Abstract
Primordial follicles dictate a female’s reproductive lifespan and therefore are central to fertility preservation for both endangered species as well as for individuals with fertility threatening conditions. Ovarian tissue containing primordial follicles can be cryopreserved and later thawed and transplanted back into individuals to restore both endocrine function and fertility. Importantly, increasing numbers of human live births have been reported following ovarian tissue cryopreservation and transplantation. A current limitation of this technology is patient access to sites that are approved or equipped to process and cryopreserve ovarian tissue – especially in larger countries or low resource settings. Here we review empirical evidence from both animal models and human studies that suggest that ovarian tissue can be transported at cold temperatures for several hours while still maintaining the integrity and reproductive potential of the primordial follicles within the tissue. In fact, several human live births have been reported in European countries using tissue that was transported at cold temperatures for up to 20 hours prior to cryopreservation and transplantation. Ovarian tissue transport, if implemented widely in clinical practice, could therefore expand both patient and provider access to emerging fertility preservation options.
Introduction
Chemotherapy and radiation therapy, although used successfully to treat a myriad of malignant and non-malignant conditions, can have the unintended long-term consequence of damaging reproductive function (De Vos, et al. 2014, Hirshfeld-Cytron, et al. 2011, Jeruss and Woodruff 2009, Lobo 2005, Meirow, et al. 2005, Wallace, et al. 2014, Woodruff 2010). In females, these treatments can negatively impact several aspects of the reproductive system, including the hypothalamic-pituitary-gonadal axis, the ovary, and the uterus (Duncan, et al. 2014, Gracia and Woodruff 2012). Impaired reproductive function can ultimately result in infertility, sterility, and other outcomes, including depression, psychological disorders, and sexuality issues (Canada and Schover 2012, Chambers, et al. 2013, Gracia and Woodruff 2012, Lawson, et al. 2014, Levine, et al. 2015, Lewis, et al. 2012, Milbury, et al. 2013, Morrow, et al. 2014). To avoid some of these consequences, fertility preservation strategies have been developed and are being implemented in clinical practice (De Vos, et al. 2014, Waimey, et al. 2013).
The American Society for Clinical Oncology (ASCO) and the American Society for Reproductive Medicine (ASRM), along with the American Academy of Pediatrics and the European Society of Human Reproduction and Embryology (ESHRE), have all recognized the need to address the potential threat of gonadotoxic treatments to fertility, each assembling expert committees and publishing guidance documents emphasizing the need for consultation at the time of diagnosis and rapid referral of patients to reproductive endocrinology and infertility specialists. (Ethics Committee of American Society for Reproductive Medicine 2013, Loren, et al. 2013). To date, female fertility preservation options that are recognized by ASCO and the ASRM/ESHRE are limited to embryo and egg cryopreservation (Ethics Committee of American Society for Reproductive Medicine 2013, Loren, et al. 2013). Although these assisted reproductive technology (ART) procedures are successful, they may not be available to all patients. For example, embryo and egg freezing are not possible or are contraindicated in prepubertal females or those who cannot delay treatment for their primary condition (Jeruss and Woodruff 2009). For these patients, one experimental fertility option is ovarian tissue cryopreservation (OTC) (Backhus, et al. 2007, Chen, et al. 2014, De Vos, et al. 2014, Dolmans, et al. 2014, Ross, et al. 2014, Stoop, et al. 2014, Suzuki, et al. 2015). OTC involves the removal of a whole ovary or cortical biopsies that are then cryopreserved by either slow freezing or vitrification (Rosendahl, et al. 2011, Suzuki, et al. 2015, Ting, et al. 2013). This tissue can be thawed at a later date and used to restore reproductive function (Backhus, et al. 2007, Chen, et al. 2014, De Vos, et al. 2014, Dolmans, et al. 2014, Ross, et al. 2014, Stoop, et al. 2014). OTC followed by orthotopic or heterotopic transplantation has resulted in restoration of endocrine function and fertility, with at least 60 live human births reported to date (Dittrich, et al. 2015, Donnez and Dolmans 2015, Gamzatova, et al. 2014, Smitz, et al. 2010). Although OTC and ovarian tissue transplantation have resulted in live human births, this technology is still considered investigational and may be associated with a reintroduction of the cancer the patient has just survived. As with all experimental technologies, this procedure can only be performed under appropriate ethical protocols.
Despite the investigational designation of OTC, there has been an upward trend in the interest in and use of this technology. In the United States, the National Physicians Cooperative (NPC), which is part of the Oncofertility Consortium, is a nationwide infrastructure of clinical and allied sites committed to providing personalized fertility preservation care (Gracia and Woodruff 2012, Gracia, et al. 2012) (http://www.oncofertility.northwestern.edu/health-professionals/npc-membership-info-and-npc-resources). Several NPC sites offer OTC under institutional review board (IRB)-approved protocols, and the number of OTC cases reported through the NPC has increased steadily in the past decade (Duncan, et al. 2015). Interestingly, and perhaps not surprisingly, the largest increase in the use of OTC in the NPC has occurred in the pediatric and young adult population, potentially because of increased awareness about this option and the expanding list of medical conditions and treatments that can compromise fertility (Duncan, et al. 2015, Hirshfeld-Cytron, et al. 2011).
The ability to perform OTC requires trained staff, specialized equipment, designated space, and time to prepare the ovarian cortex and freeze it using specified protocols (American Society for Reproductive Medicine 2014, Backhus, et al. 2007). Because of these requirements, the number of clinical sites that offer OTC for fertility preservation remains low. One approach to increasing patient access to OTC is to facilitate efficient ovarian tissue transport. The concept of preserving organ viability during transport has been instrumental for life-saving transplantation of organs, including kidney, liver, pancreas, lung, and heart. The most common organ preservation technique for transport is induced hypothermia to approximately 4°C (Cantu and Zaas 2011). Hypothermia suppresses metabolism and catabolic enzymes, such that with each 10°C drop in temperature, the metabolic rate is halved (Cantu and Zaas 2011). As a result, at 4°C, the remaining metabolic rate is approximately 10% of normal (Cantu and Zaas 2011). The lower metabolic rate reduces mitochondrial enzyme activity, which in turn reduces the accumulation of lactic acid and slows down the decrease in intracellular pH, proteolysis, lipolysis, and lipid peroxidation associated with ischemia (Guibert, et al. 2011). Induced hypothermia protects organs from damage while they are removed from the blood supply, but perhaps not surprisingly, the maximum time of preservation at cold temperatures varies depending on the specific organ (Guibert, et al. 2011). Specifically, the heart (6 hours) and the lung (8 hours) have the lowest tolerance for cold ischemia, whereas the liver (12–15 hours) and kidney (24 hours) have the highest (Guibert, et al. 2011).
