Adverse events related to hepatic ischemia may occur after transarterial chemoembolization (TACE) and are noted more frequently in patients with portosystemic shunts. The presented case describes hepatic infarction after drug-eluting embolic (DEE) TACE in a patient with a large, presumably congenital portosystemic shunt. The authors will discuss risk factors for adverse outcomes after TACE in the setting of portosystemic shunts and present strategies to minimize such complications.
Case Report
A 61-year-old woman with history of hepatitis C virus (HCV)-related cirrhosis and hepatocellular carcinoma (HCC) was diagnosed, based upon magnetic resonance (MR) imaging features, with a new 1.6 cm HCC in hepatic segment 3 (Fig. 1). She was previously diagnosed with a 2.2 cm HCC within segment 5 of the right hepatic lobe that had been treated with conventional TACE 24 months and 7 months prior, as well as being diagnosed with an additional 1.8 cm segment 5 HCC treated with percutaneous radiofrequency ablation (RFA) 21 months prior. She was without local recurrence or complications. Prior imaging was also notable for a shunt between the left portal vein and middle hepatic vein measuring 2.1 cm in diameter (Fig. 2a,b).
Fig. 1.
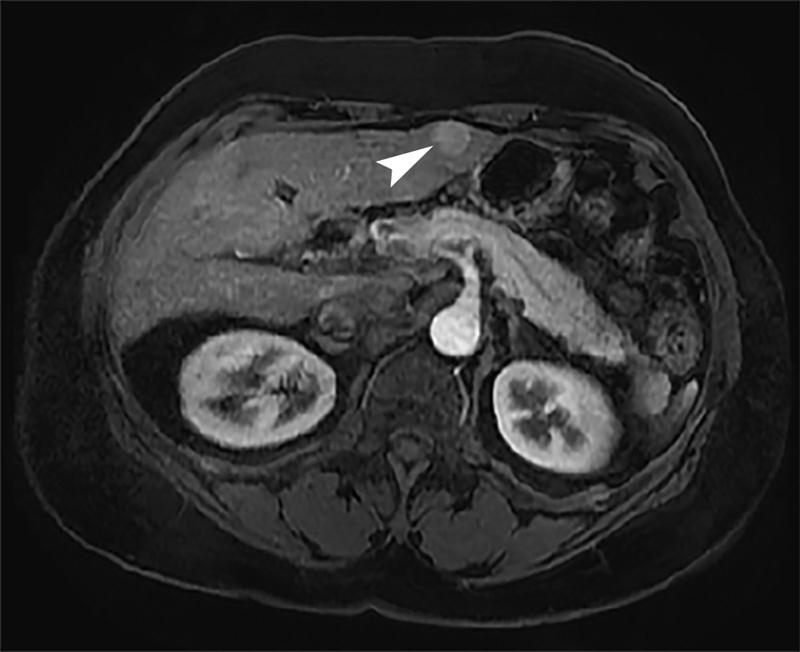
Arterial phase contrast-enhanced axial MR image demonstrates 1.6 cm arterially enhancing HCC (arrowhead) within segment 3 of left hepatic lobe.
Fig. 2.
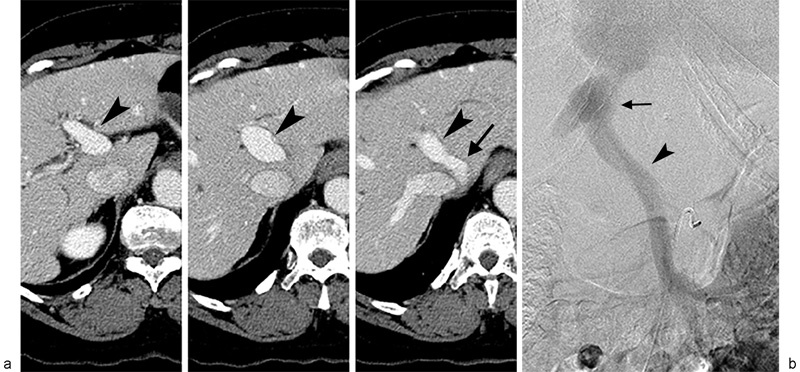
(a) Serial contrast-enhanced axial CT images from caudal (left) to cephalad (right) direction reveal middle hepatic vein (arrow) to left portal vein (arrowhead) shunt. (b) Venous phase image from superior mesenteric arteriogram confirms presence of anatomic portosystemic shunt(arrowhead - portal vein; arrow - hepatic vein).
