Abstract
Objective
The purpose of this study was to determine the incidence and clinical significance of postoperative delirium (PD) in patients with aortic stenosis undergoing surgical aortic valve replacement (SAVR) or transcatheter aortic valve replacement (TAVR).
Method
Between 2010 and 2013, 427 patients underwent TAVR (n = 168) or SAVR (n = 259) and were screened for PD using the Confusion Assessment Method for the Intensive Care Unit. The incidence of PD in both treatment groups was determined and its association with morbidity and mortality was retrospectively compared.
Results
PD occurred in 135 patients (32%) with a similar incidence between SAVR (33% [86 out of 259]) and TAVR (29% [49 out of 168]) (P = .40). TAVR by transfemoral approach had the lowest incidence of PD compared with SAVR (18% vs 33%; P = .025) or TAVR when performed by alternative access techniques (18% vs 35%; P = .02). Delirium was associated with longer initial intensive care unit stay (70 vs 27 hours), intensive care unit readmission (10% [14 out of 135] vs 2% [6 out of 292]), and longer hospital stay (8 vs 6 days) (P < .001 for all). PD was associated with increased mortality at 30 days (7% vs 1%; P < .001) and 1 year (21% vs 8%; P < .001). After multivariable adjustment, PD remained associated with increased 1-year mortality (hazard ratio, 3.02; 95% confidence interval, 1.75–5.23; P < .001). There was no interaction between PD and aortic valve replacement approach with respect to 1-year mortality (P = .12). Among propensity-matched patients (n = 170), SAVR-treated patients had a higher incidence of PD than TAVR-treated patients (51% vs 29%; P = .004).
Conclusions
PD occurs commonly after SAVR and TAVR and is associated with increased morbidity and mortality. Given the high incidence of PD and its associated adverse outcomes, further studies are needed to minimize PD and potentially improve patient outcomes.
Keywords: TAVR, delirium, cardiac surgery, aortic stenosis, aortic valve replacement
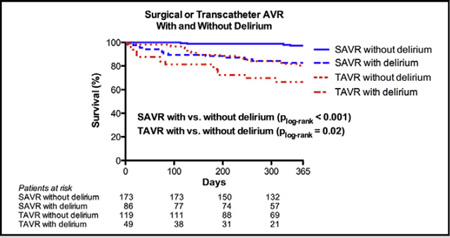
Graphical Abstract
Surgical aortic valve replacement and transcatheter aortic valve replacement with and without delirium.
Postoperative delirium (PD) is a well-recognized complication after cardiac surgery defined as an acute and fluctuating neurologic disorder that reflects a change from baseline cognition and is characterized by the cardinal features of inattention and disorganized thinking.1 PD is more common in patients older than age 65 years and has an estimated incidence between 25% and 50% after cardiac surgery, which translates into more than 120,000 patients affected per year in the United States alone.2–4 Although efforts to detect PD have increased during the past decade, it remains underestimated in many clinical settings. Moreover, as older and frailer patients increasingly undergo major procedures, the incidence of PD is likely to increase substantially during the next decade.5
PD after traditional cardiac surgery has been associated with increased morbidity, longer hospital length of stay, and greater mortality.6–8 These negative outcomes have resulted in increased health care costs estimated at $150 billion annually.9 Although the influence of PD on short-term and perioperative outcomes seems intuitive, PD has additionally been linked to late cognitive impairments that persist at intermediate and long-term follow-up.4,10 Multiple studies have focused on treating PD, but most have had limited success, resulting in a greater emphasis placed upon the prevention of PD and the identification of risk factors for its development.5
Recent advances in the treatment of patients with severe aortic stenosis (AS), specifically in the area of transcatheter aortic valve replacement (TAVR) now allow for aortic valve replacement (AVR) procedures to be performed without the need for cardiopulmonary bypass, requiring fewer transfusions, and in many cases, without even a surgical incision or general anesthesia.11,12 As such, TAVR procedures minimize or eliminate many of the traditional factors associated with the development of PD after conventional AVR surgery. Although several large trials have evaluated outcomes after TAVR in comparison to surgical aortic valve replacement (SAVR), there are fewer data available regarding the use of TAVR and its association with PD and subsequent sequelae.
