Abstract
Given the cost of sex, outcrossing populations should be susceptible to invasion and replacement by self-fertilization or parthenogenesis. However, biparental sex is common in nature, suggesting that cross-fertilization has substantial short-term benefits. The Red Queen hypothesis (RQH) suggests that coevolution with parasites can generate persistent selection favoring both recombination and outcrossing in host populations. We tested the prediction that coevolving parasites can constrain the spread of self-fertilization relative to outcrossing. We introduced wild-type Caenorhabditis elegans hermaphrodites, capable of both self-fertilization and outcrossing, into C. elegans populations that were fixed for a mutant allele conferring obligate outcrossing. Replicate C. elegans populations were exposed to the parasite Serratia marcescens for 33 generations under three treatments: a control (avirulent) parasite treatment, a fixed (non-evolving) parasite treatment, and a copassaged (potentially coevolving) parasite treatment. Self-fertilization rapidly invaded C. elegans host populations in the control and the fixed-parasite treatments, but remained rare throughout the entire experiment in the copassaged treatment. Further, the frequency of the wild-type allele (which permits selfing) was strongly positively correlated with the frequency of self-fertilization across host populations at the end of the experiment. Hence, consistent with the RQH, coevolving parasites can limit the spread of self-fertilization in outcrossing populations.
Keywords: Coevolution, Red Queen hypothesis, host-parasite coevolution, evolution, breeding system, sex
Introduction
Substantial costs are associated with biparental sexual reproduction relative to uniparental forms of reproduction such as self-fertilization and parthenogenesis (Maynard Smith 1978). For example, the inability of males to directly produce their own offspring reduces the per-capita growth rate of biparental sexual populations by as much as one half (Maynard Smith 1978). Given this cost, populations that reproduce via biparental outcrossing should be susceptible to invasion and replacement by mutants capable of uniparental reproduction (Fisher 1941; Maynard Smith 1978; Charlesworth 1980; Agrawal and Lively 2001). However, asexual reproduction and obligate self-fertilization are rare in nature, relative to outcrossing. For example, obligate asexuality occurs in less than 1% of animal species (Bell 1982; Judson and Normark 1996), and very few plant or animal species exhibit obligate self-fertilization (Goodwillie et al. 2005; Jarne and Auld 2006). Although these patterns suggest that the costs of cross-fertilization are outweighed by compensatory short-term advantages, the nature of these advantages has been debated for at least several decades.
The Red Queen hypothesis (RQH) proposes that coevolving parasites can impose selection against common host genotypes, resulting in an advantage to producing outcrossed, genetically variable progeny (Jaenike 1978; Hamilton 1980). This idea has received support from studies of natural host populations of both plants and animals (Lively 1987; King et al. 2011; Verhoeven and Biere 2013; Vergara et al. 2014). In addition, recent laboratory experiments have shown that coevolving parasites can favor increased rates of outcrossing in mixed-mating (outcrossing and self-fertilizing) host populations. For example, Morran et al. (2011) found that androdioecious mixed-mating populations of the nematode host Caenorhabditis elegans evolved and maintained higher outcrossing rates when experimentally exposed to the coevolving bacterial parasite, Serratia marcescens. Morran et al. (2011) also found found that obligately selfing populations of C. elegans went extinct within 20 generations of coevolution with S. marcescens, while mixed-mating and obligately outcrossing populations coevolving with S. marcescens parasites persisted for the entire experiment (30 generations).
While there is now evidence that coevolutionary interactions with parasites can provide a selective benefit to outcrossing, it is unknown whether parasite-mediated selection is, by itself, sufficient to explain the long-term maintenance of obligate outcrossing, which depends on the ability of host populations to resist invasion by mutants that reproduce via self-fertilization. A critical question thus remains open: can coevolving parasites constrain the spread of self-fertilization into obligately outcrossing host populations? Here we provide a direct test of this question by introducing an allele that confers the ability for self-fertilization into replicate outcrossing host populations. We then measure the effects of coevolutionary and non-coevolutionary interactions with a parasite on the spread of self-fertilization in the experimental host populations.
