Abstract
Background
Few studies have tested whether cellular processes directly associated with cardiovascular disease risk can be influenced by a psychological inoculation.
Purpose
This study investigated whether values affirmation, a psychological procedure designed to reduce stress and threat perception, would prevent endothelial injury to social evaluative threat (SET).
Methods
Participants (N=32) were randomized to SET, SET with values affirmation, or Control. SET was induced with the Trier Social Stress Test, and participants performed values affirmation prior to SET induction. Using flow cytometry, endothelial injury was assessed by measuring circulating levels of endothelial cell-derived microparticles (EMPs) phenotypic for endothelial cell activation (CD62E+), apoptosis (CD31+) or both (CD51+).
Results
Social threat caused expected increases in circulating EMPs phenotypic of endothelial cell injury, a response completely attenuated in those receiving values affirmation.
Conclusions
This study, as proof of principle due to small sample size, shows cellular level, cardiovascular disease-relevant effects of social stress and provides the first evidence of inoculation against such effects by a psychological procedure.
Keywords: social evaluative threat, stress, values affirmation, endothelial injury, cardiovascular disease
The link between stress and disease has been of paramount interest to researchers and clinicians for decades. Hans Selye was among the first to recognize the effect of stress on disease relevant processes [1], and in tandem, Paul MacLean noted the associations among emotion, the brain, and health [2]. To date, some of the strongest associations have been established between stress and cardiovascular disease (e.g., Dimsdale [3]). For example, psychological stress can provoke acute cardiac events [4] and induce transient myocardial ischemia [5], and a number of epidemiological studies have linked anger, hostility and chronic perceived stress to cardiovascular disease mortality (e.g., Haynes, Feinleib, & Kannel [6]; Rosengren et al. [7]).
Critical to cardiovascular disease is the health of vascular endothelial cells, which play an essential role in maintaining vascular tone and the integrity of blood vessels. Endothelial injury causes endothelial cells to lose integrity, progress to senescence and detach into the circulation [8,9]. Endothelial cell-derived microparticles (EMPs) are phospholipid rich, submicron particles derived and released from the membranes of activated or apoptotic endothelial cells [8,10], and can serve as markers of endothelial cell injury in humans. EMPs modulate inflammation via leukocyte activation and transendothelial migration and have procoagulant activity [11]. Thus, EMPs may have a direct role in atherosclerosis-related cardiovascular disease onset. EMPs may also contribute to other vascular disorders, including stroke [12] and preeclampsia [13].
Although many studies have investigated stress effects on health-related physiology (e.g., cortisol [14], immune [15] and autonomic [16] responses), few studies have tested whether psychological treatments can influence cellular processes directly relevant for disease. EMPs are a particularly important target in that regard; stress-induced EMP activity represents one plausible mechanism underlying the association between psychological stress and cardiovascular disease. In support of this hypothesis, experimentally provoked anger has previously been shown to elevate circulating EMPs [17], suggesting that psychological stress can cause endothelial cell injury.
If psychological stressors can impact cellular processes relevant for cardiovascular disease risk, then psychological processes may be able to prevent them, and demonstrating such effects could lead to enhanced understanding and clinical deployment of psychological interventions. However, no prior research has investigated whether endothelial cell injury can be mitigated by a psychological inoculation. Among such procedures, values affirmation has emerged as a practice that is both effective at reducing stress—including cortisol reactivity [18], catecholamine secretion [19] and performance improvement [20–23]—and deployable on a large scale in diverse populations [20,21,23].
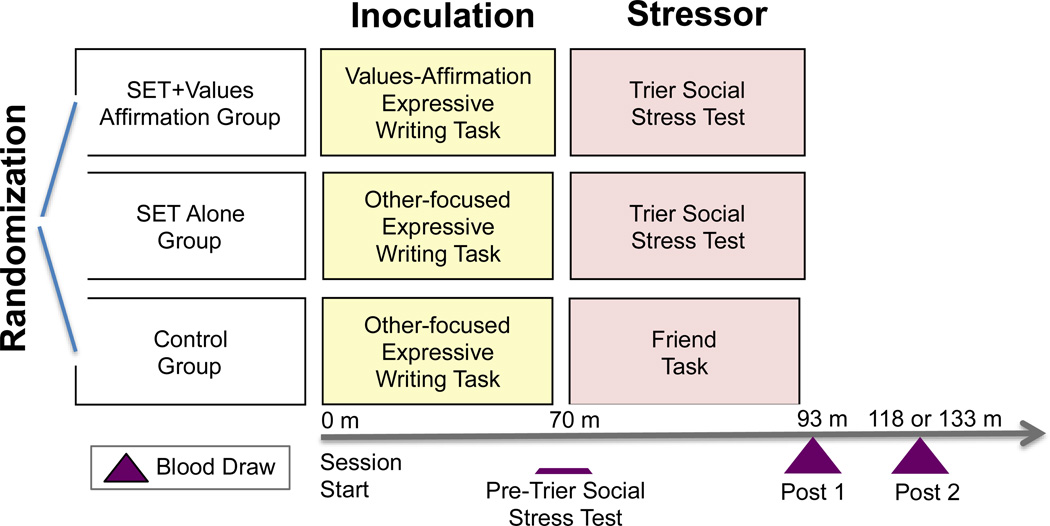
Here we present a proof of principle study that tested whether SET and values affirmation would have opposing effects on markers of endothelial cell injury. We recruited healthy participants who were randomized to one of three conditions: Social evaluative threat (SET) Alone, SET preceded by a buffering values affirmation task (SET+Values Affirmation) or a non-stressful Control task. Blood was sampled immediately prior to the implementation of the SET or Control task, immediately post-SET/Control task (Post 1) and approximately 25 or 40 minutes later (Post 2) (Fig. 1). Circulating EMPs were assessed by flow cytometry as previously described [24,25]. EMPs expressing CD62E (CD62E+ EMPs) reflect endothelial cell activation, EMPs expressing CD31 (CD31+ EMPs) reflect endothelial cell apoptosis and EMPs expressing CD51 (CD51+ EMPs) reflect both endothelial cell activation and apoptosis.