Ovarian transplantation as a clinical concept has been in existence since the late 1800s, yet it has lagged behind other organ systems (Rodriguez and Campo-Engelstein 2011). Although cold storage transport is used widely for kidney, liver, pancreas, lung, and heart, it is not yet standard of care for ovaries in the setting of fertility preservation. However, two European countries – first Denmark and subsequently Germany – have successfully pioneered the clinical use of ovarian tissue transport (Bastings, et al. 2014, Dittrich, et al. 2015, Dittrich, et al. 2012, Jensen, et al. 2015, Muller, et al. 2012). They describe this model, which has been ongoing for 10 years, as “the woman stays – the tissue moves” (Jensen, et al. 2015). In this process, ovarian tissue is removed and prepared at a local hospital and then transported to an approved site where the cryopreservation procedure is performed. Following cryopreservation, the tissue is stored for the individual’s future use. Ovarian tissue transport is particularly challenging because there are several structures and cell types within the tissue that need to be maintained in a healthy state. The ovarian cortex is of particular interest because it is enriched in primordial follicles, which comprise a female’s ovarian reserve and dictate her reproductive lifespan (Schmidt, et al. 2003a). Once the thawed tissue is transplanted, it is these dormant follicles that will eventually grow, produce hormones, and restore reproductive function and fertility. The success of clinical ovarian tissue transport in Europe demonstrates that cold storage of ovarian tissue is efficacious.
Integrating ovarian tissue transport into fertility preservation protocols would expand access to OTC by allowing remote or small clinical practices that may not have resources or staff to offer this option to their patients. This is especially important in large countries and/or in low resource settings. Additionally, some patients may not have the time to delay treatment for their primary condition in order to travel to a site that offers OTC, or they may not want to seek fertility preservation at a different and/or distant institution. Ovarian tissue transport would overcome these barriers and expand the fertility preservation options available to these patients. In this review, we highlight the current status of ovarian tissue transport, first discussing research findings in domestic animals and preclinical studies with human tissue, and then presenting clinical results documenting live births following ovarian tissue transport, OTC, and transplantation of the thawed tissue. Taken together, there is strong empirical evidence supporting the safe and efficient use of ovarian tissue transport for fertility preservation.
Evidence from Animal Studies
The mouse is a critical research model, and recently a comprehensive study was performed which interrogated the effect of storage temperature and duration on ovarian tissue function (Kamoshita, et al. 2016). Storage at 4°C for up to 24 hours did not significantly impact the histological morphology of the tissue or the number of mature gametes that could be collected or fertilized post-orthotopic transplantation (Kamoshita, et al. 2016). However, cold storage did significantly reduce the incidence of implantation and live offspring (Kamoshita, et al. 2016). The findings from this mouse study, while important, should be interpreted with caution because the mouse ovary is fundamentally different from ovaries in large mammalian species – especially in terms of architecture and size.
Studies in large animal models have demonstrated that the transport of ovarian tissue is possible without harming the pool of primordial follicles (Alves, et al. 2015, Baird, et al. 2004, Baird, et al. 1999, Onions, et al. 2008, Salih, et al. 2015). Although the majority of studies focus on the health of fully-grown oocytes as an endpoint of ovarian tissue transport (see Section on Effect of Ovarian Tissue Transport on Oocytes from Antral Follicles), some have analyzed the effect of the transport time and temperature specifically on the population of preantral follicles (Gomes, et al. 2012, Lima, et al. 2010, Lopes, et al. 2009, Lucci, et al. 2004, Silva, et al. 2000). For example in goats, transport of ovarian tissue at 4°C for up to 24 hours maintained morphologically normal primordial follicles (Silva, et al. 2000). Once the temperature was increased to 20°C or 39°C, or the time was extended past 24 hours, however, there was a significant loss of primordial follicles (Silva, et al. 2000). These results are consistent with work done on zebu cow ovaries. When ovarian tissue was stored at 4°C for up to 18 hours, more than 90% of the preantral follicles exhibited normal structure and morphology (Lucci, et al. 2004). When the ovarian tissue was stored at 20°C and for longer than 6 hours, the morphology of preantral follicles in the ovarian tissue significantly diminished (Lucci, et al. 2004). In the canine model, one study concluded that ovarian storage at 4°C for up to 12 hours provides optimal preservation conditions for preantral follicles and ensures the maintenance of morphology and viability of these follicles (Lopes, et al. 2009). This observation is supported by another study that found that when ovarian tissue was held between 3°C and 9°C, more than 80% of the primordial follicles appeared normal and viable. After 36 hours at these temperatures, however, only 59.8% of the primordial follicles survived (Lima, et al. 2010). More recently, a study with equine ovarian tissue concluded that increasing the time and temperature of tissue transport significantly reduced the percentage of morphologically normal follicles (Gomes, et al. 2012). The greatest percentage of viable follicles was found in equine ovarian tissue that was transported at 4°C for 4 hours.
Ongoing work through the NPC is consistent with this published data in large animal models. For example, we have examined the transport of bovine, feline, and canine tissue at 4°C, 25°C, and 37°C for 24 hours. In each of the ovarian tissue types, our histological analysis found that the structure and morphology of primordial and preantral follicles were best maintained when the ovarian tissue was kept at 4°C or 25°C (Figures 1 and 2). In our experience, antral follicles were compromised under all transport conditions as evidenced histologically by the disruption of the integrity of cumulus-oocyte-complex (COC) (Figure 2; see Section on Effect of Ovarian Tissue Transport on Oocytes from Antral Follicles).
Figure 1. Ovarian tissue transport at colder temperatures maintains primordial follicle morphology in multiple species.
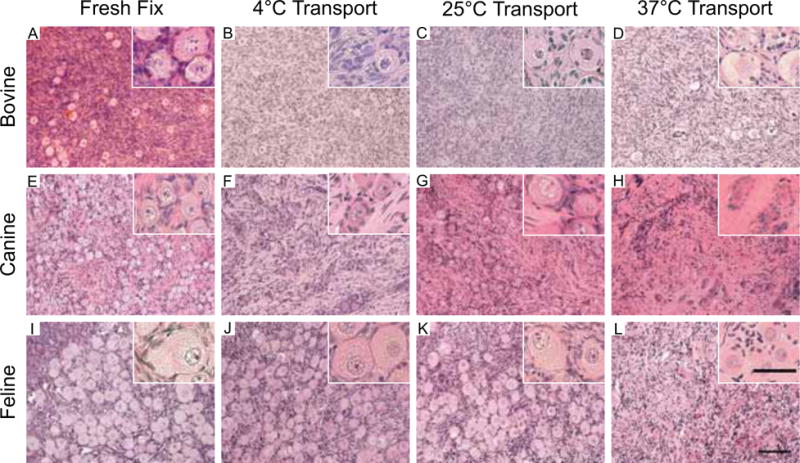
Bovine (A–D), canine (E–H), and feline (I–L) ovarian tissue was harvested from individual animals and divided. One piece of tissue was fixed immediately upon organ removal (A, E, I), and the remaining pieces were transported at 4°C (B, F, J), 25°C (C, G, K), and 37°C (D, H, L) for 24 hours. In this manner, the only variable was transport temperature. Following transport, the ovarian tissue was fixed and the morphology of the primordial follicles within the ovarian cortex was examined by standard histological evaluation by hematoxylin and eosin staining. Primordial follicle integrity was compromised in all species following transport at 37°C. However, primordial follicles at both 4°C and 25°C looked similar to fresh fixed controls. These experiments were repeated a minimum of three times and representative images are shown. Scale bar = 50 μm. The insets are higher magnification images, which highlight the morphology of the primordial follicles.
Figure 2. Ovarian tissue transport at colder temperatures maintains preantral follicle morphology in multiple species.