The nematode host Caenorhabditis elegans is an ideal model organism for studying the effect of host-parasite interactions on breeding-system evolution, because C. elegans utilizes several modes of reproduction, and it is a genetically and experimentally tractable system. The nematode has two sexes: hermaphrodites (XX) and males (XØ) (Brenner 1974). Hermaphrodites reproduce either by self-fertilization (selfing) or by cross-fertilization with males (outcrossing); hermaphrodites cannot mate with each other. Outcrossing produces a 1:1 ratio of males to hermaphrodites, while almost 100% of selfed offspring are hermaphrodites (Brenner 1974). Because of this relationship between reproductive mode and male frequency in the offspring, the frequency of outcrossing and the frequency of selfing in C. elegans populations can be accurately estimated based on male frequencies (Stewart and Phillips 2002). Additionally, C. elegans has been shown to coevolve with the virulent bacterial parasite Serratia marcescens (Morran et al. 2011; Morran et al. 2013; Morran et al. 2014; Gibson et al. 2015).
The mutant allele fog-2(q71) can transform the C. elegans breeding system from mixed mating to obligately outcrossing. The fog-2(q71) allele (hereafter called the “obligate-outcrossing allele”) is an autosomal recessive allele with a loss-of-function mutation that prevents sperm production in hermaphrodites, thereby functionally transforming hermaphrodites into females (Schedl and Kimble 1988). The obligate-outcrossing allele has no known effects on males (Schedl and Kimble 1988). In contrast, hermaphrodites harboring the fog-2(wt) allele (which we will refer to as the “mixed-mating allele”) are capable of both self-fertilization and of outcrossing with any males, regardless of the males’ genotypes at the fog-2 locus. Hence the mixed-mating breeding system and the obligately outcrossing breeding system can freely intermix whenever they occur together in the same population. Previous work in this study system has shown that the mixed-mating allele rapidly outcompetes the obligate-outcrossing allele in C. elegans populations under standard laboratory conditions (i.e. in the absence of a parasite) (Stewart and Phillips 2002; Katju et al. 2008). This result is consistent with the prediction that the 2-fold cost of sex should make outcrossing populations susceptible to invasion and replacement by self-fertilizing mutants (Fisher 1941; Charlesworth 1980; Agrawal and Lively 2001).
In the present study, we tested the prediction that coevolutionary interactions with parasites can constrain the spread of self-fertilization, and prevent self-fertilization from rising to fixation in outcrossing host populations. We introduced homozygous mixed-mating (i.e. fog-2(wt/wt)) C. elegans nematodes into obligately outcrossing C. elegans populations that were fixed for the obligate-outcrossing allele. Following introduction of the mixed-mating individuals, we tracked the spread of self-fertilization in the C. elegans host populations under three parasite treatment regimes. In the control treatment (1) hosts were exposed to avirulent, heat-killed S. marcescens bacteria. In the fixed-parasite treatment (2) hosts were exposed to the same isogenic stock population of the highly virulent S. marcescens parasite every generation. In the copassaged treatment (3) C. elegans hosts were exposed every generation to potentially coevolving S. marcescens. We predicted that in the control treatment self-fertilization would rapidly spread into host populations, due to the 2-fold cost of outcrossing. In the fixed-parasite treatment we predicted that self-fertilization would initially remain rare while the host populations adapted to the novel parasite environment, but that self-fertilization would eventually spread once the hosts had adapted to the fixed-parasite population. In the copassaged treatment, we predicted that negative frequency-dependent selection induced by the coevolving parasite would constrain the spread of self-fertilization into host populations. Finally, we predicted that, following experimental evolution, the frequency of the mixed-mating allele (which restores hermaphroditism and permits self-fertilization) should be tightly positively correlated with the frequency of self-fertilization across experimental populations.
Materials and methods
Establishment of the starting populations
Caenorhabditis elegans strains are derived from a single individual nematode isolated from a natural population and maintained as stock populations. Our laboratory group previously obtained the CB4856 strain (originally from Hawaii, USA) from the Caenorhabditis Genetics Center (University of Minnesota, Minneapolis, MN) (Morran et al. 2011). Prior to our experiment the obligate-outcrossing allele, fog-2(q71), was backcrossed into a systematically inbred strain of CB4856 (PX382) to generate the obligate-outcrossing strain PX386 (fixed for the obligate-outcrossing allele) (Morran et al. 2009b). The obligate-outcrossing allele contains a nonsense mutation G → A, which results in a non-functional gene relative to the wild-type, preventing the production of viable sperm in hermaphrodites (Katju et al. 2008)
Prior to our experiment, during three consecutive generations, four near-isogenic replicate populations of (obligately outcrossing) PX386 were independently mutagenized at 40mM of EMS for 4 hours (Morran et al. 2011). While being maintained separately, the populations received random and independent mutation loads (Morran et al. 2011). This procedure induced approximately 1,000 point mutations per lineage in each population (Epstein and Shakes 1995; Morran et al. 2011). Following EMS mutagenesis, as part of another experiment, (Morran et al. 2011), each population was then divided into two different treatments, a control treatment and a fixed-parasite treatment and underwent 30 generations of experimental evolution prior to our use of the experimental lines in the present experiment. The resulting experimental populations, following 30 generations of selection in the prior experiment, as well as the mutagenized ancestral population (pre-experimental evolution) were used as the starting populations in the current study. See our supplementary material for the details of the experimental evolution our nematode populations underwent prior to the start of our experiment. Experimental treatment in the prior study did not significantly affect the evolution of selfing rates in our experiment.