The purpose of this study was to compare the incidence of PD after TAVR and SAVR, examine the adverse outcomes associated with PD, and explore possible risk factors for the development of PD after either procedure. We hypothesized that the incidence of PD would be lower after TAVR than SAVR, would be associated with increased mortality after either procedure, and would be associated with different risk factors for its development within each treatment population.
METHODS
In this retrospective study from 2010 to 2013, we included all patients with severe AS who underwent an isolated AVR procedure at Barnes-Jewish Hospital in whom PD was assessed with the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU). Severe AS was defined as an indexed aortic valve area <0.6 cm2/m2, a mean gradient >40 mm Hg, or a maximum jet velocity >4.0 m/sec. Patients were offered TAVR in the setting of a clinical trial or if they met TAVR eligibility criteria once TAVR became approved for use by the US Food and Drug Administration. A total of 427 patients were treated with either TAVR (n = 168) or SAVR (n = 259).
This study was written in compliance with Strengthening the Reporting of Observational studies in Epidemiology guidelines and approved by the local institutional review board from which a waiver of written informed consent was obtained given the retrospective design of the study.
TAVR and SAVR Approaches
The TAVR procedures have previously been described11 and were performed via a transfemoral (TF) approach when femoral access was suitable (n = 57). In cases where TAVR-TF was not possible, TAVR was performed either by a direct aortic (TAo) (n = 37) or transapical (TA) (n = 74) approach. All procedures were performed under general anesthesia and all TF approaches were performed using surgical femoral exposure. SAVR procedures were performed via limited or full sternotomy using cardiopulmonary bypass and myocardial arrest.
Anesthetic Approach for TAVR and SAVR
General anesthesia was typically induced with propofol, fentanyl, and rocuronium. A limited use of benzodiazepines was not uncommon. All patients had endotracheal tubes placed and were mechanically ventilated for the procedure. Anesthesia was maintained with a balanced anesthetic technique, including a potent inhaled agent and titration of opioid analgesics. Transesophageal echocardiography was performed in all cases. Intraoperative data, including vital signs and medications, were automatically archived every minute by an electronic anesthetic record system (Metavision; IMDsoft, Needham, Mass). Volatile anesthetic concentrations were converted into age-adjusted minimum alveolar concentration equivalents,13 summed across all volatile anesthetic agents.
Delirium Assessment
After the AVR procedure, all patients were admitted to an intensive care unit (ICU), where clinical nurses trained in the administration of the CAM-ICU assessed patients for PD every 12 hours using the CAM-ICU. The CAM-ICU assessment has been a routine part of the standard postoperative nursing assessment of all cardiac surgical patients since its introduction in 2010. Although the CAM-ICU was developed for nonverbal ICU patients, it has also been shown to be specific and reasonably sensitive in nonintubated ICU patients.14,15 The tool is based on operationalized criteria derived from the Diagnostic and Statistical Manual of Mental Disorders and assesses 4 features: acute-onset and fluctuating course, inattention, disorganized thinking, and altered level of consciousness. The CAM-ICU assessment was positive for delirium when both acute-onset and fluctuating course and inattention, and either disorganized thinking or altered level of consciousness were present (http://www.icudelirium.org/docs/CAM_ICU_training.pdf). Patients were identified as having PD if they had at least 1 positive CAM-ICU assessment during their ICU stay.
Delirium episodes were further classified as hypoactive, hyperactive, or mixed by using the Richmond Agitation and Sedation Scale (RASS) documented at the time of the delirium assessment.16,17 The RASS is a 10-point sedation scale that measures level of consciousness. A RASS of 0 reflects an alert and calm patient, whereas scores >0 indicate increasing agitation, and scores <0 indicate deepening sedation. RASS scores were documented every 4 hours in the ICU and the corresponding RASS score within 12 hours of a given CAM-ICU assessment (the 6 hours before and after the assessment) was used to assess delirium phenotype. When patients were CAM-ICU positive, a RASS consistently >0 was indicative of hyperactive delirium, a RASS consistently <0 was indicative of hypoactive delirium, and for patients exhibiting scores both >0 and <0, the delirium phenotype was considered mixed.
