Abstract
The role of percutaneous cholecystostomy (PC) in the management of acute cholecystitis and cholangitis is outlined in the revised 2013 Tokyo Guidelines. These two emergencies constitute the vast majority of PC performed today for therapeutic purposes, and research has repeatedly shown the utility of PC in these conditions. PC is typically employed in the management of critically ill patients who are not surgical candidates. Indications and contraindications to PC are reviewed. Additional innovative applications of PC have been developed since it was first described in 1980. These include biliary drainage, dilation of biliary strictures, and stenting of the biliary tree including the common bile duct. Special consideration must be given to the patient selection criteria when deciding who can benefit from PC. Patient comorbidities can also influence the PC technique employed. Both transhepatic and transperitoneal approaches have distinct advantages and disadvantages. The technical success rate for PC is 95 to 100% and the complication rate is extremely low. Most complications are minor.
Keywords: percutaneous cholecystostomy, gallbladder, gallstones, cholecystitis, interventional radiology
Objectives: Upon completion of this article, the reader will be able to (1) identify the evidence-based indications, techniques, and outcomes of percutaneous cholecystostomy (PC) and (2) also describe the unique advantages, patient selection criteria, and risks/benefits of different technical approaches of PC.
Accreditation: This activity has been planned and implemented in accordance with the Essential Areas and Policies of the Accreditation Council for Continuing Medical Education (ACCME) through the joint providership of Tufts University School of Medicine (TUSM) and Thieme Medical Publishers, New York. TUSM is accredited by the ACCME to provide continuing medical education for physicians.
Credit: Tufts University School of Medicine designates this journal-based CME activity for a maximum of 1 AMA PRA Category 1 Credit™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Percutaneous cholecystostomy (PC) is employed for the treatment of various gallbladder conditions including biliary emergencies such as cholecystitis or cholangitis, malignant or benign biliary obstruction, gallbladder perforation, and percutaneous biliary stone removal. Although it is seldom a first-line treatment, cholecystostomy is often preferred in patients too ill to tolerate alternative procedures. Since its introduction in 1980, novel technical innovations have redefined the role of PC in the management of gallbladder pathology.1 Research in the past decade has resulted in ongoing evolution of the indications, techniques, and complications of the procedure.
PC definition: “Therapeutic procedure that involves the sterile placement of a needle into the gallbladder with use of imaging guidance to aspirate bile. This is commonly followed by sterile placement of a tube for external drainage of gallbladder contents, which completes the procedure.”2
Indications for Percutaneous Cholecystostomy
Practice guidelines introduced by the Society of Interventional Radiology in 2010 recommend that 95% of PC procedures be performed for direct gallbladder access to either manage cholecystitis or remove gallstones.2 Other indications for PC are as a second-line means of biliary tract access (when direct intrahepatic biliary tract access is not available) to decompress the biliary tract, dilate biliary strictures, and stent malignant lesions, among others.
Acute Cholecystitis
Acute cholecystitis can be divided into two groups: acute calculous cholecystitis and acute acalculous cholecystitis. Gallstones are present in approximately 90% of cases of cholecystitis and are thought to incite the process by obstructing outflow of bile from the gallbladder.3 In the remaining 10% of cases, gallstones are not present and therefore termed “acalculous cholecystitis.”