Prior to our experiment, we established mixed-mating strains by introgressing the mixed-mating allele into each of our 12 replicate obligately outcrossing genetic backgrounds (described above) through a series of 7 backcrosses. In this way, we established 12 mixed-mating strains, each associated with an obligately outcrossing population with the same genetic background. This introgression approach made it unlikely that any differences in the fitness of the mixed-mating allele and the obligate-outcrossing allele in our experimental populations would be confounded by differences in the genetic backgrounds of the mixed-maters and the obligate outcrossers. See supplementary material for details of our establishment of the mixed-mating strains.
Introduction of the mixed-mating invaders into obligately outcrossing populations
At the initiation of our experiment, approximately 38 wild-type (mixed-mating) nematodes from each of 12 replicate mixed-mating lines were liquid transferred onto the first generation Serratia selection plates (SSPs) with approximately 713 nematodes from the obligate-outcrossing populations from which the mixed-maters were derived to produce a mixed (obligate outcrossers and mixed-maters) population with a starting frequency of about 5% mixed-maters. See figure 1 for a depiction of the sexes, fog-2 genotypes, and modes of reproduction available to hosts at the start of our experiment. Following introduction of the mixed-maters into our replicate obligately outcrossing populations, each replicate was passaged separately on SSPs for 33 host generations.
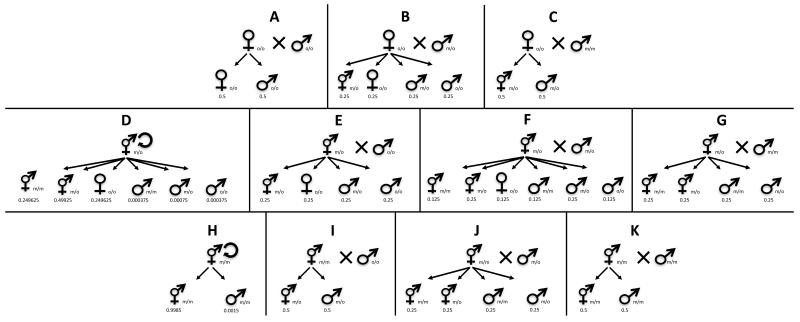
Figure 1.
A conceptual diagram depicting all of the possible reproductive combinations that can occur between different genotypes of males, females, and hermaphrodites in our experiment, and the expected frequencies of sexes and genotypes in the offspring that would result from each of these possible crosses. The expected frequencies of sexes and genotypes in the offspring of selfing hermaphrodites include males which are produced by nondisjunction of the X chromosome during meiosis (Brenner 1974). Our figure depicts males produced at a nondisjunction rate of 0.0015 (panels D and H). 95% of the individuals in our generation zero host populations were obligately outcrossing males and females which were homozygous for the obligate-outcrossing allele, designated as “o” in the figure. 5% of the individuals in our generation zero host populations were homozygous for the mixed-mating allele (designated as ‘m’ in the figure). Almost all of the mixed-mating homozygotes at the start of our experiment were hermaphrodites, although there may also have been rare males homozygous for the mixed-mating allele. Because mixed-mating hermaphrodites can mate with males carrying the obligate-outcrossing allele (panels E, F, I, and J), and because obligately outcrossing females can mate with males carrying the mixed-mating allele (panels B and C), the mixed-mating and obligately outcrossing breeding systems could freely mix in our experimental populations. The mixed-mating allele is dominant, so hermaphrodites heterozygous at the fog-2 locus (i.e. hermaphrodites with the ‘m/o’ genotype) were capable of both self-fertilization (panel D) and outcrossing (panels E, F, and G).