All perioperative data, including Society of Thoracic Surgeons database variables, intraoperative monitoring data, delirium, and RASS assessments were prospectively collected within institutional databases and subsequently retrospectively reviewed for perioperative outcomes.
Statistical Analyses
Descriptive statistics were determined for TAVR and SAVR patients separately. Comparisons between groups were done using Student t test for continuous variables and Fisher exact test for categorical variables. Nonnormal and ordinal variables were summarized as median (first quartile, third quartile) and compared via the Kruskal-Wallis test.
Propensity score matching was used to compare PD rates between TAVR and SAVR patients. A logistic regression model was used to create the probability that patient was assigned to TAVR for treatment using the following variables: age, gender, Society of Thoracic Surgeons score, New York Heart Association (NYHA) functional class III or IV (vs I or II), diabetes mellitus, coronary artery disease, peripheral vascular disease, cerebrovascular disease, moderate/severe lung disease, glomerular filtration rate, and hemoglobin. An optimal matching algorithm with caliper set to 0.05 was used to create a 1:1 matched sample. Delirium rates were compared within the matched sample using McNemar test (Figures E1 and E2).
Mortality was evaluated using time to event analysis. Start time was date of the AVR procedure and patients were followed until event occurrence or last available follow-up, to 1 year. Kaplan-Meier curves were created by delirium status and compared using the log-rank test. Additional multivariable Cox proportional hazards regression models were created to evaluate the association between delirium and 1-year mortality while adjusting for AVR approach, age, sex, and STS predicted risk of mortality (STS-PROM) score; the interaction between PD and AVR approach was assessed. These covariates were selected a priori based on their known association with mortality. Within AVR approach, the rates of occurrence for prolonged ventilation, stroke, acute kidney injury (AKI), and ICU readmission were individually compared between delirium groups using Fisher exact test. AKI was defined in accordance with Valve Academic Research Consortium-2 criteria.18 Thirty-day and 1-year Kaplan-Meier estimates for survival were compared using a z test. The Kruskal-Wallis test was used to compare initial ICU and hospital length of stay.
Multivariable logistic regression models were built to explore predictors of delirium within TAVR and SAVR patients separately. Variables for inclusion within the multivariable models were selected based on a univariable cutoff P value of <.1. The number of variables included in the models was limited based on the incidence of PD in each cohort. The variables age, STS-PROM, steroid use, TAVR approach, and intraoperative packed red blood cell (PRBC) transfusions, were entered into the TAVR model. For SAVR patients, the variables included age, STS-PROM, previous percutaneous coronary intervention, NYHA functional class ≥III, moderate/severe lung disease, hemoglobin, moderate/severe mitral regurgitation, intraoperative PRBC use, intra-aortic balloon pump, and intubation time. These models evaluated the association between each variable and PD while adjusting for other model variables.
All analyses were conducted using SAS version 9.4 (SAS Institute Inc, Cary, NC).
RESULTS
Demographic and Intraoperative Differences Between TAVR and SAVR
When compared with SAVR, patients undergoing TAVR were significantly older, had greater frequencies of comorbidities of nearly all measured variables, were of worse functional class by American Society of Anesthesiologists score, and had higher predicted risks of mortality by the STS-PROM (Table 1).
TABLE 1.
