus the DNA MMR testing may be restricted to MLH1 immunohistochemical analysis, followed by MLH1 methylation analysis if MLH1 protein expression is absent (Fig. 2). It should be noted however, that in the setting of Lynch syndrome, 14-35% of ECs are not of endometrioid histology (44,45), hence the proposed scheme is only applicable for sporadic ECs. Studies to validate the proposed scheme in independent datasets are warranted.
Fig. 2. A combination of immunohistochemical and mutational analysis may aid in the distinction of sporadic high-grade TP53-mutant endometrial carcinomas of serous endometrial and FIGO grade 3 endometrioid histologic subtypes.
Histologic features characteristic of MSI endometrial cancers or endometrial carcinomas harboring POLE hotspot mutations include enrichment in tumor-infiltrating lymphocytes and/or peri-tumoral lymphocytes, among others. *DNA MMR markers may be limited to MLH1 immunohistochemistry and/or MLH1 promoter methylation analysis. DNA MMR, DNA mismatch repair; IHC, immunohistochemistry; MSI, microsatellite instability.
It is important to note that genetic alterations affecting PTEN and FBXW7 may not only be useful for the histologic typing of high-grade TP53-mutant ECs (Fig. 2), but may also have therapeutic implications. Given that the vast majority of ECs have been found to harbor somatic genetic alterations in the PI3K/AKT/mTOR pathway (4), inhibition of this pathway is of great therapeutic interest (46). In particular, there is pre-clinical evidence to suggest that ECs harboring PIK3CA or PTEN mutations may be sensitive to inhibitors targeting different components of the PI3K/AKT/mTOR pathway (47-51). FBXW7 is a tumor suppressor gene, and its protein product FBXW7 promotes the ubiquitination and degradation of numerous oncoproteins (52). Histone deacetylase (HDAC) inhibition (53) or targeting of FBXW7 regulators in FBXW7-mutant cancers, such as NOTCH1 (54) or mTOR (55,56), may also have potential for therapeutic interventions in ECs harboring FBXW7 inactivating mutations.
The genomic subtypes of ECs as proposed by TCGA, which are defined through gene copy number and exome-wide mutational analysis, have yet to be incorporated into clinical practice. It is likely that the integration of both genomics and histopathology may lead to a classification system of ECs that helps define biologically and clinically relevant subsets of the disease, and may facilitate development of therapies tailored to specific histologic and genomic subgroups (6) as outlined above. Recent studies have confirmed that ECs of POLE ultramutated genomic subtype have a good prognosis (57,58), despite these tumors commonly being of high grade and frequently harboring TP53 mutations (42). This POLE subgroup of ECs is currently only reliably identified by sequencing of the POLE gene. In this context it is worth mentioning that in other disease types the integration of histologic and molecular information is already being performed. For example, neuropathologists recently reported on consensus guidelines that will incorporate molecular information in the next World Health Organization (WHO) classification (59), where distinct tumor entities will be derived from an “integrated” diagnosis, seeking to define biologically and clinically uniform groups precisely and objectively on the basis of a combination of molecular information, histologic features and WHO grade (59). The therapy of patients with high-grade ECs is currently primarily based on clinical parameters rather than on the biological characteristics of the tumors, and the incorporation of molecular features of these cancers will be essential for the realization of the potentials of precision medicine for patients with high-grade ECs.
In conclusion, the type and pattern of TP53 mutations varies according to histologic and genomic subtypes of EC. Importantly, TP53 mutations are not restricted to SECs, FIGO grade 3 and/or CN-high lesions. Given that a subset of TP53-mutant ECs harbor nonsense or frameshift mutations, for which interpretation of immunohistochemistry results may be challenging, and that EECs and SECs display different patterns, types and distributions of TP53 mutations, p53 immunohistochemical analysis alone may not be sufficient for the differential diagnosis of EEC and SECs, and a panel comprising additional genes, including FBXW7, PPP2R1A and PTEN, may provide further diagnostic accuracy in challenging cases.
