Abstract
Objective
Although both high and low levels of total and low-density lipoprotein (LDL) cholesterol have been associated with poor neuropsychological function, little research has examined nonlinear effects. We examined quadratic relations of cholesterol to performance on a comprehensive neuropsychological battery.
Method
Participants were 190 older adults (53% men, ages 54–83) free of major medical, neurologic, and psychiatric disease. Measures of fasting plasma total and high-density lipoprotein (HDL) cholesterol were assayed, and LDL cholesterol was calculated. Participants completed neuropsychological measures of attention, executive function, memory, visuospatial judgment, and manual speed/dexterity. Multiple regression analyses examined cholesterol levels as quadratic predictors of each measure of cognitive performance, with age (dichotomized as <70 vs. 70+) as an effect modifier.
Results
A significant quadratic effect of total cholesterol2 × age was identified for Logical Memory II (b=−.0013, p=.039), such that the 70+ group performed best at high and low levels of total cholesterol than at mid-range total cholesterol (U-shaped), and the <70 group performed worse at high and low levels of total cholesterol than at mid-range total cholesterol (inverted U-shape). Similarly, significant U- and J-shaped effects of LDL cholesterol2 × age were identified for Visual Reproduction II (b=−.0020, p=.026) and log of Trails B (b=.0001, p=.044). Quadratic associations between HDL cholesterol and cognitive performance were nonsignificant.
Conclusions
Results indicate differential associations between cholesterol and neuropsychological function across different ages and domains of function. High and low total and LDL cholesterol may confer both risk and benefit for suboptimal cognitive function at different ages.
Keywords: lipids, cognitive function, aging
An extensive yet conflicting literature has documented linear associations between total and low-density lipoprotein (LDL) cholesterol and cognitive functioning. For example, high levels of total and/or LDL cholesterol have been associated with poor performance on the Mini-Mental State Examination (MMSE) (Carlsson et al., 2009) and the Modified Mini-Mental State Examination (Yaffe, Barrett-Connor, Lin, & Grady, 2002). High serum levels of cholesterol precursors (lathosterol and lanosterol) have also been associated with poor 5-trial learning and delayed recall of a 15-word list (Teunissen et al., 2003). However, low levels of total and/or LDL cholesterol have been associated with poor cognitive functioning as well. For instance, low total and/or LDL cholesterol has been associated with slowed psychomotor speed in university students (D. Benton, 1995) and healthy adults (Muldoon, Ryan, Matthews, & Manuck, 1997; Zhang, Muldoon, & McKeown, 2004). Low total and LDL cholesterol were associated with worse performance on a memory factor score in a non-demented elderly sample (West et al., 2008), and similarly, low LDL cholesterol was related to poorer verbal memory performance among 52–63-year-old women (Henderson, Guthrie, & Dennerstein, 2003).
In the longitudinal literature, growing evidence suggests that elevations in midlife total and LDL cholesterol increase risk of accelerated cognitive decline and dementia, as demonstrated in a recent meta-analysis (Anstey, Lipnicki, & Low, 2008). However, longitudinal data from the Framingham Heart Study cohort (Elias, Elias, D'Agostino, Sullivan, & Wolf, 2005) and the National Heart, Lung, and Blood Institute Twin Study (Swan, LaRue, Carmelli, Reed, & Fabsitz, 1992) show associations between lower total cholesterol values and accelerated declines in performance on measures of abstract reasoning, attention/concentration, executive function, word fluency, and psychomotor speed.
Researchers have devoted less attention to HDL cholesterol and cognition, but the few studies that exist seem to suggest a cognitive advantage of higher HDL levels. In cross-sectional studies, higher HDL levels have been associated with higher MMSE scores in centenarians (Atzmon et al., 2002) and better working memory, MMSE, and global composite performance in 60–98-year-olds in the Maine-Syracuse Study (Crichton, Elias, Davey, Sullivan, & Robbins, 2014). Longitudinally, low baseline HDL levels have predicted poor future memory performance across two studies, at 12- and 5-year follow-up, respectively (Komulainen et al., 2007; Singh-Manoux et al., 2008). In contrast to these patterns, one notable inconsistency involves a study in which higher late-life HDL levels were correlated with increased plaques and neurofibrillary tangles at autopsy in a population-based sample (Launer, White, Petrovitch, Ross, & Curb, 2001).
Multiple explanations could account for the seemingly conflicting findings that occur in the literature. Omitted effect modifiers such as statin use may obscure true patterns of association (Benito-Leon, Louis, Vega, & Bermejo-Pareja, 2010), and pathologic declines in cholesterol can occur in the years prior to diagnosis of mild cognitive impairment (Tukiainen et al., 2012) and dementia (Mielke et al., 2005). Findings from studies with truncated age ranges, interpreted in isolation, may also appear to contradict one another, and important differences in sample characteristics are plentiful (e.g., demographic, geographic, and health-related differences). However, none of these possibilities, either alone or in concert, presents an entirely satisfactory explanation for the apparent discrepancies of the current cholesterol-cognition literature.
Notably, separate literatures have detected nonlinear associations between other cardiovascular risk factors and cognitive performance. For example, inverse U-shaped or J-shaped cross-sectional associations between blood pressure and cognitive function have been noted, such that both high and low pressures are related to poorer performance on screening and domain-specific cognitive measures (Molander, Gustafson, & Lovheim, 2010; Morris et al., 2002). Highly similar findings have been noted in the obesity literature, such that both underweight and overweight individuals show the poorest cognitive functioning (Sabia, Kivimaki, Shipley, Marmot, & Singh-Manoux, 2009; Sturman et al., 2008). It follows that the cholesterol-cognition literature could benefit from corresponding analysis. In that regard, nonlinear longitudinal relations of total cholesterol to cognitive decline were identified among >1,600 participants in the Baltimore Longitudinal Study of Aging (Wendell, Waldstein, & Zonderman, 2014). Specifically, higher cholesterol levels were associated with cognitive decline in the middle-aged (40–59 years) and young-old (60–69 years), whereas lower cholesterol levels were related to cognitive decline among old-old (80–89 years) participants. Another study has implied a similar nonlinear pattern of findings, albeit without direct examination of nonlinear effects (Reynolds, Gatz, Prince, Berg, & Pedersen, 2010).
