Abstract
Wheat blast is a serious disease caused by the fungus Magnaporthe oryzae (Triticum pathotype) (MoT). The objective of this study was to determine the effect of the 2NS translocation from Aegilops ventricosa (Zhuk.) Chennav on wheat head and leaf blast resistance. Disease phenotyping experiments were conducted in growth chamber, greenhouse, and field environments. Among 418 cultivars of wheat (Triticum aestivum L.), those with 2NS had 50.4 to 72.3% less head blast than those without 2NS when inoculated with an older MoT isolate under growth chamber conditions. When inoculated with recently collected isolates, cultivars with 2NS had 64.0 to 80.5% less head blast. Under greenhouse conditions when lines were inoculated with an older MoT isolate, those with 2NS had a significant head blast reduction. With newer isolates, not all lines with 2NS showed a significant reduction in head blast, suggesting that the genetic background and/or environment may influence the expression of any resistance conferred by 2NS. However, when near-isogenic lines (NILs) with and without 2NS were planted in the field, there was strong evidence that 2NS conferred resistance to head blast. Results from foliar inoculations suggest that the resistance to head infection that is imparted by the 2NS translocation does not confer resistance to foliar disease. In conclusion, the 2NS translocation was associated with significant reductions in head blast in both spring and winter wheat.
Wheat blast is a serious disease caused by a host-specialized population of the ascomycete Magnaporthe oryzae B.C. Couch and L.M. Kohn (synonym Pyricularia oryzae). It was first reported on wheat (Triticum aestivum L.) in 1985 in Paraná, Brazil (Igarashi et al., 1986) and has since spread throughout many of the important wheat-producing areas of Brazil and to the neighboring countries of Bolivia and Paraguay. Blast is now considered a major threat to wheat production in South America (Goulart et al., 1992; Goulart and Paiva, 2000; Goulart et al., 2007; Kohli et al., 2011).
There are several strains of M. oryzae and they tend to display a degree of host specificity. Therefore, they have been divided into pathotypes based on their host preference. The strains that commonly occur on wheat in South America have been placed into the Triticum pathotype (MoT). Nevertheless, recent evidence shows that there are US native strains of the closely related Lolium pathotype from ryegrass (Lolium perenne L.) that can cause severe disease on wheat at the head stage. It has been suggested that a US strain of M. oryzae Lolium pathogen (Farman, 2002) may have mutated and gained virulence to wheat to become the pathogen recently found in Kentucky (Pratt, 2012). In addition, under greenhouse host range inoculations, a number of US M. oryzae Lolium isolates produced typical blast symptoms on wheat heads (G. Peterson, unpublished data, 2015). Although related, the Oryza pathotype on rice (Oryza sativa L.) is genetically distinct from MoT and is not known to cause disease on wheat (Prabhu et al., 1992; Orbach et al., 1996; Urashima et al., 1999; Farman, 2002; Maciel et al., 2014).
The MoT can infect all aboveground parts of the wheat plant (Igarashi, 1991). While infections on leaves on certain cultivars may play an important role epidemiologically (Cruz et al., 2015), the most significant symptom of wheat blast in the field is the premature bleaching of spikelets (Igarashi, 1991; Urashima, 2010). In severe cases, the entire head is killed. If infections on heads occur early in head development, grain production is eliminated. Later infections reduce yields because the plant produces shriveled grain. In either case, overall production can be greatly reduced with losses in extreme cases approaching 100% infection (Goulart et al., 1992; Goulart and Paiva, 2000).
Given the importance of wheat in the United States, blast is regarded as a potential major threat. Strains of M. oryzae that can cause significant disease on wheat could become established in the United States based on three independent scenarios. First, isolates of MoT may be introduced from South America via man-mediated transport of MoT-contaminated plant materials (e.g., seeds) into US agroecosystems. Second, strains of other pathotypes that already exist in the United States may become problematic under changing environmental factors that could favor epidemics. Third, new strains of M. oryzae could arise from strains that already exist in the United States that could cause severe disease on wheat. Therefore, as a prelude to its possible introduction or development in the United States, a preliminary survey of US wheat genotypes for resistance to head blast was conducted (Cruz et al., 2012). The evidence provided by Cruz et al. (2012) indicated a continuum of reaction, from highly susceptible to highly resistant, among US winter wheat genotypes.
Although most wheat cultivars in South America are susceptible to blast, useful levels of resistance to the head phase have been reported for some cultivars (Urashima et al., 2004; Prestes et al., 2007; Maciel et al., 2008; Cruz et al., 2010). However, some of these previously resistant cultivars (e.g., ‘BR-18 Terena’) now display susceptibility in certain environments (Urashima et al., 2001; Urashima et al., 2005). This is evidence that new races have developed in the field that render deployed resistance ineffective. Furthermore, Maciel et al. (2014) reported that none of the cultivars found to possess resistance to the head phase were resistant to all 27 isolates used in their studies. In addition, wheat cultivars showing high levels of resistance to isolates in greenhouse and/or growth chamber conditions may not show resistance under natural field conditions (Kohli et al., 2011). Cultivars derived from the CIMMYT line Milan appear to contain high levels of resistance under field conditions (Kohli et al., 2011). The genetic basis of the resistance in Milan has not yet been established (Kohli et al., 2011). Other cultivars with this resistance source are now being widely deployed, but it remains to be seen how long this resistance will be effective (Kohli et al., 2011). Thus, there is a critical need for identification of new sources of resistance to wheat blast.
The objective of this study was to determine the effect of the 2NS translocation from Aegilops ventricosa (Zhuk.) Chennav on wheat head and leaf blast resistance. This translocation carries a 25 to 38 cM distal segment of chromosome arm 2NS from Aegilops ventricosa to the distal region of chromosome arm 2AS in wheat (Maia, 1967; Helguera et al., 2003). The Ae. ventricosa 2NS/2AS translocation carries resistance genes Rkn3 against root-knot nematodes (Meloidogyne spp.) (Williamson et al., 2013), Cre5 against the French pathotype Ha12 of the cereal cyst nematode (Heterodera avenae Wollenweber) (Jahier et al., 2001), and Lr37, Sr38, and Yr17 against some races of wheat leaf, stem and stripe rust (Helguera et al., 2003).
MATERIALS AND METHODS
Disease phenotyping experiments were conducted in growth chamber and greenhouse environments in the United States and in the field in South America.
Growth Chamber and Greenhouse Experiments
Phenotyping of wheat cultivars were performed in biosafety-level-3 (BSL-3) facilities on the campus of Kansas State University at the Biosecurity Research Institute (BRI) in Manhattan, KS, or the Agricultural Research Service (ARS), Foreign Disease-Weed Science Research Unit (FDWSRU) at Ft. Detrick, MD. Isolates of M. oryzae were obtained from infected wheat heads from South America. Infected material was transported to the BSL-3 laboratories under conditions authorized by permits from the USDA Animal and Plant Health Inspection Service. Routine isolation techniques were used to obtain single-spore isolates from this infected tissue. Isolates used in this study included B-2, collected in Quirusillas, Bolivia, in 2011; B-71, collected in Okinawa, Bolivia, in 2012; P-3, collected in Canindeyu, Paraguay, in 2012; and T-25, originally collected by Seiji Igarashi at São Jorge do Ivaí, Paraná, Brazil, in 1988 (Cruz et al., 2012). Isolates were catalogued and stored on colonized, dried cellulose filter paper discs at −20°C (Tuite, 1969; Valent et al., 1986).