Supplementary Material
Acknowledgements/ Sources of Support
AMS is funded by a stipend from the German Cancer Aid (Dr. Mildred Scheel Stiftung), and SP by a Susan G Komen Postdoctoral Fellowship Grant (PDF14298348).
Footnotes
CONFLICT OF INTEREST STATEMENT
The authors have no conflicts of interest to declare in relation to this study.
REFERENCES
- 1.Siegel R, Ma J, Zou Z, et al. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Zaino RC, Ellenson SG, Eng LH, et al. Tumors of the uterine corpus. In: Kurman RJ, Carcangiu ML, Herrington CS, Young RH, editors. WHO Classification of Tumors of Female Reproductive Organs. International Agency for Research on Cancer; Lyon: 2014. pp. 121–67. C. [Google Scholar]
- 3.Bokhman JV. Two pathogenetic types of endometrial carcinoma. Gynecol Oncol. 1983;15:10–7. doi: 10.1016/0090-8258(83)90111-7. [DOI] [PubMed] [Google Scholar]
- 4.Cancer Genome Atlas Research Network. Kandoth C, Schultz N, et al. Integrated genomic characterization of endometrial carcinoma. Nature. 2013;497:67–73. doi: 10.1038/nature12113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matias-Guiu X, Prat J. Molecular pathology of endometrial carcinoma. Histopathology. 2013;62:111–23. doi: 10.1111/his.12053. [DOI] [PubMed] [Google Scholar]
- 6.Murali R, Soslow RA, Weigelt B. Classification of endometrial carcinoma: more than two types. Lancet Oncol. 2014;15:e268–78. doi: 10.1016/S1470-2045(13)70591-6. [DOI] [PubMed] [Google Scholar]
- 7.Cancer Genome Atlas Network Comprehensive molecular portraits of human breast tumours. Nature. 2012;490:61–70. doi: 10.1038/nature11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cancer Genome Atlas Research Network Integrated genomic analyses of ovarian carcinoma. Nature. 2011;474:609–15. doi: 10.1038/nature10166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Freed-Pastor WA, Prives C. Mutant p53: one name, many proteins. Genes Dev. 2012;26:1268–86. doi: 10.1101/gad.190678.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Muller PA, Vousden KH. p53 mutations in cancer. Nat Cell Biol. 2013;15:2–8. doi: 10.1038/ncb2641. [DOI] [PubMed] [Google Scholar]
- 11.Olivier M, Hollstein M, Hainaut P. TP53 mutations in human cancers: origins, consequences, and clinical use. Cold Spring Harb Perspect Biol. 2010;2:a001008. doi: 10.1101/cshperspect.a001008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brosh R, Rotter V. When mutants gain new powers: news from the mutant p53 field. Nat Rev Cancer. 2009;9:701–13. doi: 10.1038/nrc2693. [DOI] [PubMed] [Google Scholar]
- 13.Rivlin N, Brosh R, Oren M, et al. Mutations in the p53 Tumor Suppressor Gene: Important Milestones at the Various Steps of Tumorigenesis. Genes Cancer. 2011;2:466–74. doi: 10.1177/1947601911408889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hall PA, McCluggage WG. Assessing p53 in clinical contexts: unlearned lessons and new perspectives. J Pathol. 2006;208:1–6. doi: 10.1002/path.1913. [DOI] [PubMed] [Google Scholar]
- 15.Soussi T, Leroy B, Taschner PE. Recommendations for analyzing and reporting TP53 gene variants in the high-throughput sequencing era. Hum Mutat. 2014;35:766–78. doi: 10.1002/humu.22561. [DOI] [PubMed] [Google Scholar]