The present study thus aimed to augment the current understanding of cholesterol-cognition associations. While the study serves as an extension of our group’s prior nonlinear longitudinal examination of total cholesterol (Wendell et al., 2014), to our knowledge, no prior study has directly addressed cholesterol subcomponents nonlinearly. We examined potential quadratic relations of multiple cholesterol levels (total, LDL, and HDL cholesterol) to performance across a relatively comprehensive neuropsychological battery, including measures of attention, executive function, memory, visuospatial judgment, and manual dexterity/speed. Based on the implications of prior literature, we hypothesized that lower total and LDL cholesterol would be associated with the poorest cognitive performance in older participants (>70 years), with the opposite pattern appearing among middle-aged and young-old participants (<70 years). However, we expected these associations to show inflection points at mid-range cholesterol levels, such that a simple linear dose-response curve would not best describe the associations. Less evidence points toward potential HDL cholesterol nonlinearity (Wendell, Katzel, & Waldstein, 2013), so we anticipated either linear or marginal nonlinear effects to arise in analyses examining this cholesterol subcomponent.
Method
Participants
Participants were 190 stroke- and dementia-free, community-dwelling older adults enrolled in a cross-sectional study of the relation of cardiovascular risk factors to subtle brain abnormalities and cognition (Waldstein & Katzel, 2006; Waldstein, Lefkowitz, et al., 2010). Participants were recruited by local advertisement, from the Geriatric Research Education and Clinical Center at the Baltimore Veterans Affairs Medical Center (B-VAMC), and by general advertisement at the B-VAMC. Participants gave a full medical history and underwent extensive physical, medical, neuroradiological, and neuropsychological examinations over the course of multiple study visits occurring within a 3-month window. Medical exclusions were: self-reported history or clinical evidence of cardiovascular disease (except mild-to-moderate hypertension), diabetes mellitus, other major medical disease (e.g., renal, hepatic, pulmonary), neurologic disease (including stroke, transient ischemic attack, and known or suspected dementia, with MMSE<24), psychiatric disorder, severe head injury (loss of consciousness >30 minutes), or medications affecting central nervous system function. Participants provided written informed consent according to the guidelines of the Institutional Review Boards of University of Maryland, Baltimore and University of Maryland, Baltimore County.
Biomedical Assessment
Participants underwent a comprehensive medical evaluation that included history, physical examination, blood chemistries, and an oral glucose tolerance test. Age and education were assessed in years. Blood for assay was drawn in the General Clinical Research Center at the University of Maryland Medical Center in Baltimore, Maryland after an overnight fast. Total cholesterol, HDL cholesterol, and glucose levels were determined enzymatically. LDL cholesterol concentrations were calculated using the Friedewald equation [LDL = total cholesterol – HDL – triglycerides/5.0 (mg/dL)]. Clinical assessment of blood pressure was performed on two to three occasions with participants in a seated position using an automated vital signs monitor (Dinamap Model #1846SX, Critikon, Tampa, FL) and appropriately sized occluding cuff. The readings were averaged to yield an estimate of participants’ resting systolic and diastolic blood pressures. Alcohol consumption was assessed as the average number of drinks consumed per week over the past month, with one drink defined as 12 oz. beer, 4 oz. wine, or 1 oz. hard liquor. Smoking status was assessed as ever vs. never. Current antihypertensive and lipid-lowering medication use was recorded.
Neuropsychological Assessment
Participants underwent neuropsychological testing in the Cardiovascular Behavioral Medicine Laboratory at University of Maryland, Baltimore County. Tests of attention and executive function included the Trail Making Test, Parts A and B (Reitan, 1992) and Digit Span of the Wechsler Adult Intelligence Scales-Revised (WAIS-R) (Wechsler, 1981). Tests of memory included the Logical Memory and Visual Reproduction subtests from the Wechsler Memory Scales-Revised (WMS-R) (Wechsler, 1987). WAIS-R Block Design (Wechsler, 1981) and Judgment of Line Orientation (A. L. Benton, Sivian, & Hamsher, 1983) assessed visual spatial abilities. Psychomotor speed and manual dexterity were assessed with the Grooved Pegboard Test (Matthews & Kløve, 1964). Lastly, participants completed the self-report Beck Depression Inventory to assess current depressive symptoms (Beck, 1987).