Experimental evolution on Serratia Selection Plates (SSPs)
The details of our experimental evolution protocol on SSPs have been previously described in (Morran et al. 2011). Briefly, we constructed SSPs by pouring 24 mL of autoclaved NGM Lite (US Biological, Swampscott, MA) into a 10 cm Petri dish. One side of the plate was seeded with 50 μl of E. coli (the food source) while the other side of the plate was seeded with 50 μl of live S. marcescens (for the fixed-parasite and copassaged populations) or with 50 μl of heat-killed S. marcescens (for the control populations). The bacteria solutions used to seed our SSPs were grown in 5 mL test tubes of Lysogeny broth (LB) for 24 hours at 28 degrees C. The E. coli and S. marcescens inocula were both spread across their respective sides of the plate using a sterile spreader. The plate was then incubated for 24 hours at 28 degrees to allow the bacteria to grow. Following incubation, 20 μl of ampicillin (100 mg/mL) was streaked across the plate between the lawns of E. coli and S. marcescens. Approximately 750 nematodes were liquid transferred in M9 buffer onto the S. marcescens side of the plate. Relatively constant nematode population sizes were maintained across all three treatments by counting the number of individual nematodes in random 10 μl samples of our nematodes/buffer liquid transfer solutions prior to transfers to estimate the density of nematodes in the transfer solutions, and by adjusting the volume of solution transferred to the fresh SSP every generation to ensure that approximately 750 individual nematodes were transferred. When necessary, to avoid transferring large volumes of nematode/buffer solution to fresh SSPs, we would concentrate the nematodes in our transfer buffer solution by leaving the solution in an Eppendorf tube for a couple of minutes to allow the nematodes to settle to the bottom of the tube, and then discarding the buffer solution off the top of the tube, leaving a concentrated solution of nematodes at the bottom of the tube for transfer. We maintained populations on SSPs at 20°C.
Our experimental setup required C. elegans to crawl through the S. marcescens parasite (live or heat-killed) to reach their food source of Escherichia coli (Morran et al. 2009b). Four days after the nematodes were introduced onto a SSP, a random sample of approximately seven-hundred and fifty individual nematodes were selected from the E. coli side of the plate and transferred to repeat the process. Most of the transferred nematodes were L4 larva, however, our protocol did not explicitly synchronize our populations during experimental evolution. In the copassaged treatment, bacterial cells were harvested every generation from the bodies of 20 dead nematodes (per line) for passage to the next SSP. Bacterial cells were harvested from the carcasses of nematodes that died on the S. marcescens lawn by picking the carcasses into 1mL of M9 buffer, and centrifuging the solution at 3,000 RPM for 3 minutes, discarding the supernatant, and rinsing 5 times with 1mL of M9 (Morran et al. 2011). Harvested bacterial cells were streaked on NGM lite agar which was incubated for 24 hours at 28°C. A random sample of 10 bacterial colonies which grew on the agar plate were used to inoculate 5mL of Lysogeny broth (LB). Bacterial communities were grown in the LB solution for 24 hours at 28 degrees. Following bacterial growth 50 μl of bacterial culture was then used to seed the next SSP onto which the copassaged nematode population would be plated. A thick lawn of S. marcescens grew up on the SSPs prior to nematode exposure. The whole bacterial community (S. marcescens parasites as well as contaminating E. coli) harvested from the bodies of dead nematodes was used to grow the next generation of parasites. However, when the recycled parasite lineages became visibly contaminated with E. coli, each line was purified by streaking the bacterial communities on an agar plate, and by selecting a random sample of 20 S. marcescens colonies (as determined by their red color) for passage to the next generation. In this way our copassaged treatment protocol selected for resistance in the host and infectivity and virulence in the coevolving parasite (Morran et al. 2011). Fixed-parasite treatment SSPs were always seeded with the same ancestral stock population of S. marcescens (SM2170), while control SSPs were always seeded with heat-killed S. marcescens. The same stock population of S. marcescens (SM2170) that served as the non-evolving parasite in our fixed treatment, was the starting parasite population in all of our copassaged lines.
Male frequency was measured at generations 10, 15, 21, and 33 following the protocol described in Stewart and Phillips (2002). Specifically, we measured male frequencies by following a transect on the plates and scoring the sex of each nematode in a random sample of 200 adult and L4 larva individuals (Stewart and Phillips 2002). Outcrossing rates were calculated from male frequency data by multiplying the male frequency by 2 and adjusting for males produced by nondisjunction of the X chromosome during meiosis (Stewart and Phillips 2002). Selfing rates were calculated by subtracting the outcrossing rate from 1 (Stewart and Phillips 2002). In order to estimate the selfing rates of our combined (mixed-maters + obligate outcrossers) populations at generation zero we calculated the selfing rates of our mixed-mating lines (prior to combining the mixed-mating lines with the obligately-outcrossing lines) based on male frequencies, and then we multiplied the selfing rate of each mixed-mating line by 0.05 (the starting frequency of mixed-maters in our combined populations) to determine the selfing rate in the overall (mixed-mating and obligately-outcrossing) populations. Samples from all replicate populations were cryogenically frozen at generations 0, 10, 15, 21, and 33.