Fig. 1. Study Procedure.
Participants were randomized to one of three groups after which they completed two tasks, one in the Inoculation phase and one in the Stressor phase. Blood was drawn at three timepoints including: Pre-Trier Social Stress Test, Post 1 and Post 2. SET = Social Evaluative Threat; m = minutes.
Those assigned to the SET+Values Affirmation condition completed a self-affirmation of values task immediately prior to SET, which consisted of reflecting on and writing about aspects of one’s life for which one is grateful, including family and social support, social roles and important values or prized skills [26,27]. A key ingredient of values affirmation is thought to be affirmation of overall perceptions of personal self-worth, efficacy and adequacy. It is thought to bolster internal resources for coping with threat, thus reducing threat appraisal when faced with a challenging situation [28]. Prior research has shown that values affirmation attenuates stress and defensiveness and improves performance among those contending with racial [20,21] and gender identity [23] threat. Participants assigned to the SET+Values Affirmation group wrote about personally important values, whereas those assigned to SET Alone and Control groups wrote about values least important to them but important to others; this was not expected to confer benefit.
SET was induced via the well-established Trier Social Stress Test [14,29], a task in which participants are instructed to prepare a public speech and perform mental math problems before a panel of ‘expert judges’ (here, two paid actors). The Trier Social Stress Test reliably increases sympathetic activity and reduces parasympathetic cardiac control [30] via prefrontal-brainstem connections [31] and is similar to other procedures found to influence vascular endothelial function [32,33]. The Control group performed a task that involved planning and cognition with no evaluative threat.
Methods
Participants
Participants were healthy, English-speaking adults (N=32) (23 male; mean age (SD) = 22.6 (3.8) years) randomized into one of three groups: SET Alone (n=7; 4 male), SET+Values Affirmation (n=11; 9 male) and Control (n=14; 10 male). Exclusion criteria relevant to the current study included personal history of cardiovascular disease, use of anti-hypertensive medication and smoking. Three participants from the SET+Values Affirmation group missed one blood draw each due to random equipment failure, leaving full data sets available from 29 participants (SET Alone (n=7), SET+Values Affirmation (n=8) and Control (n=14)) (Fig. 1). Data were not complete for one participant in the SET Alone group for cortisol (n=6) and one participant in the Control group for the psychological stress measure (n=13). In the SET+Values Affirmation group, three participants were added to the analysis of cortisol (n=11), and two were added to the analysis of psychological stress (n=10). All participants gave written informed consent, and study procedures were approved by the Institutional Review Board of Columbia University and carried out in accordance with the provisions of the World Medical Association Declaration of Helsinki.
Study Procedure
Participants reported to the laboratory between 1–2 PM, having refrained from consuming caffeine, eating a large meal and participating in any form of exercise for 2 hours prior to the session and from consuming alcohol and participating in heavy exercise for 48 hours prior to the session. Also, participants obtained a normal night’s sleep on the preceding night. Participant compliance was confirmed with self-report.
Upon arrival, an indwelling catheter (21-gauge) was inserted into the non-dominant forearm. Next, participants completed the values affirmation task. Blood samples (4.5 mL) were drawn into citrated tubes 70 min after catheter insertion and a resting baseline condition, at the end of the SET/Control condition, and then 25–40 minutes after completion of additional cognitive tasks (Fig. 1). At the end of the study, participants were fully debriefed and paid.
Values Affirmation Task
The values affirmation task was designed to have two versions: self-focused and other-focused. In the self-focused task, participants were instructed to select one of the following six values that was most important to them: athletic ability, creativity, relationship with family and friends, religious values, sense of humor, music or art. They then wrote a short description and answered brief questions concerning why that value was important to them.
In the other-focused task, participants were presented the same list of values, but instead, were instructed to select one that was least important to them. They then wrote a short description and answered brief questions concerning why that value might be important to someone else, with the brief questions being the same as the self-focused task, but reoriented toward other people.
Trier Social Stress Test
Two confederates (paid actors) wearing white lab coats were introduced to participants as professors. The confederates informed participants that they would be required to give a 5 minute speech, with 3 minutes to prepare the speech. Participants were told that the topic of the speech would be revealed shortly. At the onset of the speech preparation period, the speech topic––describing their strengths and weaknesses as a candidate for their dream job ––was displayed on a computer screen. The job was selected from a questionnaire completed previously. At the end of the 3 minute speech preparation period, a conspicuous video camera was turned on, and participants were instructed to begin delivery of their speeches to the confederates. Throughout speech delivery, the confederates used neutral facial expressions and frequently interrupted the participants with reminders to adhere to instructions. Following speech delivery, the confederates administered a serial subtraction task, instructing participants to count backwards by 13s from the number 1022 and to start over when mistakes were made.
Control Task
In this task, the experimenter informed participants that they would have a few minutes to imagine a day spent with a friend who was visiting New York City, and following this period, they would be asked to describe the day verbally to the experimenter. Participants were told that there were no correct answers, and all instructions were designed to minimize social threat. Equivalent time was allotted for the speech preparation and delivery periods as in the Trier Social Stress Test.