Acute calculous cholecystitis patients are approximately 60% women, but cases in men are disproportionately more severe in comparison. The pathogenesis is related to gallstone impaction within the gallbladder or the cystic duct, causing bile outflow obstruction and subsequent distention and wall edema followed by ischemia and necrosis in severe cases (gangrenous cholecystitis). Initial sterile inflammation is commonly followed by bacterial superinfection, most commonly with Escherichia coli, Klebsiella, and Enterococcus species. Infection with gas-forming organisms results in gas within the wall or lumen of the gallbladder (emphysematous cholecystitis). Untreated cases may progress to perforation of the wall, abscess formation, or generalized peritonitis.3 Uncommonly, inflammation or ischemia can lead to intraluminal hemorrhage within the gallbladder (hemorrhagic cholecystitis), which can also be seen in cases of perforated cholecystitis and lead to hemorrhage within the peritoneal cavity. Mortality rates in cases of perforated cholecystitis range from 12 to 16%.4
Acute acalculous cholecystitis is defined as an “acute necroinflammatory disease of the gallbladder in the absence of cholelithiasis”5 and is clinically indistinguishable from acute calculous cholecystitis; however, the pathogenesis is multifactorial and is thought to occur secondary to bile stasis or ischemia in the setting of critical illness. The most common risk factors in descending order are trauma, recent surgery, shock, burn injury, sepsis, intensive care unit admission, total parenteral nutrition (TPN), and prolonged fasting. Complications including gangrene, perforation, and empyema occur in approximately 40% of cases and mortality ranges from 10 to 90% depending on early or late diagnosis. Imaging is often the best diagnostic test in these patients due to multiple confounding factors. Cholecystectomy is generally considered the definitive treatment but is often impractical in this patient population. Many studies have concluded that PC is often necessary and may also be definitive.5
Studies have shown PC to be an effective treatment for resolving acute cholecystitis in up to 90% of patients6 and the definitive treatment in up to 54% of patients7 with acute calculous cholecystitis. Laparoscopic cholecystectomy (LC) is the standard of care, but perioperative mortality has been shown to approach 19% in the critically ill and elderly.8 For these populations, PC can be considered a safer management option9 with a significantly lower reported complication rate.10 Critically ill patients with acalculous cholecystitis similarly fare better when managed with PC rather than LC.9 Further investigative studies are ongoing11 to clarify the role of PC and LC in acute cholecystitis.
The 2013 revised Tokyo Guidelines12 integrate diagnostic imaging such as ultrasonography, computed tomography (CT), and scintigraphy (HIDA scan) with blood tests and local inflammatory signs for the definite diagnosis of acute cholecystitis. In addition to these diagnostic criteria, the Tokyo Guidelines also present a severity grading scale from grade I (mild) to grade III (severe). These guidelines single out patients with grade II (moderate) and grade III (severe) acute cholecystitis for percutaneous intervention. Percutaneous transhepatic gallbladder drainage (PTGBD) with placement of a drainage catheter is recommended for all “surgically unfit patients with cholecystitis,” while percutaneous transhepatic gallbladder aspiration without catheter placement and endoscopic drainage are secondary alternatives without sufficient evidence to recommend them over PTGBD.
Early PC (<24 hours after symptom onset) for patients with inoperable acute severe cholecystitis has been shown to reduce length of hospital stay and procedure-related bleeding when compared with PC performed after 24 hours.13 Although evidence suggests that delayed LC after PC is associated with longer hospitalization and greater morbidity,14 these patients are not surgical candidates and PC is therefore recommended per the Tokyo Guidelines. Another study that examined outcomes in critically ill patients with acute cholecystitis concluded that surgical results in survivors managed with PC are better than those managed without drainage.15 A study randomizing patients with acute cholecystitis to either LC or PC (CHOCOLATE trial11) is currently underway in the Netherlands and is designed to provide evidence-based guidelines on management.
Acute Cholangitis
Acute cholangitis, defined as acute ascending infection of the biliary tree, ranges on a continuum from mild infection to biliary sepsis and shock.
The 2013 revised Tokyo Guidelines recommend endoscopic biliary drainage as the preferred treatment due to its decreased invasiveness, while surgical drainage is discouraged due to increased mortality. Percutaneous transhepatic cholangial drainage (PTCD) as the second-line therapy is recommended in cases where endoscopic intervention is not an option when the papilla is absent or inaccessible due to prior surgery (Whipple or Roux-en-Y anastomosis), upper gastrointestinal tract obstruction, or inability to pass an endoscope. The presence of intrahepatic bile duct stones or peripheral obstruction may also favor PTCD over ERCP. Additionally, PTCD can be offered in cases when a skilled endoscopist is not available or when endoscopic intervention has failed. The preferred route of drainage is via transhepatic biliary tract access, but transcholecystic access can be considered in patients as a second-line option.16
Biliary Tract Access
Access to the biliary tract through the gallbladder (transcholecystic access) is an alternate route that can be considered when usual transhepatic or endoscopic routes are not available. This can occur in cases of malignancy or benign conditions such as diffuse hepatic cysts or nondilated intrahepatic bile ducts.16 Once access is obtained, various therapeutic procedures can be completed, such as stenting of the common bile duct for malignant or benign strictures, or stone removal.