Baseline characteristics of patients undergoing transcatheter aortic valve replacement (TAVR) and surgical aortic valve replacement (SAVR)
| Characteristic | TAVR (n = 168) |
SAVR (n = 259) |
P value |
|---|---|---|---|
| Age (y) | 81 ± 8 | 71 ± 11 | <.001 |
| Female | 92 (55) | 108 (42) | .010 |
| Body mass index | 27.5 ± 6.3 | 31 ± 8 | <.001 |
| Society of Thoracic Surgeons predicted risk of mortality score |
9.3 (6.1, 13.5) | 3.0 (1.5, 6.0) | <.001 |
| American Society of Anesthesiologists class ≥ 4 |
162 (96) | 180 (70) | <.001 |
| New York Heart Association functional class III–IV |
147 (88) | 139 (54) | <.001 |
| Diabetes mellitus | 72 (43) | 95 (37) | .220 |
| Hypertension | 156 (93) | 209 (81) | <.001 |
| Coronary artery disease | 143 (85) | 114 (44) | <.001 |
| Cerebrovascular disease | 52 (31) | 57 (22) | .040 |
| Atrial fibrillation | 65 (39) | 49 (19) | <.001 |
| Chronic lung disease* | 59 (35) | 39 (15) | <.001 |
| Peripheral vascular disease | 113 (67) | 71 (27) | <.001 |
| Ejection fraction (%) | 53 ± 15 | 58 ± 14 | .002 |
| Mean aortic valve gradient (mm Hg) |
44 ± 14 | 43 ± 14 | .700 |
| Preoperative glomerular filtration rate (mL/min) |
64 ± 27 | 75 ± 26 | <.001 |
| Hemoglobin (g/dL) | 11.3 ± 1.5 | 12.5 ± 1.9 | <.001 |
| Albumin (g/dL) | 4.0 ± 0.5 | 4.1 ± 4 | .120 |
Values are presented as mean ± standard deviation, n (%), or median (first quartile, third quartile).
TAVR, Transcatheter aortic valve replacement; SAVR, surgical aortic valve replacement.
Moderate/severe.
Intraoperatively, whereas TAVR procedures were shorter in duration (153 ± 58 minutes vs 253 ± 73 minutes; P = .001) these patients received a higher mean minimum alveolar concentration of volatile anesthetic than patients undergoing SAVR (0.95 ± 0.13 vs 0.86 ± 0.11; P < .001). PRBC transfusions (67% vs 40%; P < .001) and tranexamic acid administration (93% vs 2%; P < .001) were more frequent during SAVR procedures.
Delirium Incidence in TAVR and SAVR
For the entire study, PD was identified in 135 patients (32%; 95% confidence interval [CI], 27%–36%). Hypoactive delirium was the most common subtype, encountered in 71 patients (53%); 64 patients (47%) presented with a mixed phenotype; and no patient consistently had hyperactive delirium. The incidence of PD after TAVR was not significantly different than after SAVR (29% vs 33%; odds ratio [OR], 0.82; 95% CI, 0.54–1.26; P = .40); however within the subset of TAVR, patients undergoing TAVR-TF had an 18% incidence of PD, the lowest incidence of any AVR approach. The incidence of PD after TAVR-TF was significantly lower when compared with SAVR (18% vs 33%; OR, 0.43; 95% CI, 0.21–0.89; P = .025) or TAVR when performed using alternative access (ie, TA or TAo) techniques (18% vs 35%; OR, 0.39; 95% CI, 0.18–0.86; P = .020). After propensity matching, 29% (25 out of 85) of the TAVR-treated patients and 51% (43 out of 85) of the SAVR-treated patients developed PD (P = .004).
Perioperative Complications and Mortality
Table 2 shows univariable associations between delirium and postoperative complications. For both TAVR and SAVR patients, PD was associated with a longer ICU and hospital Maniar et al Perioperative Management length of stay. For SAVR specifically, there were additional complications associated with PD that included prolonged ventilation, and a greater need for readmission to the ICU. PD after TAVR and SAVR was associated with an increased likelihood that at hospital discharge, patients required transfer to an extended care facility, and were less likely to be discharged to home. These associations between PD and AKI, discharge location, ICU time and hospital length of stay all remained unchanged even after adjustment by STS-PROM score.
TABLE 2.