The patient's disease status was Barcelona Clinic Liver Cancer (BCLC) stage A, with Child-Pugh class A liver function and Eastern Cooperative Oncology Group performance grade 0. She was listed for liver transplant. The subcapsular location of the tumor adjacent to the stomach was considered unfavorable for RFA; therefore, doxorubicin DEE TACE was planned.
Initial angiography was performed of the celiac artery with a 5 Fr catheter, demonstrating conventional celiac arterial anatomy. Selective arteriography of the left hepatic artery (Fig. 3a) and segment 3 hepatic artery (Fig. 3b) with a 2.8 French microcatheter (Renegade HI-FLO, Boston Scientific, Natick, Massachusetts, USA) opacified the hypervascular tumor noted on MR imaging. Two vials of 100–300 micron LC Bead microspheres (Biocompatibles UK, Franham, United Kingdom) loaded with 50 mg doxorubicin per vial and suspended in 10 mL iodinated contrast (Ominipaque-300; GE Healthcare, Little Chalfont, United Kingdom) were administered into the segment 3 hepatic artery. Embolotherapy was performed to angiographic stasis (Fig. 3c). Levofloxacin was administered intravenously during the procedure and continued orally for 5 days after DEE TACE.
Fig. 3.
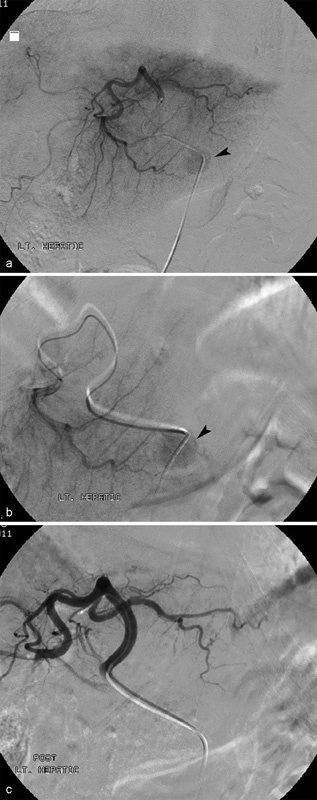
(a) Digital subtraction left lateral segment arteriogram performed during DEE-TACE demonstrates hypervascular segment 3 tumor (arrowhead). (b) Hypervascular tumor (arrowhead) better delineated on subsegmental arteriography. (c) Digital subtraction arteriogram performed after DEE-TACE shows complete devascularization of treated tumor as well as liver segment 3.
Within 24 hours of the procedure, the patient had severe epigastric pain that was inadequately controlled with oral medications. In addition, she developed National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE) grade 4 elevations of aspartate aminotransferase (AST) and alanine aminotransferase (ALT). Her serum AST peaked at 2,341 U/L on day 2, and ALT peaked at 1,577 U/L on day 3. She also developed grade 3 elevation of total bilirubin, from a baseline level of 1.9 mg/dL to 3.5 mg/dL on day 4. Her symptoms and LFT elevations improved during inpatient observation with supportive care. She was discharged 5 days after DEE TACE.
The patient returned to the Emergency Department 6 days after discharge (11 days after DEE TACE) with persistent abdominal pain and increased abdominal distention. Multiphase contrast-enhanced computed tomography (CT) demonstrated complete response of the treated HCC, but also lack of enhancement on all contrast phases of liver parenchyma in the segment 3 territory treated with DEE TACE, reflecting evolving infarction (Fig. 4). She had no clinical signs or symptoms to suggest concurrent infection. With supportive management, her LFTs normalized and symptoms resolved within 14 days of DEE TACE. Follow-up CT and MR demonstrated progressive involution of the infarcted portion of the left hepatic lobe (Fig. 5). She ultimately developed tumor progression with bone and lung metastases and expired 33 months after DEE TACE.
Fig. 4.
