Abstract
Purpose
We characterized the early gestation development of the female external genitalia using optical projection tomography to visualize anatomical structures at high resolution.
Materials and Methods
First and early second trimester human female fetal external genitalia were collected with consent after voluntary termination. Specimens labeled with anti-E-Cadherin antibody underwent analysis with optical projection tomography. Histological sections were immunostained for androgen receptor, 5α-reductase, Ki67 for proliferation and Caspase 3 for apoptosis.
Results
Three-dimensional reconstructions demonstrated proximal to distal canalization of the epithelial vestibular plate and formation of a vestibular groove, which remained open. Ki67 was observed throughout with greatest density in the dorsal vestibular plate at the level of the opening groove. Staining for Caspase 3 was minimal in all sections. Androgen receptor staining was seen throughout the mesenchyme and in the apical epithelium of the dorsal vestibular groove. Throughout the epithelium and epidermis 5α-reductase staining was observed.
Conclusions
Early development of the external genitalia in the female is analogous to that in the male, demonstrating a similar opening zipper driving canalization of the vestibular plate with localized epithelial proliferation in the absence of significant apoptosis. Thus we hypothesize that the mechanism underlying the opening zipper must be androgen independent and the absence of androgen driven urethral fusion characterizes the normal development of the human clitoris.
Keywords: genitalia, female, clitoris, embryonic and fetal development, tomography, optical, androgens
While a large body of work has been published describing fetal development of the human penis,1–5 far less is known about the development of the female external genitalia in humans or even laboratory animals. The few studies of the development of the human clitoris were based on the study of a small number of gross fetal specimens and serial sections of the specimens.1,6,7 What little is known about the molecular mechanisms underlying patterning of the external genitalia comes primarily from animal studies. For example, expression of the morphogen Shh (sonic hedgehog) is required during distinct temporal windows for the early outgrowth and ambisexual development of the genital tubercle in mice.8 However, to our knowledge analogous mechanisms have not yet been found in human development.
We recently described the development of the human male genital tubercle and penile urethra using OPT.9 Two key observations were made, including 1) canalization of the urethral plate or the opening zipper followed by 2) fusion of the urethral folds or the closing zipper. We hypothesized that androgen independent canalization of the vestibular plate (opening zipper) occurs in the same fashion in female development as it does in male development. In contrast, the androgen dependent fusion process (closing zipper) remains quiescent in normal females with the potential for closure under the influence of abnormal exogenous or endogenous androgen exposure.
We performed OPT to determine early fetal development of the human external female genitalia. OPT allows for visualization of internal structures in whole mount specimens.9 Specimens are labeled with fluorescent antibodies and unlabeled tissues are rendered optically clear. Hundreds of projection images are integrated into a 3-dimensional reconstruction, which can be virtually rotated and sectioned to elucidate relationships among intact internal structures at a level of detail previously not possible. After OPT the same specimens can be sectioned for standard immunohistochemical staining. Thus, 3-dimensional structures visualized by OPT can be correlated with subcellular patterns of protein expression.
METHODS
First and early second trimester human fetal specimens were collected after elective termination of pregnancy with approval from the committee for human research at University of California-San Francisco (IRB 12-08813). Fetal age was estimated using heel-toe length.10 We report fetal age from the time of fertilization and not from the last menstrual period (ie gestational age minus 2 weeks).
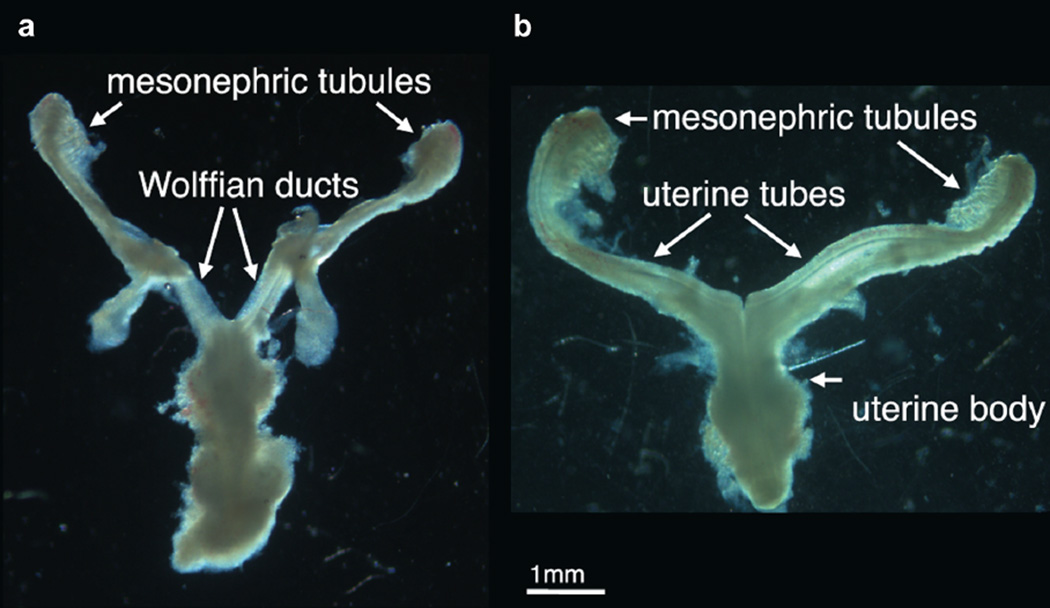
Gender was determined using PCR to detect X and Y chromosomal sequences. When available, gender was confirmed by identifying wolffian and müllerian duct morphology (fig. 1). External genitalia were identified under a dissecting microscope. A total of 18 female genital tubercle specimens were processed for OPT as previously described.9
Figure 1.