Perioperative morbidity and mortality for patients undergoing transcatheter aortic valve replacement (TAVR) and surgical aortic valve replacement (SAVR)
| TAVR | SAVR | |||||
|---|---|---|---|---|---|---|
| Event | No delirium (n = 119) | Delirium (n = 49) | P value | No delirium (n = 173) | Delirium (n = 86) | P value |
| Prolonged ventilation | 2 (2) | 0 (0) | >.999 | 10 (6) | 18 (21) | <.001 |
| Stroke | 1 (1) | 1 (2) | .500 | 1 (1) | 1 (1) | >.999 |
| Acute kidney injury | 8 (7) | 12 (24) | .003 | 25 (14) | 15 (17) | .580 |
| Initial intensive care unit stay (h) | 26 | 50 | <.001 | 31 | 72 | <.001 |
| Readmission to the intensive care unit | 4 (3) | 5 (10) | .120 | 2 (1) | 9 (10) | .001 |
| Hospital length of stay (d) | 5 | 7 | <.001 | 7 | 9 | <.001 |
| Discharged to home | 87 (73) | 26 (53) | .018 | 146 (84) | 49 (57) | <.001 |
| 30-d survival (%) | 98 | 88 | .029 | 100 | 95 | .040 |
| 1-y survival (%) | 80 | 66 | .090 | 97 | 83 | .001 |
Values are presented as n (%) unless otherwise noted.
TAVR, Transcatheter aortic valve replacement; SAVR, surgical aortic valve replacement.
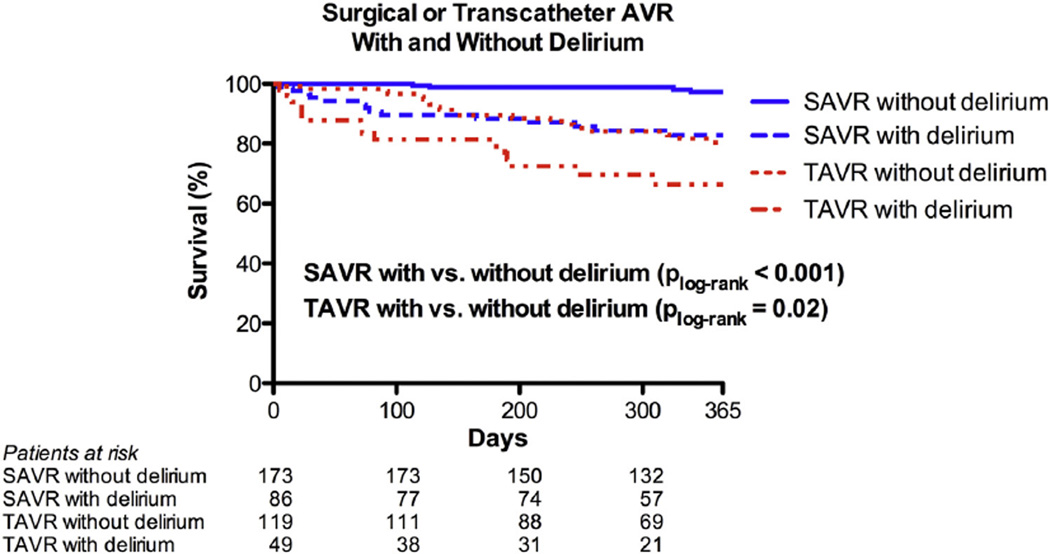
In unadjusted analyses, the development of PD after SAVR or TAVR was associated with increased mortality over the first year for either procedure (Figure 1). For TAVR patients without PD, survival was better at 30 days (98% vs 88%; P = .029) and tended to be better at 1 year (80% vs 66%; P = .09) based on Kaplan-Meier estimates. Similarly for SAVR, Kaplan-Meier estimates of survival were better for patients without PD at both 30 days (100% vs 95%; P = .04) and 1 year (97% vs 83%; P = .001), respectively. By multivariable analysis, after adjusting for age, sex, STS-PROM score, and AVR type, PD was associated with increased risk of mortality through the first year after AVR for the entire study population (n = 427) (hazard ratio [HR], 3.02; 95% CI, 1.75–5.23; P < .001) (Table 3). PD additionally remained significantly associated with increased risk of mortality within both TAVR (HR, 2.02; 95% CI, 1.12–4.35) and SAVR groups (HR, 6.28; 95% CI, 2.04–19.31) after similarly adjusting for age, sex, and STS-PROM score. There was also no significant interaction between AVR type (TAVR vs SAVR) and PD with respect to 1-year mortality (interaction P = .12), indicating that the relationship between PD and post-AVR mortality was similar for patients treated with TAVR and SAVR.