Experiments in growth chambers at the BRI consisted of phenotypic assays of winter wheat cultivars. Cultivars were selected based on preliminary studies using MoT (Cruz et al., 2012). In those studies, 23 out of 85 cultivars showed <5% severity whereas the susceptible cultivars showed >90% severity. Initial experiments focused on those 23 cultivars and subsets of 7 cultivars from that group were re-tested in later experiments. In addition to the resistant cultivars, the highly susceptible check ‘Everest’ was included in all experiments. Seeds were germinated in soil in 2.5 by 13 cm plastic tubes (Stuewe and Sons, Tangent, OR) and grown for 2 wk in a greenhouse (28:15°C day/night, 14:10 h light/dark) with sunlight supplemented by high-pressure sodium lamps. The seedlings were then vernalized for a minimum of 7 wk in a cold room (4°C and 9:15 h light/dark) and transplanted into 15-cm diameter pots for growth in the greenhouse to the heading stage. Pots contained a commercial potting medium (Metro-Mix 360, Hummert International, Earth City, MO) and standard fertilization practices were used. When plants began to head, they were transferred to the BSL-3 laboratory where they were inoculated and grown in growth chambers (23 ± 2°C and 150 μmol m−2 s−1) until rated for disease severity (Cruz et al., 2012). Spores of M. oryzae were produced from cultures grown on homemade oatmeal agar (Valent et al., 1991) (50 g of rolled oats in 500 mL water heated at 70°C for 1 h and then filtered through four layers of cheese cloth, adjusted to 1L, 1.5% agar added, and autoclaved). Agar plates were incubated at 23 to 25°C under continuous fluorescent illumination (25 μmol m−2 s−1). Colonies of 5- to 7-d age were flooded with sterile deionized water containing 0.42% w/w gelatin and 0.01% v/v Tween-20 (Sigma-Aldrich) surfactant and gently scraped with an inoculation loop to dislodge conidia from conidiophores. The spore suspension was then filtered through two layers of cheesecloth and adjusted to 2 × 104 conidia mL−1 in a solution of deionized water, gelatin, and Tween-20. Disease phenotyping of winter wheat in growth chambers consisted of completely randomized design experiments, with four or five pots (replicates) per cultivar and 10 to 20 inoculated heads (experimental unit) per isolate by cultivar treatment. Controls consisted of non-inoculated heads and the highly susceptible cultivar Everest. Heads inoculated with isolates T-25 or B-71 were tagged and sprayed (0.75 mL per head with 2 × 104 spores mL−1) within 2 d of full head emergence from the boot and then individually covered with black, 7.5 by 13 cm plastic bags with zipper closure (Uline, Coppell, TX). The bags had been moistened with water on the inside to maintain high humidity around the inoculated heads. Bags were removed 48 h after inoculation and plants remained inside the growth chamber until rated for disease severity. Each head was visually rated 14 d after inoculation for the percentage of the total number of diseased spikelets (Cruz et al., 2012). When isolate B-71 had a significant difference in aggressiveness and/or virulence from isolate T-25, the finding was verified in repeat experiments.
Experiments in the greenhouse at the FDWSRU consisted of phenotypic assays of spring wheat adult plants selected from the Uniform Spring Wheat Nurseries. One hundred seventy five spring wheat entries consisting of hard red spring, soft white spring and club types were phenotyped. In all experiments, spring wheat ‘Glenn’ was included as a susceptible control. The soil mix consisted of 0.18 m3 peat, 0.11 m3 vermiculite, 0.03 m3 perlite, 1.06 L of wetting agent, 2.4 kg Osmocote 14–14–14 fertilizer (Scotts Co., Marysville, OH), 2.3 kg lime, 144 kg soil, and 171 kg sand. Biweekly, 40 entries were planted in 6-cm clay pots (five pots per entry and four seeds per pot) and maintained in a greenhouse (25 ± 4°C, 16:8 h light/dark) in which natural light was supplemented with high pressure sodium lamps. Routine fertilization practices were used. At the onset of head emergence, plants were transported to the BSL-3 plant disease containment facility of the USDA-ARS in Frederick, MD, where they were inoculated and maintained in a containment greenhouse until rated. Procedures for inoculum production followed at the FDWSRU were similar to those at the BRI. Sporulating cultures grown under continuous fluorescent light (33 μmol m−2 s−1) were flooded with sterile water with 0.01% v/v Tween-20 and gently scraped using a 1.3 cm flat, soft nylon paint brush to dislodge the conidia. The spore suspension was filtered through a 53-μm mesh sieve and the conidial concentration adjusted to 5 × 104 conidia mL−1. Disease phenotyping of spring wheat in the greenhouse consisted of completely randomized design experiments, with 5 pots (replicates) per cultivar and 14 to 21 inoculated heads (experimental unit) per entry. All heads were inoculated at Feekes growth stage 10.5. Negative controls consisted of bagged non-inoculated heads, whereas the highly susceptible cultivar Cavalier was used as a positive control. For each of the entries, heads were spray inoculated to run off (~1 mL per wheat head) with a MoT monosporic isolate (T-25) with a conidial concentration of 5 × 104 spores mL−1 using an atomizer (Devilbliss Manufacturing Co., Toledo, OH). Individual heads were covered with a 7.5 by 13 cm clear plastic bag with a zipper closure (Uline, Coppell, TX) and placed in a shaded portion of the greenhouse. Bags were removed 24 h after inoculation, and plants moved to a greenhouse bench. Greenhouse temperature was 25 ± 4°C and supplemental light was used to extend the day length to 16 h. Disease severity was visually rated 14 to 16 d after inoculation based on the percentage of blasted spikelets per head. Entries that exhibited <10% disease severity were tested again using T-25 and if consistent results were obtained, tested two more times with the isolate B-2.
In addition near isogenic lines (NILs) with and without the 2NS chromosome segment were phenotyped at the adult and seedling stages in greenhouse experiments. To test the specific effect of the 2NS translocation in different genetic backgrounds, we used six independent pairs of NILs in which the 2NS translocation was introgressed by six backcross generations into the hard red spring cultivars Anza, Yecora Rojo, Express, Kern and the UC Davis breeding lines UC1037 and UC1041. The resulting NILs with the segment were designated by the cultivar name followed by “-2NS”. The Anza-2NS NIL was previously deposited in the National Small Grain Collection under PI 638742 (Chicaiza et al., 2006). In each backcross generation, the 2NS segment was selected using a cleaved amplification polymorphic sequence marker (Helguera et al., 2003). After BC6, plants were self-pollinated and lines homozygous for the 2NS translocation were selected. This 2NS segment does not recombine with the wheat chromosomes in the presence of the Ph1b gene and is transmitted as a single block (Helguera et al., 2003). Wheat entries were planted, grown, and inoculated under the same conditions described for phenotypic assays of spring wheat adult plants. Disease phenotyping of NILs at the adult plant stage consisted of completely randomized design experiments. For each of the 13 entries, which included spring wheat cultivar Glenn as a susceptible control, 20 pots (replicates) were planted with four to five seeds per six cm clay pot, grown to head emergence and transferred to the ARS BSL-3 greenhouse. Plants were segregated into six groups, each containing at least seven heads (experimental unit) for each NIL and Glenn (check and control group). Five groups were inoculated as before with MoT isolates T-25, B-2, B-71 or P-3. The sixth group was used as a non-inoculated control. This experiment was repeated three times. Disease severity was visually rated 12 to 14 d after inoculation based on the percentage of blasted spikelets per head.