Images of reproductive tract show subtle morphology differences between genders. Morphology of male and female specimens is distinctive and diagnostic of gender, which was further verified by PCR. a, 9-week male human fetus. b, 9-week female human fetus.
Briefly, genital tubercles were fixed in 10% formalin, bleached with hydrogen peroxide, stained using a whole mount immunofluorescence protocol with EP700Y anti-E-Cadherin monoclonal antibody (Abcam®) and Alexa Fluor® 488 anti-rabbit secondary antibody, optically cleared in benzyl alcohol and benzyl benzoate, embedded in agarose and imaged with the 3001M OPT scanner (Bioptonics, Edinburgh, Scotland). Similar to the principles of xray computerized tomography 800 projected images from each channel were constructed into 3-dimensional voxel data sets with in-house software. They were then visualized with the Volocity software suite (PerkinElmer®). Measurements were made using the caliper function in Volocity.
After OPT the specimens were recovered, formalin fixed, paraffin embedded and serially sectioned for immunohistochemistry. An additional 6 specimens were directly processed for immunohistochemistry. Using standard antigen retrieval and immunoperoxidase techniques 7 µm sections were stained for Ki67 (Leica Biosystems®), Caspase 3 (Cell Signaling Technology®), AR (EPR1535, Abcam) and rabbit polyclonal 5α-reductase type II protein expression. Micrographs were background subtracted and contrast adjusted with ImageJ (http://imagej.nih.gov/ij/).
RESULTS
Human Clitoris
Ontogeny
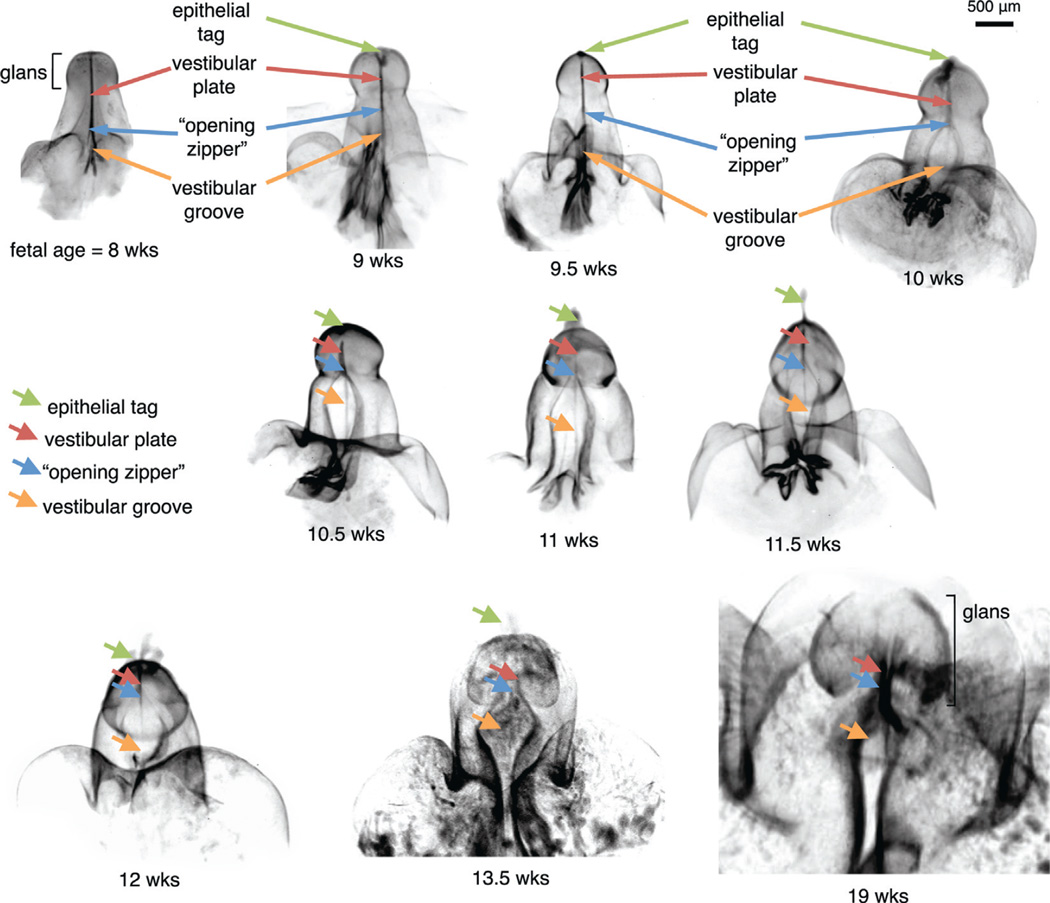
Figure 2 shows OPT ontogeny of the human clitoris from 8 to 19 weeks in utero. As described 18 specimens confirmed female by PCR were processed for OPT. Planar cross sections of the 3-dimensional reconstructions were reviewed for relationships among the internal anatomical structures. The fluorescent E-Cadherin signal was observed throughout the epidermis and in the midline epithelial vestibular plate.