- 16.Gao J, Aksoy BA, Dogrusoz U, et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal. 2013;6:pl1. doi: 10.1126/scisignal.2004088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Petitjean A, Mathe E, Kato S, et al. Impact of mutant p53 functional properties on TP53 mutation patterns and tumor phenotype: lessons from recent developments in the IARC TP53 database. Hum Mutat. 2007;28:622–9. doi: 10.1002/humu.20495. [DOI] [PubMed] [Google Scholar]
- 18.Silwal-Pandit L, Vollan HK, Chin SF, et al. TP53 mutation spectrum in breast cancer is subtype specific and has distinct prognostic relevance. Clin Cancer Res. 2014;20:3569–80. doi: 10.1158/1078-0432.CCR-13-2943. [DOI] [PubMed] [Google Scholar]
- 19.Muller PA, Vousden KH. Mutant p53 in cancer: new functions and therapeutic opportunities. Cancer Cell. 2014;25:304–17. doi: 10.1016/j.ccr.2014.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garcia-Dios DA, Lambrechts D, Coenegrachts L, et al. High-throughput interrogation of PIK3CA, PTEN, KRAS, FBXW7 and TP53 mutations in primary endometrial carcinoma. Gynecol Oncol. 2013;128:327–34. doi: 10.1016/j.ygyno.2012.11.037. [DOI] [PubMed] [Google Scholar]
- 21.Lee EJ, Kim TJ, Kim DS, et al. p53 alteration independently predicts poor outcomes in patients with endometrial cancer: a clinicopathologic study of 131 cases and literature review. Gynecol Oncol. 2010;116:533–8. doi: 10.1016/j.ygyno.2009.11.018. [DOI] [PubMed] [Google Scholar]
- 22.Stelloo E, Bosse T, Nout RA, et al. Refining prognosis and identifying targetable pathways for high-risk endometrial cancer; a TransPORTEC initiative. Mod Pathol. 2015;28:836–44. doi: 10.1038/modpathol.2015.43. [DOI] [PubMed] [Google Scholar]
- 23.Allo G, Bernardini MQ, Wu RC, et al. ARID1A loss correlates with mismatch repair deficiency and intact p53 expression in high-grade endometrial carcinomas. Mod Pathol. 2014;27:255–61. doi: 10.1038/modpathol.2013.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McCluggage WG, Soslow RA, Gilks CB. Patterns of p53 immunoreactivity in endometrial carcinomas: 'all or nothing' staining is of importance. Histopathology. 2011;59:786–8. doi: 10.1111/j.1365-2559.2011.03907.x. [DOI] [PubMed] [Google Scholar]
- 25.Garg K, Leitao MM, Jr., Wynveen CA, et al. p53 overexpression in morphologically ambiguous endometrial carcinomas correlates with adverse clinical outcomes. Mod Pathol. 2010;23:80–92. doi: 10.1038/modpathol.2009.153. [DOI] [PubMed] [Google Scholar]
- 26.Tashiro H, Isacson C, Levine R, et al. p53 gene mutations are common in uterine serous carcinoma and occur early in their pathogenesis. Am J Pathol. 1997;150:177–85. [PMC free article] [PubMed] [Google Scholar]
- 27.Lax SF, Kendall B, Tashiro H, et al. The frequency of p53, K-ras mutations, and microsatellite instability differs in uterine endometrioid and serous carcinoma: evidence of distinct molecular genetic pathways. Cancer. 2000;88:814–24. [PubMed] [Google Scholar]
- 28.Weigelt B, Reis-Filho JS. Epistatic interactions and drug response. J Pathol. 2014;232:255–63. doi: 10.1002/path.4265. [DOI] [PubMed] [Google Scholar]
- 29.McConechy MK, Ding J, Cheang MC, et al. Use of mutation profiles to refine the classification of endometrial carcinomas. J Pathol. 2012;228:20–30. doi: 10.1002/path.4056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Le Gallo M, O'Hara AJ, Rudd ML, et al. Exome sequencing of serous endometrial tumors identifies recurrent somatic mutations in chromatin-remodeling and ubiquitin ligase complex genes. Nat Genet. 2012;44:1310–5. doi: 10.1038/ng.2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kuhn E, Wu RC, Guan B, et al. Identification of molecular pathway aberrations in uterine serous carcinoma by genome-wide analyses. J Natl Cancer Inst. 2012;104:1503–13. doi: 10.1093/jnci/djs345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hoang LN, McConechy MK, Kobel M, et al. Histotype-genotype correlation in 36 high-grade endometrial carcinomas. Am J Surg Pathol. 2013;37:1421–32. doi: 10.1097/PAS.0b013e31828c63ed. [DOI] [PubMed] [Google Scholar]