Statistical Analyses
All statistical analyses were performed using SAS version 9.2 (Cary, NC). Data were carefully evaluated for normality and homoscedasticity. Independent samples t-tests and chi-square tests assessed presence of any significant differences across age groups. Logarithmic transformations were indicated for Visual Reproduction I, Trail Making Test, and the Grooved Pegboard Test (bilaterally) due to non-normal distributions. Separate general linear model (GLM) multiple regression analyses were constructed for each neuropsychological outcome variable. Age was dichotomized at 70 (<70 = 0; ≥70 = 1) to examine group differences between younger and older participants according to Burnside, Ebersole, and Monea’s (1979) widely-used age labels. The former age category included middle-aged (ages 40–60) and young-old (ages 60–69) participants, while the latter category included middle-aged old (ages 70–79) and old-old (ages 80–89) participants. This cut-off represents a meaningful transition period among older adults with respect to cognitive decline, and prior research has identified cholesterol-cognition differences using this threshold (Wendell et al., 2014). Quadratic terms of interest included total cholesterol2 × age, LDL cholesterol2 × age, and HDL cholesterol2 × age. Linear cholesterol terms were also included to ensure models were fully specified (i.e., all models included cholesterol, cholesterol × age, cholesterol2, cholesterol2 × age terms). Covariates included sex, education (years), systolic blood pressure (mm Hg), fasting plasma glucose (mg/dL), alcohol (drinks per week over last month), smoking (ever = 1; never = 0), depressive symptoms (Beck Depression Inventory total score), antihypertensive medication use (yes = 1; no = 0), and lipid-lowering medication use (yes = 1, no = 0). To explore the relative role(s) of covariates in these analyses, secondary hierarchical analyses were also computed including model (A): age group and cholesterol terms only, and model (B): model (A) + sex and education. Please refer to the online supplement for these results.
Results
Participants were 53% men, 88% white, and ranged in age from 54 to 83 years (mean = 66 years). Age-stratified sample characteristics (Table 1) show that participants were relatively healthy and well educated. Additional cholesterol-stratified sample characteristics can be found in the online supplement. Chi-square tests and two-tailed independent samples tests t-tests revealed no significant group differences according to age group on demographic or health status variables (all ps > .05). Not unexpectedly, younger participants performed significantly better on multiple neuropsychological tests, including Logical Memory (I and II), Visual Reproduction (I and II), Trail Making Test (Parts A and B), Block Design, and the Grooved Pegboard Test (both hands).
Table 1.
Sample Characteristics
| Age <70 (n=126) | Age ≥70 (n=64) | ||
|---|---|---|---|
| Variable | Mean (SD) or % | Mean (SD) or % | Effect size (d or φ)† |
| Age | 62.2 (4.4) | 74.4 (3.2) | 3.17** |
| Sex (% male) | 52 | 56 | 0.04 |
| Smoking (% ever) | 50 | 56 | 0.06 |
| Antihypertensive medications (% currently taking) | 24 | 30 | 0.06 |
| Lipid-lowering medications (% currently taking) | 8 | 13 | 0.07 |
| Education (years) | 16.4 (2.7) | 15.9 (3.2) | 0.17 |
| Systolic blood pressure (mm Hg) | 128 (16.5) | 129 (16.1) | 0.06 |
| Glucose (mg/dL) | 94.7 (11.1) | 94.1 (9.0) | 0.06 |
| Alcohol (drinks/week) | 2.7 (3.0) | 2.5 (3.8) | 0.06 |
| Beck Depression Inventory (total score) | 4.4 (4.2) | 3.8 (4.4) | 0.14 |
| Low-density lipoprotein cholesterol (mg/dL) | 121 (25.4) | 122 (31.8) | 0.04 |
| High-density lipoprotein cholesterol (mg/dL) | 53 (16.7) | 53 (15.1) | <0.01 |
| Total cholesterol (mg/dL) | 196 (30.5) | 197 (36.2) | 0.03 |
| Digit Span Forward (total score) | 8.2 (2.0) | 8.1 (2.1) | 0.05 |
| Digit Span Backward (total score) | 7.3 (2.5) | 6.8 (2.5) | 0.20 |
| Logical Memory I (total score) | 25.7 (6.1) | 23.5 (7.2) | 0.33* |
| Logical Memory II (total score) | 20.9 (7.3) | 17.8 (8.5) | 0.39* |
| Visual Reproduction I (total score) | 33.3 (5.3) | 31.6 (5.3) | 0.32* |
| Visual Reproduction II (total score) | 26.8 (7.8) | 21.6 (9.1) | 0.61** |
| Trail Making Test, Part A (seconds) | 30.1 (9.8) | 35.4 (11.9) | 0.49** |
| Trail Making Test, Part B (seconds) | 75.4 (44.3) | 88.6 (31.3) | 0.34* |
| Judgment of Line Orientation (total score) | 24.5 (4.3) | 23.7 (4.6) | 0.18 |
| Block Design (total score) | 27.0 (9.6) | 24.2 (7.5) | 0.33* |
| Grooved Pegboard Test, dominant (seconds) | 74.6 (10.7) | 89.0 (16.2) | 1.05** |
| Grooved Pegboard Test, non-dominant (seconds) | 83.3 (15.6) | 103.4 (51.8) | 0.53** |
T-tests performed for mean comparisons, with Cohen’s d effect sizes reported. Chi-square tests performed for percentage/categorical comparisons, with phi coefficients reported.