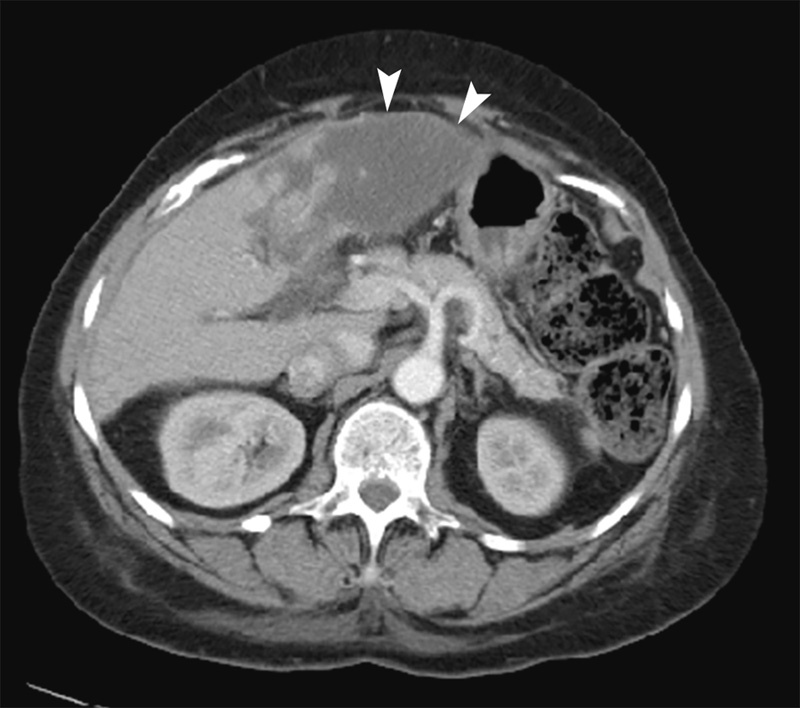
11-day follow-up contrast-enhanced axial CT image after DEE-TACE displays geographic non-enhancement and expansion (arrowhead) of left hepatic lobe indicative of hepatic infarction.
Fig. 5.
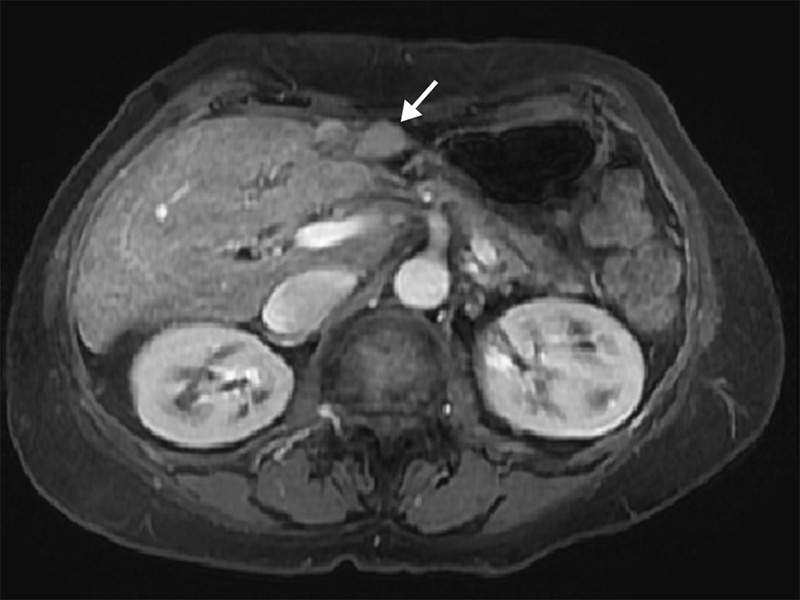
30-month follow-up contrast-enhanced axial MR image after DEE-TACE reveals marked atrophy (arrow) of left hepatic lobe. The treated tumor has been eradicated and is no longer evident.
Discussion
TACE treatment of HCC has conferred a survival benefit over supportive therapies in randomized controlled trials and is the standard therapy for BCLC intermediate stage disease.1 However, treatment-related hepatic ischemia may result in deterioration of liver function and other adverse events.2 3 Hepatotoxicity after TACE is often transient, but a significant number of patients may experience irreversible elevation of creatinine, bilirubin, AST and ALT, or develop new or worsening encephalopathy and ascites.4 5
Several risk factors are associated with hepatotoxicity after TACE.4 Patients with poor liver function prior to TACE, as evidenced by elevated LFTs or refractory ascites, are at higher risk of irreversible deterioration of liver function.6 7 Tumor size is also predictive of severe TACE-related toxicity.8 Risk factors related to procedural technique include TACE administration via a lower order branch of the hepatic artery, and embolization to a greater degree of angiographic stasis.9 10
Diminished hepatic perfusion by the portal vein caused by portosystemic shunting, as in the setting of transjugular intrahepatic portosystemic shunts (TIPS), may also increase risk of poor outcomes after conventional TACE. However, current evidence for this is limited to small, retrospective observational studies that differ in important variables such as baseline liver function and TACE technique.