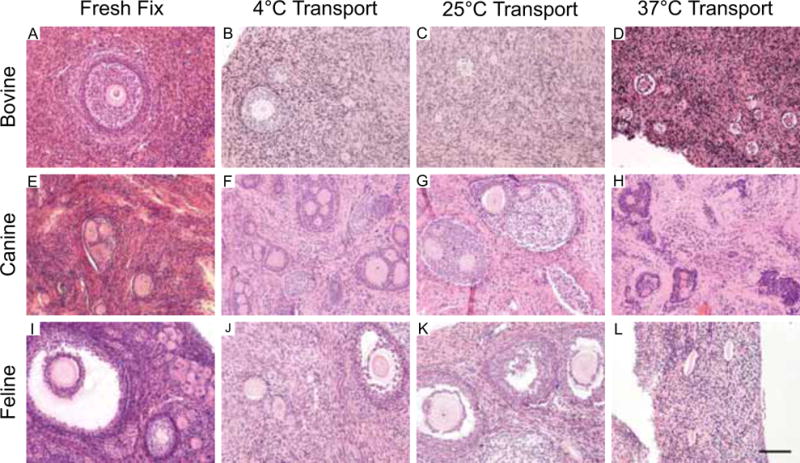
Bovine (A–D), canine (E–H), and feline (I–L) ovarian tissue was harvested from individual animals and divided. One piece of tissue was fixed immediately upon organ removal (A, E, I), and the remaining pieces were transported at 4°C (B, F, J), 25°C (C, G, K), and 37°C (D, H, L) for 24 hours. In this manner, the only variable was transport temperature. Following transport, the ovarian tissue was fixed and the morphology of the primordial follicles within the ovarian tissue was examined by standard histological evaluation by hematoxylin and eosin staining. Preantral follicle integrity was significantly compromised in all species following transport at 37°C. However, preantral follicles at both 4°C and 25°C looked similar to fresh fixed controls. Antral follicles did not appear to tolerate any transport conditions. This is highlighted in the feline sample where disruption of the cumulus layer surrounding the oocyte (arrows) was evident following transport (J, K) but not in the control (I). These experiments were repeated a minimum of three times and representative images are shown. Scale bar = 50 μm.
Some of the most convincing evidence supporting the clinical use of ovarian tissue transport in the setting of fertility preservation comes from the nonhuman primate model. A recent study determined that transport of primate ovarian tissue at 4°C for up to 24 hours did not compromise the quality of the tissue or the health of follicles (Hornick, et al. 2012). When compared to freshly fixed controls, morphology markers in the transported ovarian tissue were indistinguishable and there were no differences in the number of apoptotic cells (Hornick, et al. 2012). In addition, follicles isolated from these transported tissues survived and grew in an encapsulated in vitro follicle growth (IVFG) system that recapitulates oogenesis and folliculogenesis, demonstrating the functionality of these cells post-transport (Hornick, et al. 2012). Thus taken together, there was better preservation of primordial follicles than of developing follicles at colder temperatures in ovarian tissue from most large animal models. Because the majority of primordial follicles are presumably quiescent, it is possible that they may be less affected by the reduced cellular metabolism rate at cooler temperatures (Gomes, et al. 2012, Silva, et al. 2000). In addition, the less complex cellular structure as well as the smaller oocyte volume may also contribute to the relative resilience of primordial follicle to cold temperatures.
Evidence from Preclinical Studies with Human Ovarian Tissue
The ovarian tissue transport studies performed in animal models have been replicated successfully with human ovarian tissue by several groups (Isachenko, et al. 2009, Laronda, et al. 2014, Schmidt, et al. 2003b). The transport of human ovarian tissue at cold temperatures and within a period of 4 to 26 hours maintains functional parameters of the tissue as assessed by morphology, viability, and follicle development in vitro and in vivo (Isachenko, et al. 2009, Laronda, et al. 2014, Schmidt, et al. 2003b). In one recent study, ovarian cortical tissue from 24 individuals was transported for up to 22 hours at 4°C, and cell quality and morphology were compared in histological and TUNEL analyses to pre-transport control tissue from the same individual (Laronda, et al. 2014). In more than half of the samples, the transported tissue was histologically similar to the freshly fixed control tissue, and TUNEL analysis demonstrated a similar percentage of apoptotic cells in the pre- and post-transport tissue. In some samples, significantly more TUNEL-positive cells were observed in the post-transport tissue compared to freshly fixed control tissue. These TUNEL-positive cells, however, were primarily constrained to the edge of the tissue. Preantral follicles at the secondary follicle stage isolated from this transported human ovarian tissue were able to survive and grow to the antral stage in culture and to develop with normal morphology (Laronda, et al. 2014).
The findings from the study by Laronda et al. (2014) are consistent with another study that also evaluated the quality of transported human ovarian tissue using an IVFG assay (Isachenko, et al. 2009). In that study, storage of ovarian cortical fragments at suprazero temperatures for up to 26 hours did not compromise the in vitro development of follicles during subsequent culture of intact tissue pieces (Isachenko, et al. 2009). Interestingly, the storage of human ovarian cortex at 5°C for 24 hours before cryopreservation increased the viability of the follicles after thawing as assessed by culture in the chorioallantoic membrane system followed by immunohistochemical analysis of proliferation marker expression within the cultured tissue (Isachenko, et al. 2013). Immunohistochemical analysis of ovarian tissue after 24 hours at 5°C also showed that neo-vascularization was increased compared to non-cooled control tissue, which is important because this process is critical for implantation and function of transplanted ovarian tissue (Isachenko, et al. 2012). The physiologic basis for these phenomena, however, is unclear. Moreover, without evaluation of the number and viability of follicles as well as the extent of vascularization in the tissue pieces prior to experimental manipulation, it is impossible to conclude whether cooling per se had a significant impact on these parameters.
Reports of consistent histology and IVFG following transport are robustly supported by the successful xenotransplantation of human ovarian tissue that was transported prior to cryopreservation (Schmidt, et al. 2003b). Following transport on ice for approximately 4 hours, ovarian tissue was cryopreserved, then thawed and xenotransplanted into a mouse. After 4 weeks, each of the transplanted pieces of ovarian tissue was recovered and found to contain healthy follicles at the primordial, primary, and secondary stages (Schmidt, et al. 2003b). This work is of particular significance because it demonstrates the function of the transported, cryopreserved, thawed, and transplanted tissue in an in vivo model. Taken together, this research demonstrates that transport of human ovarian tissue at or around 4°C prior to cryopreservation maintains the primordial follicle pool and may even have a positive effect on the subsequent growth and development of the follicles within the tissue (Isachenko, et al. 2013, Laronda, et al. 2014, Schmidt, et al. 2003b).
Clinical Studies
The strongest unequivocal support of the safety and efficacy of ovarian tissue transport are the live births that have been reported after transplantation of transported ovarian tissue. Transplantation of ovarian tissue has led to at least 60 live births to date, and several of these were achieved after transplantation of ovarian tissue that was transported for up to 22 hours prior to cryopreservation (Andersen, et al. 2008, Dittrich, et al. 2015, Dittrich, et al. 2012, Donnez and Dolmans 2015, Ernst, et al. 2010, Jensen, et al. 2015, Muller, et al. 2012, Rosendahl, et al. 2011). These cases indicate that transport prior to cryopreservation does not compromise the functional potential of the tissue. Moreover, these reports demonstrate that (1) live births can be obtained following cold tissue transport at 4°C, (2) tissue can be transported up to 20 hours and remain viable, and (3) multiple live births can be obtained from the same tissue.