p<.05,
p<.01
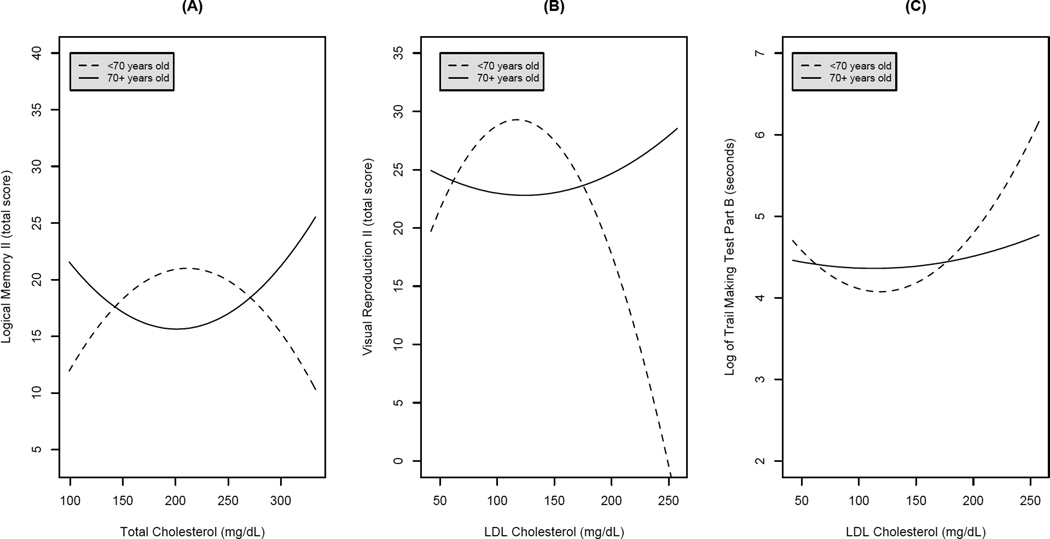
Regression equations, adjusted for sex, education, systolic blood pressure, glucose, alcohol use, smoking, depressive symptoms, antihypertensive use, and lipid-lowering medication use, revealed significant cholesterol2 × age interactive effects for select neuropsychological tests. A significant quadratic effect of total cholesterol2 × age was identified for Logical Memory II (b = −.0013, p = .039), such that the 70+ group performed best at high and low levels of total cholesterol than at mid-range total cholesterol (U-shaped), and the <70 group performed worse at high and low levels of total cholesterol than at mid-range total cholesterol (inverted U-shape). Similarly, significant U- and J-shaped effects of LDL cholesterol2 × age were identified for Visual Reproduction II (b = −.0020, p = .026) and log of Trails B (b = .0001, p = .044). The Figure illustrates these patterns graphically for each of these significant measures. Beta coefficients and associated significance levels for all quadratic total and LDL cholesterol terms are presented in Table 2. Note that total and LDL cholesterol were examined in separate models; the quadratic cholesterol terms are presented alongside one another only for brevity. Quadratic associations between HDL cholesterol and cognitive performance were nonsignificant.
Figure 1.
Significant nonlinear associations between cholesterol and cognitive performance. (A) Total cholesterol and Logical Memory II performance. (B) LDL cholesterol and Visual Reproduction II performance. (C) LDL cholesterol and log of Trail Making Test, Part B performance.
Table 2.
Results from Regression Models Predicting Neuropsychological Test Performance from Cholesterol and Covariates*
| Neuropsychological Test | Total cholesterol2 × Age | LDL cholesterol2 × Age | ||||||
|---|---|---|---|---|---|---|---|---|
| b | SE | p | r2 † | b | SE | p | r2 † | |
| Digit Span Forward (total score) | −.00002 | .0002 | .90 | .0001 | .00001 | .0002 | .96 | <.0001 |
| Digit Span Backward (total score) | −.0004 | .0002 | .057 | .0192 | −.0005 | .0003 | .057 | .0194 |
| Logical Memory I (total score) | −.0005 | .0005 | .31 | .0051 | −.0005 | .0007 | .50 | .0023 |
| Logical Memory II (total score) | −.0013 | .0006 | .039 | .0202 | −.0012 | .0008 | .14 | .0106 |
| Visual Reproduction I (log total score) | −.00001 | .00001 | .61 | .0013 | −.00002 | .00002 | .27 | .0062 |
| Visual Reproduction II (total score) | −.0009 | .0007 | .21 | .0075 | −.0020 | .0009 | .026 | .0236 |
| Trail Making Test, Part A (log seconds) | .00001 | .00003 | .62 | .0013 | .00004 | .00004 | .22 | .0080 |
| Trail Making Test, Part B (log seconds) | .00004 | .00003 | .19 | .0081 | .0001 | .00004 | .044 | .0195 |
| Judgment of Line Orientation (total score) | −.0002 | .0003 | .50 | .0019 | −.0004 | .0004 | .32 | .0041 |
| Block Design (total score) | −.0009 | .0007 | .19 | .0078 | −.0017 | .0009 | .059 | .0158 |
| Grooved Pegboard Test, dominant (log seconds) | .00002 | .00001 | .14 | .0091 | .00002 | .00002 | .16 | .0083 |
| Grooved Pegboard Test, non-dominant (log seconds) | .00003 | .00002 | .073 | .0152 | .00004 | .00002 | .068 | .0157 |
Models adjusted for age, sex, education, systolic blood pressure, glucose, smoking, alcohol use, depressive symptoms, antihypertensive medication use, and lipid-lowering medication use. Total and LDL cholesterol examined in separate models. Only quadratic cholesterol terms are presented here for brevity; all models included linear cholesterol terms and were fully specified (i.e., cholesterol, cholesterol × age, cholesterol2, cholesterol2 × age).
Values represent covariate-adjusted squared semi-partial correlation coefficients.
Abbreviation: LDL = low-density lipoprotein
Quadratic cholesterol terms approached significance for multiple other neuropsychological tests, including Digit Span Backward (p = .057) and Grooved Pegboard non-dominant hand (p = .073) for total cholesterol2 and Digit Span Backward (p = .057), Block Design (p = .059), and Grooved Pegboard non-dominant hand (p = .068) for LDL cholesterol2. For all non-significant findings, plots of the regression curves revealed nonlinear patterns highly similar to those described above.