Measuring the mixed-mating allele frequencies
In order to determine whether the frequency of the mixed-mating allele was correlated with the frequency of selfing across our experimental populations, we thawed and reanimated a subsample of 12 host populations that had been frozen at host generation 33 (the experimental endpoint). We calculated the post-thaw selfing rate based on male frequencies (using the same selfing-rate estimation methods described above). We used molecular techniques to measure the frequency of the mixed-mating allele in our reanimated host populations. See supplementary material for our detailed protocol for measuring fog-2 genotype frequencies.
Statistical methods
We used an ANOVA to test whether the generation 10 selfing rates differed by treatment. Because our starting populations were distributed evenly among our three treatments, the selfing rate was the same across all three treatments at generation zero. Therefore, any significant differences observed in the selfing rates across treatments at generation 10 would reflect differences in the evolution of selfing rates during the first 10 generations of evolution. The assumption of homogeneity of variances was violated in the generation 10 selfing rate data, as assessed by Levene's test for equality of variances (P = 0.015), so we report the results of a Welch’s ANOVA on the generation 10 selfing rates. We used Games-Howell post hoc tests on all pairwise treatment comparisons to determine which treatments differed significantly in their mean selfing rates.
Similarly, we used an ANOVA to test whether treatment significantly affected the selfing rates at generation 33 (our experimental endpoint). We used Tukey post hoc tests across all treatment pairwise comparisons to determine which treatments means differed significantly. Finally, we used a one sample t-test to assess whether the mean selfing rate in the copassaged treatment populations at generation 33 (our experimental endpoint) was significantly greater than zero.
We used a linear regression analysis to test whether the frequency of individuals with mixed-mating genotypes was associated with the self-fertilization frequency (as measured post-thaw) across our reanimated nematode populations at generation 33 (our experimental endpoint). All p-values reported are 2-tailed unless otherwise specified. All statistical tests were run using SPSS version 23.
Results
The selfing rates were not equal across all treatments at generation 10 of the experiment (Welch’s F2, 18.331 = 24.497, P < 0.001), indicating that treatment significantly affected the evolution of selfing rates during the first 10 generations of experimental evolution. Specifically, post hoc Games-Howell tests indicated that the mean selfing rate in the control treatment was significantly greater than the fixed-parasite treatment (P = 0.005), and the copassaged treatment (P < 0.001). This indicates that selfing spread more quickly in the controls than in either parasite treatment during the first 10 generations of evolution. There was no significant difference between the copassaged and fixed-parasite treatment selfing rates at generation 10 (P = 0.645) (figure 2).
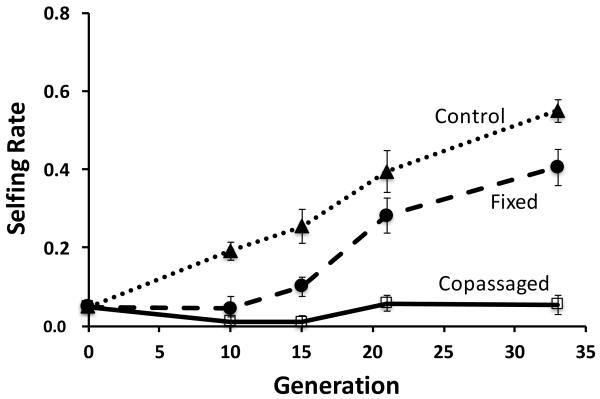
Figure 2.
Mean selfing rates (± one standard error) over the course of the experiment. Host populations were exposed to three different treatments: control (heat-killed S. marcescens; dotted line and triangular markers), fixed-parasite treatment (fixed strain of S. marcescens; dashed line and circle markers), copassaged (copassaged S. marcescens; solid line and square markers) for 33 generations.
The selfing rates were not equal across all treatments at generation 33, the experimental endpoint (F2, 33 = 54.864, P < 0.001). The selfing rate was significantly lower in the copassaged treatment than in the fixed-parasite treatment (Tukey HSD P < 0.001), and the selfing rate was significantly lower in the fixed-parasite treatment than in the control treatment (Tukey HSD P = 0.015) (figure 2).