Figure 2.
OPT reveals fetal ontogeny of human clitoris from 8 to 19 weeks (wks) of fetal age. Note opening zipper, which facilitates vestibular plate opening to form vestibular groove, and lack of closing zipper with vestibular groove remaining open.
To our knowledge we introduce for the first time the term vestibular plate, indicating the female homologue of the urethral plate in males. This new term is justified by virtue of the fact that canalization of the vestibular plate is involved in the development of the mature vestibule.
By 8 weeks of development the vestibular plate extended to near the tip of the genital tubercle. At this age canalization of the vestibular plate formed the beginning of the vestibular groove at the proximal base of the genital tubercle. By 9 weeks the vestibular groove extended halfway to the distal tip of the tubercle. By 10 weeks a widely opened vestibular groove almost reached the glans clitoris. By 11 weeks the open vestibular groove extended into the glans clitoris. By 13.5 weeks the vestibular plate was almost completely canalized and it was widely opened. At 19 weeks the vestibular groove remained open and the glans clitoris had begun to bend caudal to resemble the orientation in the adult female.
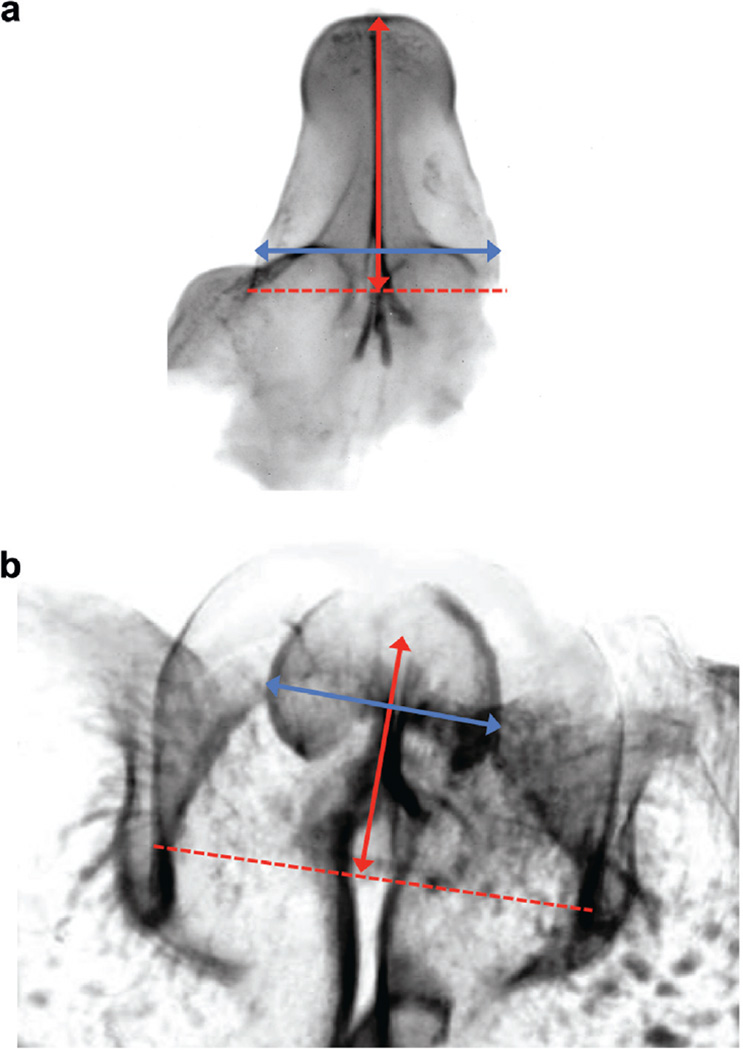
The length of the genital tubercle ranged from 1.25 mm at 8 weeks to 2.75 mm at 19 weeks when measured from the base of the genital tubercle to the tip of the glans (fig. 3). The width of the clitoris ranged from 0.70 mm at 8 weeks to 1.73 mm at 19 weeks (fig. 3). No evidence of a closing zipper or fusion process was observed in any of the specimens.