Discussion
This study examined associations between multiple cholesterol measurements (total, LDL, and HDL cholesterol) and performance on tests of attention, executive function, auditory-verbal memory, visuospatial memory, visuospatial judgment, and manual speed/dexterity. We found significant quadratic associations between total cholesterol and delayed story recall, as well as LDL cholesterol and delayed figure recall and Trails B performance. Each of these effects was modified by age. Specifically, for Logical Memory II performance, individuals aged 70+ years old performed best at high and low levels of total cholesterol (U-shaped), whereas individuals <70 years old performed best at mid-range total cholesterol (inverted-U-shape). A nearly identical pattern arose for LDL cholesterol and Visual Reproduction II performance, with the exception that individuals <70 years old performed particularly poorly at high levels of LDL cholesterol. For LDL cholesterol and Trails B performance, younger participants (<70 years old) again performed particularly poorly at high levels of LDL cholesterol. However, degree of nonlinearity was less pronounced among older participants (70+ years old). Among younger participants (<70 years old) in particular, the performance difference between mid-range and extreme levels of total or LDL cholesterol approximated or exceeded 1 SD. We found no significant nonlinear effects for HDL cholesterol, or for tests of simple attention, visuospatial judgment, or manual speed/dexterity.
Both high and low levels of total and LDL cholesterol have been associated with cognitive performance across different ages (Wendell et al., 2013; Wendell et al., 2014), but we are unaware of any other study that has directly examined cholesterol subcomponents in a nonlinear manner. Thus, select elements of our findings have been previously identified, but to our knowledge, never within the same study using nonlinear analysis. For instance, Henderson and colleagues (2003) identified associations between low LDL cholesterol and poorer recall of a 10-item supraspan word list among 52- to 63-year-old women, similar to our finding of poorer delayed story and figure recall among 54- to 69-year-olds with lower LDL or total cholesterol. The particularly poor performance of our middle-aged/young-old individuals with high LDL cholesterol on Visual Reproduction II and Trails B is consistent with our prior findings (Wendell et al., 2014) and extends rapidly accumulating evidence relating midlife cardiovascular risk factors to poorer cognitive performance and decline (Anstey et al., 2008; Yaffe et al., 2014). The nonlinearity among our older participants (70+ years) is less striking, and yet there does appear to be at least a slight performance advantage for older participants with higher LDL or total cholesterol across cognitive measures, again similar to prior findings regarding total cholesterol (Wendell et al., 2014). This pattern is consistent with prior studies showing associations between low cholesterol and poorer cognitive performance in the elderly (West et al., 2008).
While the directionality of findings in prior studies has been variable, the broad domains of memory and psychomotor speed have been most often associated with cholesterol levels (Muldoon & Conklin, 2015). This pattern is consistent with our significant findings for Logical Memory II, Visual Reproduction II, and Trail Making Test, Part B. Our results imply that delayed, rather than immediate, recall may be more strongly related to cholesterol levels, and that stimulus modality (auditory-verbal vs. visuospatial) may be immaterial. Our non-significant findings for Trail Making Test, Part A and the Grooved Pegboard Test are surprising in light of previous literature, but it is possible that Part B of the Trail Making Test was more sensitive to speed decrements than these other measures in our relatively healthy sample. Alternatively, a higher-order executive function such as set-shifting may be the pertinent domain, rather than psychomotor speed per se.
Mechanistic explanations for nonlinear patterns in cholesterol-cognition associations are complex yet plausible, in part because different processes likely explain different aspects of the findings. Cholesterol is a critical structural component of neuronal membranes, glial membranes, and myelin sheaths and is also involved in numerous neuronal functions including synaptogenesis, synaptic transmission, and nutrient delivery (Muldoon & Conklin, 2015). While the brain synthesizes its own cholesterol, it also interacts continuously with peripheral lipids via oxysterol molecules such as 24S and 27 hydroxycholesterol (Bjorkhem, 2006). Low total and LDL cholesterol may thus be cognitively detrimental due to adverse effects on brain microstructure and function. Indeed, mice fed a high cholesterol diet have shown less cognitive decline on water maze testing and less hippocampal pathological changes than normally fed mice (Li et al., 2012). Low cholesterol has also been associated with increased risk of mortality in humans (Schatz et al., 2001; Schupf et al., 2005). Conversely, high levels of total and LDL cholesterol are formidably associated with atherosclerosis and its multitudinous health consequences, including micro- and macrovascular cerebrovascular disease, coronary disease, and peripheral vascular disease (Navab et al., 1995). High total and LDL cholesterol are also strongly correlated with other potent cardiovascular risk factors such as elevated blood pressure, obesity, and poor aerobic fitness, all of which are independently associated with structural and functional brain outcomes (Waldstein, Wendell, Hosey, Seliger, & Katzel, 2010).
We would therefore hypothesize that among middle-aged and young-old individuals, high total and LDL cholesterol may be cognitively detrimental due to correlated cerebrovascular risk factors and disease, whereas low total and LDL cholesterol may be correspondingly detrimental due to problems with nutrient delivery and harmful effects on brain structure and function. Among the elderly, nonlinearity seems less pronounced. High total and LDL cholesterol are likely beneficial in this age group for the reasons outlined above, but we also suspect that these findings may reflect selective survival. Individuals aged 70+ are more likely to be resistant to the effects of correlated cerebrovascular risk factors and disease. For example, centenarians with diabetes mellitus have relatively fewer complications than younger individuals with diabetes (Davey et al., 2012). Further, old-old individuals may be less prone to age-related cognitive decline (Perls, Morris, Ooi, & Lipsitz, 1993) and at a minimum, may show more variability in cognitive functioning (Miller et al., 2010). As such, results in our older age group may simply reflect these patterns, rather than a direct association between higher total and LDL cholesterol and cognition.