Several case series have reported outcomes of conventional TACE in patients with TIPS. Tesdal et al retrospectively evaluated 6 patients with TIPS who underwent a total of 17 conventional TACE procedures with (n = 3) or without (n = 3) percutaneous ethanol injection (PEI). In this series, 50% of patients developed encephalopathy, fever, or abdominal pain after TACE. These authors suggested that patients with preserved baseline liver function had better outcomes and therefore may be more appropriate candidates for TACE.11 In a study of 20 patients with TIPS who underwent segmental or subsegmental conventional TACE, Kang et al reported spontaneous bacterial peritonitis in one patient (5%). No other severe adverse events (SAEs) were reported, though this may be attributed to the definition of major complications in this study, as NCI CTCAE was not used.12 In contrast, Wang et al reported SAEs in 31.6% of 17 patients with TIPS who underwent at least two conventional TACE procedures.13 Another retrospective cohort study of 16 patients with TIPS who underwent conventional TACE procedures reported grade 3 or 4 hepatobiliary SAEs in 25% of patients.14
Kohi et al retrospectively compared the incidence of hepatotoxicity within 30 days of conventional TACE for HCC between 10 patients with TIPS and 148 controls. BCLC stage was 0/A in 60% of patients in the TIPS group and 63% of the control group; however, a high proportion of patients in both groups had baseline Child-Pugh class B or C liver function (50% in the TIPS group, 72% in the control group). One or more grade 3 or 4 hepatobiliary SAEs occurred in 70% of patients with TIPS compared with 36% of patients without TIPS (p = 0.046).15 Most SAEs were transient, and there was no statistical difference between the groups for irreversible hepatotoxicity.
The high incidence of SAEs in TIPS patients in the Kohi study (15) compared with other studies may be secondary to a high prevalence of limited hepatic reserve in the patient population, as well as the procedural endpoint of angiographic stasis using gelfoam slurry. The current authors reported a lower rate (11%) of grade 3 bilirubin and AST toxicity in 9 TACE procedures for 7 patients with TIPS; however, all patients in this cohort had Child-Pugh Class A or B liver function, the majority of patients had BCLC stage A disease (n = 6), and TACE was not administered to angiographic stasis.16 Comparison of outcomes between these studies is inherently problematic because of their small sample sizes, heterogeneous patient populations, and study design. However, these studies suggest that SAEs are relatively common after conventional TACE in patients with TIPS.
It is currently unknown whether DEE TACE is safer or more effective than conventional TACE for patients with significant portosystemic shunting. Two prospective randomized controlled trials have demonstrated a lower incidence of toxicity after DEE TACE compared with conventional TACE for patients without TIPS.17 18 However, a retrospective comparison of DEE TACE and conventional TACE demonstrated no difference in toxicity for HCC patients with portal vein thrombosis.19 The reported case illustrates that DEE TACE in the setting of significant portosystemic shunting can result in severe hepatoxity and infarction and should be approached with as much caution as with conventional TACE.
