Abstract
We report the early growth and neurologic findings of 48 infants in Brazil diagnosed with probable congenital Zika virus syndrome and followed to age 1–8 months. Most of these infants had microcephaly (86.7%) and craniofacial disproportion (95.8%). The clinical pattern included poor head growth with increasingly negative z-scores, pyramidal/extrapyramidal symptoms, and epilepsy.
Keywords: Zika virus infection, growth, birthweight, epilepsy, microcephaly, congenital abnormalities, neurologic, outcomes, infants, viruses
The first reports of Zika virus infection in Brazil were in early 2015 (1). Shortly thereafter, Zika virus was associated with microcephaly (2). In February 2016, the World Health Organization (WHO) declared the potential association between Zika virus and microcephaly, a public health emergency of international concern (3).
Zika virus is able to cross the placental barrier. A growing body of evidence suggests that Zika virus causes cell death in neurons in vitro (4), brain anomalies, and microcephaly, resulting in what has been called congenital Zika virus syndrome (5). Cortical and subcortical atrophy, brain calcifications, ventriculomegaly, cerebellum anomalies, and abnormal neuronal migration have been described (6). The main reported signs and symptoms include abnormalities in neurologic examination, dysphagia, microcephaly (7–9), and a phenotype characterized as fetal brain disruption sequence (10).
Because this congenital infection is newly recognized, its full spectrum is not completely described, and little is known about the growth and neurologic outcomes of infants with congenital Zika virus syndrome in the first months of life. We reviewed the records of 48 infants born from September 2015 onwards that were enrolled at the Reference Center for Neurodevelopment, Assistance, and Rehabilitation of Children during January–May 2016 in Sao Luis, Brazil.
The Study
Because isolating Zika virus from human tissues is difficult, we used the following definition by Franca et al. (6), which was developed based on a protocol of the Brazil Ministry of Health (11) to identify highly probable cases of congenital Zika virus syndrome: 1) central nervous system abnormalities detected by cranial computed tomography (CT) scan, with or without microcephaly; and 2) negative results for syphilis, toxoplasmosis, rubella, cytomegalovirus, and herpes (STORCH) on serologic tests of the infant after delivery. Microcephaly was defined as head circumference (HC) 2 SD below the mean for gestational age and sex based on the INTERGROWTH-21st standards (12). Severe microcephaly was defined as HC 3 SD below the mean (12). The mothers were asked about the month of appearance of rashes during pregnancy. Birthweight and birth length z-scores were also classified according to the INTERGROWTH-21st criterion (12). The weight, length and HC after birth were classified according to the WHO standards (13). The initial status and rate of change of weight, length, and HC were estimated in a random-intercept multilevel linear regression model by using age in months as an explanatory variable. The Research Ethics Board of the Federal University of Maranhão approved the study (1510305).
Rash during pregnancy was reported by 73.9% (34/46) of mothers, mostly in the first trimester (52.2%). Most infants (52.1%) were male, and 87.2% were born at term. The HC z-score at birth was considered normal for 13.3% of the infants, whereas for 22.2% of the infants, the HC was >2 but <3 SD below the mean. However, most infants had an HC >3 SD below the mean (64.5%). The birth length z-score was compromised for 43.2%, and the birthweight was >2 SD below the mean for 19.6% of infants. The mean age at last visit to the reference center was 4.4 months. Nearly all infants had a phenotype characteristic of fetal brain disruption sequence (Figure 1), including craniofacial disproportion (95.8%), biparietal depression (83.3%), prominent occiput (75.0%), and excess nuchal skin (47.9%) (Table).
Figure 1.
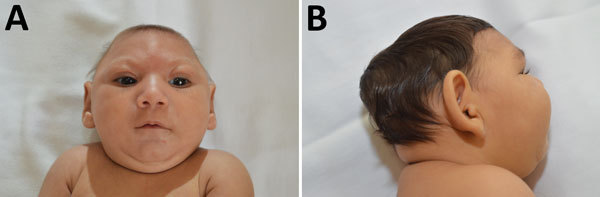
Characteristic phenotype of fetal brain disruption sequence in infants with probable congenital Zika virus syndrome, Sao Luís, Brazil, 2015–2016. A) Craniofacial disproportion and biparietal depression. B) Prominent occiput.