Percutaneous transcholecystic common bile duct stenting can be performed in the setting of malignant lesions compressing the bile duct when both first-line endoscopic retrograde biliary drainage (ERBD) and second-line PTCD options are unavailable. In cases where the intrahepatic biliary tree is not identified with ultrasound or fluoroscopy, PTCD may be deferred in favor of percutaneous transcholecystic access. One study demonstrated a 100% technical success rate for transcholecystic stenting of the common bile duct in patients who were not candidates for ERBD or PTCD.17 The technique involves transhepatic cholecystic access followed by cannulation of the CBD through the cystic duct with a guidewire and the deployment of a metallic stent at the stenotic segment.
Contraindications
No absolute contraindications to PC have been described,18 but relative contraindications may preclude either the transhepatic or transperitoneal route. Coagulopathy is the most common contraindication, and attempts to correct it with platelet and plasma transfusions appear to show benefit when target goals (platelets >50,000 and international normalized ratio of <1.5) prior to intervention are achieved. Allergy to iodinated contrast may preclude any fluoroscopic procedure, but ultrasound-guided PC can still be considered. Patients with ascites can be treated with paracentesis prior to biliary intervention. However, new data suggest that complication rates are low and not significantly different when comparing patients with and without ascites undergoing transhepatic PC tube placement.19 A gallbladder tightly packed with gallstones may also preclude the secure placement of a drainage catheter.18
Preprocedural Considerations
Coagulation abnormalities should be corrected and prophylactic antibiotics are usually given 12 to 24 hours prior to the procedure.20 Preprocedural preparation involves reviewing the patient's cross-sectional MR and CT images to evaluate the anatomy. Ultrasound is also helpful to examine focal gallbladder wall thickening and to determine if bowel may interfere with the procedure. These findings may weigh heavily in the decision to take a transhepatic or transperitoneal approach.
The two access routes for PC are the transhepatic approach where the gallbladder is accessed through the surface in contact with the liver and the transperitoneal approach through the exposed surface of the gallbladder lined by visceral peritoneum. Each approach has distinct advantages. Transhepatic access provides greater catheter stability by anchoring the catheter in the liver parenchyma, reduces bile leakage by tamponade against the liver, and results in quicker maturation of the catheter tract by promoting fibrin sheath formation. It is also preferred in cases where there is ascites or unfavorable anatomy with interposed bowel. Disadvantages of this technique include greater bleeding from passage through the highly vascular liver. Cases of fistula formation and pneumothorax have also been reported.
The transperitoneal route is preferred in cases of diffuse liver disease (including metastases) or coagulopathy. However, a retrospective study did not demonstrate any significant difference in the complication rates for coagulopathic patients who underwent ultrasound-guided cholecystostomy versus patients with a normal coagulation profile.21 The potential risks of pneumothorax, intrahepatic hemorrhage, or hemobiliary fistula are also reduced.22 However, the presence of a friable or emphysematous gallbladder wall generally precludes this approach. Reported complication rates between the two routes of access are not significantly different.16
Technique
PC is performed using radiological guidance. Ultrasound is the modality of choice because of the readily availability of the device in all radiology departments, the portability of the machine, and real-time imaging during the procedure. If the patient is stable enough to be transported out of the ICU to the radiology department, the procedure could also be performed using a CT scanner, which would have the advantage of excellent spatial resolution. Compared with ultrasound, however, using CT guidance can be more cumbersome. CT-guided intervention, however, is often used in patients where ultrasound is unable to visualize the gallbladder due to anatomy (under-distended gallbladder, overlying bowel loops, or body habitus) or pathology (wall edema or gallstones).23
Sedation with intravenous midazolam and fentanyl is usually administered at the start. The gallbladder is punctured with an access needle (22–18 gauge) and appropriate needle position can be confirmed by bile aspiration or contrast injection under fluoroscopy. An 8 or 10F drain catheter, generally with multiple side holes to provide adequate drainage, is most commonly used and is advanced over the wire into the gallbladder. The gallbladder is decompressed, the bile sent for appropriate cultures and antibiotic sensitivity, and the catheter left to gravity drainage. A cholecystogram can be performed at the time of the procedure if using fluoroscopy. Cholecystography at a later date is always useful and performed to evaluate catheter position and cystic duct patency. Following gallbladder decompression, the 2013 Tokyo guidelines recommend drain placement over simple aspiration for all cases meriting percutaneous intervention.