In several European countries, primarily Denmark and Germany, ovarian tissue transport has been combined with OTC and transplantation with successful clinical outcomes. In Denmark, OTC is only performed at the Laboratory of Reproductive Biology at Copenhagen University Hospital, Rigshospitalet, which provides a centralized localization (Rosendahl, et al. 2011). Ovaries removed from patients at other centers around the country must be placed on ice and transported by airfreight, train, or car within 4–5 hours to the Copenhagen University Hospital for cryopreservation. As of 2015, this program performed 53 transplantations to 41 patients over the course of 10 years, and nine of these women had tissue that was transported on ice prior to cryopreservation (Jensen, et al. 2015). This group reports a pregnancy rate of ~30% using this technology, again supporting the combined use of ovarian tissue transport with OTC and transplantation (Jensen, et al. 2015).
Two case reports from the Danish and German experiences are described in more detail below because they highlight the longevity and functionality of ovarian tissue grafts post-transport and illustrate the relative resistance of ovarian tissue to cold transport. In the first case from Denmark, a 27-year-old woman who had been diagnosed with Ewing’s sarcoma had undergone OTC for fertility preservation. The patient’s ovarian tissue was transported on ice for 5 hours and then cryopreserved for 21 months. For the ovarian transplantation procedure, tissue corresponding to approximately 15–20% of one entire ovary was transplanted into subperitoneal pockets in the patient’s parietal peritoneum (Andersen, et al. 2008, Ernst, et al. 2010). This woman had two healthy girls from this tissue, making her the first patient to have two separate pregnancies and births resulting from a single OTC-autotransplantation procedure. At the time of OTC, the woman only had her right ovary because the left one had been removed previously due to a dermoid cyst. A part of the right ovary was removed for OTC. Although it is possible that the pregnancies resulted from residual ovarian tissue rather than the transplanted tissue, a biopsy of the remaining ovary was performed at the time of transplantation, and no follicles were found by histological examination.
In the second case from Germany, a live birth following ovarian tissue cryopreservation and transplantation was reported after 20 hours of cold transport, suggesting that the ovary is similar to the kidney and liver in terms of its tolerance to cold temperatures (Dittrich, et al. 2012, Guibert, et al. 2011). In this case, a 25-year-old woman with nodular sclerosing Hodgkin lymphoma had two-thirds of the ovarian cortex from each ovary removed, and the tissue was transported at 5°C to 8°C for 20 hours from Dresden University Hospital to the Reproductive Medicine Laboratory in Bonn for cryopreservation (Dittrich, et al. 2012). After 5 years of cryopreservation, the tissue was transplanted into a deep pouch of the peritoneum in the region of the broad ligament. The patient had a spontaneous pregnancy and delivered a healthy male (Dittrich, et al. 2012). During the cesarean section, the research team stated that healthy follicles were clearly visible on the surface of the transplanted tissue (Muller, et al. 2012). Further, biopsy samples taken from the transplanted ovarian tissue revealed numerous follicles in all stages of development. This histological analysis confirms the viability of ovarian tissue that had been transported up to 20 hours at 4°C prior to cryopreservation (Muller, et al. 2012).
Thus it is clear that in the short time that ovarian tissue transport protocols have been employed in Europe, there have been multiple live births following autotransplantation (Andersen, et al. 2008, Dittrich, et al. 2015, Dittrich, et al. 2012, Ernst, et al. 2010, Jensen, et al. 2015, Muller, et al. 2012, Rosendahl, et al. 2011). These impressive clinical results illustrate the potential for integrating ovarian tissue transport prior to cryopreservation as a way to expand global access to OTC.
Effect of Ovarian Tissue Transport on Oocytes from Antral Follicles
The majority of this review has focused on ovarian tissue transport conditions that favor the preservation of preantral follicles. However, ovarian tissue also contains antral follicles at various stages of development. Cumulus-oocyte-complexes (COCs) from both small and large antral follicles can be isolated directly from harvested ovarian tissue and matured in vitro to obtain mature gametes. In fact, COCs in the ovarian medulla are often released from antral follicles during cortical ovarian tissue processing for OTC and can be used for fertility preservation if they are from follicles that have reached adequate maturity and if they are of high quality (Duncan, et al. 2012, Revel, et al. 2003). Mature eggs derived from IVM can be cryopreserved or fertilized and then cryopreserved as embryos. In vitro maturation (IVM) is another promising fertility preservation option, and importantly, pregnancies and live births have been obtained using this method (Chian, et al. 2013, Guzman, et al. 2012, Segers, et al. 2015, Uzelac, et al. 2015).
In animal studies, the data regarding how well COCs withstand cold transport are not consistent. In one study, bovine ovaries were transported for 3–4 hours at 15°C, 25°C, and 35°C; oocytes from the ovaries transported at 15°C had a higher developmental competence and lower apoptotic index compared to the other transport groups (Wang, et al. 2010). In the canine model, oocytes obtained from ovaries transported for up to 4 hours at 4°C had a significantly higher MII maturation rate than those transported at 35°C to 38°C (Tas, et al. 2006). However, in another study, transport at 37°C for up to 8 hours resulted in the greatest oocyte viability, although this study did not directly compare outcomes with oocytes from ovarian tissue transported at 4°C (Hanna, et al. 2008). Conflicting results regarding COC viability after ovarian tissue transport have been seen in other animal models as well. One study concluded that when equine ovaries were transported within 7 hours, temperature played no role in determining the developmental competence of retrieved oocytes (Ribeiro, et al. 2008), while another group reported that transport at 4°C for up to 4 hours was optimal for oocyte viability (Gomes, et al. 2012). Experimental data from the feline model showed that oocytes from ovaries transported for up to 24 hours at 4°C maintained were capable of producing blastocysts after in vitro fertilization (Evecen, et al. 2009, Naoi, et al. 2007, Wolfe and Wildt 1996). The effect of cooler transport temperatures was also seen in a study with Iberian red deer, which showed that ovarian transport for up to 12 hours at 5–8°C produced oocytes that had a higher embryonic cleavage rate than those transported at 20–25°C (Garcia-Alvarez, et al. 2011).
In humans, the data appears to be more conclusive that COCs do not appear to withstand extended transport at cold temperatures well. Duncan et al. (2012) reported that, although COCs can be isolated directly from fresh human ovarian tissue, there were no viable COCs recovered from tissue that had been transported for 22 hours at 4°C. Short-term transport prior to OTC has also been shown to significantly compromise the oocyte maturation rate (Wilken-Jensen, et al. 2014). Cryopreserved ovarian tissue from a cohort of 61 patients (0–38 years of age) from Denmark’s centralized cryopreservation program was examined; the tissue transport time for these samples was between 2 and 5 hours at 4°C. A total of 682 immature oocytes within cumulus-oocyte-complexes (COCs) were collected; 11% of oocytes from non-transported tissue were successfully matured in vitro to the MII stage, compared to 1.4% and 4.6% of oocytes from tissue that had been transported 2–3 hours and 4–5 hours, respectively.
Taken together these results suggest that there are species-specific and follicle-class dependent differences in optimal ovarian tissue transport conditions. For human ovarian tissue, cold temperatures favor the preservation of preantral follicles but may not be optimal for antral follicles.