Table. Clinical characteristics of probable congenital Zika virus syndrome in infants from birth to 1–8 months of age, Sao Luis, Brazil, 2015–2016.
| Characteristic |
No. (%) |
|---|---|
| Rash in mother during pregnancy, n = 46 | |
| First trimester | 24 (52.2) |
| First month | 1 (2.2) |
| Second month | 12 (26.1) |
| Third month | 11 (23.9) |
| Second trimester | 10 (21.7) |
| Fourth month | 9 (19.6) |
| Sixth month | 1 (2.2) |
| No rash |
12 (26.1) |
| Sex, n = 48 | |
| M | 25 (52.1) |
| F |
23 (47.9) |
| Gestational age at birth, n = 47 | |
| Preterm | 4 (8.5) |
| Term | 41 (87.2) |
| Postterm |
2 (4.3) |
| Head circumference z-score at birth,* n = 45 | |
| >–2 | 6 (13.3) |
| Microcephaly, <–2 | 10 (22.2) |
| Severe microcephaly, <–3 |
29 (64.5) |
| Birth length z-score,* n = 3 | |
| >–2 | 21 (56.8) |
| <–2 | 11 (29.7) |
| <–3 |
5 (13.5) |
| Birthweight z-score,* n = 46 | |
| >–2 | 37 (80.4) |
| <–2 | 8 (17.4) |
| <–3 |
1 (2.2) |
| Age at last visit, mo, n = 48 | |
| 1 | 2 (4.2) |
| 2 | 6 (12.5) |
| 3 | 7 (14.6) |
| 4 | 10 (20.8) |
| 5 | 10 (20.8) |
| 6 | 7 (14.6) |
| 7 | 5 (10.4) |
| 8 |
1 (2.1) |
| Phenotype, n = 48 | |
| Craniofacial disproportion | 46 (95.8) |
| Biparietal depression | 40 (83.3) |
| Prominent occiput | 36 (75.0) |
| Excess nuchal skin |
23 (47.9) |
| Signs and symptoms, n = 48 | |
| Irritability | 41 (85.4) |
| Pyramidal/extrapyramidal syndrome | 27 (56.3) |
| Epileptic seizures | 24 (50.0) |
| Dysphagia | 7 (14.6) |
| Congenital clubfoot | 5 (10.4) |
| Arthrogryposis | 5 (10.4) |
| Cleft lip/cleft palate |
1 (2.1) |
| Electroencephalogram findings, n = 27 | |
| Abnormal activity, no epileptiform discharges | 13 (48.1) |
| Focal epileptiform discharges | 8 (29.6) |
| Multifocal epileptiform discharges |
6 (22.2) |
| Cranial computed tomography imaging findings, n = 48 | |
| Calcifications in the brain parenchyma | 44 (91.7) |
| Malformation of cortical development | 42 (87.5) |
| Ventriculomegaly | 37 (77.1) |
| White matter attenuation | 15 (31.3) |
| Brain stem and cerebellum hypoplasia | 6 (12.5) |
*Reported as deviations of the raw z-score from the mean measured in SD units.
Of the 48 infants, 85.4% had irritability, making irritability the most common symptom described, followed by pyramidal/extrapyramidal syndrome (56.3%), epileptic seizures (50.0%), and dysphagia (14.6%). Pyramidal syndrome included hypertonia, clonus, hyperreflexia, and increased archaic reflexes. Extrapyramidal symptoms were characterized by tonus fluctuation and asymmetric dyskinesias in the extremities that were absent during sleep. Some infants also had clubfoot (10.4%) and arthrogryposis (10.4%), and 1 infant (2.1%) had cleft lip/cleft palate. Among the 27 infants who underwent electroencephalography, 48.1% had abnormal brain activity without epileptiform discharges, 29.6% had focal discharges, and 22.2% had multifocal epileptiform discharges. All infants had abnormal cranial CT scan imaging findings. The most common were brain calcifications (91.7%), cortical malformations (87.5%), and secondary ventriculomegaly (77.1%). Brain stem and cerebellum hypoplasia and white matter attenuation were less common (Table).
For each infant, we noted weight, length, and HC z-scores at birth and each postnatal visit up to 8 months of age (Figure 2). The mean HC z-score at birth was −3.61, and it decreased −0.46 per month. The mean weight z-score was −1.12 at birth, and it decreased −0.08 per month. The mean length z-score was −1.57 at birth, and it decreased −0.16 per month.
Figure 2.
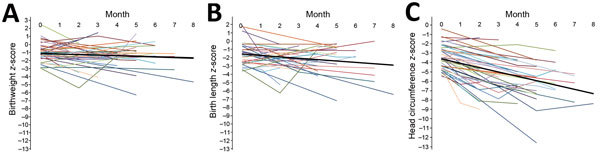
Weight (A), length (B), and head circumference (C) z-scores from birth to 1–8 months of age among infants with probable congenital Zika virus syndrome, Sao Luís, Brazil, 2015–2016. The thick black line depicts the mean z-score at birth and the mean rate of change in the z-score over time, estimated in a random-intercept multilevel linear regression model.