The Seldinger technique is often employed in conjunction with the transhepatic route. After needle puncture, a guidewire is inserted into the gallbladder lumen followed by serial dilation and placement of a drainage tube. Minor bile leaks may be encountered during exchange maneuvers (Figs. 1 2 3).
Fig. 1.
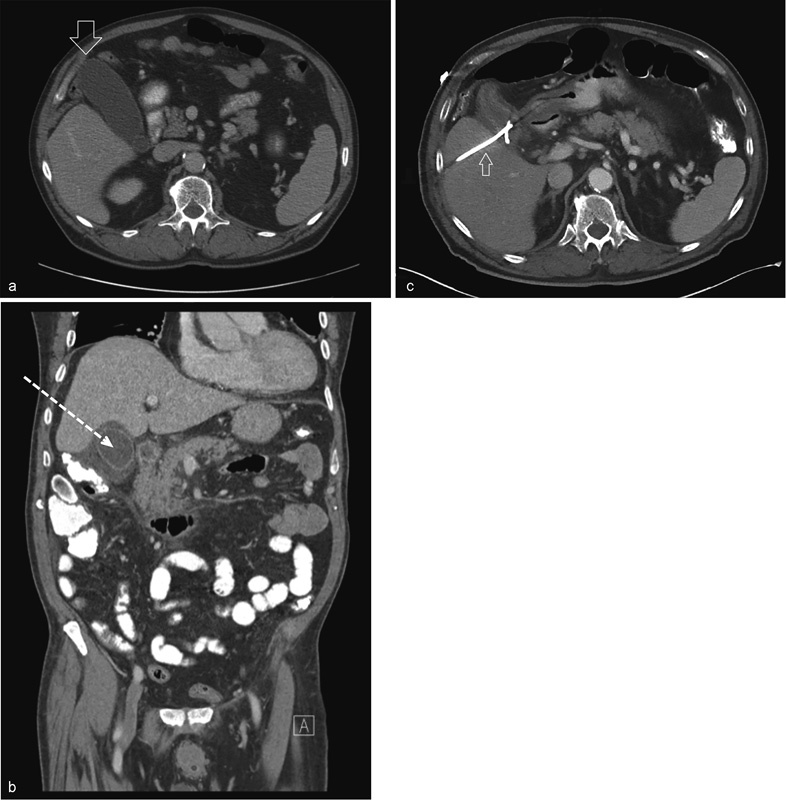
Computed tomography of (a) axial image of an unremarkable gallbladder (open arrow) with normal wall thickness, no pericholecystic fluid, and no adjacent fat stranding; (b) coronal image of acute acalculous cholecystitis with wall thickening, pericholecystic fluid, and fat stranding; the dotted arrow indicates an adequate transhepatic window for percutaneous cholecystostomy (PC); (c) axial image of a decompressed gallbladder after adequate placement of a drainage catheter (small arrow) status post–PC.
Fig. 2.