Earlier research (Cruz et al., 2012) found low correlation between the reaction to blast at the adult and seedling plant stages. In a replicated study, foliar inoculations were conducted using the above set of NILs at the fourth leaf stage to determine if the presence of the 2NS fragment had an effect on foliar disease expression. Glenn and Cavalier wheat, which are highly susceptible to head blast, were included in the group. Disease phenotyping of NILs at the seedling stage consisted of completely randomized design experiments, with 10 pots (replicates) per NIL and seven inoculated plants (experimental unit) per line. Plants for foliar inoculation were grown in individual clay pots, segregated into four groups per NIL set and controls, 7 to 10 plants for each line. Approximately 2 wk after seeding, seven plants for each NIL per isolate were transferred to the BSL-3 containment facility for inoculation and rating. Each group was inoculated to run off with a conidia suspension of 2 × 104 conidia mL−1 with either MoT isolate T-25, B-2, or B-71, placed in a dew chamber at 25°C for 24 h, and then moved to the greenhouse (25 ± 4°C). Plants were visually rated for leaf blast severity 7 d after inoculation. Data were only collected on the assessment of symptoms on the third leaf of each plant. Ratings were based on a scale of 0 to 9 (IRRI, 1996) where 0 = no lesions (highly resistant); 1 = small, brown, specks of pinhead size (resistant); 2 = Small roundish to slightly elongated, necrotic gray spots, about one-two mm in diameter, with a distinct brown margin (moderately resistant); 3 = small, roundish to slightly elongated, necrotic, gray spots about one-two mm in diameter (moderately resistant); 4 = Typical susceptible blast lesions, three mm or longer infecting less than four percent of leaf area (moderately susceptible); 5 = typical blast lesions infecting <10% of the leaf area (moderately susceptible); 6 = Typical susceptible blast lesions of three mm or longer infecting 11 to 25% of the leaf area (susceptible); 7 = typical blast lesions infecting 26 to 50% of the leaf area (susceptible); 8 = Typical susceptible blast lesions of three mm or longer infecting 51 to 75% of the leaf area and many leaves are dead (highly susceptible); and 9 = typical blast lesions infecting >51% leaf area and many dead leaves (highly susceptible). The experiment was repeated twice. For each NIL, the mean scores of two experiments were used to rate the level of foliar disease severity.
DNA Extraction and 2NS Molecular Marker Analysis
Tissue samples from 418 of the 431 phenotyped (230 of the 243 winter, all 175 spring, and all 12 NILs plus a spring check) wheat entries were collected and tested for the presence of the 2NS segment from Ae. ventricosa using a dominant PCR marker corresponding to this segment (Helguera et al., 2003). For each wheat line, DNA was extracted using the Qiagen DNeasy 96 Plant Kit from 50 mg of plant tissue, which was frozen in liquid nitrogen. DNA was quantified using a fluorescence-based PicoGreen procedure. PCR amplification was performed on samples in 96-well microtiter plates using the GoTaq Green Kit (Promega Corporation, Madison, WI) and the 2NS specific primers VENTRIUP (5′-AGG GGC TAC TGA CCA AGG CT-3′) and LN2 (5′-TGC AGC TAC AGC AGT ATG TAC ACA AAA-3′). PCR was performed as described by Helguera et al. (2003) (see also http://maswheat.ucdavis.edu/protocols/Lr37/index.htm) using an Eppendorf thermocycler. Specifically, PCR cycling conditions included: denaturing at 94°C for 45 s; amplification at 94°C for 45 s, 65°C for 30 s, and 72°C for 60 s repeated for 30 cycles; and extension at 72°C for 7 min. The presence or absence of the 259-bp 2NS fragment of DNA was observed after electrophoresis on 1.5% agarose gels.
Field Experiments
Seeds of selected spring wheat cultivars with and without the 2NS translocation (Williamson et al., 2013) were shipped to Bolivia under conditions authorized by permits from the National Service of Food Health and Safety of Bolivia. Seeds were sown in two field locations near Quirusillas on 7 Dec. 2013 and 22 Dec. 2014. South American and North American spring wheat lines with various levels of susceptibility to head blast were used as checks. South American checks included Urubó (moderately resistant), Motacú (moderately resistant), BR-18 (moderately susceptible/moderately resistant), and San Marcos (susceptible) (Urashima et al., 2004; Kohli et al., 2011; E. Guzman Arnez, CIAT, Santa Cruz, Bolivia, personal communication, 2015). North American checks included Glenn (susceptible), UC-1617 (resistant), UC-1618 (resistant), WA-8123 (resistant), and Lassik (resistant), selected based on results from previously tested entries against isolate T-25 under greenhouse conditions at FDWSRU.
Checks were used across locations and years according to seed availability. All entries were planted in a randomized complete block design with 10 (2013) or 5 (2014) replications per cultivar. Each replication consisted of a hill plot seeded with 10 (2013) or 50 (2014) seeds of the appropriate cultivar and separated from other hill plots by about 50 cm in all directions. Natural epidemics of head blast developed at both locations during 2014 and 2015. At about the medium milk-to-dough growth stage, hill plots on each corresponding experiment were visually rated for the percentage of the total number of diseased spikelets (Cruz et al., 2012).
Statistical Analyses
In growth chamber and greenhouse experiments, analyses were performed using datasets combined from several experiments using the same protocol under controlled conditions described by Cruz et al. (2012). Each pot was treated as a replication in a completely randomized design. Analyses of variance of head or leaf blast severity were determined using the MIXED procedure of SAS v.9.3 (SAS Institute, 2011) or one-way ANOVA of StatPlus:mac v.5 (AnalystSoft, 2012), respectively. These procedures were used to determine the p-value for pairwise comparisons of cultivars, using blocks as the random effect and disease as fixed effect. Analysis of variance was also used to test for differences in head blast severity among entries with and without the 2NS segment. Combined means of entries with and without 2NS were separated using Fisher’s least significant difference at p < 0.05. For the field experiments, data were also analyzed with the MIXED procedure of SAS, but with a randomized complete block design.