Summary and Future Directions
In summary, critical advances have been made in ovarian tissue transport. Preclinical studies support the use of this technique in the setting of fertility preservation for both endangered species as well as patients with fertility-threatening conditions (Figure 3). Studies are ongoing to further optimize ovarian tissue transport conditions. For example, in studies using sheep ovaries as a model, addition of anti-apoptotic agents in the transport, processing, and cryopreservation media improved the quality of primordial follicles post-thaw as assessed by culture and histologic evaluation (Henry, et al. 2016). It is especially encouraging for the field of fertility preservation that multiple human live births have been reported following transplantation of transported ovarian tissue. This technique, should it be implemented in clinical practice in larger countries or in those with limited resources, will increase the options for patients who are in geographic areas that lack an oncofertility program to have ovarian tissue preserved for their later use for transplantation or other emerging fertility preservation options (Figure 3).
Figure 3. Schematic detailing how ovarian tissue transport integrates into clinical fertility preservation.
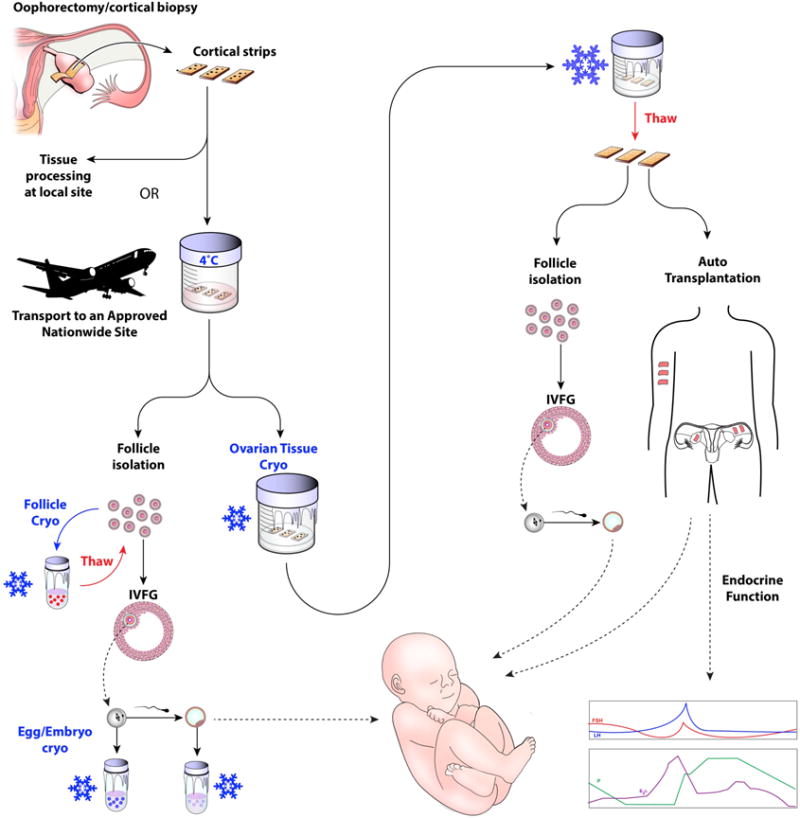
Ovarian tissue transport would fully expand the fertility preservation options available to those individuals who currently do not have access to such technologies. Ovarian tissue that is removed as an entire organ or as a biopsy would be prepared into cortical strips upon harvesting and transported to an approved processing site. Upon arrival at the processing site, the ovarian tissue could be cryopreserved by slow freezing or vitrification methods. Following thawing, the ovarian tissue could be used for transplantation to restore endocrine function and/or fertility. Ovarian follicles could also be isolated from the thawed ovarian tissue and used for in vitro follicle growth (IVFG) to obtain mature eggs that can be used to generate embryos following in vitro fertilization or intracytoplasmic sperm injection. Another option is that follicles could be isolated directly from the transported tissue without the need for tissue cryopreservation. These isolated follicles could be cryopreserved or used for IVFG.
Acknowledgments
We would like to thank Dr. Dominique Keller for useful insight into ovarian tissue transport studies in domestic species. We also acknowledge the Aurora Packing Company, Dr. Jessica Hornick, and Jennifer Nagashima for technical assistance with the bovine, canine, and feline experiments. We appreciate the work of the National Physicians Cooperative and particularly Kristin Smith and Brigid Martz. Finally, we would like to thank Stacey Tobin for editorial assistance and Stanton Fernald for his expertise in medical illustration.
Funding
This work was funded through the Thomas J. Watkins Endowment (TKW, Northwestern University, Chicago, IL) and by the Center for Reproductive Health After Disease (P50HD076188) from the National Institutes of Health National Center for Translational Research in Reproduction and Infertility (NCTRI).
Footnotes
Declaration of Interest
The authors declare no conflicts of interest.
References
- Alves KA, Alves BG, Rocha CD, Visonna M, Mohallem RF, Gastal MO, Jacomini JO, Beletti ME, Figueiredo JR, Gambarini ML, Gastal EL. Number and density of equine preantral follicles in different ovarian histological section thicknesses. Theriogenology. 2015;83:1048–1055. doi: 10.1016/j.theriogenology.2014.12.004. [DOI] [PubMed] [Google Scholar]
- American Society for Reproductive Medicine. Ovarian tissue cryopreservation: a committee opinion. Fertil Steril. 2014;101:1237–1243. doi: 10.1016/j.fertnstert.2014.02.052. [DOI] [PubMed] [Google Scholar]
- Andersen CY, Rosendahl M, Byskov AG, Loft A, Ottosen C, Dueholm M, Schmidt KL, Andersen AN, Ernst E. Two successful pregnancies following autotransplantation of frozen/thawed ovarian tissue. Hum Reprod. 2008;23:2266–2272. doi: 10.1093/humrep/den244. [DOI] [PubMed] [Google Scholar]
- Backhus LE, Kondapalli LA, Chang RJ, Coutifaris C, Kazer R, Woodruff TK. Oncofertility consortium consensus statement: guidelines for ovarian tissue cryopreservation. Cancer Treat Res. 2007;138:235–239. doi: 10.1007/978-0-387-72293-1_17. [DOI] [PubMed] [Google Scholar]
- Baird DT, Campbell B, de Souza C, Telfer E. Long-term ovarian function in sheep after ovariectomy and autotransplantation of cryopreserved cortical strips. Eur J Obstet Gynecol Reprod Biol. 2004;113(Suppl 1):S55–59. doi: 10.1016/j.ejogrb.2003.11.023. [DOI] [PubMed] [Google Scholar]
- Baird DT, Webb R, Campbell BK, Harkness LM, Gosden RG. Long-term ovarian function in sheep after ovariectomy and transplantation of autografts stored at −196 C. Endocrinology. 1999;140:462–471. doi: 10.1210/endo.140.1.6453. [DOI] [PubMed] [Google Scholar]
- Bastings L, Liebenthron J, Westphal JR, Beerendonk CC, van der Ven H, Meinecke B, Montag M, Braat DD, Peek R. Efficacy of ovarian tissue cryopreservation in a major European center. J Assist Reprod Genet. 2014;31:1003–1012. doi: 10.1007/s10815-014-0239-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canada AL, Schover LR. The psychosocial impact of interrupted childbearing in long-term female cancer survivors. Psychooncology. 2012;21:134–143. doi: 10.1002/pon.1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantu E, Zaas DW. Organ donor management and procurement. In: Klein AA, Lewish CJ, Madsen CJ, editors. Organ Transplantation: A Clinical Guide. 2011. p. 53. [Google Scholar]
- Chambers SK, Schover L, Nielsen L, Halford K, Clutton S, Gardiner RA, Dunn J, Occhipinti S. Couple distress after localised prostate cancer. Support Care Cancer. 2013;21:2967–2976. doi: 10.1007/s00520-013-1868-6. [DOI] [PubMed] [Google Scholar]
- Chen CH, Tan SJ, Tzeng CR. In vivo fate mapping of cryopreserved murine ovarian grafts. J Ovarian Res. 2014;7:81. doi: 10.1186/s13048-014-0081-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chian RC, Uzelac PS, Nargund G. In vitro maturation of human immature oocytes for fertility preservation. Fertil Steril. 2013;99:1173–1181. doi: 10.1016/j.fertnstert.2013.01.141. [DOI] [PubMed] [Google Scholar]