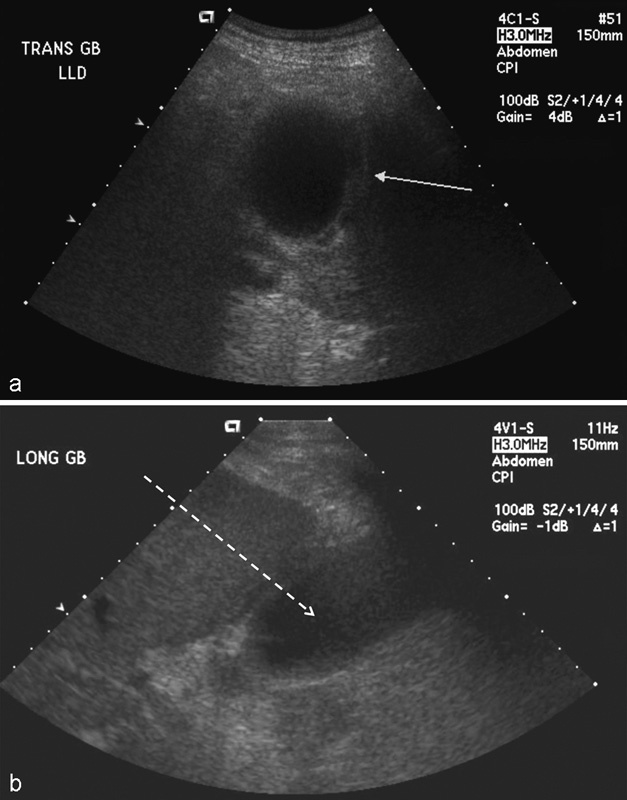
Ultrasound of (a) acute acalculous cholecystitis with gallbladder wall thickening (white arrow) and pericholecystic fluid in this patient with a positive sonographic Murphy sign; (b) longitudinal view of the inflamed gallbladder and potential transhepatic route (dotted arrow) for percutaneous cholecystostomy access.
Fig. 3.
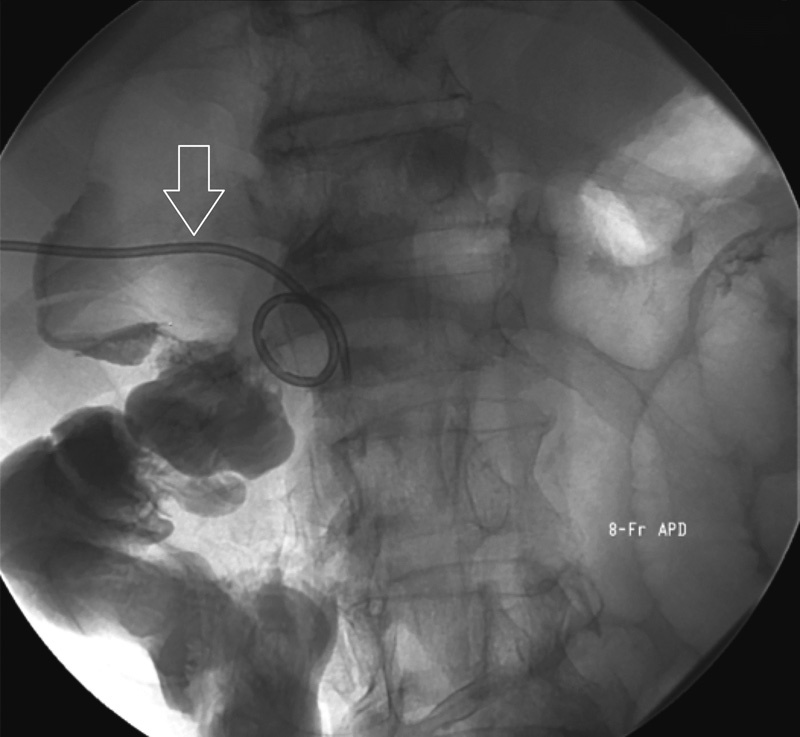
Fluoroscopic image of a cholecystogram after technically successful percutaneous cholecystostomy. A newly placed 8F all-purpose drain (APD) is seen within the right upper quadrant (open arrow), placed under ultrasonographic guidance. Contrast is confirmed to flow through the cystic duct, common bile duct, and into the duodenum without obstruction.
The trocar technique is often used in conjunction with the transperitoneal route in a well-distended gallbladder, where the catheter is loaded onto the trocar and inserted into the gallbladder as one unit. This approach is often performed along the long axis of the gallbladder to allow for further interventions such as gallstone removal or stenting.23
Outcomes and Complications
Technical success rates for PC range from 95 to 100%.24 The overwhelming majority of failures result from thick biliary aspirate and difficult access. Failure to access the gallbladder usually occurs in patients with decompressed gallbladders, impacted stones, and gallbladder wall thickening or calcification.