- 33.Soslow RA. High-grade endometrial carcinomas - strategies for typing. Histopathology. 2013;62:89–110. doi: 10.1111/his.12029. [DOI] [PubMed] [Google Scholar]
- 34.Bartosch C, Manuel Lopes J, Oliva E. Endometrial carcinomas: a review emphasizing overlapping and distinctive morphological and immunohistochemical features. Adv Anat Pathol. 2011;18:415–37. doi: 10.1097/PAP.0b013e318234ab18. [DOI] [PubMed] [Google Scholar]
- 35.Soslow RA. Endometrial carcinomas with ambiguous features. Semin Diagn Pathol. 2010;27:261–73. doi: 10.1053/j.semdp.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 36.Alkushi A, Kobel M, Kalloger SE, et al. High-grade endometrial carcinoma: serous and grade 3 endometrioid carcinomas have different immunophenotypes and outcomes. Int J Gynecol Pathol. 2010;29:343–50. doi: 10.1097/PGP.0b013e3181cd6552. [DOI] [PubMed] [Google Scholar]
- 37.Hussein YR, Broaddus R, Weigelt B, et al. The genomic heterogeneity of FIGO grade 3 endometrioid carcinoma impacts diagnostic accuracy and reproducibility. Int J Gynecol Pathol. 2016;35:16–24. doi: 10.1097/PGP.0000000000000212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Joerger AC, Fersht AR. Structure-function-rescue: the diverse nature of common p53 cancer mutants. Oncogene. 2007;26:2226–42. doi: 10.1038/sj.onc.1210291. [DOI] [PubMed] [Google Scholar]
- 39.Leroy B, Anderson M, Soussi T. TP53 mutations in human cancer: database reassessment and prospects for the next decade. Hum Mutat. 2014;35:672–88. doi: 10.1002/humu.22552. [DOI] [PubMed] [Google Scholar]
- 40.Chiang S, Soslow RA. Updates in diagnostic immunohistochemistry in endometrial carcinoma. Semin Diagn Pathol. 2014;31:205–15. doi: 10.1053/j.semdp.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 41.Djordjevic B, Hennessy BT, Li J, et al. Clinical assessment of PTEN loss in endometrial carcinoma: immunohistochemistry outperforms gene sequencing. Mod Pathol. 2012;25:699–708. doi: 10.1038/modpathol.2011.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hussein YR, Weigelt B, Levine DA, et al. Clinicopathological analysis of endometrial carcinomas harboring somatic POLE exonuclease domain mutations. Mod Pathol. 2015;28:505–14. doi: 10.1038/modpathol.2014.143. [DOI] [PubMed] [Google Scholar]
- 43.Garg K, Leitao MM, Jr., Kauff ND, et al. Selection of endometrial carcinomas for DNA mismatch repair protein immunohistochemistry using patient age and tumor morphology enhances detection of mismatch repair abnormalities. Am J Surg Pathol. 2009;33:925–33. doi: 10.1097/PAS.0b013e318197a046. [DOI] [PubMed] [Google Scholar]
- 44.Carcangiu ML, Radice P, Casalini P, et al. Lynch syndrome--related endometrial carcinomas show a high frequency of nonendometrioid types and of high FIGO grade endometrioid types. Int J Surg Pathol. 2010;18:21–6. doi: 10.1177/1066896909332117. [DOI] [PubMed] [Google Scholar]