Modifications to the authors approach to locoregional therapy (LRT) in this patient may have mitigated hepatotoxicity. The authors typically administer DEE TACE to an embolic endpoint of angiographic stasis to maximize tumor ischemia, but this approach may have been too aggressive for the patient. Conventional TACE has been reported as being safer without the subsequent administration of gelfoam or particle embolics, minimizing angiographic stasis among patients with TIPS.14 However, further research is needed to determine whether lower levels of angiographic stasis after TACE adversely impact tumor response due to decreased tumor ischemia, and whether clinical outcomes are affected. Indeed, tumor response to TACE may be generally poorer in patients with TIPS because of TIPS-associated increased arterioportal shunting.20
Other LRTs may impose less risk of hepatotoxic complications for patients with portosystemic shunts and should be considered. A study by Padia et al suggested that RFA and PEI are associated with lower incidence of abdominal pain (p = 0.003) and grade 2 or greater AST/ALT level elevation (p < 0.02/0.004) in comparison to TACE.21 Similarly, Park et al reported no SAEs among 19 patients with TIPS who underwent RFA.22 Yttrium-90 (Y90) radioembolization is less embolic than TACE, and may be associated with lower rates of hepatotoxicity in patients with TIPS.23 Safety profiles between TACE and other LRTs in the setting of TIPS have yet to be evaluated in randomized controlled studies, but these alternative therapies should be considered in the clinical management of HCC patients with significant portosystemic shunting.
In summary, the case presented here demonstrates that DEE TACE can cause severe hepatotoxicity and irreversible hepatic infarction in the setting of portosystemic shunting, and the safety of DEE TACE in this patient group needs further investigation. Locoregional treatment options for HCC should be carefully considered in all patients with TIPS in an effort to minimize complications.
References
- 1.Bruix J Sherman M; American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update Hepatology 20115331020–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tarazov P G, Polysalov V N, Prozorovskij K V, Grishchenkova I V, Rozengauz E V. Ischemic complications of transcatheter arterial chemoembolization in liver malignancies. Acta Radiol. 2000;41(2):156–160. doi: 10.1080/028418500127344966. [DOI] [PubMed] [Google Scholar]
- 3.Fujiwara H, Kanazawa S, Hiraki T. et al. Hepatic infarction following abdominal interventional procedures. Acta Med Okayama. 2004;58(2):97–106. doi: 10.18926/AMO/32095. [DOI] [PubMed] [Google Scholar]
- 4.Raoul J L, Sangro B, Forner A. et al. Evolving strategies for the management of intermediate-stage hepatocellular carcinoma: available evidence and expert opinion on the use of transarterial chemoembolization. Cancer Treat Rev. 2011;37(3):212–220. doi: 10.1016/j.ctrv.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 5.A comparison of lipiodol chemoembolization and conservative treatment for unresectable hepatocellular carcinoma. Groupe d'Etude et de Traitement du Carcinome Hépatocellulaire. N Engl J Med. 1995;332(19):1256–1261. doi: 10.1056/NEJM199505113321903. [DOI] [PubMed] [Google Scholar]
- 6.Garwood E R, Fidelman N, Hoch S E, Kerlan R K Jr, Yao F Y. Morbidity and mortality following transarterial liver chemoembolization in patients with hepatocellular carcinoma and synthetic hepatic dysfunction. Liver Transpl. 2013;19(2):164–173. doi: 10.1002/lt.23552. [DOI] [PubMed] [Google Scholar]
- 7.Hsin I F, Hsu C Y, Huang H C. et al. Liver failure after transarterial chemoembolization for patients with hepatocellular carcinoma and ascites: incidence, risk factors, and prognostic prediction. J Clin Gastroenterol. 2011;45(6):556–562. doi: 10.1097/MCG.0b013e318210ff17. [DOI] [PubMed] [Google Scholar]
- 8.Boulin M, Adam H, Guiu B. et al. Predictive factors of transarterial chemoembolisation toxicity in unresectable hepatocellular carcinoma. Dig Liver Dis. 2014;46(4):358–362. doi: 10.1016/j.dld.2013.12.012. [DOI] [PubMed] [Google Scholar]