Figure 3.
Representative fetal clitoris. a, 8 weeks of development. b, 19 weeks of development. Red double arrow indicates genital tubercle length. Blue double arrow indicates clitoral width.
Proliferation and Apoptosis Markers
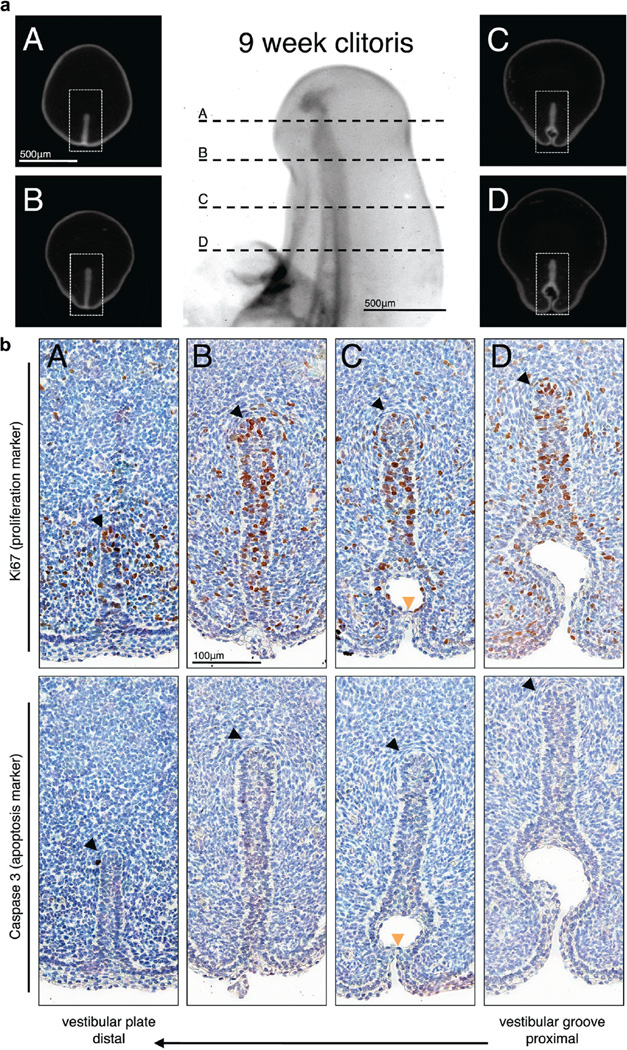
Ki67 labeling was noted in 48 sections of the developing female external genitalia in the solid vestibular plate and at the site of canalization of the vestibular plate to form the vestibular groove. At 9 weeks in utero the vestibular plate expressed Ki67 in relatively uniform fashion (fig. 4). There was a notable paucity of Ki67 expression at the area of canalization (opening zipper) (fig. 4, b, C). At 10.5 weeks Ki67 expression was localized to the dorsal vestibular plate and groove (fig. 5). Histological appearance of the epithelial layer also changed from a thin undifferentiated layer in the dorsal aspect of the vestibular plate to a thick stratified layer along the walls of the urethral groove (fig. 5, a, D and E, and b, D and E). At 13.5 weeks in utero the expression of Ki67 was less intense (fig. 6). Stratified epithelium lined the entire vestibular groove in contrast to the younger specimens (fig. 6, b, B).
Figure 4.
Human 9-week fetal clitoris. a, closed vestibular plate (A and B), opening zipper (C) and vestibular groove (D). Scale bar indicates 500 µm. b, immunohistochemical staining reveals closed vestibular plate (A and B), opening zipper (C) and vestibular groove (D). Black arrowheads indicate vestibular plate dorsal margin. Orange arrowheads indicate opening zipper at canalized vestibular plate level. Scale bar indicates 100 µm.