Other indirect mechanisms warrant mention. Cholesterol serves as a precursor molecule of steroid hormones (e.g., cortisol, testosterone, estrogen) that have age- and sex-moderated effects on the brain and cognitive function (Hogervorst, 2013; Wingenfeld & Wolf, 2014). Apolipoprotein E genotype may also play a role given its known associations with lipid metabolism, cognitive decline, and risk of Alzheimer’s disease (Hauser, Narayanaswami, & Ryan, 2011; van den Kommer et al., 2012). In in vitro studies, increased cholesterol has been associated with increased synthesis of beta-amyloid (Stefani & Liguri, 2009), a known pathological component of Alzheimer’s disease.
Strengths of this study include its direct examination of nonlinear effects, inclusion of multiple cholesterol measurements, and use of a well-characterized, relatively healthy sample. The latter strength limits confounding by the known cognitive sequelae of many clinical medical, neurologic, and psychiatric diseases. Study limitations include use of WAIS-R and WMS-R subtests (for consistency with prior research protocols and years of testing) instead of current WAIS-IV and WMS-IV subtests and absence of tests directly assessing language functions. A word-list learning test would also have been useful for comparison with prior literature and analysis of learning efficiency. The study’s cross-sectional design prohibits causal inferences, and its relatively well-educated, non-representative sample limits its generalizability. Lastly, we consider this an exploratory rather than confirmatory study. As such, we conducted many hypothesis tests without regard to adjustments for multiple testing. We are hopeful that our results will lead to hypothesis-driven confirmatory analyses with strict type I error control.
In sum, results from this investigation indicate nonlinear age-modified associations between total and LDL cholesterol levels and performance on measures of memory and speeded executive functioning. Middle-aged and young-old individuals showed inverse U- or J-shaped associations between cholesterol and cognitive performance, whereas middle-old and old-old individuals showed more attenuated U- or J-shaped associations. These findings imply that mid-range total and LDL cholesterol levels may be cognitively “ideal” among the middle-aged/young-old, whereas mid-range cholesterol levels may actually confer cognitive disadvantage among the middle-aged-old and old-old participants. Quadratic associations involving HDL cholesterol or measures of simple attention, visuospatial judgment, or manual speed/dexterity were not identified. These results suggest that ostensibly conflicting findings across prior studies may not be truly contradictory. Rather, it may be the case that abbreviated age ranges, variable covariate inclusion, and omission of nonlinear analysis have obscured true cholesterol-cognition associations. Such patterns require future replication but could have important clinical implications if causal relations are identified. For instance, younger (<70 years old) individuals may benefit cognitively from mid-range cholesterol levels. In contrast, among 70+ year-olds, lowering to mid-range cholesterol may be cognitively detrimental in the absence of other cardiovascular risk factors. Additional longitudinal and intervention work will clarify presence of causality, and if present, whether these associations operate via direct and/or indirect pathways.
Supplementary Material
Acknowledgments
Funding: This work was supported by National Institutes of Health (NIH) grants [grant numbers R29 AG15112, 5RO1 AG015112, P30 AG028747, K24 AG00930], a Veterans Affairs Merit Grant, and the Department of Veterans Affairs Baltimore Geriatric Research Education and Clinical Center. The National Institute on Aging Intramural Research Program of the National Institutes of Health also supported a portion of this research.
References
- Anstey KJ, Lipnicki DM, Low LF. Cholesterol as a risk factor for dementia and cognitive decline: A systematic review of prospective studies with meta-analysis. Am J Geriatr Psychiatry. 2008;16:343–354. doi: 10.1097/JGP.0b013e31816b72d4. [DOI] [PubMed] [Google Scholar]
- Atzmon G, Gabriely I, Greiner W, Davidson D, Schechter C, Barzilai N. Plasma HDL levels highly correlate with cognitive function in exceptional longevity. J Gerontol A Biol Sci Med Sci. 2002;57:M712–M715. doi: 10.1093/gerona/57.11.m712. [DOI] [PubMed] [Google Scholar]
- Beck AT. Beck Depression Inventory: Manual. San Antonio, TX: Psychological Corporation; 1987. [Google Scholar]
- Benito-Leon J, Louis ED, Vega S, Bermejo-Pareja F. Statins and cognitive functioning in the elderly: A population-based study. J Alzheimers Dis. 2010;21:95–102. doi: 10.3233/JAD-2010-100180. [DOI] [PubMed] [Google Scholar]
- Benton AL, Sivian AB, Hamsher K. Contributions to neuropsychological assessment. Orlando, FL: Psychological Assessment Resources; 1983. [Google Scholar]
- Benton D. Do low cholesterol levels slow mental processing? Psychosom Med. 1995;57:50–53. doi: 10.1097/00006842-199501000-00008. [DOI] [PubMed] [Google Scholar]
- Bjorkhem I. Crossing the barrier: Oxysterols as cholesterol transporters and metabolic modulators in the brain. J Intern Med. 2006;260:493–508. doi: 10.1111/j.1365-2796.2006.01725.x. [DOI] [PubMed] [Google Scholar]