- 9.Bouvier A, Ozenne V, Aubé C. et al. Transarterial chemoembolisation: effect of selectivity on tolerance, tumour response and survival. Eur Radiol. 2011;21(8):1719–1726. doi: 10.1007/s00330-011-2118-2. [DOI] [PubMed] [Google Scholar]
- 10.Jin B, Wang D, Lewandowski R J. et al. Chemoembolization endpoints: effect on survival among patients with hepatocellular carcinoma. AJR Am J Roentgenol. 2011;196(4):919–928. doi: 10.2214/AJR.10.4770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tesdal I K, Wikström M, Flechtenmacher C, Filser T, Dueber C. Percutaneous treatment of hepatocellular carcinoma in patients with transjugular intrahepatic portosystemic shunts. Cardiovasc Intervent Radiol. 2006;29(5):778–784. doi: 10.1007/s00270-005-0063-7. [DOI] [PubMed] [Google Scholar]
- 12.Kang J W, Kim J H, Ko G Y, Gwon D I, Yoon H K, Sung K B. Transarterial chemoembolization for hepatocellular carcinoma after transjugular intrahepatic portosystemic shunt. Acta Radiol. 2012;53(5):545–550. doi: 10.1258/ar.2012.110476. [DOI] [PubMed] [Google Scholar]
- 13.Wang Z, Zhang H, Zhao H. et al. Repeated transcatheter arterial chemoembolization is safe for hepatocellular carcinoma in cirrhotic patients with transjugular intrahepatic portosystemic shunt. Diagn Interv Radiol. 2014;20(6):487–491. doi: 10.5152/dir.2014.13493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miura J T, Rilling W S, White S B. et al. Safety and efficacy of transarterial chemoembolization in patients with transjugular intrahepatic portosystemic shunts. HPB (Oxford) 2015;17(8):707–712. doi: 10.1111/hpb.12433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kohi M P, Fidelman N, Naeger D M, LaBerge J M, Gordon R L, Kerlan R K Jr. Hepatotoxicity after transarterial chemoembolization and transjugular intrahepatic portosystemic shunt: do two rights make a wrong? J Vasc Interv Radiol. 2013;24(1):68–73. doi: 10.1016/j.jvir.2012.08.032. [DOI] [PubMed] [Google Scholar]
- 16.Gaba R C, Rim C M, Parvinian A. Re: Hepatotoxicity after transarterial chemoembolization and transjugular intrahepatic portosystemic shunt: do two rights make a wrong? J Vasc Interv Radiol. 2013;24(7):1075–1076. doi: 10.1016/j.jvir.2013.03.008. [DOI] [PubMed] [Google Scholar]
- 17.Lammer J, Malagari K, Vogl T. et al. Prospective randomized study of doxorubicin-eluting-bead embolization in the treatment of hepatocellular carcinoma: results of the PRECISION V study. Cardiovasc Intervent Radiol. 2010;33(1):41–52. doi: 10.1007/s00270-009-9711-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Malenstein H, Maleux G, Vandecaveye V. et al. A randomized phase II study of drug-eluting beads versus transarterial chemoembolization for unresectable hepatocellular carcinoma. Onkologie. 2011;34(7):368–376. doi: 10.1159/000329602. [DOI] [PubMed] [Google Scholar]
- 19.Gorodetski B, Chapiro J, Schernthaner R. et al. Advanced-stage hepatocellular carcinoma with portal vein thrombosis: conventional versus drug-eluting beads transcatheter arterial chemoembolization. Eur Radiol. 2016 doi: 10.1007/s00330-016-4445-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuo Y C, Kohi M P, Naeger D M. et al. Efficacy of TACE in TIPS patients: comparison of treatment response to chemoembolization for hepatocellular carcinoma in patients with and without a transjugular intrahepatic portosystemic shunt. Cardiovasc Intervent Radiol. 2013;36(5):1336–1343. doi: 10.1007/s00270-013-0698-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Padia S A, Chewning R H, Kogut M J. et al. Outcomes of Locoregional Tumor Therapy for Patients with Hepatocellular Carcinoma and Transjugular Intrahepatic Portosystemic Shunts. Cardiovasc Intervent Radiol. 2015;38(4):913–921. doi: 10.1007/s00270-014-1009-8. [DOI] [PubMed] [Google Scholar]
- 22.Park J K, Al-Tariq Q Z, Zaw T M, Raman S S, Lu D S. Radiofrequency Ablation for the Treatment of Hepatocellular Carcinoma in Patients with Transjugular Intrahepatic Portosystemic Shunts. Cardiovasc Intervent Radiol. 2015;38(5):1211–1217. doi: 10.1007/s00270-015-1050-2. [DOI] [PubMed] [Google Scholar]
- 23.Donahue L A, Kulik L, Baker T. et al. Yttrium-90 radioembolization for the treatment of unresectable hepatocellular carcinoma in patients with transjugular intrahepatic portosystemic shunts. J Vasc Interv Radiol. 2013;24(1):74–80. doi: 10.1016/j.jvir.2012.09.030. [DOI] [PubMed] [Google Scholar]