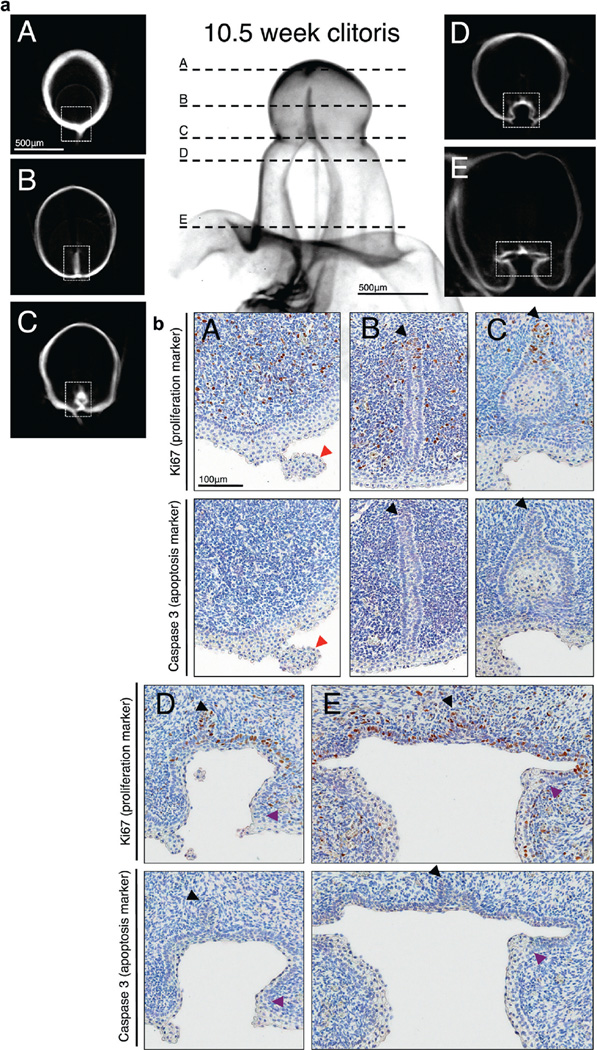
Figure 5.
Human 10.5-week fetal clitoris. a, clitoris distal tip (A), closed vestibular plate (B), opening zipper (C) and vestibular groove (D and E). Scale bar indicates 500 µm. b, immunohistochemical staining shows clitoris distal tip (A), closed vestibular plate (B), opening zipper (C) and vestibular groove (D and E). Black arrowheads indicate vestibular plate dorsal margin. Red arrowheads indicate epithelial tag. Purple arrowheads indicate transition from dorsal vestibular groove columnar epithelium to lateral vestibular groove squamous epithelium. Scale bar indicates 100 µm.
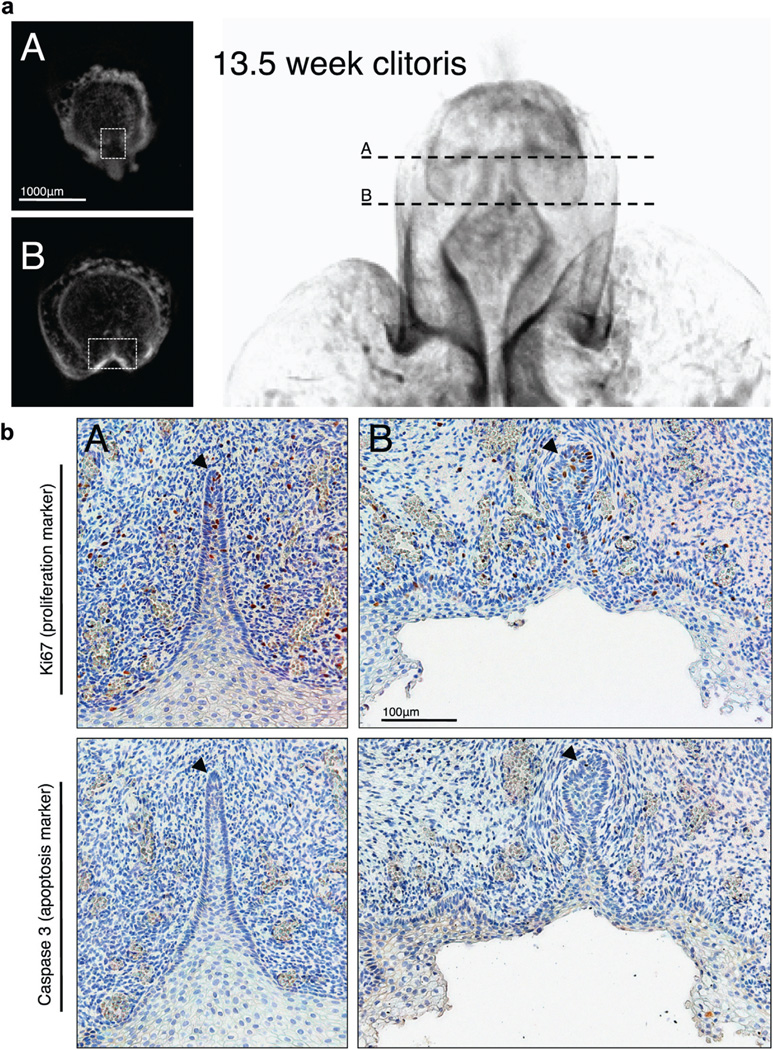
Figure 6.