- Burnside IM, Ebersole P, Monea HE. Psychosocial caring throughout the life span. New York: McGraw Hill; 1979. [Google Scholar]
- Carlsson CM, Nondahl DM, Klein BE, McBride PE, Sager MA, Schubert CR, Cruickshanks KJ. Increased atherogenic lipoproteins are associated with cognitive impairment: Effects of statins and subclinical atherosclerosis. Alzheimer Dis Assoc Disord. 2009;23:11–17. doi: 10.1097/wad.0b013e3181850188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crichton GE, Elias MF, Davey A, Sullivan KJ, Robbins MA. Higher HDL cholesterol is associated with better cognitive function: The Maine-Syracuse Study. J Int Neuropsychol Soc. 2014;20:961–970. doi: 10.1017/S1355617714000885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey A, Lele U, Elias MF, Dore GA, Siegler IC, Johnson MA, Georgia Centenarian S. Diabetes mellitus in centenarians. J Am Geriatr Soc. 2012;60:468–473. doi: 10.1111/j.1532-5415.2011.03836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias PK, Elias MF, D'Agostino RB, Sullivan LM, Wolf PA. Serum cholesterol and cognitive performance in the Framingham Heart Study. Psychosom Med. 2005;67:24–30. doi: 10.1097/01.psy.0000151745.67285.c2. [DOI] [PubMed] [Google Scholar]
- Hauser PS, Narayanaswami V, Ryan RO. Apolipoprotein e: From lipid transport to neurobiology. Prog Lipid Res. 2011;50:62–74. doi: 10.1016/j.plipres.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson VW, Guthrie JR, Dennerstein L. Serum lipids and memory in a population based cohort of middle age women. J Neurol Neurosurg Psychiatry. 2003;74:1530–1535. doi: 10.1136/jnnp.74.11.1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogervorst E. Effects of gonadal hormones on cognitive behaviour in elderly men and women. J Neuroendocrinol. 2013;25:1182–1195. doi: 10.1111/jne.12080. [DOI] [PubMed] [Google Scholar]
- Komulainen P, Lakka TA, Kivipelto M, Hassinen M, Helkala EL, Haapala I, Rauramaa R. Metabolic syndrome and cognitive function: A population-based follow-up study in elderly women. Dement Geriatr Cogn Disord. 2007;23:29–34. doi: 10.1159/000096636. [DOI] [PubMed] [Google Scholar]
- Launer LJ, White LR, Petrovitch H, Ross GW, Curb JD. Cholesterol and neuropathologic markers of AD: A population-based autopsy study. Neurology. 2001;57:1447–1452. doi: 10.1212/wnl.57.8.1447. [DOI] [PubMed] [Google Scholar]
- Li L, Xiao N, Yang X, Gao J, Ding J, Wang T, Xiao M. A high cholesterol diet ameliorates hippocampus-related cognitive and pathological deficits in ovariectomized mice. Behav Brain Res. 2012;230:251–258. doi: 10.1016/j.bbr.2012.02.024. [DOI] [PubMed] [Google Scholar]
- Mielke MM, Zandi PP, Sjogren M, Gustafson D, Ostling S, Steen B, Skoog I. High total cholesterol levels in late life associated with a reduced risk of dementia. Neurology. 2005;64:1689–1695. doi: 10.1212/01.WNL.0000161870.78572.A5. [DOI] [PubMed] [Google Scholar]
- Miller LS, Mitchell MB, Woodard JL, Davey A, Martin P, Poon LW, Siegler IC. Cognitive performance in centenarians and the oldest old: Norms from the Georgia Centenarian Study. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn. 2010;17:575–590. doi: 10.1080/13825585.2010.481355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molander L, Gustafson Y, Lovheim H. Low blood pressure is associated with cognitive impairment in very old people. Dement Geriatr Cogn Disord. 2010;29:335–341. doi: 10.1159/000289821. [DOI] [PubMed] [Google Scholar]
- Morris MC, Scherr PA, Hebert LE, Bennett DA, Wilson RS, Glynn RJ, Evans DA. Association between blood pressure and cognitive function in a biracial community population of older persons. Neuroepidemiology. 2002;21:123–130. doi: 10.1159/000054809. [DOI] [PubMed] [Google Scholar]
- Muldoon MF, Conklin SM. Effects of cholesterol and n-3 fatty acids on cognitive functioning, decline, and dementia. In: Waldstein SR, Elias MF, editors. Neuropsychology of cardiovascular disease. Vol. 2. New York: Taylor & Francis; 2015. pp. 150–183. [Google Scholar]
- Muldoon MF, Ryan CM, Matthews KA, Manuck SB. Serum cholesterol and intellectual performance. Psychosom Med. 1997;59:382–387. doi: 10.1097/00006842-199707000-00008. [DOI] [PubMed] [Google Scholar]
- Navab M, Fogelman AM, Berliner JA, Territo MC, Demer LL, Frank JS, Lusis AJ. Pathogenesis of atherosclerosis. American Journal of Cardiology. 1995;76:18C–23C. doi: 10.1016/s0002-9149(99)80466-4. [DOI] [PubMed] [Google Scholar]
- Perls TT, Morris JN, Ooi WL, Lipsitz LA. The relationship between age, gender and cognitive performance in the very old: The effect of selective survival. J Am Geriatr Soc. 1993;41:1193–1201. doi: 10.1111/j.1532-5415.1993.tb07302.x. [DOI] [PubMed] [Google Scholar]
- Reitan R. Trail Making Test: Manual for administration and scoring. Tucson, AZ: Reitan Neuropsychological Laboratory; 1992. [Google Scholar]
- Reynolds CA, Gatz M, Prince JA, Berg S, Pedersen NL. Serum lipid levels and cognitive change in late life. J Am Geriatr Soc. 2010;58:501–509. doi: 10.1111/j.1532-5415.2010.02739.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabia S, Kivimaki M, Shipley MJ, Marmot MG, Singh-Manoux A. Body mass index over the adult life course and cognition in late midlife: The Whitehall II cohort study. Am J Clin Nutr. 2009;89:601–607. doi: 10.3945/ajcn.2008.26482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatz IJ, Masaki K, Yano K, Chen R, Rodriguez BL, Curb JD. Cholesterol and all-cause mortality in elderly people from the Honolulu Heart Program: A cohort study. Lancet. 2001;358:351–355. doi: 10.1016/S0140-6736(01)05553-2. [DOI] [PubMed] [Google Scholar]