Circulating EMP Measures
Flow cytometry was used to differentiate EMP phenotypic surface marker expression associated with endothelial cell activation (CD62+ EMPs), apoptosis (CD31+ EMPs) or both (CD52+ EMPs), based on a technique of Jimenez and colleagues [34]. Sample preparation occurred within two hours of each blood draw. Citrated blood was centrifuged at 160 × g for 10 min to prepare platelet-rich plasma, and the platelet-rich plasma was further centrifuged for 8 min at 1000 × g to obtain platelet-poor plasma, and 50 µL of the platelet-poor plasma was then incubated with 5 µL of phycoerythrin-conjugated monoclonal antibody to CD62E (BD Biosciences). This antigen is specific for endothelial cells, obviating the need for a second antibody labeling. Unlike CD62E, the expression of CD31 and CD51 occurs on both platelet microparticles (PMPs) and EMPs. Thus, as CD42b is only present on platelets, fluorescein isothiocyante-labeled anti-CD42b was additionally used to distinguish between PMPs and EMPs expressing CD31 and separately EMPs expressing CD51. As such, 50 µL of platelet-poor plasma was incubated with 4 µL of phycoerythrin-conjugated monoclonal antibody to CD31 (AbD Serotec) and separately CD51 (AbD Serotec), along with 4 µL of fluorescein isothiocyante-conjugated monoclonal antibody to CD42b (AbD Serotec). After incubation, 1 mL of 0·01 M PBS buffer was added, and the samples were placed in polypropylene tubes for analysis by flow cytometry (BD FACSCalibur) at a medium flow rate for a 30 second period. EMPs are defined as the number of particles with size < 1·5 um, which are positively labeled by CD62E (CD62E+ EMPs), positively labeled by CD31 and negatively labeled by CD42 (CD31+ EMPs), and positively labeled by CD51 and negatively labeled by CD42 (CD51+ EMPs). For all experiments, appropriate fluorescein isothiocyante- and phycoerythrin-labeled isotype-matched IgG were also used to determine non-specific binding. Using standard beads, total flow cytometry counts for each experiment were converted to the number of EMPs per µL as previously described.
Manipulation Check: Salivary Cortisol and Psychological Stress
Participants provided saliva samples and psychological stress ratings (i.e. ‘How stressful was the task you just completed?’) on a Likert scale of 0 to 8 over seven timepoints during the session. The three timepoints coinciding with EMP collection will be presented here. Saliva samples, collected with Salivettes, were sent to the Kirschbaum Laboratory (Dresden, Germany) to be assayed for free circulating cortisol (nmol/L).
Data Analysis
EMPs
Total counts (counts/µL) of each measure (CD31+ EMPs, CD51+ EMPs and CD62E+ EMPs) were log-transformed due to skewed distributions identified with Kolmogorov-Smirnov tests. A 3 × 3 repeated measures ANOVA was conducted on each EMP marker including the within subjects factor of TIME (Pre-Trier Social Stress Test, Post 1, Post 2) and the between subjects factor of GROUP (SET Alone, SET+Values Affirmation, Control). The critical test of these ANOVAS was the interaction of TIME × GROUP.
Next, the following planned contrasts were tested on each EMP marker: 1) Post 1 and Pre-Trier Social Stress Test levels within each group were compared using a paired t-test, 2) a change score between Post 1 and Pre-Trier Social Stress Test was calculated, and then this score was compared as a function of group with an independent t-test, 3) baseline effects were assessed by comparing Pre-Trier Social Stress Test values as a function of group with an independent t-test and 4) group differences were assessed at Post 1, controlling for Pre-Trier Social Stress Test levels by employing multiple linear regression where the outcome was Post 1 values and the predictors were Group and Pre-Trier Social Stress Test values.
Salivary Cortisol and Psychological Stress
These data were submitted to the same ANOVA as above. Post hoc t-tests were conducted accounting for multiple comparisons with Bonferroni correction.
Results
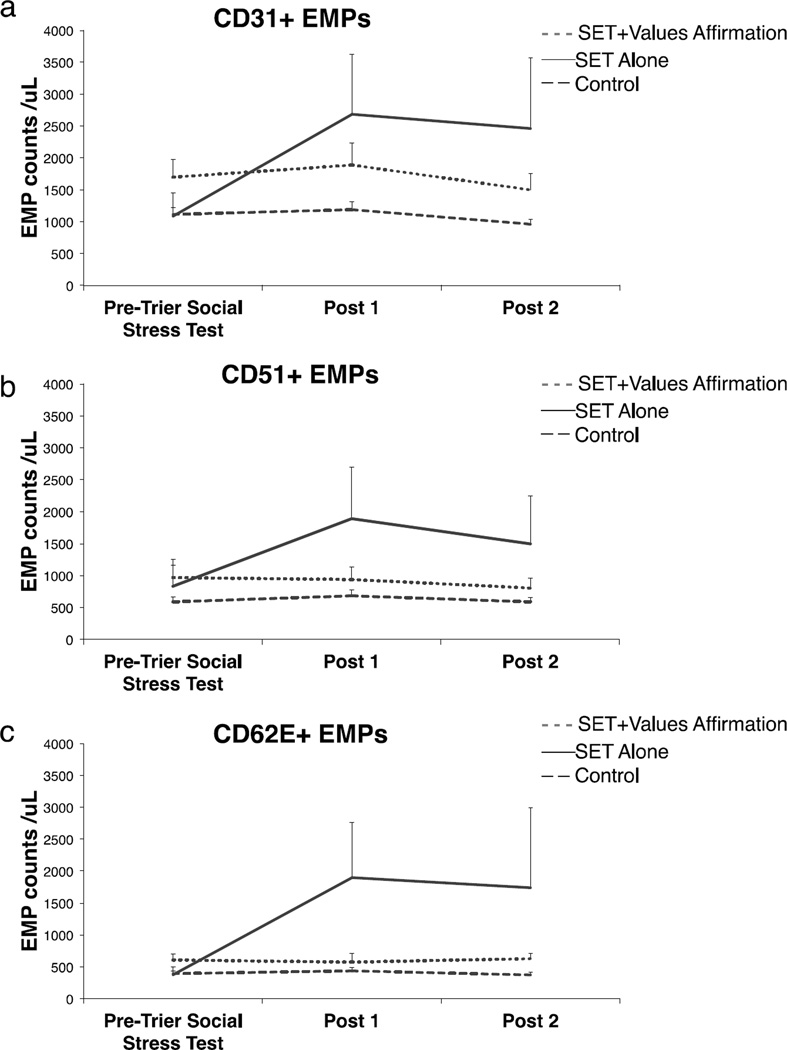
As predicted, the interaction of TIME × GROUP from ANOVA was significant for each EMP marker (CD31+: F(4,52) = 4.88 p = .002; CD51+: F(4,52) = 3.24, p = .019; CD62E+: F(4,52) = 3.89, p = .008). Planned contrasts revealed that the SET Alone group demonstrated significantly increased circulating levels of all three EMP populations from Pre-Trier Social Stress Test to Post 1 relative to the Control group (CD31+: t(19) = 3.06, p = 0.006; CD51+: t(19) = 2.74, p = 0.013; CD62E+: t(19) = 3.08, p = 0.006). EMP levels did not change in the Control group (all p > 0.10). In addition, levels of all three EMPs increased significantly less in the SET+Values Affirmation group relative to the SET Alone group (CD31+: t(13) = 2.79, p = 0.015; CD51+: t(13) = 2.81, p = 0.015; CD62E+: t(13) = 2.89, p = 0.013). EMP changes in the SET+Values Affirmation and Control groups were statistically equivalent (all p > 0.10). Thus, values affirmation prevented the endothelial injury caused by SET. There were no significant differences on the three measures of EMPs between groups at Pre-Trier Social Stress Test (see Table 1 and Fig. 2). Averaging Post 1 and Post 2 timepoints yielded similar results for all EMPs.