- De Vos M, Smitz J, Woodruff TK. Fertility preservation in women with cancer. Lancet. 2014;384:1302–1310. doi: 10.1016/S0140-6736(14)60834-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dittrich R, Hackl J, Lotz L, Hoffmann I, Beckmann MW. Pregnancies and live births after 20 transplantations of cryopreserved ovarian tissue in a single center. Fertil Steril. 2015;103:462–468. doi: 10.1016/j.fertnstert.2014.10.045. [DOI] [PubMed] [Google Scholar]
- Dittrich R, Lotz L, Keck G, Hoffmann I, Mueller A, Beckmann MW, van der Ven H, Montag M. Live birth after ovarian tissue autotransplantation following overnight transportation before cryopreservation. Fertil Steril. 2012;97:387–390. doi: 10.1016/j.fertnstert.2011.11.047. [DOI] [PubMed] [Google Scholar]
- Dolmans MM, Marotta ML, Pirard C, Donnez J, Donnez O. Ovarian tissue cryopreservation followed by controlled ovarian stimulation and pick-up of mature oocytes does not impair the number or quality of retrieved oocytes. J Ovarian Res. 2014;7:80. doi: 10.1186/s13048-014-0080-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnez J, Dolmans MM. Ovarian cortex transplantation: 60 reported live births brings the success and worldwide expansion of the technique towards routine clinical practice. J Assist Reprod Genet. 2015;32:1167–1170. doi: 10.1007/s10815-015-0544-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan FE, Brannigan RE, Woodruff TK. Fertility preservation. In: Strauss JF, Barbieri RL, editors. Yen & Jaffe’s Reproductive Endocrinology: Physiology, Pathophysiology, and Clinical Management. 7th. Philadelphia, PA: Saunders; 2014. [Google Scholar]
- Duncan FE, Hornick JE, Lampson MA, Schultz RM, Shea LD, Woodruff TK. Chromosome cohesion decreases in human eggs with advanced maternal age. Aging Cell. 2012;11:1121–1124. doi: 10.1111/j.1474-9726.2012.00866.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan FE, Pavone ME, Gunn AH, Badawy S, Gracia C, Ginsberg JP, Lockart B, Gosiengfiao Y, Woodruff TK. Pediatric and Teen Ovarian Tissue Removed for Cryopreservation Contains Follicles Irrespective of Age, Disease Diagnosis, Treatment History, and Specimen Processing Methods. J Adolesc Young Adult Oncol. 2015;4:174–183. doi: 10.1089/jayao.2015.0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst E, Bergholdt S, Jorgensen JS, Andersen CY. The first woman to give birth to two children following transplantation of frozen/thawed ovarian tissue. Hum Reprod. 2010;25:1280–1281. doi: 10.1093/humrep/deq033. [DOI] [PubMed] [Google Scholar]
- Ethics Committee of American Society for Reproductive Medicine. Fertility preservation and reproduction in patients facing gonadotoxic therapies: a committee opinion. Fertil Steril. 2013;100:1224–1231. doi: 10.1016/j.fertnstert.2013.08.041. [DOI] [PubMed] [Google Scholar]
- Evecen M, Cirit U, Demir K, Karaman E, Hamzaoglu AI, Bakirer G. Developmental competence of domestic cat oocytes from ovaries stored at various durations at 4 degrees C temperature. Anim Reprod Sci. 2009;116:169–172. doi: 10.1016/j.anireprosci.2009.01.006. [DOI] [PubMed] [Google Scholar]
- Gamzatova Z, Komlichenko E, Kostareva A, Galagudza M, Ulrikh E, Zubareva T, Sheveleva T, Nezhentseva E, Kalinina E. Autotransplantation of cryopreserved ovarian tissue–effective method of fertility preservation in cancer patients. Gynecol Endocrinol. 2014;30(Suppl 1):43–47. doi: 10.3109/09513590.2014.945789. [DOI] [PubMed] [Google Scholar]
- Garcia-Alvarez O, Maroto-Morales A, Berlinguer F, Fernandez-Santos MR, Esteso MC, Mermillod P, Ortiz JA, Ramon M, Perez-Guzman MD, Garde JJ, Soler AJ. Effect of storage temperature during transport of ovaries on in vitro embryo production in Iberian red deer (Cervus elaphus hispanicus) Theriogenology. 2011;75:65–72. doi: 10.1016/j.theriogenology.2010.07.011. [DOI] [PubMed] [Google Scholar]
- Gomes RG, Andrade ER, Lisboa LA, Ciquini A, Barreiros TR, Fonseca NA, Seneda MM. Effect of holding medium, temperature and time on structural integrity of equine ovarian follicles during the non-breeding season. Theriogenology. 2012;78:731–736. doi: 10.1016/j.theriogenology.2012.03.019. [DOI] [PubMed] [Google Scholar]
- Gracia C, Woodruff TK. Oncofertility Medical Practice. New York, NY: Springer Science+Business Media; 2012. [Google Scholar]
- Gracia CR, Chang J, Kondapalli L, Prewitt M, Carlson CA, Mattei P, Jeffers S, Ginsberg JP. Ovarian tissue cryopreservation for fertility preservation in cancer patients: successful establishment and feasibility of a multidisciplinary collaboration. J Assist Reprod Genet. 2012;29:495–502. doi: 10.1007/s10815-012-9753-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guibert EE, Petrenko AY, Balaban CL, Somov AY, Rodriguez JV, Fuller BJ. Organ Preservation: Current Concepts and New Strategies for the Next Decade. Transfus Med Hemother. 2011;38:125–142. doi: 10.1159/000327033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzman L, Ortega-Hrepich C, Albuz FK, Verheyen G, Devroey P, Smitz J, De Vos M. Developmental capacity of in vitro-matured human oocytes retrieved from polycystic ovary syndrome ovaries containing no follicles larger than 6 mm. Fertil Steril. 2012;98:503–507. e501–502. doi: 10.1016/j.fertnstert.2012.01.114. [DOI] [PubMed] [Google Scholar]
- Hanna C, Long C, Hinrichs K, Westhusin M, Kraemer D. Assessment of canine oocyte viability after transportation and storage under different conditions. Anim Reprod Sci. 2008;105:451–456. doi: 10.1016/j.anireprosci.2007.12.009. [DOI] [PubMed] [Google Scholar]
- Henry L, Fransolet M, Labied S, Blacher S, Masereel MC, Foidart JM, Noel A, Nisolle M, Munaut C. Supplementation of transport and freezing media with anti-apoptotic drugs improves ovarian cortex survival. J Ovarian Res. 2016;9:4. doi: 10.1186/s13048-016-0216-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirshfeld-Cytron J, Gracia C, Woodruff TK. Nonmalignant diseases and treatments associated with primary ovarian failure: an expanded role for fertility preservation. J Womens Health (Larchmt) 2011;20:1467–1477. doi: 10.1089/jwh.2010.2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornick JE, Duncan FE, Shea LD, Woodruff TK. Isolated primate primordial follicles require a rigid physical environment to survive and grow in vitro. Hum Reprod. 2012;27:1801–1810. doi: 10.1093/humrep/der468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isachenko E, Isachenko V, Nawroth F, Rahimi G, Weiss JM. Effect of long-term exposure at suprazero temperatures on activity and viability of human ovarian cortex. Fertil Steril. 2009;91:1556–1559. doi: 10.1016/j.fertnstert.2008.09.068. [DOI] [PubMed] [Google Scholar]