Predictions of worse outcomes after PC are higher APACHE II and CCI scores and a longer interval between diagnosis and PC.23 Early intervention has been shown to reduce the rate of complications and length of hospitalization.13
The total rate of major and minor complications ranges from 2.4 to 16% of cases.23 24 Most complications related to PC are minor, but major complications include procedure-related mortality, sepsis, and significant bleeding requiring transfusion. Mortality related directly to the procedure is difficult to assess due to significant patient comorbidity, but it is reportedly extremely low ranging from 0 to 1.4%.23 24 Biliary infection and sepsis is not uncommon after instrumentation, but it is usually present before intervention and similarly difficult to evaluate. The reported incidence of sepsis attributable to PC is 0.9%.23 Preprocedure antibiotics help reduce this incidence.
Tube dislodgement is the most common minor complication ranging from 4.5 to 15% followed by minor bleeding reported in 0 to 1.2% of patients.20 23 25 Other complications that have been described such as pneumothorax, abscess formation, bowel injury, and bile leak with or without peritonitis are rare.13
Conclusion
The advantages of PC in the management of patients with acute cholecystitis are well documented and a part of the treatment algorithm of the 2013 Tokyo guidelines. This procedure has a high rate of clinical and technical success with low reported complications. The ongoing CHOCOLATE trial will shed more light on evidence-based guidelines for the future role of PC in the management of acute cholecystitis. At the same time, new developments are expanding the role of PC in the treatment of biliary conditions from malignant and benign strictures to biliary stone removal.
References
- 1.Radder R W. Ultrasonically guided percutaneous catheter drainage for gallbladder empyema. Diagn Imaging. 1980;49(6):330–333. [PubMed] [Google Scholar]
- 2.Saad W E, Wallace M J, Wojak J C, Kundu S, Cardella J F. Quality improvement guidelines for percutaneous transhepatic cholangiography, biliary drainage, and percutaneous cholecystostomy. J Vasc Interv Radiol. 2010;21(6):789–795. doi: 10.1016/j.jvir.2010.01.012. [DOI] [PubMed] [Google Scholar]
- 3.Strasberg S M. Clinical practice. Acute calculous cholecystitis. N Engl J Med. 2008;358(26):2804–2811. doi: 10.1056/NEJMcp0800929. [DOI] [PubMed] [Google Scholar]
- 4.Derici H, Kara C, Bozdag A D, Nazli O, Tansug T, Akca E. Diagnosis and treatment of gallbladder perforation. World J Gastroenterol. 2006;12(48):7832–7836. doi: 10.3748/wjg.v12.i48.7832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huffman J L, Schenker S. Acute acalculous cholecystitis: a review. Clin Gastroenterol Hepatol. 2010;8(1):15–22. doi: 10.1016/j.cgh.2009.08.034. [DOI] [PubMed] [Google Scholar]
- 6.Baron T H, Grimm I S, Swanstrom L L. Interventional approaches to gallbladder disease. N Engl J Med. 2015;373(4):357–365. doi: 10.1056/NEJMra1411372. [DOI] [PubMed] [Google Scholar]
- 7.Horn T, Christensen S D, Kirkegård J, Larsen L P, Knudsen A R, Mortensen F V. Percutaneous cholecystostomy is an effective treatment option for acute calculous cholecystitis: a 10-year experience. HPB (Oxford) 2015;17(4):326–331. doi: 10.1111/hpb.12360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Winbladh A, Gullstrand P, Svanvik J, Sandström P. Systematic review of cholecystostomy as a treatment option in acute cholecystitis. HPB (Oxford) 2009;11(3):183–193. doi: 10.1111/j.1477-2574.2009.00052.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Simorov A Ranade A Parcells J et al. Emergent cholecystostomy is superior to open cholecystectomy in extremely ill patients with acalculous cholecystitis: a large multicenter outcome study Am J Surg 20132066935–940., discussion 940–941 [DOI] [PubMed] [Google Scholar]