Table 1.
Results for each planned contrast and endothelial cell-derived microparticle (EMP) marker.
| CD31+ EMPs | CD51+ EMPs | CD62E+ EMPs | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Contrast | Effect | STE | 95%Cl | t | P | Sig. | Effect | STE | 95%Cl | t | P | Sig. | Effect | STE | 95%Cl | t | P | Sig. | |||
| Post 1 vs. Pre-Trier Social Stress Test | |||||||||||||||||||||
| Control | 0.07 | 0.10 | −0.14 | 0.27 | 0.67 | 0.512 | 0.13 | 0.08 | −0.05 | 0.31 | 1.54 | 0.147 | 0.06 | 0.11 | −0.17 | 0.30 | 0.58 | 0.575 | |||
| SET Alone | 0.71 | 0.23 | 0.15 | 1.28 | 3.08 | 0.022 | * | 0.71 | 0.25 | 0.09 | 1.32 | 2.81 | 0.031 | * | 1.11 | 0.44 | 0.04 | 2.19 | 2.53 | 0.044 | * |
| SET+Values Affirmation | 0.09 | 0.05 | −0.03 | 0.21 | 1.78 | 0.118 | −0.05 | 0.12 | −0.33 | 0.24 | −0.38 | 0.714 | −0.21 | 0.20 | −0.68 | 0.25 | −1.09 | 0.313 | |||
| Change scores (Post 1 - Pre-Trier Social Stress Test) | |||||||||||||||||||||
| SET Alone vs. Control | 0.65 | 0.21 | 0.20 | 1.09 | 3.06 | 0.006 | ** | 0.58 | 0.21 | 0.14 | 1.02 | 2.74 | 0.013 | * | 1.05 | 0.34 | 0.34 | 1.76 | 3.08 | 0.006 | ** |
| SET Alone vs. SET+Values Affirmation | 0.62 | 0.22 | 0.14 | 1.10 | 2.79 | 0.015 | * | 0.75 | 0.27 | 0.17 | 1.33 | 2.81 | 0.015 | * | 1.32 | 0.46 | 0.33 | 2.32 | 2.89 | 0.013 | * |
| SET+Values Affirmation vs. Control | 0.03 | 0.14 | −0.26 | 0.31 | 0.20 | 0.846 | −0.18 | 0.14 | −0.48 | 0.12 | −1.22 | 0.236 | −0.28 | 0.21 | −0.71 | 0.15 | −1.34 | 0.196 | |||
| Pre-Trier Social Stress Test | |||||||||||||||||||||
| SET Alone vs. Control | −0.20 | 0.25 | −0.73 | 0.33 | −0.79 | 0.440 | −0.09 | 0.40 | −0.92 | 0.74 | −0.24 | 0.816 | −0.19 | 0.23 | −0.68 | 0.31 | −0.79 | 0.437 | |||
| SET Alone vs. SET+Values Affirmation | −0.58 | 0.30 | −1.23 | 0.07 | −1.92 | 0.075 | −0.55 | 0.46 | −1.53 | 0.43 | −1.20 | 0.248 | −0.57 | 0.31 | −1.22 | 0.08 | −1.86 | 0.083 | |||
| SET+Values Affirmation vs. Control | 0.38 | 0.20 | −0.02 | 0.79 | 1.95 | 0.064 | 0.46 | 0.29 | −0.13 | 1.05 | 1.61 | 0.122 | 0.38 | 0.20 | −0.04 | 0.81 | 1.88 | 0.074 | |||
| Post 1 controlling for Pre-Trier Social Stress Test | |||||||||||||||||||||
| SET Alone vs. Control | 0.32 | 0.11 | 0.09 | 0.55 | 2.91 | 0.009 | ** | 0.29 | 0.11 | 0.06 | 0.52 | 2.67 | 0.016 | * | 0.51 | 0.18 | 0.14 | 0.88 | 2.89 | 0.010 | * |
| SET Alone vs. SET+Values Affirmation | 0.36 | 0.13 | 0.08 | 0.64 | 2.76 | 0.017 | * | 0.39 | 0.15 | 0.07 | 0.71 | 2.68 | 0.020 | * | 0.74 | 0.26 | 0.17 | 0.31 | 2.84 | 0.015 | * |
| SET+Values Affirmation vs. Control | 0.05 | 0.08 | −0.11 | 0.20 | 0.61 | 0.552 | −0.09 | 0.08 | −0.25 | 0.08 | −1.10 | 0.283 | −0.17 | 0.11 | −0.40 | 0.07 | −1.49 | 0.153 | |||
SET = Social Evaluative Threat; STE = standard error of the mean; CI = confidence interval
p < ·01;
p < ·05
Fig. 2. Average counts/µL as a function of Time and Group for each endothelial cell-derived microparticle (EMP) marker.