- Isachenko V, Isachenko E, Mallmann P, Rahimi G. Increasing follicular and stromal cell proliferation in cryopreserved human ovarian tissue after long-term precooling prior to freezing: in vitro versus chorioallantoic membrane (CAM) xenotransplantation. Cell Transplant. 2013;22:2053–2061. doi: 10.3727/096368912X658827. [DOI] [PubMed] [Google Scholar]
- Isachenko V, Mallmann P, Petrunkina AM, Rahimi G, Nawroth F, Hancke K, Felberbaum R, Genze F, Damjanoski I, Isachenko E. Comparison of in vitro- and chorioallantoic membrane (CAM)-culture systems for cryopreserved medulla-contained human ovarian tissue. PLoS One. 2012;7:e32549. doi: 10.1371/journal.pone.0032549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen AK, Kristensen SG, Macklon KT, Jeppesen JV, Fedder J, Ernst E, Andersen CY. Outcomes of transplantations of cryopreserved ovarian tissue to 41 women in Denmark. Hum Reprod. 2015;30:2838–2845. doi: 10.1093/humrep/dev230. [DOI] [PubMed] [Google Scholar]
- Jeruss JS, Woodruff TK. Preservation of fertility in patients with cancer. N Engl J Med. 2009;360:902–911. doi: 10.1056/NEJMra0801454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamoshita K, Okamoto N, Nakajima M, Haino T, Sugimoto K, Okamoto A, Sugishita Y, Suzuki N. Investigation of in vitro parameters and fertility of mouse ovary after storage at an optimal temperature and duration for transportation. Hum Reprod. 2016;31:774–781. doi: 10.1093/humrep/dew023. [DOI] [PubMed] [Google Scholar]
- Laronda MM, Duncan FE, Hornick JE, Xu M, Pahnke JE, Whelan KA, Shea LD, Woodruff TK. Alginate encapsulation supports the growth and differentiation of human primordial follicles within ovarian cortical tissue. J Assist Reprod Genet. 2014;31:1013–1028. doi: 10.1007/s10815-014-0252-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson AK, Klock SC, Pavone ME, Hirshfeld-Cytron J, Smith KN, Kazer RR. Prospective study of depression and anxiety in female fertility preservation and infertility patients. Fertil Steril. 2014;102:1377–1384. doi: 10.1016/j.fertnstert.2014.07.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine JM, Kelvin JF, Quinn GP, Gracia CR. Infertility in reproductive-age female cancer survivors. Cancer. 2015;121:1532–1539. doi: 10.1002/cncr.29181. [DOI] [PubMed] [Google Scholar]
- Lewis PE, Sheng M, Rhodes MM, Jackson KE, Schover LR. Psychosocial concerns of young African American breast cancer survivors. J Psychosoc Oncol. 2012;30:168–184. doi: 10.1080/07347332.2011.651259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima GL, Costa LL, Cavalcanti DM, Rodrigues CM, Freire FA, Fontenele-Neto JD, Silva AR. Short-term storage of canine preantral ovarian follicles using a powdered coconut water (ACP)-based medium. Theriogenology. 2010;74:146–152. doi: 10.1016/j.theriogenology.2010.01.025. [DOI] [PubMed] [Google Scholar]
- Lobo RA. Potential options for preservation of fertility in women. N Engl J Med. 2005;353:64–73. doi: 10.1056/NEJMra043475. [DOI] [PubMed] [Google Scholar]
- Lopes CA, dos Santos RR, Celestino JJ, Melo MA, Chaves RN, Campello CC, Silva JR, Bao SN, Jewgenow K, de Figueiredo JR. Short-term preservation of canine preantral follicles: Effects of temperature, medium and time. Anim Reprod Sci. 2009;115:201–214. doi: 10.1016/j.anireprosci.2008.12.016. [DOI] [PubMed] [Google Scholar]
- Loren AW, Mangu PB, Beck LN, Brennan L, Magdalinski AJ, Partridge AH, Quinn G, Wallace WH, Oktay K, American Society of Clinical Oncology Fertility preservation for patients with cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2013;31:2500–2510. doi: 10.1200/JCO.2013.49.2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucci CM, Kacinskis MA, Rumpf R, Bao SN. Effects of lowered temperatures and media on short-term preservation of zebu (Bos indicus) preantral ovarian follicles. Theriogenology. 2004;61:461–472. doi: 10.1016/s0093-691x(03)00226-7. [DOI] [PubMed] [Google Scholar]
- Meirow D, Levron J, Eldar-Geva T, Hardan I, Fridman E, Zalel Y, Schiff E, Dor J. Pregnancy after transplantation of cryopreserved ovarian tissue in a patient with ovarian failure after chemotherapy. N Engl J Med. 2005;353:318–321. doi: 10.1056/NEJMc055237. [DOI] [PubMed] [Google Scholar]
- Milbury K, Cohen L, Jenkins R, Skibber JM, Schover LR. The association between psychosocial and medical factors with long-term sexual dysfunction after treatment for colorectal cancer. Support Care Cancer. 2013;21:793–802. doi: 10.1007/s00520-012-1582-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow PK, Broxson AC, Munsell MF, Basen-Enquist K, Rosenblum CK, Schover LR, Nguyen LH, Hsu L, Castillo L, Hahn KM, Litton JK, Kwiatkowski DN, Hortobagyi GN. Effect of age and race on quality of life in young breast cancer survivors. Clin Breast Cancer. 2014;14:e21–31. doi: 10.1016/j.clbc.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller A, Keller K, Wacker J, Dittrich R, Keck G, Montag M, Van der Ven H, Wachter D, Beckmann MW, Distler W. Retransplantation of cryopreserved ovarian tissue: the first live birth in Germany. Dtsch Arztebl Int. 2012;109:8–13. doi: 10.3238/arztebl.2012.0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naoi H, Otoi T, Shimamura T, Karja NW, Agung B, Shimizu R, Taniguchi M, Nagai T. Developmental competence of cat oocytes from ovaries stored at various temperature for 24 h. J Reprod Dev. 2007;53:271–277. doi: 10.1262/jrd.18115. [DOI] [PubMed] [Google Scholar]
- Onions VJ, Mitchell MR, Campbell BK, Webb R. Ovarian tissue viability following whole ovine ovary cryopreservation: assessing the effects of sphingosine-1-phosphate inclusion. Hum Reprod. 2008;23:606–618. doi: 10.1093/humrep/dem414. [DOI] [PubMed] [Google Scholar]