- Schupf N, Costa R, Luchsinger J, Tang MX, Lee JH, Mayeux R. Relationship between plasma lipids and all-cause mortality in nondemented elderly. J Am Geriatr Soc. 2005;53:219–226. doi: 10.1111/j.1532-5415.2005.53106.x. [DOI] [PubMed] [Google Scholar]
- Singh-Manoux A, Britton A, Kivimaki M, Gueguen A, Halcox J, Marmot M. Socioeconomic status moderates the association between carotid intima-media thickness and cognition in midlife: Evidence from the Whitehall II study. Atherosclerosis. 2008;197:541–548. doi: 10.1016/j.atherosclerosis.2007.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefani M, Liguri G. Cholesterol in Alzheimer's disease: Unresolved questions. Curr Alzheimer Res. 2009;6:15–29. doi: 10.2174/156720509787313899. [DOI] [PubMed] [Google Scholar]
- Sturman MT, de Leon CF, Bienias JL, Morris MC, Wilson RS, Evans DA. Body mass index and cognitive decline in a biracial community population. Neurology. 2008;70:360–367. doi: 10.1212/01.wnl.0000285081.04409.bb. [DOI] [PubMed] [Google Scholar]
- Swan GE, LaRue A, Carmelli D, Reed TE, Fabsitz RR. Decline in cognitive performance in aging twins. Heritability and biobehavioral predictors from the National Heart, Lung, and Blood Institute Twin Study. Arch Neurol. 1992;49:476–481. doi: 10.1001/archneur.1992.00530290058012. [DOI] [PubMed] [Google Scholar]
- Teunissen CE, De Vente J, von Bergmann K, Bosma H, van Boxtel MP, De Bruijn C, Lutjohann D. Serum cholesterol, precursors and metabolites and cognitive performance in an aging population. Neurobiol Aging. 2003;24:147–155. doi: 10.1016/s0197-4580(02)00061-1. [DOI] [PubMed] [Google Scholar]
- Tukiainen T, Jylanki P, Makinen VP, Grohn O, Hallikainen M, Soininen H, Ala-Korpela M. Mild cognitive impairment associates with concurrent decreases in serum cholesterol and cholesterol-related lipoprotein subclasses. J Nutr Health Aging. 2012;16:631–635. doi: 10.1007/s12603-011-0341-9. [DOI] [PubMed] [Google Scholar]
- van den Kommer TN, Dik MG, Comijs HC, Lutjohann D, Lips P, Jonker C, Deeg DJ. The role of extracerebral cholesterol homeostasis and apoe e4 in cognitive decline. Neurobiol Aging. 2012;33:622, e617–e628. doi: 10.1016/j.neurobiolaging.2011.02.019. [DOI] [PubMed] [Google Scholar]
- Waldstein SR, Katzel LI. Interactive relations of central versus total obesity and blood pressure to cognitive function. Int J Obes (Lond) 2006;30:201–207. doi: 10.1038/sj.ijo.0803114. [DOI] [PubMed] [Google Scholar]
- Waldstein SR, Lefkowitz DM, Siegel EL, Rosenberger WF, Spencer RJ, Tankard CF, Katzel L. Reduced cerebral blood flow in older men with higher levels of blood pressure. J Hypertens. 2010;28:993–998. doi: 10.1097/hjh.0b013e328335c34f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldstein SR, Wendell CR, Hosey MM, Seliger SL, Katzel LI. Cardiovascular disease and neurocognitive function. In: Armstrong C, Morrow LA, editors. Handbook of medical neuropsychology: Applications of cognitive neuroscience. New York: Springer; 2010. [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale-Revised manual. San Antonio, TX: The Psychological Corporation; 1981. [Google Scholar]
- Wechsler D. Manual for the Wechsler Memory Scale-Revised. San Antonio, TX: The Psychological Corporation; 1987. [Google Scholar]
- Wendell CR, Katzel LI, Waldstein SR. Nonlinear relations of cardiovascular risk factors to neuropsychological function and dementia. In: Sturmberg JP, Martin CM, editors. Handbook of systems and complexity in health. New York: Springer; 2013. pp. 379–396. [Google Scholar]
- Wendell CR, Waldstein SR, Zonderman AB. Nonlinear longitudinal trajectories of cholesterol and neuropsychological function. Neuropsychology. 2014;28:106–112. doi: 10.1037/neu0000002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West R, Beeri MS, Schmeidler J, Hannigan CM, Angelo G, Grossman HT, Silverman JM. Better memory functioning associated with higher total and low-density lipoprotein cholesterol levels in very elderly subjects without the apolipoprotein e4 allele. Am J Geriatr Psychiatry. 2008;16:781–785. doi: 10.1097/JGP.0b013e3181812790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingenfeld K, Wolf OT. Stress, memory, and the hippocampus. Front Neurol Neurosci. 2014;34:109–120. doi: 10.1159/000356423. [DOI] [PubMed] [Google Scholar]
- Yaffe K, Barrett-Connor E, Lin F, Grady D. Serum lipoprotein levels, statin use, and cognitive function in older women. Arch Neurol. 2002;59:378–384. doi: 10.1001/archneur.59.3.378. [DOI] [PubMed] [Google Scholar]
- Yaffe K, Vittinghoff E, Pletcher MJ, Hoang TD, Launer LJ, Whitmer R, Sidney S. Early adult to midlife cardiovascular risk factors and cognitive function. Circulation. 2014;129:1560–1567. doi: 10.1161/CIRCULATIONAHA.113.004798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Muldoon MF, McKeown RE. Serum cholesterol concentrations are associated with visuomotor speed in men: Findings from the Third National Health and Nutrition Examination Survey, 1988–1994. Am J Clin Nutr. 2004;80:291–298. doi: 10.1093/ajcn/80.2.291. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.