In general, (a) CD31+ EMPs (b) CD51+ EMPs and (c) CD62E+ EMPs increased as a function of the Trier Social Stress Test for the Social Evaluative Threat (SET) Alone group, but did not for the SET+Values Affirmation and Control groups, indicating that 1) SET affects EMPs and 2) a psychological inoculation can block such effects. Error bars represent one standard error of the mean.
Salivary Cortisol and Psychological Stress
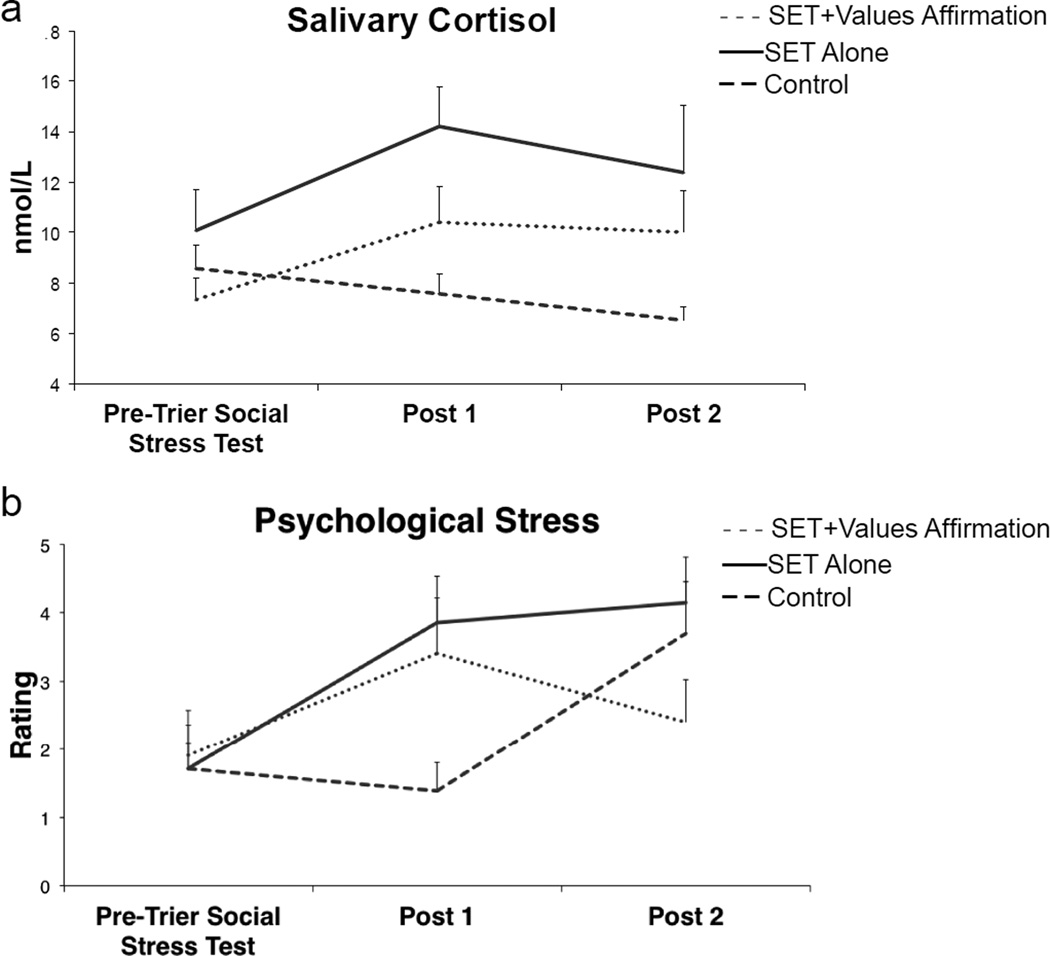
The interaction of TIME × GROUP on cortisol and psychological stress was significant (F(4,56) = 3.54, p = .012 and F(4,54) = 3.74, p = .009, respectively), as shown in Fig. 3. For cortisol, post hoc t-tests revealed higher values at Post 1 relative to the Pre-Trier Social Stress Test timepoint in the SET Alone (Mdifference = 4.15, p = .015) and SET+Values Affirmation (Mdifference = 3.08, p = .015) groups and higher values for the SET Alone group relative to Controls at Post 1 (Mdifference = 6.65, p = .004) and Post 2 (Mdifference = 5.88, p = .037), with no other effects being significant. Of note, no differences were found among groups at Pre-Trier Social Stress Test, and no difference was found among the SET+Values Affirmation and Control groups at Post 1. Finally, no difference was found among the SET Alone and SET+Values Affirmation groups at Post 1. Taken together, these results indicate the SET+Values Affirmation group fell in between the other two groups.
Fig. 3. Manipulation Check: Salivary Cortisol and Psychological Stress measures as a function of Time and Group.
A manipulation check was conducted using (a) salivary cortisol and (b) psychological stress measures to provide support for the novel findings of the Social Evaluative Threat (SET) and values affirmation manipulations on endothelial cell-derived microparticles (EMPs). In general, cortisol and stress ratings for the SET+Values Affirmation group fell between those for SET Alone and Control with the exception of the Post 2 timepoint where psychological stress ratings for the SET+Values Affirmation group fell to baseline levels and those of the Control group increased.