Conclusions
We describe the early growth and neurologic outcomes of infants with probable congenital Zika virus syndrome in the first 8 months of age. In total, 64.5% of infants were born with severe microcephaly, and 95.8% had a phenotype of fetal brain disruption sequence.
The most common clinical symptom noted was irritability, characterized by hyperexcitability (clonus following external stimulation), irritable and impatient cry, and sleep disorders. The infants were difficult to calm down even when fed. As the infants aged, neurologic symptoms began to emerge, usually from the second to the third month onwards with pyramidal/extrapyramidal syndrome, epileptic seizures, and dysphagia, although some infants had >1 of these symptoms much earlier. All infants who underwent electroencephalography had some abnormality, including brain activity maturation disorders and focal or multifocal epileptiform discharges. In 9 infants, brain activity maturation disorders evolved into focal or multifocal epileptiform discharge patterns over time. Focal or multifocal patterns were associated with epileptic seizures that did not respond to medication. Five infants initially had hypsarrhythmia, indicating highly disorganized brain activity, and had spasms and neuromotor delays. These 5 infants subsequently had a multifocal epileptiform pattern.
Early head growth was severely compromised, suggesting a very disruptive brain insult (10). In addition, as the infants aged, the HC z-scores dropped even further, suggesting that most of these infants would not be able to show catch-up growth. The HC z-score was substantially compromised (−5.45) at 4 months of age, whereas the weight z-score was in the normal range (−1.44), and the length z-score was affected (−2.21) but not as substantially.
Notably, 6 infants with probable congenital Zika virus syndrome who had abnormal imaging findings and a characteristic phenotype were not born with microcephaly. However, 3 infants had microcephaly postnatally. This finding suggests that microcephaly at birth is only 1 of the manifestations of this syndrome (5). Therefore, screening should be based not only on HC measurement at birth but also on the phenotype associated with fetal brain disruption sequence and cranial CT scan imaging findings.
Our findings are subject to a few limitations. For some infants, data were missing for some variables. A higher likelihood of selection bias exists because infants with more severe cases tended to be referred to the rehabilitation center. Zika virus infection was not confirmed in any mother, and only 1 infant was IgM positive. Because specific laboratory tests were still ongoing, the case definition might have included patients without Zika virus infection. However, we ruled out the 5 most common causes of congenital infection. Chikungunya incidence was low in the area in 2015 (1.3 cases/100.000) (14), and congenital infection caused by this pathogen occurs almost exclusively peripartum and is associated with maternal viremia (15). No mother in our case series reported fever or arthralgia near delivery.
Acknowledgments
We are thankful for the support of the Maranhão State Health Department.
The authors received partial support from Brazil’s National Research Council (CNPq) (scholarship to A.S.) and the Maranhão State Research Foundation (FAPEMA) (scholarships to R.Q., V.S., Z.L., and M.A.).
Biography
Dr. Silva is a senior health scientist at the Postgraduate Program in Public Health, Federal University of Maranhão, Maranhão, Brazil. His primary research interests are perinatal and life-course epidemiology.