RESULTS
Growth Chamber and Greenhouse Experiments
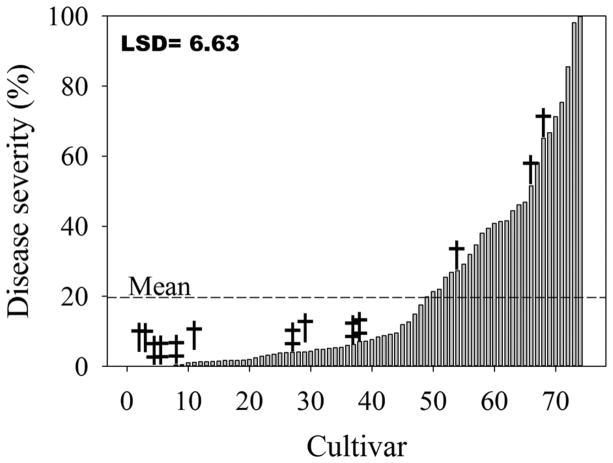
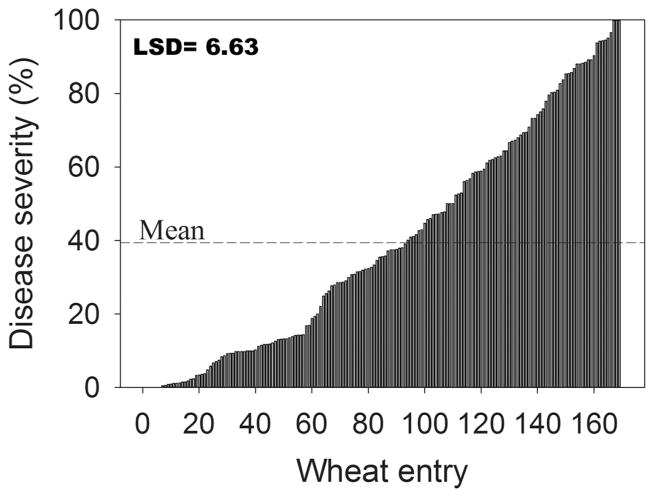
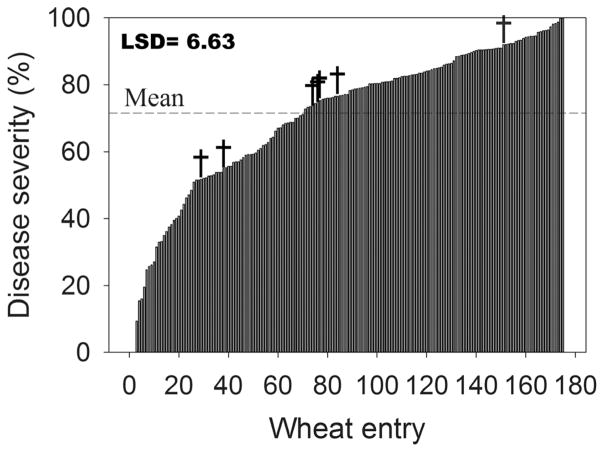
A total of 418 randomly selected wheat cultivars (243 winter and 175 spring) were phenotyped for reactions to MoT isolate T-25, adding 333 additional cultivars to the 85 that had already been reported (Cruz et al., 2012). Of the 418 cultivars, 405 were tested for the presence of the molecular marker completely linked to the 2NS chromosome segment from Ae. ventricosa (Helguera et al., 2003). Cultivars were separated into winter and spring wheat with or without the marker (Fig. 1, 2, and 3). Those that were positive for the presence of the 2NS marker included 61 winter, 7 spring entries, and 6 NILs (Fig. 1). Entries without the 2NS marker included 169 winter (Fig. 2) and 175 spring wheat (Fig. 3). Entries with the 2NS marker showed significantly (p < 0.0001) higher levels of resistance to head blast (Fig. 1) compared to those that did not have the marker (Fig. 2 and 3). The average disease severity for 74 cultivars with the marker was 19.7% (Fig. 1), which was lower than the average severity for the 169 winter (average severity was 39.7%) and 175 spring cultivars without the marker (average severity was 71.1%) (Fig. 2 and 3). Of the 175 spring wheat entries inoculated with T-25 (Fig. 3), only 5 averaged <10% head blast severity when inoculated with T-25 and B-2 isolates. The five entries included Lassik, WA8123 (DAYN), UC1617, UC1618, OR4071004. When the average percent of head blast severity of these entries was compared to highly susceptible checks, analysis of variance for the two isolates showed significant differences (p < 0.001) only between the checks and all other test entries (data not shown). The presence of the 2NS segment was found in the pedigree of Lassik, WA8123, UC1617, UC1618, and OR4071004.
Fig. 1.
Head blast reaction caused by isolate T-25 of Magnaporthe oryzae for 61 winter, 7 spring wheat cultivars (†), and 6 near isogenic lines (‡). All entries contain the marker for the 2NS segment from Aegilops ventricosa. Entries are sorted by disease reaction from lowest to highest. Mean reaction was 19.7%.
Fig. 2.
Head blast reaction caused by isolate T-25 of Magnaporthe oryzae for 169 winter wheat lines that do not contain the marker for the 2NS segment from Aegilops ventricosa. Mean was 39.7%.
Fig. 3.
Head blast reaction caused by isolate T-25 of Magnaporthe oryzae for 175 spring wheat lines that do not contain the marker for the 2NS segment from Aegilops ventricosa. Six parents of isogenic lines and one susceptible check Glenn without the 2NS segment are labeled with †. Mean reaction was 71.1%.
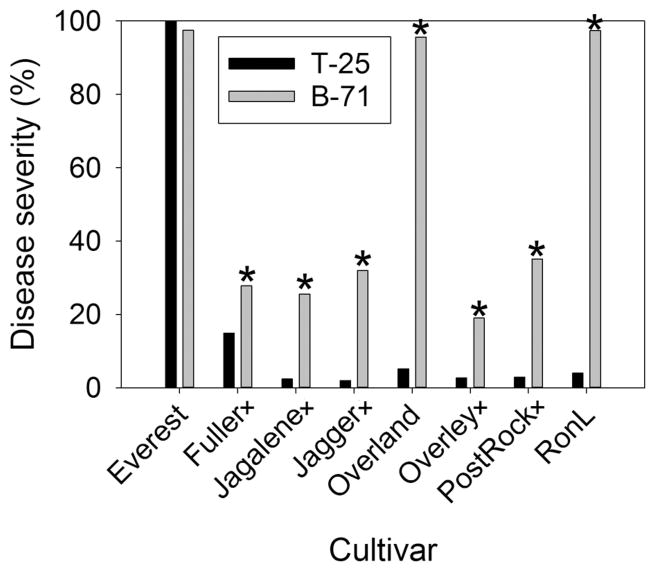
The initial phenotyping experiments involved several isolates that were obtained in the late 1980s (Cruz et al., 2012), therefore additional experiments were conducted using more recent MoT isolates obtained from severely affected wheat fields in South America between 2011 and 2014. Many of these newer isolates showed increased aggressiveness and/or virulence on wheat cultivars when compared with the earlier isolates. The most aggressive and/or virulent isolate obtained was B-71 which was isolated from an infected wheat head collected in 2012 at the CAICO Field Research Facility, Bolivia. This new isolate caused nearly complete death of heads on some cultivars that had displayed high levels of resistance to the earlier isolate (T-25) obtained in 1988 (Fig. 4). In fact, B-71 caused significantly higher disease (p < 0.01) than T-25 on all cultivars except for the susceptible check Everest, which was already at 100% (Fig. 4). On the two cultivars that did not contain the 2NS marker (Overland and RonL), the drastic increase in susceptibility could be the results of gene-for-gene relationship (Flor, 1971) in a race-specific interaction. Disease from T-25 was <6% for those cultivars, but B-71 caused >95% disease on them (Fig. 4). The high levels of disease using B-71 were similar to those seen on the susceptible cultivar Everest. Even though B-71 increased disease on cultivars with the 2NS segment, those levels were all significantly lower than levels on the susceptible cultivar Everest or cultivars Overland or RonL, which lacked the 2NS marker (p < 0.0001).
Fig. 4.
Head blast reaction of selected winter wheat cultivars to two isolates (T-25 and B-71) of the Triticum pathotype of Magnaporthe oryzae; cultivars followed by a plus sign (+) contain the 2NS chromosome segment. Means with an asterisk (*) showed that B-71 caused significantly (p < 0.05) higher disease than T-25 on all cultivars except the susceptible check Everest, which was already at 100%.