The copassaged treatment selfing rate at our experimental endpoint was significantly greater than zero, suggesting that some selfing persisted throughout the entire experiment in at least some of the copassaged treatment host populations (mean selfing rate = 0.054, stdv = 0.086, t11 = 2.185, P (1-tailed) = 0.026) (figure 2).
Overall, the frequency of self-fertilization increased rapidly throughout the entire experiment in our control treatment populations. In our fixed-parasite treatment populations, selfing remained low initially, and then increased rapidly. In our copassaged populations, the selfing rate remained low throughout the entire experiment, although some selfing persisted in at least some of our copassaged populations (figure 2).
Evolution of the mixed-mating allele frequency
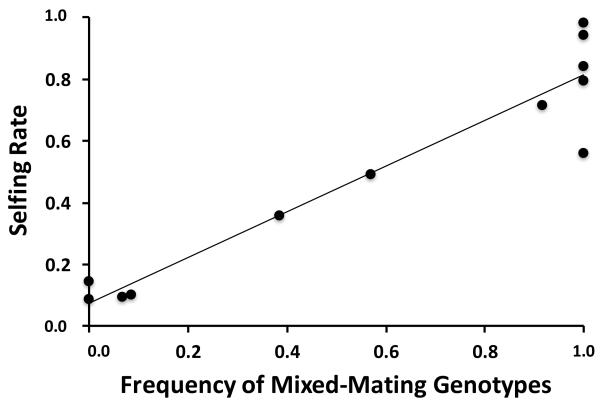
At generation 33 (our experimental endpoint), we found a highly significant positive relationship between the frequency of individuals with mixed-mating genotypes and the frequency of selfing across experimental populations (r2 = 0.911, F1,11 = 101.949, P < 0.001) (figure 3).
Figure 3.
A bivariate plot showing the frequency of individuals with mixed-mating genotypes (i.e. the frequency of individuals with genotypes that permit both outcrossing and selfing) plotted against selfing rates for 12 host populations at generation 33 (our experimental endpoint).
Discussion
We tested a key prediction of the Red Queen hypothesis: coevolutionary interactions with parasites can constrain the spread of self-fertilization within outcrossing host populations. In our experimental laboratory system, we found that, in the absence of a coevolving parasite, self-fertilization rapidly invaded outcrossing populations of the nematode host C. elegans. Repeated exposure to a fixed (non-evolving) strain of the virulent bacterial parasite S. marcescens delayed, but ultimately did not prevent the spread of self-fertilization into host populations. However, the spread of self-fertilization into host populations was constrained throughout our entire 33-generation experiment in our copassaged parasite treatment (Fig. 1), suggesting that antagonistic coevolutionary interactions with a virulent parasite can render obligate outcrossing resistant to invasion and replacement by self-fertilization. We also found that the spread of self-fertilization into outcrossing host populations was tightly linked with the spread of an allele that restored hermaphroditism and permitted self-fertilization (i.e. the mixed-mating allele fog-2(wt)).
Our observation that self-fertilization spread rapidly in control (no parasite) conditions is consistent with theoretical models which predict that biparental sex is costly (Fisher 1941; Maynard Smith 1978; Charlesworth 1980; Agrawal and Lively 2001). Furthermore, our results are consistent with previous empirical work in this study system which has demonstrated that self-fertilization rapidly invades outcrossing C. elegans populations under standard laboratory conditions (Stewart and Phillips 2002; Cutter 2005; Katju et al. 2008).
Our results also indicate that exposure to a novel environment may temporarily impede the invasion of self-fertilization. In our fixed-parasite treatment lines, the selfing rate remained low for the first 10 generations of our experiment, and then increased rapidly. We believe that the most likely explanation for this result is that outcrossing may have been favored in the fixed-parasite treatment populations at the beginning of the experiment, because it facilitated host adaptation to their novel parasite environment. However, after the fixed-parasite treatment host populations adapted to their static parasite environment, selection may have shifted. Selection could have favored selfing following adaptation because, as the host populations adapted to the fixed parasite, resistant host genotypes may have become common in the host populations. Once resistant host genotypes became common, outcrossing may have been selected against because it breaks up resistant host gene combinations. Additionally, selection may have favored selfing following adaptation to the novel environment because of the numerical cost of outcrossing (Maynard Smith 1978).