Footnotes
Suggested citation for this article: Silva AAM, Ganz JSS, Sousa PS, Doriqui MJC, Ribeiro MRC, Branco MRFC, et al. Early growth and neurologic outcomes of infants with probable congenital Zika virus syndrome. Emerg Infect Dis. 2016 Nov [date cited]. http://dx.doi.org/10.3201/eid2211.160956
References
- 1.Zanluca C, Melo VC, Mosimann AL, Santos GI, Santos CN, Luz K. First report of autochthonous transmission of Zika virus in Brazil. Mem Inst Oswaldo Cruz. 2015;110:569–72. 10.1590/0074-02760150192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martines RB, Bhatnagar J, Keating MK, Silva-Flannery L, Muehlenbachs A, Gary J, et al. Notes from the field: evidence of Zika virus infection in brain and placental tissues from two congenitally infected newborns and two fetal losses—Brazil, 2015. MMWR Morb Mortal Wkly Rep. 2016;65:159–60. 10.15585/mmwr.mm6506e1 [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization. WHO statement on the first meeting of the International Health Regulations (2005) (IHR 2005) Emergency Committee on Zika virus and observed increase in neurological disorders and neonatal malformations [cited 2016 Jun 9]. http://www.who.int/mediacentre/news/statements/2016/1st-emergency-committee-zika/en
- 4.Garcez PP, Loiola EC, Madeiro da Costa R, Higa LM, Trindade P, Delvecchio R, et al. Zika virus impairs growth in human neurospheres and brain organoids. Science. 2016;352:816–8. 10.1126/science.aaf6116 [DOI] [PubMed] [Google Scholar]
- 5.França GV, Schuler-Faccini L, Oliveira WK, Henriques CM, Carmo EH, Pedi VD, et al. Congenital Zika virus syndrome in Brazil: a case series of the first 1501 livebirths with complete investigation. Lancet. 2016;pii: S0140-6736(16)30902-3. 10.1016/S0140-6736(16)30902-3 [DOI] [PubMed]
- 6.de Fatima Vasco Aragao M, van der Linden V, Brainer-Lima AM, Coeli RR, Rocha MA, Sobral da Silva P, et al. Clinical features and neuroimaging (CT and MRI) findings in presumed Zika virus related congenital infection and microcephaly: retrospective case series study. BMJ. 2016;353:i1901. 10.1136/bmj.i1901 [DOI] [PMC free article] [PubMed]
- 7.Schuler-Faccini L, Ribeiro EM, Feitosa IM, Horovitz DD, Cavalcanti DP, Pessoa A, et al. ; Brazilian Medical Genetics Society–Zika Embryopathy Task Force. Possible association between Zika virus infection and microcephaly—Brazil, 2015. MMWR Morb Mortal Wkly Rep. 2016;65:59–62. 10.15585/mmwr.mm6503e2 [DOI] [PubMed] [Google Scholar]
- 8.Microcephaly Epidemic Research Group1. Microcephaly in infants, Pernambuco State, Brazil, 2015. Emerg Infect Dis. 2016;22:1090–3. 10.3201/eid2206.160062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brasil P, Pereira JP Jr, Raja Gabaglia C, Damasceno L, Wakimoto M, Ribeiro Nogueira RM, et al. Zika virus infection in pregnant women in Rio de Janeiro—preliminary report. N Engl J Med. 2016;NEJM:oa1602412. 10.1056/NEJMoa1602412 [DOI] [PMC free article] [PubMed]
- 10.Corona-Rivera JR, Corona-Rivera E, Romero-Velarde E, Hernández-Rocha J, Bobadilla-Morales L, Corona-Rivera A. Report and review of the fetal brain disruption sequence. Eur J Pediatr. 2001;160:664–7. 10.1007/s004310100813 [DOI] [PubMed] [Google Scholar]
- 11.Brazilian Ministry of Health. Surveillance and response protocol to the occurrence of microcephaly and/or central nervous system (CNS) abnormalities [in Portuguese] [cited 2016 Jun 9]. http://combateaedes.saude.gov.br/images/sala-de-situacao/Microcefalia-Protocolo-de-vigilancia-e-resposta-10mar2016-18h.pdf
- 12.Villar J, Cheikh Ismail L, Victora CG, Ohuma EO, Bertino E, Altman DG, et al. ; International Fetal and Newborn Growth Consortium for the 21st Century (INTERGROWTH-21st). International standards for newborn weight, length, and head circumference by gestational age and sex: the Newborn Cross-Sectional Study of the INTERGROWTH-21st Project. Lancet. 2014;384:857–68. 10.1016/S0140-6736(14)60932-6 [DOI] [PubMed] [Google Scholar]
- 13.WHO Multicentre Growth Reference Study Group. WHO child growth standards: length/height-for-age, weight-for-age, weight-for-length, weight-for-height and body mass index-for-age: methods and development. Geneva: World Health Organization; 2006. http://www.who.int/childgrowth/standards/technical_report/en
- 14.Brazilian Ministry of Health. Epidemiologic bulletin monitoring of cases of dengue, chikungunya fever and fever by Zika virus through epidemiologic week 23, 2016. [in Portuguese] [cited 2016 Jul 20]. http://combateaedes.saude.gov.br/images/boletins-epidemiologicos/2016-Dengue_Zika_Chikungunya-SE23.pdf
- 15.Gérardin P, Barau G, Michault A, Bintner M, Randrianaivo H, Choker G, et al. Multidisciplinary prospective study of mother-to-child chikungunya virus infections on the island of La Réunion. PLoS Med. 2008;5:e60. 10.1371/journal.pmed.0050060 [DOI] [PMC free article] [PubMed] [Google Scholar]