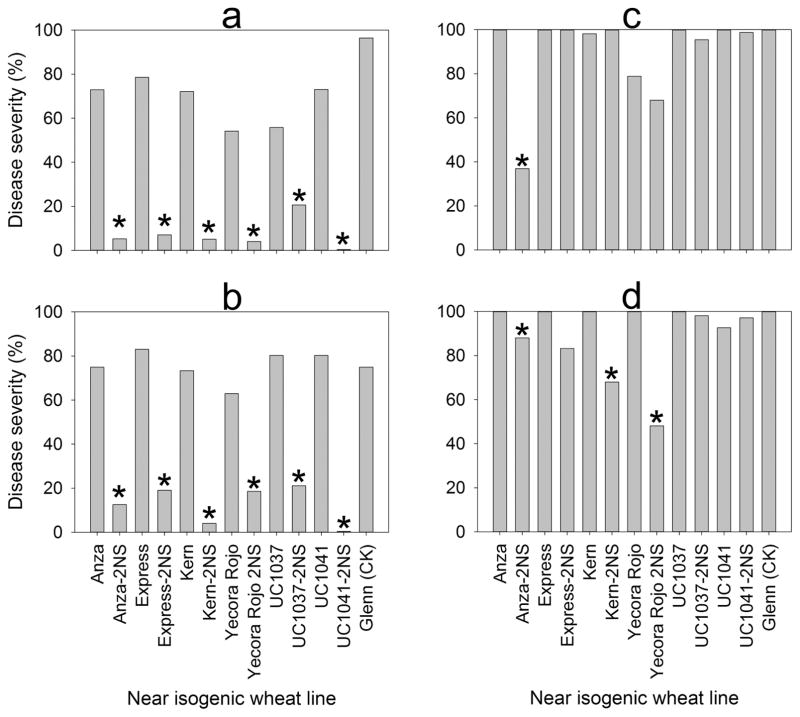
When NILs of spring wheat with and without the 2NS segment were inoculated with isolates T-25 and B-2, all lines with the 2NS segment showed significantly lower head blast severity than the corresponding line without 2NS (Fig. 5a and 5b). When two newer, more aggressive and virulent isolates were used, head blast severity was significantly reduced only on Anza-2NS for isolate B-71 (p < 0.0001) and on Anza-2NS, Kern-2NS, and Yecora Rojo-2NS for isolate P-3 (p < 0.05) (Fig. 5c and 5d).
Fig. 5.
Head blast reaction of near-isogenic spring wheat lines with or without the 2NS chromosome segment to inoculation with isolates T-25 (a), B-2 (b), B-71 (c), and P-3 (d) of Magnaporthe oryzae Triticum pathotype under greenhouse conditions. Means with an asterisk (*) on isolines with 2NS are significantly (p < 0.05) different from their corresponding isogenic parent.
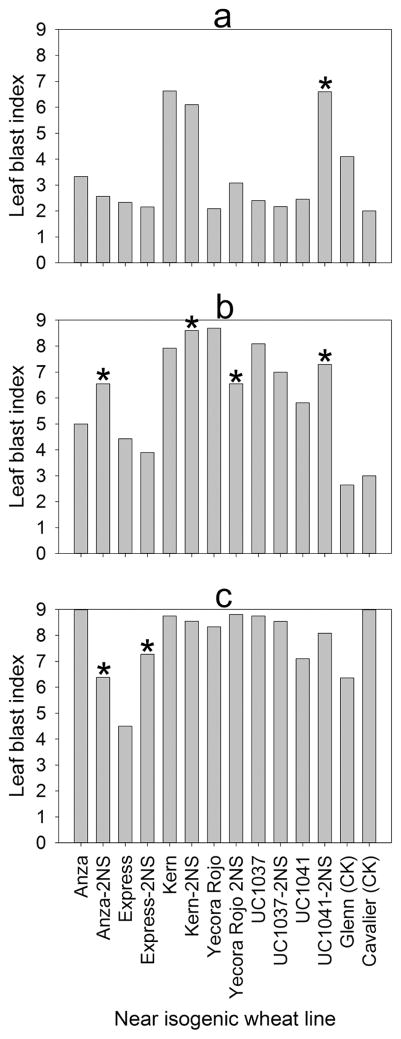
Leaves of NILs at the fourth-leaf stage were inoculated with isolates T-25, B-2, and B-71 to determine if the presence of the 2NS fragment impacted foliar disease severity. In a comparison of foliar infection of corresponding 2NS paired NILs inoculated with isolate T-25, only UC1041 and UC1041-2NS were significantly different (p < 0.001) with more disease on UC1041-2NS (Fig. 6a). When inoculated with isolate B-2, significant differences (p < 0.05) were detected for all 2NS paired NILs, except Express and UC-1037 (Fig. 6b). However ANZA-2NS, Kern-2NS and UC1041-2NS had greater disease severity than their associated non-2NS lines (Fig. 6b). No NILs inoculated with B-71 were rated better than mildly resistant with foliar inoculation. The ANOVA displayed significant differences only between the NILs of ANZA (p < 0.001) and Express (p < 0.05) pairs (Fig. 6c). However, Express-2NS was rated more susceptible than Express (Fig. 6c). Overall ratings of the NILs showed a difference in virulence among the three isolates used in the study where 25% of the NILs inoculated with T-25, 75% with B-2, and 91.6% inoculated with B-71 were susceptible (data not shown).
Fig. 6.
Foliar blast reaction of near-isogenic spring wheat lines with or without the 2NS chromosome segment after inoculation with isolates T-25 (a), B-2 (b), and B-71 (c) of Magnaporthe oryzae Triticum pathotype under greenhouse conditions. Means with an asterisk (*) were significantly (p < 0.05) different from their corresponding isogenic parent.
Field Experiments
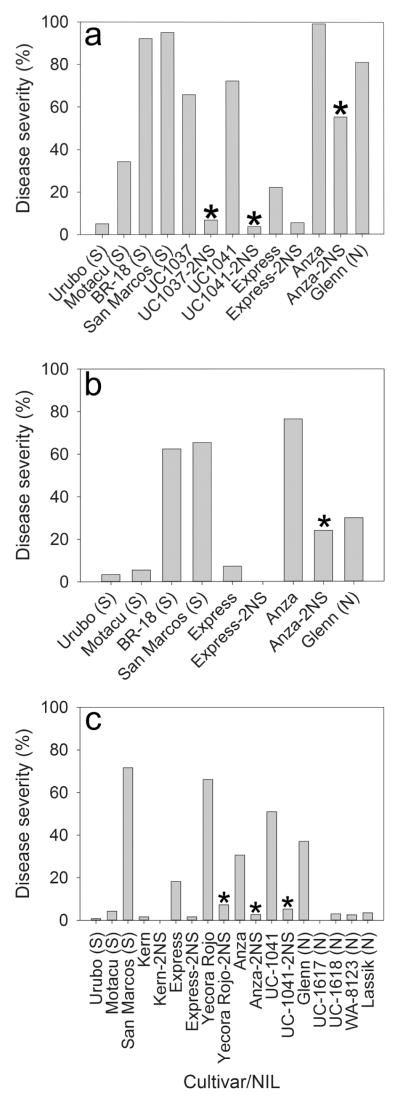
Data from field experiments involving the NILs mimicked what was seen in the growth chamber and greenhouse experiments. At the first location in 2014 (Fig. 7a), all of the lines with 2NS showed significantly lower (p <0.0001) head blast severity than those without the segment, although Express and Express-2NS were at the border of the significance level (p = 0.06). At the second location in 2014 (Fig. 7b), Anza-2NS showed significantly lower (p < 0.0001) head blast severity than Anza but the isogenic lines of Express were not significantly different (p = 0.57). In 2015 (Fig. 7c), all of the lines with 2NS, except Kern-2NS and Express-2NS, showed significantly lower (p < 0.01) head blast severity than those without the segment.