Results for ratings of psychological stress are shown in Fig. 3. Post hoc t-tests revealed higher values at Post 1 relative to Pre-Trier Social Stress Test (Mdifference = 2.14, p = .056) and Post 2 (Mdifference = 2.43, p = .024) in the SET Alone group; higher values at Post 1 only (Mdifference = 1.90, p = .040) in the SET+Values Affirmation group, indicating their stress levels fell to baseline at Post 2; and higher values at Post 2 only (Mdifference = 2.00, p = .010) for the Control group. This finding is not surprising in that the Control group likely perceived the cognition tasks occurring prior to the Post 2 measurement as more stressful relative to the Control task they performed prior to the Post 1 measurement. Additionally, higher values were observed in the SET Alone vs. Control group at Post 1 (Mdifference = 2.47, p = .039) and a marginally significant difference was observed between the SET+Values Affirmation and Control groups at Post 1 (Mdifference = 2.02, p = .068). No other effects were significant. As with cortisol, stress ratings for the SET+Values Affirmation group fell between those for SET Alone and Control.
Discussion
This proof of principle study is the first demonstration that a psychological manipulation designed to reduce psychological threat appraisals in vulnerable populations can prevent endothelial damage provoked by psychological stress. In addition to corroborating our earlier finding that this form of stress dynamically elevates circulating EMPs [17]—a biological marker of endothelial damage and apoptosis—we now show that a brief psychological inoculation designed to alter the appraisal of stress eliminates this effect. Together, these findings show that psychological events related to one’s social and interpersonal setting have immediate and direct influences on vascular health. These findings were paralleled by consistent and predicted ones with salivary cortisol and self-report psychological stress measures, serving as manipulation checks.
Conceptually, values affirmation engages social cognitive processes that likely originate in higher cortical areas related to the construction of self-concept [35], appraisals of self-worth and social standing [36] and working memory [37]. Priming negative social, cultural, or racial associations that threaten one’s self-concept—a process known as stereotype threat [38]—has long been thought to impair performance. The active psychological ingredients of our SET challenge likely involved similar threats to self-concept, as it has been shown to activate areas of the ventromedial prefrontal cortex [31,35,39] important for stress [31,38], appraisals of self-concept [40,41], social standing [36] and emotional meaning [35]. Our findings extend the concepts of stereotype threat and SET into physiological systems directly relevant for health. The psychological effects on endothelial health we describe here may be mediated by autonomic control of vascular performance and regulation. This is consistent with other studies of self-affirmation that have shown effects on autonomic and neuroendocrine responses to stressful tasks [18,19]. By linking the effects of stress and self-affirmation to factors directly relevant for incident cardiovascular disease risk, the current findings provide new bridges between psychological science and medical science, or mind-brain-body connections.
We note some important limitations of this study. First, it may be asked whether actual and irreversible endothelial injury occurred in our study. Although we use the term endothelial injury throughout this report, free circulating EMPs arguably are characterized more precisely as markers of endothelial damage. As for the duration of the effect, like many aspects of routine daily living, the Trier Social Stress Test is believed to have produced small, but reversible, damage to the endothelium, but not permanent damage. What remains critical for future research is to understand the cumulative effects of many such instances, i.e. those that lead to measurable changes in health.
Similarly, whether exaggerated acute reactivity of endothelial function predicts long-term damage is also unknown. Several studies demonstrate that various markers of endothelial dysfunction are associated with an increased risk of subclinical cardiovascular disease and cardiovascular disease events [4]. These studies assessed markers of endothelial function at rest and did not consider the level of stress that each individual was experiencing at the time of the assessment. In addition, studies have demonstrated acute negative effects of various probes - e.g., a high fat meal, acute mental stress, smoking a cigarette – on endothelial function however, these effects have not been linked to longer term outcomes. Therefore, it is unclear whether stress-induced endothelial dysfunction predicts subclinical cardiovascular disease and cardiovascular disease events. Moreover, our study was conducted with healthy individuals, and it could be that those at risk for cardiovascular disease have more prolonged effects. This is another important area of future research.
Second, the values affirmation intervention produced acute, short-term changes in this study. The long-term effects of the intervention on physiology are largely unknown, though there appear to be advantageous long-term effects on academic performance [21,42] and health [42]. Therefore its viability as a stress inoculation treatment with respect to cardiovascular health remains unknown.
Third, these data are considered preliminary due to the relatively small sample size. They serve as a proof of principle, providing a promising foundation for future larger-scale investigations. At the same time, it should be noted that within small samples, robust effect sizes can be found (as in the case of the current study) whereas small effect sizes rarely reach significance. Thus, such significant findings should be interpreted with caution in particular when determining power for future studies [43]. However, overestimation of effect sizes is often related to by (1) multiple testing and picking the “winning” tests, and (2) altering the analyses, e.g., by changing covariates included or median splits on individual difference variables until significance is obtained, and (3) increasing the sample size until significance is reached and then stopping. The current study did not implement any of these problematic approaches.
Finally, future work should characterize the mind-brain-body connections implicated in the current study. For example, self-affirmation of values in the face of threatening information is thought to have multiple potential psychological effects, including boosting self-resources, broadening perspective and decoupling the individual from the threat (cf. Sherman & Hartson [28]). It is possible that any or all of these psychological processes could have downstream effects on physiological risk factors for cardiovascular disease, with multiple potential mediating brain-peripheral pathways.
Acknowledgments
Funding: This work was supported by the National Institutes of Health grants NIH R21 MH082308 (T.D.W), NIH R01 MH076136 (T.D.W.), and NIH R01 HL116470 (D.S.).
Footnotes
Conflict of Interest: The authors declare that they have no conflict of interest.
Ethical approval: All procedures performed in this study were in accordance with the ethical standards of the Institutional Review Board of Columbia University and the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent: Informed consent was obtained from all individual participants included in the study.