Human 13.5-week fetal clitoris. a, closed vestibular plate (A) and vestibular groove (B). Scale bar indicates 1,000 µm. b, immunohistochemical staining demonstrates closed vestibular plate (A) and vestibular groove (B). Black arrowheads indicate vestibular plate dorsal margin. Scale bar indicates 100 µm.
Occasional Ki67 positive cells were noted in the epidermis at all 3 ages. As revealed by Caspase 3 expression in 48 sections apoptosis was largely absent throughout the developing clitoris (figs. 4 to 6).
AR and 5α-Reductase Expression
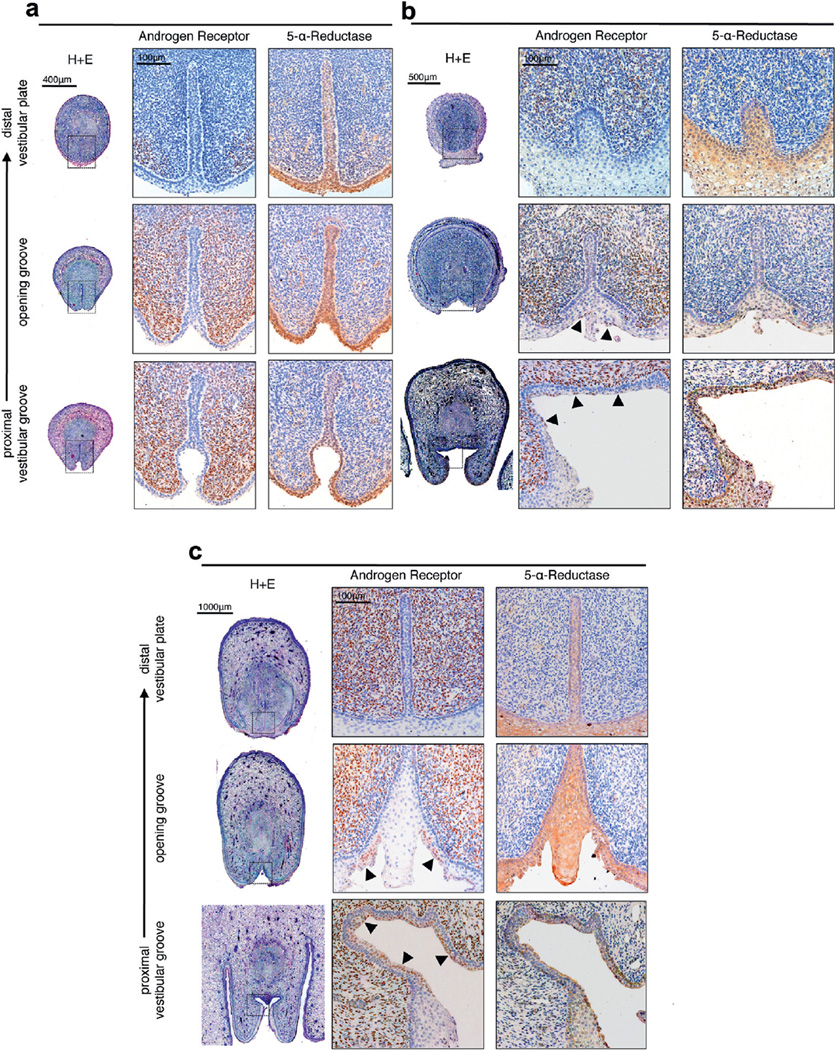
AR staining, which was observed diffusely in 48 sections throughout the mesenchyme of the developing female external genitalia, was most dense in the dorsal aspect of the tubercle but undetectable in the epithelium. At 9 weeks AR expression was restricted to the mesenchyme with a greater density of AR positive cells seen in the ventral aspect of the tubercle (fig. 7, a). Sparse expression was seen in the distal sections. An abundance of AR positive cells was noted in the mesenchyme surrounding the vestibular plate at the level of the opening zipper and at the open vestibular groove. At 11.5 weeks prominent AR expression was again noted throughout the mesenchyme with additional positive cells in the epithelium, predominantly in the apical layer at the level of the opening zipper and in the dorsal aspect of the open vestibular groove (fig. 7, b). A similar distribution was noted at 16.5 weeks with AR expressing cells in the apical epithelium at the level of the opening zipper again but notably sparing the midline epithelial protrusion into the opening vestibular groove (fig. 7, c). Distinct AR positive cells were observed in the apical cells of the epithelium in the most dorsal portion of the open vestibular groove and at the level of the opening zipper at all ages. In contrast, 5α-reductase staining was observed in 48 sections primarily in the epithelium of the solid vestibular plate and epidermis, and in the floor of the vestibular groove (fig. 7).