- 45.Broaddus RR, Lynch HT, Chen LM, et al. Pathologic features of endometrial carcinoma associated with HNPCC: a comparison with sporadic endometrial carcinoma. Cancer. 2006;106:87–94. doi: 10.1002/cncr.21560. [DOI] [PubMed] [Google Scholar]
- 46.Slomovitz BM, Coleman RL. The PI3K/AKT/mTOR pathway as a therapeutic target in endometrial cancer. Clin Cancer Res. 2012;18:5856–64. doi: 10.1158/1078-0432.CCR-12-0662. [DOI] [PubMed] [Google Scholar]
- 47.Weigelt B, Warne PH, Lambros MB, et al. PI3K pathway dependencies in endometrioid endometrial cancer cell lines. Clin Cancer Res. 2013;19:3533–44. doi: 10.1158/1078-0432.CCR-12-3815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cheung LW, Hennessy BT, Li J, et al. High frequency of PIK3R1 and PIK3R2 mutations in endometrial cancer elucidates a novel mechanism for regulation of PTEN protein stability. Cancer Discov. 2011;1:170–85. doi: 10.1158/2159-8290.CD-11-0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shoji K, Oda K, Kashiyama T, et al. Genotype-dependent efficacy of a dual PI3K/mTOR inhibitor, NVP-BEZ235, and an mTOR inhibitor, RAD001, in endometrial carcinomas. PLoS One. 2012;7:e37431. doi: 10.1371/journal.pone.0037431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Myers AP. New strategies in endometrial cancer: targeting the PI3K/mTOR pathway--the devil is in the details. Clin Cancer Res. 2013;19:5264–74. doi: 10.1158/1078-0432.CCR-13-0615. [DOI] [PubMed] [Google Scholar]
- 51.Weigelt B, Downward J. Genomic Determinants of PI3K Pathway Inhibitor Response in Cancer. Front Oncol. 2012;2:109. doi: 10.3389/fonc.2012.00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang L, Ye X, Liu Y, et al. Aberrant regulation of FBW7 in cancer. Oncotarget. 2014;5:2000–15. doi: 10.18632/oncotarget.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.He L, Torres-Lockhart K, Forster N, et al. Mcl-1 and FBW7 control a dominant survival pathway underlying HDAC and Bcl-2 inhibitor synergy in squamous cell carcinoma. Cancer Discov. 2013;3:324–37. doi: 10.1158/2159-8290.CD-12-0417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Aydin IT, Melamed RD, Adams SJ, et al. FBXW7 mutations in melanoma and a new therapeutic paradigm. J Natl Cancer Inst. 2014;106:dju107. doi: 10.1093/jnci/dju107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mao JH, Kim IJ, Wu D, et al. FBXW7 targets mTOR for degradation and cooperates with PTEN in tumor suppression. Science. 2008;321:1499–502. doi: 10.1126/science.1162981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Villaruz LC, Socinski MA. Temsirolimus therapy in a patient with lung adenocarcinoma harboring an FBXW7 mutation. Lung Cancer. 2014;83:300–1. doi: 10.1016/j.lungcan.2013.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Meng B, Hoang LN, McIntyre JB, et al. POLE exonuclease domain mutation predicts long progression-free survival in grade 3 endometrioid carcinoma of the endometrium. Gynecol Oncol. 2014;134:15–9. doi: 10.1016/j.ygyno.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 58.Church DN, Stelloo E, Nout RA, et al. Prognostic significance of POLE proofreading mutations in endometrial cancer. J Natl Cancer Inst. 2015;107:402. doi: 10.1093/jnci/dju402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Louis DN, Perry A, Burger P, et al. International Society Of Neuropathology--Haarlem consensus guidelines for nervous system tumor classification and grading. Brain Pathol. 2014;24:429–35. doi: 10.1111/bpa.12171. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.