References
- 1.Selye H. The stress of life. New York: McGraw-Hill; 1956. [Google Scholar]
- 2.MacLean PD. Psychosomatic disease and the visceral brain; recent developments bearing on the Papez theory of emotion. Psychosom Med. 1949;11(6):338–353. doi: 10.1097/00006842-194911000-00003. [DOI] [PubMed] [Google Scholar]
- 3.Dimsdale JE. Psychological stress and cardiovascular disease. J Am Coll Cardiol. 2008;51(13):1237–1246. doi: 10.1016/j.jacc.2007.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wilbert-Lampen U, Leistner D, Greven S, et al. Cardiovascular events during World Cup soccer. N Engl J Med. 2008;358(5):475–483. doi: 10.1056/NEJMoa0707427. [DOI] [PubMed] [Google Scholar]
- 5.Rozanski A, Bairey CN, Krantz DS, et al. Mental stress and the induction of silent myocardial ischemia in patients with coronary artery disease. N Engl J Med. 1988;318(16):1005–1012. doi: 10.1056/NEJM198804213181601. [DOI] [PubMed] [Google Scholar]
- 6.Haynes SG, Feinleib M, Kannel WB. The relationship of psychosocial factors to coronary heart disease in the Framingham Study. III. Eight-year incidence of coronary heart disease. Am J Epidemiol. 1980;111(1):37–58. doi: 10.1093/oxfordjournals.aje.a112873. [DOI] [PubMed] [Google Scholar]
- 7.Rosengren A, Hawken S, Ounpuu S, et al. Association of psychosocial risk factors with risk of acute myocardial infarction in 11119 cases and 13648 controls from 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364(9438):953–962. doi: 10.1016/S0140-6736(04)17019-0. [DOI] [PubMed] [Google Scholar]
- 8.Boulanger CM, Amabile N, Tedgui A. Circulating microparticles: a potential prognostic marker for atherosclerotic vascular disease. Hypertension. 2006;48(2):180–186. doi: 10.1161/01.HYP.0000231507.00962.b5. [DOI] [PubMed] [Google Scholar]
- 9.Deanfield JE, Halcox JP, Rabelink TJ. Endothelial function and dysfunction: testing and clinical relevance. Circulation. 2007;115(10):1285–1295. doi: 10.1161/CIRCULATIONAHA.106.652859. [DOI] [PubMed] [Google Scholar]
- 10.VanWijk MJ, VanBavel E, Sturk A, Nieuwland R. Microparticles in cardiovascular diseases. Cardiovasc Res. 2003;59(2):277–287. doi: 10.1016/s0008-6363(03)00367-5. [DOI] [PubMed] [Google Scholar]
- 11.Lynch SF, Ludlam CA. Plasma microparticles and vascular disorders. Br J Haematol. 2007;137(1):36–48. doi: 10.1111/j.1365-2141.2007.06514.x. [DOI] [PubMed] [Google Scholar]
- 12.Simak J, Gelderman MP, Yu H, Wright V, Baird AE. Circulating endothelial microparticles in acute ischemic stroke: a link to severity, lesion volume and outcome. J Thromb Haemost. 2006;4(6):1296–1302. doi: 10.1111/j.1538-7836.2006.01911.x. [DOI] [PubMed] [Google Scholar]
- 13.Vanwijk MJ, Svedas E, Boer K, Nieuwland R, Vanbavel E, Kublickiene KR. Isolated microparticles, but not whole plasma, from women with preeclampsia impair endothelium-dependent relaxation in isolated myometrial arteries from healthy pregnant women. Am J Obstet Gynecol. 2002;187(6):1686–1693. doi: 10.1067/mob.2002.127905. [DOI] [PubMed] [Google Scholar]
- 14.Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: a theoretical integration and synthesis of laboratory research. Psychol Bull. 2004;130(3):355–391. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- 15.Kemeny ME, Schedlowski M. Understanding the interaction between psychosocial stress and immune-related diseases: a stepwise progression. Brain Behav Immun. 2007;21(8):1009–1018. doi: 10.1016/j.bbi.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 16.Berntson GG, Cacioppo JT, Binkley PF, Uchino BN, Quigley KS, Fieldstone A. Autonomic cardiac control. III. Psychological stress and cardiac response in autonomic space as revealed by pharmacological blockades. Psychophysiology. 1994;31(6):599–608. doi: 10.1111/j.1469-8986.1994.tb02352.x. [DOI] [PubMed] [Google Scholar]
- 17.Shimbo D, Rosenberg LB, Chaplin W, et al. Endothelial cell activation, reduced endothelial cell reparative capacity, and impaired endothelial-dependent vasodilation after anger provocation. Int J Cardiol. 2013;167(3):1064–1065. doi: 10.1016/j.ijcard.2012.10.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Creswell JD, Welch WT, Taylor SE, Sherman DK, Gruenewald TL, Mann T. Affirmation of personal values buffers neuroendocrine and psychological stress responses. Psychol Sci. 2005;16(11):846–851. doi: 10.1111/j.1467-9280.2005.01624.x. [DOI] [PubMed] [Google Scholar]
- 19.Sherman DK, Bunyan DP, Creswell JD, Jaremka LM. Psychological vulnerability and stress: the effects of self-affirmation on sympathetic nervous system responses to naturalistic stressors. Health Psychol. 2009;28(5):554–562. doi: 10.1037/a0014663. [DOI] [PubMed] [Google Scholar]
- 20.Cohen GL, Garcia J, Apfel N, Master A. Reducing the racial achievement gap: a social-psychological intervention. Science. 2006;313(5791):1307–1310. doi: 10.1126/science.1128317. [DOI] [PubMed] [Google Scholar]
- 21.Cohen GL, Garcia J, Purdie-Vaughns V, Apfel N, Brzustoski P. Recursive processes in self-affirmation: intervening to close the minority achievement gap. Science. 2009;324(5925):400–403. doi: 10.1126/science.1170769. [DOI] [PubMed] [Google Scholar]