Our fixed-parasite treatment results are consistent with previous work in androdioecious (wild-type) C. elegans populations which showed that exposure to non-evolving Serratia marcescens parasites initially results in increases in C. elegans host outcrossing rates, but that outcrossing is only temporarily favored over selfing in a static parasite environment (Morran et al. 2009b; Morran et al. 2011). Additionally, our results are consistent with experimental work in the facultatively sexual rotifer Brachionus calyciflorus, which showed that, when rotifers were initially exposed to a novel environment selection favored sexual reproduction, however, following adaptation to the novel environment selection favored asexual reproduction (Becks and Agrawal 2012).
While adaptation to a novel environment temporarily impeded the spread of self-fertilization in the fixed-parasite treatment, the spread of self-fertilization was constrained throughout the entire experiment in our copassaged treatment in which both the host and the parasite could evolve, and potentially coevolve. This result suggests that a coevolving parasite may provide the persistent selection necessary to maintain outcrossing in host populations in the long term. However, while the spread of selfing was constrained in the copassaged populations, a low frequency of self-fertilization did persist over the course of the entire experiment in at least some of our copassaged host populations. This persistence of some selfing, even in an environment with a coevolving parasite, suggests that parasite-mediated selection may favor some degree of self-fertilization in hosts. This result is consistent with theoretical models predicting that, when parasite virulence is high, parasites can select for mixtures of selfed and outcrossed host progeny (Agrawal and Lively 2001).
The rate at which self-fertilization spread in our experimental populations was probably highly dependent on the frequency with which mixed-mating hermaphrodites within our experimental populations self-fertilized their own offspring rather than reproduced by outcrossing with males. The selfing rate of hermaphrodites within C. elegans populations composed of both obligate outcrossers and mixed-maters is unknown. However, (Morran et al. 2009a) manipulated male frequencies in the C. elegans strain CB4856 (the strain from which the experimental populations in the present study were derived), and found that the selfing rates of hermaphrodites increases as the male frequency in the population decreases. Furthermore, parasite treatment may have affected the selfing rate of hermaphrodites within our experimental populations. Perhaps differences in the selfing rates of hermaphrodites could help explain treatment differences in the rate at which self-fertilization spread into experimental host populations.
Overall, our results provide strong evidence that coevolutionary interactions with parasites can constrain the spread of self-fertilization and prevent selfing from rising to fixation in outcrossing host populations in a controlled laboratory setting. Furthermore, the spread of the mixed-mating allele, which restores hermaphroditism and permits selfing, was tightly linked with the spread of selfing in our experimental populations. Taken together with other laboratory and field studies, our results strongly support the Red Queen hypothesis.
Supplementary Material
Acknowledgements
We thank the Center for the Integrative Study of Animal Behavior lab at Indiana University for use of equipment, Laura Weingartner for helping establish the molecular protocols, and Julie Xu for helping with PCRs. We thank Michael Frisby and Stephanie Dickinson for help with our statistical analyses. Funding provided by the NIH-NICHD (1F32GM096482 to LTM and 5T32HD049336 to SPS), the Indiana University Center for the Integrative Study of Animal Behavior (Fellowship to SPS), the NSF (DEB-0640639 to CML and DEB-1120417 to PCP), the IU Hutton Honors College (Research Grant to EC), and Emory University (LTM).