Fig. 7.
Field reaction of spring wheat lines with or without the 2NS chromosome segment to head blast in Bolivia during 2014 in two locations at the Quirusillas municipality (a and b), and 2015 in one location at Quirusillas (c). South American (S) and North American (N) cultivars with known reaction to wheat head blast were included as checks. Means with an asterisk (*) were significantly (p < 0.05) different from their corresponding isogenic parent.
DISCUSSION
Among randomly selected wheat cultivars, those with the 2NS chromosome segment from Ae. ventricosa (Fig. 1) had 50.4 to 72.3% less head blast than cultivars without 2NS (Fig. 2 and 3). This provided preliminary evidence that the 2NS segment conferred resistance to head blast. However, those phenotypic data were obtained using an older isolate (1988) of M. oryzae. Therefore, the question remained as to whether the 2NS translocation would provide some level of resistance to more recent isolates. When newer isolates were obtained from infected wheat in South America from 2011 to 2014, selected cultivars with the 2NS segment had 64.0 to 80.5% less head blast than cultivars without the 2NS translocation (Fig. 4). This finding was further evidence that 2NS provided a significant level of resistance to head blast even against new, more aggressive and/or virulent isolates.
Additional evidence of the impact of 2NS on head blast was provided by the experiments using NILs of spring wheat with and without 2NS. Under greenhouse conditions when lines were inoculated with an older isolate of M. oryzae, those with the 2NS translocation had a significant (p < 0.05) reduction in head blast (Fig. 5a). With newer isolate B-2, significant (p < 0.05) reduction in head blast was observed in all lines with 2NS (Fig. 5b). However, with isolates B-71 and P-3, not all lines with 2NS showed a significant reduction in head blast (Fig. 5c and 5d). Data from these newer isolates indicated that the 2NS translocation might confer useful levels of resistance to the new isolates in certain genetic backgrounds and not in others. These findings substantiated earlier results where some cultivars with the 2NS translocation displayed high levels of susceptibility (Fig. 1). It is currently not clear if there are complementary genes in the different genetic backgrounds that can enhance the positive effect of the 2NS resistance genes towards B-71 and P-3, resistance gene(s) on 2NS that are silenced, or susceptibility gene(s) in certain wheat backgrounds that override the 2NS resistance. Further research is needed to determine the cause for the reduced expression of 2NS resistance to B-71 and P-3 in some genetic backgrounds and not in others. In spite of these limitations, when the NILs were planted in the field in South America under natural blast epidemic conditions, significant reductions of 47.3 to 95.0% were seen for all four pairs of lines (Fig. 7a). In a second field experiment in South America (Fig. 7b), data from only two pairs of NILs were available. For one of those pairs (Anza) a significant reduction of 68.6% was observed. For the other pair of lines (Express), the line without the 2NS translocation had very low disease (7.2%); therefore, even though the line with 2NS had lower disease (0.0%), the difference was not statistically significant. Nevertheless, in a third field experiment in 2015 (Fig. 7c), the data mimicked what was found with the other nurseries with NILs. Significant reductions were seen for lines containing 2NS except where very low disease levels occurred on the corresponding line without the 2NS translocation. Therefore, three field locations in Bolivia evaluated in 2014 and 2015 provided strong evidence that the 2NS segment conferred resistance to head blast under natural epidemic conditions. This result was more encouraging than the greenhouse experiments with new isolates where only 10 of 18 NILs showed statistically significant decreases in head blast caused by a new isolate (Fig. 5b, 5c, and 5d). Therefore, the environment, in addition to genetic background, may be involved in the usefulness of 2NS resistance genes.
The results from foliar inoculations (Fig. 6) suggest that the resistance to spike infection imparted by the 2NS translocation does not confer resistance to foliar disease. Surprisingly, in some cases, NILs with the 2NS fragment had significantly greater leaf disease severity than those without. Differences in the aggressiveness of the three MoT isolates was observed, with foliar disease severity increasing from T-25 to B-2 to the most recently collected aggressive isolate, B-71.
Results reported here show evidence for virulence differences (physiological races) within the population of M. oryzae with regard to head blast on winter wheat. Two cultivars (Overland and RonL) showed high levels of resistance to older isolates but complete susceptibility to newer isolates (Fig. 4). The gene(s) responsible for resistance to the older isolates in these two varieties was (were) completely ineffective against the newer isolates. To our knowledge, this is the first documented case of differential and/or physiological race interactions for winter wheat to head blast. However, such interactions have been reported for spring wheat cultivars in Brazil (Maciel et al., 2014), where commercial wheat is of the spring type. In addition, on the two cultivars that did not contain the 2NS marker (Overland and RonL), the drastic increase in susceptibility may be the result of a gene-for-gene interaction (Flor, 1971), an idea that is also supported by several studies described below. The rice/M. oryzae pathosystem has been shown to follow a gene-for-gene relationship (Flor, 1971; Silué et al., 1992). Therefore, it seems likely that the wheat blast pathosystem may also display that type of relationship. If that is shown to be the case, there will be distinct virulence groups for MoT. Several publications related to MoT in South America have shown significant isolate × cultivar interactions and have reported many virulence groups (Urashima et al., 2004; Maciel et al., 2014). Two resistance genes to the seedling phase of wheat blast, Rmg2 on chromosome 7A and Rmg3 on chromosome 6B, have been identified in the wheat cultivar Thatcher (Zhan et al., 2008). A resistance gene to MoT isolates, designated Rmg7, was recently identified in tetraploid wheat (Tagle et al., 2015). Race specificity for wheat isolates has been reported in Brazil for the leaf phase of wheat blast (Urashima et al., 2004, Maciel et al., 2014) and for the head phase (Maciel et al., 2014). In Urashima et al. (2004), inoculation of 72 wheat isolates on 20 Brazilian wheat cultivars in greenhouse assays identified 54 distinct virulence patterns. In the Maciel et al. (2014) report, 14 virulence groups were recently identified for the leaf phase of wheat blast and eight virulence groups for the head phase.
In conclusion, the 2NS chromosome segment from Ae. ventricosa translocated to wheat chromosome arm 2AS was associated with significant reductions in head blast in both spring and winter wheat. Reductions were seen with older and newer isolates of the pathogen and under natural epidemic conditions in the field. However, not all cultivars and lines with 2NS showed resistance in controlled inoculations in the greenhouse. These data suggest that the genetic background and/or environment may influence the expression of the resistance conferred by the 2NS translocation. Based on the positive results in the field experiments, wheat breeders interested in increasing resistance to head blast in wheat cultivars should consider incorporation of the 2NS segment into wheat backgrounds that lack it. The Ae. ventricosa 2NS/2AS translocation brings additional value to the wheat breeding program because it also carries resistance genes Rkn3 against root-knot nematodes (Meloidogyne spp.) (Williamson et al., 2013), Cre5 against the French pathotype Ha12 of the cereal cyst nematode (Heterodera avenae Wollenweber) (Jahier et al., 2001), and Lr37, Sr38, and Yr17 against some races of wheat leaf, stem and stripe rust (Helguera et al., 2003).