- 22.Creswell JD, Dutcher JM, Klein WM, Harris PR, Levine JM. Self-affirmation improves problem-solving under stress. PLoS One. 2013;8(5):e62593. doi: 10.1371/journal.pone.0062593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miyake A, Kost-Smith LE, Finkelstein ND, Pollock SJ, Cohen GL, Ito TA. Reducing the gender achievement gap in college science: a classroom study of values affirmation. Science. 2010;330(6008):1234–1237. doi: 10.1126/science.1195996. [DOI] [PubMed] [Google Scholar]
- 24.Bernal-Mizrachi L, Jy W, Jimenez JJ, et al. High levels of circulating endothelial microparticles in patients with acute coronary syndromes. Am Heart J. 2003;145(6):962–970. doi: 10.1016/S0002-8703(03)00103-0. [DOI] [PubMed] [Google Scholar]
- 25.Jy W, Horstman LL, Jimenez JJ, et al. Measuring circulating cell-derived microparticles. J Thromb Haemost. 2004;2(10):1842–1851. doi: 10.1111/j.1538-7836.2004.00936.x. [DOI] [PubMed] [Google Scholar]
- 26.Cohen GL, Sherman DK. The psychology of change: self-affirmation and social psychological intervention. Annu Rev Psychol. 2014;65:333–371. doi: 10.1146/annurev-psych-010213-115137. [DOI] [PubMed] [Google Scholar]
- 27.Steele CM. The psychology of self-affirmation: Sustaining the integrity of the self. Advances in experimental social psychology. 1988;21:261–302. [Google Scholar]
- 28.Sherman DK, Hartson KA. Reconciling Self-Protection with Self-Improvement. In: Alicke MD, Sedikides C, editors. Handbook of Self-Enhancement and Self-Protection. New York: Guildford Press; 2011. pp. 128–151. [Google Scholar]
- 29.Kirschbaum C, Pirke KM, Hellhammer DH. The 'Trier Social Stress Test'--a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993;28(1–2):76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- 30.Cacioppo JT, Berntson GG, Binkley PF, Quigley KS, Uchino BN, Fieldstone A. Autonomic cardiac control. II. Noninvasive indices and basal response as revealed by autonomic blockades. Psychophysiology. 1994;31(6):586–598. doi: 10.1111/j.1469-8986.1994.tb02351.x. [DOI] [PubMed] [Google Scholar]
- 31.Wager TD, van Ast VA, Hughes BL, Davidson ML, Lindquist MA, Ochsner KN. Brain mediators of cardiovascular responses to social threat, part II: Prefrontal-subcortical pathways and relationship with anxiety. Neuroimage. 2009;47(3):836–851. doi: 10.1016/j.neuroimage.2009.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ghiadoni L, Donald AE, Cropley M, et al. Mental stress induces transient endothelial dysfunction in humans. Circulation. 2000;102(20):2473–2478. doi: 10.1161/01.cir.102.20.2473. [DOI] [PubMed] [Google Scholar]
- 33.Gottdiener JS, Kop WJ, Hausner E, McCeney MK, Herrington D, Krantz DS. Effects of mental stress on flow-mediated brachial arterial dilation and influence of behavioral factors and hypercholesterolemia in subjects without cardiovascular disease. Am J Cardiol. 2003;92(6):687–691. doi: 10.1016/s0002-9149(03)00823-3. [DOI] [PubMed] [Google Scholar]
- 34.Jimenez JJ, Jy W, Mauro LM, Horstman LL, Soderland C, Ahn YS. Endothelial microparticles released in thrombotic thrombocytopenic purpura express von Willebrand factor and markers of endothelial activation. Br J Haematol. 2003;123(5):896–902. doi: 10.1046/j.1365-2141.2003.04716.x. [DOI] [PubMed] [Google Scholar]
- 35.Roy M, Shohamy D, Wager TD. Ventromedial prefrontal-subcortical systems and the generation of affective meaning. Trends Cogn Sci. 2012;16(3):147–156. doi: 10.1016/j.tics.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gianaros PJ, Horenstein JA, Cohen S, et al. Perigenual anterior cingulate morphology covaries with perceived social standing. Soc Cogn Affect Neurosci. 2007;2(3):161–173. doi: 10.1093/scan/nsm013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schmader T, Johns M, Forbes C. An integrated process model of stereotype threat effects on performance. Psychol Rev. 2008;115(2):336–356. doi: 10.1037/0033-295X.115.2.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dedovic K, Renwick R, Mahani NK, Engert V, Lupien SJ, Pruessner JC. The Montreal Imaging Stress Task: using functional imaging to investigate the effects of perceiving and processing psychosocial stress in the human brain. J Psychiatry Neurosci. 2005;30(5):319–325. [PMC free article] [PubMed] [Google Scholar]
- 39.van Ast VA, Spicer J, Smith EE, et al. Brain Mechanisms of Social Threat Effects on Working Memory. Cereb Cortex. 2014 doi: 10.1093/cercor/bhu206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Denny BT, Kober H, Wager TD, Ochsner KN. A meta-analysis of functional neuroimaging studies of self- and other judgments reveals a spatial gradient for mentalizing in medial prefrontal cortex. J Cogn Neurosci. 2012;24(8):1742–1752. doi: 10.1162/jocn_a_00233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Northoff G, Heinzel A, de Greck M, Bermpohl F, Dobrowolny H, Panksepp J. Self-referential processing in our brain--a meta-analysis of imaging studies on the self. Neuroimage. 2006;31(1):440–457. doi: 10.1016/j.neuroimage.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 42.Walton GM, Cohen GL. A brief social-belonging intervention improves academic and health outcomes of minority students. Science. 2011;331(6023):1447–1451. doi: 10.1126/science.1198364. [DOI] [PubMed] [Google Scholar]
- 43.Button KS, Ioannidis JP, Mokrysz C, et al. Power failure: why small sample size undermines the reliability of neuroscience. Nat Rev Neurosci. 2013;14(5):365–376. doi: 10.1038/nrn3475. [DOI] [PubMed] [Google Scholar]