Figure 7.
Immunohistochemical staining for AR, which was localized to mesenchyme, and 5α-reductase, which was localized to epithelium, in human fetal clitoris vestibular plate, opening zipper and open vestibular groove. a, at 9 weeks. H&E, scale bar indicates 400 µm. Immunohistochemical staining, scale bar indicates 500 µm. b, at 11.5 weeks. Arrowheads indicate AR positive apical epithelium. H&E, scale bar indicates 500 µm. Immunohistochemical staining, scale bar indicates 100 µm. c, at 16.5 weeks. H&E, scale bar indicates 1,000 µm. Immunohistochemical staining, scale bar indicates 100 µm.
DISCUSSION
We describe the normal anatomy of the developing human female external genitalia using OPT. We observed a solid epithelial vestibular plate, which canalizes in a proximal to distal progression to form a widely open vestibular groove, which remains open once formed (fig. 2). Our 2-zipper hypothesis of penile development describes a first distal opening zipper followed by a second proximal closing zipper.5 We found that the opening zipper is active in female development to create the vestibule but development of the clitoris is characterized by absence of the closing zipper fusion mechanism. The analogous opening of the urethral and vestibular plates seen in development of the external genitalia of males and females suggests that this process is androgen independent in both genders, a particularly important idea in regard to development of the penile urethra.
Our immunostaining experiments demonstrated that midline cellular proliferation in the vestibular plate occurs in the female. This is analogous to epithelial proliferation in the urethral plate in the male. In both genders it may be involved in lateral expansion of the respective vestibular and urethral grooves (figs. 4 to 6). As in the male the lack of apoptosis revealed by Caspase 3 expression suggests that apoptosis may not have a major role in canalizing and opening the epithelial plate, although other forms of programmed cell death not detected by this technique may be involved.
AR expression in the developing female external genitalia is primarily mesenchymal while 5α-reductase is primarily expressed in the epithelium (fig. 7). Similar gross distribution of 5α-reductase was observed in the developing human male external genitalia (data not shown). The precise distribution of AR positive apical epithelial cells of the vestibular groove is intriguing and merits further study. Given the evidence in animal models of the role of patterning molecules such as Shh and MafB in sexually dimorphic differentiation of the mouse genital tubercle,8,11 it will also be interesting to discover whether expression correlates with the differential development of the human fetal male and female genital tubercle.
We know that differential male vs female development of external genitalia is driven by the presence or absence of androgenic action. This is demonstrated in disorders such as congenital adrenal hyperplasia and androgen insensitivity syndrome.12 As we found, AR and 5α-reductase expression is broadly analogous between developing female and male genital tubercles. The mechanism of the closing zipper is presumably activated in the developing penis by androgens. The presence of AR and 5α-reductase expression in analogous regions in the developing female genital tubercle provides a mechanistic explanation for masculinization of the external genitalia of female fetuses exposed to endogenous or exogenous androgens.
We hypothesize that the cellular response to androgens results in differential expression of a set of proteins at the leading edge of the fusing epithelium and in the surrounding tissues to activate the closing zipper. The absence of any one of the steps could result in developmental disorders such as hypospadias. To test these hypotheses further study is warranted to identify these putative molecular players and test them in a system in which androgen exposure can be manipulated. Such a system could involve subrenal capsular grafting of human fetal tissue in athymic nude hosts followed by systemic exogenous androgen exposure.13
Previously Li et al from our laboratory reported that the fetal penis almost tripled in length from 6.5 to 16.5 weeks in utero.5 In the current series we found that even in the absence of androgenic stimulation the clitoris more than doubled in length and width from 8 to 19 weeks in utero. Presumably the human external genitalia undergo 2 forms of growth, including 1) androgen independent growth before the indifferent stage and 2) androgen dependent growth. The substantial growth of the human female genital tubercle after the indifferent stage is presumably androgen independent and, thus, consistent with this hypothesis. There is a precedent for androgen independent growth of the clitoris in the spotted hyena.14 To our knowledge the mechanism remains to be determined but it is possible that estrogens have a role in this process.