The incorporation of the 2NS segment, together with other sources of resistance would be useful in areas where wheat blast is prevalent. Moving the 2NS chromosome segment into wheat backgrounds that lack it provides a short-term opportunity for breeders. However, given that there is some evidence presented here that 2NS resistance may be overcome by certain MoT isolates, additional sources of resistance should also be identified. The winter bread wheat VPM1 was the initial recipient of the 2NS translocation in a hexaploid background and has been the initial source of 2NS in several cultivars (Maia, 1967). The CIMMYT cultivar Milan (Kohli et al., 2011; Nicol et al., 2009) possesses the 2NS translocation (Dubcovsky, unpublished data, 2015), and Milan-based resistant wheat cultivars released in South America appear to contain high levels of resistance to wheat head blast under field conditions (Kohli et al., 2011). Other cultivars derived from Milan are now being widely deployed but it remains to be seen how long this resistance will be effective (Kohli et al., 2011). Thus, wheat cultivars or lines that do not contain 2NS but show resistance to wheat blast, wheat alien chromosome introgression lines, and synthetic hexaploids should be considered in future research.
Supplementary Material
Acknowledgments
The project has been funded by Agriculture and Food Research Initiative Competitive Grants No. 2009-55605-05201, 2011-68002-30029 (TCAP) and 2013-68004-20378 (Blast Integrated Project; ‘BIP’) from the USDA National Institute of Food and Agriculture. Funding was also provided by Rotary International, The Rotary Foundation, Rotary International District 5710, and the Manhattan Rotary Club of Manhattan Kansas, through the Rotary Global Grant Program. Contribution number 15-408-J from the Kansas Agricultural Experiment Station. Dr. Dubcovsky acknowledges support from the Howard Hughes Medical Institute and the Gordon and Betty Moore Foundations.
Abbreviations
- 2NS
a 25 to 38 cM distal segment of chromosome arm 2NS from Aegilops ventricosa translocated to the distal region of wheat chromosome arm 2AS
- BRI
Biosecurity Research Institute in Manhattan, KS
- BSL-3
biosafety-level-3
- FDWSRU
Foreign Disease-Weed Science Research Unit at Ft. Detrick, MD
- MoT
Magnaporthe oryzae (Triticum pathotype)
- NIL
near-isogenic line
Footnotes
Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the US Department of Agriculture. USDA is an equal opportunity provider and employer.
Supplemental Materials Available
Supplemental material containing the disease severity ratings for winter and spring wheat entries is available with the online version of this article.
Contributor Information
C.D. Cruz, Dep. of Plant Pathology, Kansas State Univ., 1712 Claflin Rd., Manhattan, KS 66506.
G.L. Peterson, USDA-ARS, Fort Detrick, MD 21702
W.W. Bockus, Dep. of Plant Pathology, Kansas State Univ., 1712 Claflin Rd., Manhattan, KS 66506
P. Kankanala, Dep. of Plant Pathology, Kansas State Univ., 1712 Claflin Rd., Manhattan, KS 66506
J. Dubcovsky, Howard Hughes Medical Institute, Chevy Chase, MD 20815
K.W. Jordan, Dep. of Plant Pathology, Kansas State Univ., 1712 Claflin Rd., Manhattan, KS 66506
E. Akhunov, Dep. of Plant Pathology, Kansas State Univ., 1712 Claflin Rd., Manhattan, KS 66506
F. Chumley, Dep. of Plant Pathology, Kansas State Univ., 1712 Claflin Rd., Manhattan, KS 66506
F.D. Baldelomar, Asociación Nacional de Productores de Oleaginosas y Trigo, Av. Ovidio Barbery esq. Jaime Mendoza, Santa Cruz de la Sierra, Bolivia
B. Valent, Dep. of Plant Pathology, Kansas State Univ., 1712 Claflin Rd., Manhattan, KS 66506
References
- AnalystSoft. AnalystSoft: Analysis made easy. StatPlus:mac version 5.0. AnalystSoft Inc; Walnut Grove, CA: 2012. [Google Scholar]
- Chicaiza O, I, Khan A, Zhang X, Brevis JC, Jackson L, Chen X, Dubcovsky J. Registration of five wheat iso-genic lines for leaf rust and stripe rust resistance genes. Crop Sci. 2006;46:485–487. doi: 10.2135/cropsci2005.04-0048. [DOI] [Google Scholar]
- Cruz CD, Bockus WW, Stack JP, Tang X, Valent B, Pedley KF, Peterson GL. Preliminary assessment of resistance among U.S. wheat cultivars to the Triticum pathotype of Magnaporthe oryzae. Plant Dis. 2012;96:1501–1505. doi: 10.1094/PDIS-11-11-0944-RE. [DOI] [PubMed] [Google Scholar]
- Cruz CD, Kiyuna J, Bockus WW, Todd TC, Stack JP, Valent B. Magnaporthe oryzae conidia on basal wheat leaves as a potential source of wheat blast inoculum. Plant Pathol. 2015;64:1491–1498. doi: 10.1111/ppa.12414. [DOI] [Google Scholar]
- Cruz MF, Prestes AM, Maciel JLN, Scheeren PL. Resistência parcial à brusone de genótipos de trigo comum e sintético nos estádios de planta jovem e de planta adulta. (In Portuguese.) Trop. Plant Pathol. 2010;35:24–31. doi: 10.1590/S1982-56762010000100004. [DOI] [Google Scholar]
- Farman ML. Pyricularia grisea isolates causing gray leaf spot on perennial ryegrass (Lolium perenne) in the United States: Relationship to P. grisea isolates from other host plants. Phytopathology. 2002;92:245–254. doi: 10.1094/PHYTO.2002.92.3.245. [DOI] [PubMed] [Google Scholar]
- Flor HH. Current status of the gene-for-gene concept. Annu Rev Phytopathol. 1971;9:275–296. doi: 10.1146/annurev.py.09.090171.001423. [DOI] [Google Scholar]
- Goulart A, Paiva F. Perdas no rendimiento de grãos de trigo causada por Pyricularia grisea, nos anos de 1991 e 1992, no Mato Grosso do Sul. Summa Phytopathol. 2000;26:279–282. (In Portuguese.) [Google Scholar]
- Goulart A, Paiva F, Mesquita N. Perdas en trigo (Triticum aestivum) causadas por Pyricularia oryzae. Fitopatol Bras. 1992;17:115–117. (In Portuguese.) [Google Scholar]
- Goulart ACP, Sousa PG, Urashima AS. Damages in wheat caused by infection of Pyricularia grisea(In Portuguese.) Summa Phytopathol. 2007;33:358–363. doi: 10.1590/S0100-54052007000400007. [DOI] [Google Scholar]
- Helguera M, I, Khan A, Kolmer J, Lijavetzky D, Zhongqi L, Dubcovsky J. PCR assays for the Lr37-Yr17-Sr38 cluster of rust resistance genes and their use to develop isogenic hard red spring wheat lines. Crop Sci. 2003;43:1839–1847. doi: 10.2135/cropsci2003.1839. [DOI] [Google Scholar]