- Revel A, Koler M, Simon A, Lewin A, Laufer N, Safran A. Oocyte collection during cryopreservation of the ovarian cortex. Fertil Steril. 2003;79:1237–1239. doi: 10.1016/s0015-0282(02)04963-4. [DOI] [PubMed] [Google Scholar]
- Ribeiro BI, Love LB, Choi YH, Hinrichs K. Transport of equine ovaries for assisted reproduction. Anim Reprod Sci. 2008;108:171–179. doi: 10.1016/j.anireprosci.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Rodriguez SB, Campo-Engelstein L. Conceiving wholeness: women, motherhood, and ovarian transplantation, 1902 and 2004. Perspect Biol Med. 2011;54:409–416. doi: 10.1353/pbm.2011.0036. [DOI] [PubMed] [Google Scholar]
- Rosendahl M, Schmidt KT, Ernst E, Rasmussen PE, Loft A, Byskov AG, Andersen AN, Andersen CY. Cryopreservation of ovarian tissue for a decade in Denmark: a view of the technique. Reprod Biomed Online. 2011;22:162–171. doi: 10.1016/j.rbmo.2010.10.015. [DOI] [PubMed] [Google Scholar]
- Ross L, Chung K, Macdonald H. Fertility preservation in the female cancer patient. J Surg Oncol. 2014;110:907–911. doi: 10.1002/jso.23754. [DOI] [PubMed] [Google Scholar]
- Salih SM, Ringelstetter AK, Elsarrag MZ, Abbott DH, Roti EC. Dexrazoxane abrogates acute doxorubicin toxicity in marmoset ovary. Biol Reprod. 2015;92:73. doi: 10.1095/biolreprod.114.119495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt KL, Byskov AG, Nyboe Andersen A, Muller J, Yding Andersen C. Density and distribution of primordial follicles in single pieces of cortex from 21 patients and in individual pieces of cortex from three entire human ovaries. Hum Reprod. 2003a;18:1158–1164. doi: 10.1093/humrep/deg246. [DOI] [PubMed] [Google Scholar]
- Schmidt KL, Ernst E, Byskov AG, Nyboe Andersen A, Yding Andersen C. Survival of primordial follicles following prolonged transportation of ovarian tissue prior to cryopreservation. Hum Reprod. 2003b;18:2654–2659. doi: 10.1093/humrep/deg500. [DOI] [PubMed] [Google Scholar]
- Segers I, Mateizel I, Van Moer E, Smitz J, Tournaye H, Verheyen G, De Vos M. In vitro maturation (IVM) of oocytes recovered from ovariectomy specimens in the laboratory: a promising “ex vivo” method of oocyte cryopreservation resulting in the first report of an ongoing pregnancy in Europe. J Assist Reprod Genet. 2015;32:1221–1231. doi: 10.1007/s10815-015-0528-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva JR, Lucci CM, Carvalho FC, Bao SN, Costa SH, Santos RR, Figueiredo JR. Effect of coconut water and Braun-Collins solutions at different temperatures and incubation times on the morphology of goat preantral follicles preserved in vitro. Theriogenology. 2000;54:809–822. doi: 10.1016/S0093-691X(00)00392-7. [DOI] [PubMed] [Google Scholar]
- Smitz J, Dolmans MM, Donnez J, Fortune JE, Hovatta O, Jewgenow K, Picton HM, Plancha C, Shea LD, Stouffer RL, Telfer EE, Woodruff TK, Zelinski MB. Current achievements and future research directions in ovarian tissue culture, in vitro follicle development and transplantation: implications for fertility preservation. Hum Reprod Update. 2010;16:395–414. doi: 10.1093/humupd/dmp056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoop D, Cobo A, Silber S. Fertility preservation for age-related fertility decline. Lancet. 2014;384:1311–1319. doi: 10.1016/S0140-6736(14)61261-7. [DOI] [PubMed] [Google Scholar]
- Suzuki N, Yoshioka N, Takae S, Sugishita Y, Tamura M, Hashimoto S, Morimoto Y, Kawamura K. Successful fertility preservation following ovarian tissue vitrification in patients with primary ovarian insufficiency. Hum Reprod. 2015;30:608–615. doi: 10.1093/humrep/deu353. [DOI] [PubMed] [Google Scholar]
- Tas M, Evecen M, Ozdas OB, Cirit U, Demir K, Birler S, Pabuccuoglu S. Effect of transport and storage temperature of ovaries on in vitro maturation of bitch oocytes. Anim Reprod Sci. 2006;96:30–34. doi: 10.1016/j.anireprosci.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Ting AY, Yeoman RR, Campos JR, Lawson MS, Mullen SF, Fahy GM, Zelinski MB. Morphological and functional preservation of pre-antral follicles after vitrification of macaque ovarian tissue in a closed system. Hum Reprod. 2013;28:1267–1279. doi: 10.1093/humrep/det032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uzelac PS, Delaney AA, Christensen GL, Bohler HC, Nakajima ST. Live birth following in vitro maturation of oocytes retrieved from extracorporeal ovarian tissue aspiration and embryo cryopreservation for 5 years. Fertil Steril. 2015;104:1258–1260. doi: 10.1016/j.fertnstert.2015.07.1148. [DOI] [PubMed] [Google Scholar]
- Waimey KE, Duncan FE, Su HI, Smith K, Wallach H, Jona K, Coutifaris C, Gracia CR, Shea LD, Brannigan RE, Chang RJ, Zelinski MB, Stouffer RL, Taylor RL, TK Woodruff. Future Directions in Oncofertility and Fertility Preservation: A Report from the 2011 Oncofertility Consortium Conference. J Adolesc Young Adult Oncol. 2013;2:25–30. doi: 10.1089/jayao.2012.0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace WH, Smith AG, Kelsey TW, Edgar AE, Anderson RA. Fertility preservation for girls and young women with cancer: population-based validation of criteria for ovarian tissue cryopreservation. Lancet Oncol. 2014;15:1129–1136. doi: 10.1016/S1470-2045(14)70334-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Al Naib A, Sun DW, Lonergan P. Membrane permeability characteristics of bovine oocytes and development of a step-wise cryoprotectant adding and diluting protocol. Cryobiology. 2010;61:58–65. doi: 10.1016/j.cryobiol.2010.05.001. [DOI] [PubMed] [Google Scholar]
- Wilken-Jensen HN, Kristensen SG, Jeppesen JV, Yding Andersen C. Developmental competence of oocytes isolated from surplus medulla tissue in connection with cryopreservation of ovarian tissue for fertility preservation. Acta Obstet Gynecol Scand. 2014;93:32–37. doi: 10.1111/aogs.12264. [DOI] [PubMed] [Google Scholar]
- Wolfe BA, Wildt DE. Development to blastocysts of domestic cat oocytes matured and fertilized in vitro after prolonged cold storage. J Reprod Fertil. 1996;106:135–141. doi: 10.1530/jrf.0.1060135. [DOI] [PubMed] [Google Scholar]
- Woodruff TK. The Oncofertility Consortium–addressing fertility in young people with cancer. Nat Rev Clin Oncol. 2010;7:466–475. doi: 10.1038/nrclinonc.2010.81. [DOI] [PMC free article] [PubMed] [Google Scholar]