CONCLUSIONS
We present data supporting our hypothesis that the opening zipper canalization process develops in analogous fashion in the male and the female fetus, implying that this process is androgen independent. In contrast the closing zipper mechanism appears quiescent in the normal female with a resulting open vestibular groove.
Acknowledgments
Supported by NIH Grant DK058105.
David Russell provided rabbit polyclonal 5α-reductase type II.
Abbreviations and Acronyms
- AR
androgen receptor
- OPT
optical projection tomography
- PCR
polymerase chain reaction
Footnotes
The corresponding author certifies that, when applicable, a statement(s) has been included in the manuscript documenting institutional review board, ethics committee or ethical review board study approval; principles of Helsinki Declaration were followed in lieu of formal ethics committee approval; institutional animal care and use committee approval; all human subjects provided written informed consent with guarantees of confidentiality; IRB approved protocol number; animal approved project number.
REFERENCES
- 1.Spaulding MH. Contributions to Embryology. Vol. 61. Baltimore: Carnegie Institution of Washington; 1921. The Development of the External Genitalia in the Human Embryo. [Google Scholar]
- 2.Altemus AR, Hutchins GM. Development of the human anterior urethra. J Urol. 1991;146:1085. doi: 10.1016/s0022-5347(17)38008-4. [DOI] [PubMed] [Google Scholar]
- 3.van der Werff JF, Nievelstein RA, Brands E, et al. Normal development of the male anterior urethra. Teratology Mar. 2000;61:172. doi: 10.1002/(SICI)1096-9926(200003)61:3<172::AID-TERA4>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 4.Baskin LS, Erol A, Jegatheesan P, et al. Urethral seam formation and hypospadias. Cell Tissue Res Sep. 2001;305:379. doi: 10.1007/s004410000345. [DOI] [PubMed] [Google Scholar]
- 5.Li Y, Sinclair A, Cao M, et al. Canalization of the urethral plate precedes fusion of the urethral folds during male penile urethral development: the double zipper hypothesis. J Urol. 2014;193:1353. doi: 10.1016/j.juro.2014.09.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ridley CM, Neill SM, Lewis FM. Ridley’s The Vulva. 3rd. Hoboken: Wiley-Blackwell; 2009. [Google Scholar]
- 7.Baskin LS, Erol A, Li YW, et al. Anatomical studies of the human clitoris. J Urol. 1999;162:1015. doi: 10.1016/S0022-5347(01)68052-2. [DOI] [PubMed] [Google Scholar]
- 8.Seifert AW, Bouldin CM, Choi KS, et al. Multiphasic and tissue-specific roles of sonic hedgehog in cloacal septation and external genitalia development. Development. 2009;136:3949. doi: 10.1242/dev.042291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sharpe J, Ahlgren U, Perry P, et al. Optical projection tomography as a tool for 3D microscopy and gene expression studies. Science. 2002;296:541. doi: 10.1126/science.1068206. [DOI] [PubMed] [Google Scholar]
- 10.Drey EA, Kang MS, McFarland W, et al. Improving the accuracy of fetal foot length to confirm gestational duration. Obstet Gynecol. 2005;105:773. doi: 10.1097/01.AOG.0000154159.75022.11. [DOI] [PubMed] [Google Scholar]
- 11.Suzuki K, Numata T, Suzuki H, et al. Sexually dimorphic expression of Mafb regulates masculinization of the embryonic urethral formation. Proc Natl Acad Sci U S A. 2014;111:16407. doi: 10.1073/pnas.1413273111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Conte FA, Grumbach MM. Disorders of sexual determination and differentiation. In: Gardner DG, editor. Greenspan’s Basic and Clinical Endocrinology. 8th. New York: McGraw-Hill; 2007. pp. 562–610. [Google Scholar]
- 13.Baskin LS, Sutherland RS, DiSandro MJ, et al. The effect of testosterone on androgen receptors and human penile growth. J Urol. 1997;158:1113. doi: 10.1097/00005392-199709000-00108. [DOI] [PubMed] [Google Scholar]
- 14.Cunha GR, Risbridger G, Wang H, et al. Development of the external genitalia: perspectives from the spotted hyena (Crocuta crocuta) Differentiation. 2014;87:4. doi: 10.1016/j.diff.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]