- Igarashi S. Update on wheat blast (Pyricularia oryzae) in Brazil. In: Saunders D, editor. Wheat for the nontraditional warm areas; Proc. Intl. Conf. Foz Do Iguaçu; Brazil. 29 July–3 Aug. 1990; 1991. pp. 480–483. [Google Scholar]
- Igarashi S, Utiamada CM, Igarashi LC, Kazuma AH, Lopes RS. Pyricularia em trigo. 1. Ocorrência de Pyricularia sp. no estado do Paraná. Fitopatol Bras. 1986;11:351–352. (In Portuguese.) [Google Scholar]
- IRRI. Standard evaluation system for rice. 4. Intl. Rice Res. Inst; Manila, Philippines: 1996. [Google Scholar]
- Jahier J, Abelard PAM, Tanguy F, Dedryver F, Rivoal R, Khatkar S, Bariana HS. The Aegilops ventricosa segment on chromosome 2AS of the wheat cultivar ‘VPM1’ carries the cereal cyst nematode resistance gene Cre5. Plant Breed. 2001;120:125–128. doi: 10.1046/j.1439-0523.2001.00585.x. [DOI] [Google Scholar]
- Kohli MM, Mehta YR, Guzman E, De Viedma L, Cubilla LE. Pyricularia blast – A threat to wheat cultivation. Czech J Genet Plant Breed. 2011;47:S130–S134. [Google Scholar]
- Maciel JLN, Ceresini PC, Castroagudin VL, Zala M, Kema GHJ, McDonald BA. Population structure and pathotype diversity of the wheat blast pathogen Magnaporthe oryzae 25 years after its emergence in Brazil. Phytopathology. 2014;104:95–107. doi: 10.1094/PHYTO-11-12-0294-R. [DOI] [PubMed] [Google Scholar]
- Maciel JLN, Paludo EA, Só e Silva M, Scheeren PL, Caierão E. Reação à brusone de genótipos de trigo do programa de melhoramento da Embrapa Trigo no estádio de planta adulta. Embrapa Trigo, Passo Fundo; RS, Brazil: 2008. (In Portuguese.) [Google Scholar]
- Maia N. Obtention des bles tendres resistants au pietin-verse par croisements interspecifiques bles x Aegilops. C R Acad Agric Fr. 1967;53:149–154. (In Portuguese.) [Google Scholar]
- Nicol JM, Bolat N, Yıldırım AF, Yorgancılar A, Kılınc AT, Elekcioglu HI, Sahin E, Erginbas-Orakcı G, Braun HJ. Identification of genetic resistance to cereal cyst nematode (Heterodera filipjevi) for international bread wheat improvement. Cereal cyst nematodes: Status, research and outlook; Proc. First Workshop Intl. Cereal Cyst Nematode Initiative. Intl. Maize Wheat Improv. Center; Antalya, Turkey. 21–23 Oct. 2009; 2009. pp. 160–165. [Google Scholar]
- Orbach J, Chumley F, Valent B. Electrophoretic karyotypes of Magnaporthe grisea pathogens of diverse grasses. Mol. Plant-Microbe Interact. 1996;9:261–271. doi: 10.1094/MPMI-9-0261. [DOI] [Google Scholar]
- Prabhu A, Filippi M, Castro N. Pathogenic variation among isolates of Pyricularia oryzae infecting rice, wheat and grasses in Brazol. Trop. Pest Manage. 1992;38:367–371. doi: 10.1080/09670879209371729. [DOI] [Google Scholar]
- Pratt K. UKAgNews. Univ. Kentucky College Agric., Food, and Environ; Lexington, KY: 2012. Apr 24, [accessed 17 Feb. 2016]. UK researchers find important new disease. 2012. http://news.ca.uky.edu/article/uk-researchers-find-important-new-disease. [Google Scholar]
- Prestes A, Arendt P, Fernandes M, Scheeren P. Resistance to Magnaporthe grisea among Brazilian wheat genotypes. In: Buck HT, Nisi JE, Salomon N, editors. Wheat production in stressed environments; Proc. 7th Intl. Wheat Conf. Mar del Plata; Argentina. 27 Nov.–2 Dec, 2005; the Netherlands: Springer; 2007. pp. 119–133. [Google Scholar]
- SAS Institute. The SAS system for Windows. Release 9.3. SAS Inst; Cary, NC: 2011. [Google Scholar]
- Silué D, Notteghem JL, Tharreau D. Evidence for a gene-for-gene relationship in the Oryza sativa–Magnaporthe grisea pathosystem. Phytopathology. 1992;82:577–580. doi: 10.1094/Phyto-82-577. [DOI] [Google Scholar]
- Tagle AG, Chuma I, Tosa Y. Rmg7, a new gene for resistance to Triticum isolates of Pyricularia oryzae identified in tetraploid wheat. Phytopathology. 2015;105:495–499. doi: 10.1094/PHYTO-06-14-0182-R. [DOI] [PubMed] [Google Scholar]
- Tuite JF. Plant pathological methods, fungi and bacteria. Burgess Publishing Company; Minneapolis, MN: 1969. [Google Scholar]
- Urashima AS. Blast. In: Bockus WW, Bowden RL, Hunger RM, Morrill WL, Murray TD, Smiley RW, editors. Compendium of wheat diseases and pests. American Phytopathological Society; Saint Paul, MN: 2010. pp. 22–23. [Google Scholar]
- Urashima AS, Bruno AC, Lavorenti NA. Análise da segregação de avirulência de Magnaporthe grisea do trigo. Fitopatol. Bras. 2001;26:644–648. doi: 10.1590/S0100-41582001000300011. [DOI] [Google Scholar]
- Urashima AS, Galbieri R, Stabili A. DNA finger-printing and sexual characterization revealed two distinct populations of Magnaporthe grisea in wheat blast from Brazil. Czech J Genet Plant Breed. 2005;41:238–245. [Google Scholar]
- Urashima AS, Hashimoto Y, Don L, Kusaba M, Tosa Y, Nakayashiki H, Mayama S. Molecular analysis of the wheat blast population in Brazil with a homolog of retrotransposon MGR583. Jpn J Phytopathol. 1999;65:429–436. doi: 10.3186/jjphytopath.65.429. [DOI] [Google Scholar]
- Urashima AS, Lavorent NA, Goulart ACP, Mehta YR. Resistance spectra of wheat cultivars and virulence diversity of Magnaporthe grisea isolates in Brazil. Fitopatol. Bras. 2004;29:511–518. doi: 10.1590/S0100-41582004000500007. [DOI] [Google Scholar]
- Valent B, Crawford MS, Weaver CG, Chumley FG. Genetic studies of fertility and pathogenicity in Magnaporthe grisea (Pyricularia oryzae) Iowa State J Res. 1986;60:569–594. [Google Scholar]
- Valent B, Farrall L, Chumley FG. Magnaporthe grisea genes for pathogenicity and virulence identified through a series of backcrosses. Genetics. 1991;127:87–101. doi: 10.1093/genetics/127.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson VM, Thomas V, Ferris H, Dubcovsky J. An Aegilops ventricosa translocation confers resistance against root-knot nematodes to common wheat. Crop Sci. 2013;53:1412–1418. doi: 10.2135/cropsci2012.12.0681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan SW, Mayama S, Tosa Y. Identification of two genes for resistance to Triticum isolates of Magnaporthe oryzae in wheat. Genome. 2008;51:216–221. doi: 10.1139/G07-094. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.