- 10.Li M, Li N, Ji W. et al. Percutaneous cholecystostomy is a definitive treatment for acute cholecystitis in elderly high-risk patients. Am Surg. 2013;79(5):524–527. doi: 10.1177/000313481307900529. [DOI] [PubMed] [Google Scholar]
- 11.Kortram K, van Ramshorst B, Bollen T L. et al. Acute cholecystitis in high risk surgical patients: percutaneous cholecystostomy versus laparoscopic cholecystectomy (CHOCOLATE trial): study protocol for a randomized controlled trial. Trials. 2012;13:7. doi: 10.1186/1745-6215-13-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Takada T, Strasberg S M, Solomkin J S. et al. TG13: Updated Tokyo Guidelines for the management of acute cholangitis and cholecystitis. J Hepatobiliary Pancreat Sci. 2013;20(1):1–7. doi: 10.1007/s00534-012-0566-y. [DOI] [PubMed] [Google Scholar]
- 13.Chou C K, Lee K C, Chan C C. et al. Early percutaneous cholecystostomy in severe acute cholecystitis reduces the complication rate and duration of hospital stay. Medicine (Baltimore) 2015;94(27):e1096. doi: 10.1097/MD.0000000000001096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mizrahi I, Mazeh H, Yuval J B. et al. Perioperative outcomes of delayed laparoscopic cholecystectomy for acute calculous cholecystitis with and without percutaneous cholecystostomy. Surgery. 2015;158(3):728–735. doi: 10.1016/j.surg.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 15.Atar E, Bachar G N, Berlin S. et al. Percutaneous cholecystostomy in critically ill patients with acute cholecystitis: complications and late outcome. Clin Radiol. 2014;69(6):e247–e252. doi: 10.1016/j.crad.2014.01.012. [DOI] [PubMed] [Google Scholar]
- 16.Hatzidakis A, Venetucci P, Krokidis M, Iaccarino V. Percutaneous biliary interventions through the gallbladder and the cystic duct: what radiologists need to know. Clin Radiol. 2014;69(12):1304–1311. doi: 10.1016/j.crad.2014.07.016. [DOI] [PubMed] [Google Scholar]
- 17.Yasumoto T, Yokoyama S, Nagaike K. Percutaneous transcholecystic metallic stent placement for malignant obstruction of the common bile duct: preliminary clinical evaluation. J Vasc Interv Radiol. 2010;21(2):252–258. doi: 10.1016/j.jvir.2009.10.010. [DOI] [PubMed] [Google Scholar]
- 18.Pomerantz B J. Biliary tract interventions. Tech Vasc Interv Radiol. 2009;12(2):162–170. doi: 10.1053/j.tvir.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 19.Duncan C, Hunt S J, Gade T, Shlansky-Goldberg R D, Nadolski G J. Outcomes of percutaneous cholecystostomy in the presence of ascites. J Vasc Interv Radiol. 2016;27(4):562–60. doi: 10.1016/j.jvir.2015.12.004. [DOI] [PubMed] [Google Scholar]
- 20.Ginat D, Saad W E. Cholecystostomy and transcholecystic biliary access. Tech Vasc Interv Radiol. 2008;11(1):2–13. doi: 10.1053/j.tvir.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 21.Dewhurst C, Kane R A, Mhuircheartaigh J N, Brook O, Sun M, Siewert B. Complication rate of ultrasound-guided percutaneous cholecystostomy in patients with coagulopathy. AJR Am J Roentgenol. 2012;199(6):W753–W760. doi: 10.2214/AJR.11.8445. [DOI] [PubMed] [Google Scholar]
- 22.Sato K T. Percutaneous management of biliary emergencies. Semin Intervent Radiol. 2006;23(3):249–257. doi: 10.1055/s-2006-948764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Katabathina V S, Zafar A M, Suri R. Clinical presentation, imaging, and management of acute cholecystitis. Tech Vasc Interv Radiol. 2015;18(4):256–265. doi: 10.1053/j.tvir.2015.07.009. [DOI] [PubMed] [Google Scholar]
- 24.Yeo C S, Tay V W, Low J K, Woon W W, Punamiya S J, Shelat V G. Outcomes of percutaneous cholecystostomy and predictors of eventual cholecystectomy. J Hepatobiliary Pancreat Sci. 2016;23(1):65–73. doi: 10.1002/jhbp.304. [DOI] [PubMed] [Google Scholar]
- 25.Furtado R, Le Page P, Dunn G, Falk G L. High rate of common bile duct stones and postoperative abscess following percutaneous cholecystostomy. Ann R Coll Surg Engl. 2016;98(2):102–106. doi: 10.1308/rcsann.2016.0004. [DOI] [PMC free article] [PubMed] [Google Scholar]