References
- Agrawal AF, Lively CM. Parasites and the evolution of self-fertilization. Evolution. 2001;55:869–879. doi: 10.1554/0014-3820(2001)055[0869:pateos]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Becks L, Agrawal AF. The evolution of sex is favoured during adaptation to new environments. Plos Biology. 2012;10:e1001317. doi: 10.1371/journal.pbio.1001317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell G. The masterpiece of nature: the evolution and genetics of sexuality. University of California Press; Berkeley: 1982. [Google Scholar]
- Brenner S. Genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlesworth B. The cost of sex in relation to mating system. Journal of Theoretical Biology. 1980;84:655–671. doi: 10.1016/s0022-5193(80)80026-9. [DOI] [PubMed] [Google Scholar]
- Cutter AD. Mutation and the experimental evolution of outcrossing in Caenorhabditis elegans. Journal of Evolutionary Biology. 2005;18:27–34. doi: 10.1111/j.1420-9101.2004.00804.x. [DOI] [PubMed] [Google Scholar]
- Epstein HF, Shakes DC. Caenorhabditis elegans: modern biological analysis of an organism. Academic Press; San Diego: 1995. pp. 31–54. [Google Scholar]
- Fisher RA. Average excess and average effect of a gene substitution. Annals of Eugenics. 1941;11:53–63. [Google Scholar]
- Gibson AK, Stoy KS, Gelarden IA, Penley MJ, Lively CM, Morran LT. The evolution of reduced antagonism–A role for host-parasite coevolution. Evolution. 2015;69:2820–2830. doi: 10.1111/evo.12785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwillie C, Kalisz S, Eckert CG. The evolutionary enigma of mixed mating systems in plants: Occurrence, theoretical explanations, and empirical evidence. Annual Review of Ecology Evolution and Systematics; 2005. pp. 47–79. [Google Scholar]
- Hamilton WD. Sex versus non-sex versus parasite. Oikos. 1980;35:282–290. [Google Scholar]
- Jaenike J. An hypothesis to account for the maintenance of sex within populations. Evolutionary Theory. 1978;3:191–194. [Google Scholar]
- Jarne P, Auld JR. Animals mix it up too: The distribution of self-fertilization among hermaphroditic animals. Evolution. 2006;60:1816–1824. doi: 10.1554/06-246.1. [DOI] [PubMed] [Google Scholar]
- Judson OP, Normark BB. Ancient asexual scandals. Trends in Ecology and Evolution. 1996;11:41–46. doi: 10.1016/0169-5347(96)81040-8. [DOI] [PubMed] [Google Scholar]
- Katju V, LaBeau EA, Lipinski KJ, Bergthorsson U. Sex change by gene conversion in a Caenorhabditis elegans fog-2 mutant. Genetics. 2008;180:669–672. doi: 10.1534/genetics.108.090035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King KC, Jokela J, Lively CM. Parasites, sex, and clonal diversity in natural snail populations. Evolution. 2011;65:1474–1481. doi: 10.1111/j.1558-5646.2010.01215.x. [DOI] [PubMed] [Google Scholar]
- Lively CM. Evidence from a New Zealand snail for the maintenance of sex by parasitism. Nature. 1987;328:519–521. [Google Scholar]
- Maynard Smith J. The evolution of sex. New York, Cambridge [Eng.]: 1978. [Google Scholar]
- Morran LT, Cappy BJ, Anderson JL, Phillips PC. Sexual partners for the stressed: facultative outcrossing in the self-fertilizing nematode Caenorhabditis elegans. Evolution. 2009a;63:1473–1482. doi: 10.1111/j.1558-5646.2009.00652.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morran LT, Parmenter MD, Phillips PC. Mutation load and rapid adaptation favour outcrossing over self-fertilization. Nature. 2009b;462:350–352. doi: 10.1038/nature08496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morran LT, Parrish RC, 2nd, Gelarden IA, Allen MB, Lively CM. Experimental coevolution: rapid local adaptation by parasites depends on host mating system. The American naturalist. 2014;184(Suppl 1):S91–100. doi: 10.1086/676930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morran LT, Parrish RC, II, Gelarden IA, Lively CM. Temporal dynamics of outcrossing and host mortality rates in host-pathogen experimental coevolution. Evolution. 2013;67:1860–1868. doi: 10.1111/evo.12007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morran LT, Schmidt OG, Gelarden IA, Parrish RC, II, Lively CM. Running with the Red Queen: host-parasite coevolution selects for biparental sex. Science. 2011;333:216–218. doi: 10.1126/science.1206360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schedl T, Kimble J. Fog-2, a germ-line-specific sex determination gene required for hermaphrodite spermatogenesis in Caenorhabditis elegans. Genetics. 1988;119:43–61. doi: 10.1093/genetics/119.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart AD, Phillips PC. Selection and maintenance of androdioecy in Caenorhabditis elegans. Genetics. 2002;160:975–982. doi: 10.1093/genetics/160.3.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theologidis I, Chelo IM, Goy C, Teotonio H. Reproductive assurance drives transitions to self-fertilization in experimental Caenorhabditis elegans. Bmc Biology. 2014;12:93–114. doi: 10.1186/s12915-014-0093-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergara D, Jokela J, Lively CM. Infection dynamics in coexisting sexual and asexual host populations: support for the Red Queen hypothesis. The American naturalist. 2014;184(Suppl 1):S22–30. doi: 10.1086/676886. [DOI] [PubMed] [Google Scholar]
- Verhoeven KJF, Biere A. Geographic parthenogenesis and plant-enemy interactions in the common dandelion. BMC Evolutionary Biology. 2013;13:23–32. doi: 10.1186/1471-2148-13-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.