FIGURE 1.
Time to even curves for 1-year death from any cause. One year time to event curves are shown for patients treated with surgical aortic valve replacement (SAVR) and transcatheter aortic valve replacement (TAVR) with and without delirium. Within each treatment group, patients with and without delirium were compared with the use of the log-rank test. AVR, Aortic valve replacement.
TABLE 3.
Multivariable analysis for 1-year mortality for patients undergoing transcatheter aortic valve replacement (TAVR) and surgical aortic valve replacement (SAVR)
| Variable | Hazard ratio |
95% Confidence interval |
P value |
|---|---|---|---|
| Delirium (yes) | 3.023 | 1.748–5.228 | <.001 |
| TAVR (yes) | 2.360 | 1.269–4.389 | .007 |
| Age (per 1 year) | 1.006 | 0.976–1.036 | .710 |
| Female (yes) | 0.580 | 0.329–1.023 | .060 |
| Society of Thoracic Surgeons predicted risk of mortality score (per 1 unit) |
1.099 | 1.054–1.146 | <.001 |
TAVR, Transcatheter aortic valve replacement.
Predictors of Delirium After TAVR and SAVR
Univariable associations with PD for patients undergoing both TAVR and SAVR are shown in Table 4. The use of a non-TF approach (P = .013) and preoperative steroid use (8% vs 24%; P = .004) were associated with the development of PD after TAVR, whereas for SAVR, the development of PD was associated with increasing age, elevated STS-PROM score, worse American Society of Anesthesiologists score, NYHA functional class III or IV, and the presence of specific comorbidities such as coronary artery disease and chronic lung disease. PD was seen more frequently in patients with lower preoperative hemoglobin levels and, similar to TAVR, PD was more frequent when PRBC transfusions were required intraoperatively. Specifically for SAVR, there were no differences in PD with respect to cardiopulmonary bypass (110 ± 37 vs 108 ± 25; P = .63) or aortic crossclamp times (76 ± 23 vs 72 ± 18; P = .15). Procedural time was not a factor for PD for either TAVR or SAVR.
TABLE 4.
Univariable analysis of delirium among patients undergoing transcatheter aortic valve replacement (TAVR) and surgical aortic valve replacement (SAVR)
| TAVR | SAVR | |||||
|---|---|---|---|---|---|---|
| Characteristic | No delirium (n = 119) |
Delirium (n = 49) |
P value |
No delirium (n = 173) |
Delirium (n = 86) |
P value |
| Age (y) | 82 ± 8 | 81 ± 8 | .410 | 69 ± 11 | 74 ± 10 | <.001 |
| Female | 68 (57) | 24 (49) | .390 | 69 (40) | 39 (45) | .420 |
| Body mass index | 28 ± 6 | 27 ± 5 | .360 | 31 ± 7 | 31 ± 9 | .990 |
| Society of Thoracic Surgeons predicted risk of mortality score | 9.5 (5.7, 14.0) | 9.2 (6.4, 12.0) | .920 | 2.2 (1.2, 4.7) | 4.2 (2.3, 7.5) | <.001 |
| American Society of Anesthesiologists class ≥ 4 | 115 (97) | 47 (96) | >.999 | 110 (64) | 70 (81) | .004 |
| New York Heart Association functional class III–IV | 103 (87) | 44 (90) | .800 | 81 (47) | 58 (67) | .002 |
| Diabetes mellitus | 50 (42) | 22 (45) | .740 | 59 (34) | 36 (42) | .270 |
| Hypertension | 111 (93) | 45 (92) | .750 | 138 (80) | 71 (83) | .620 |
| Coronary artery disease | 100 (84) | 43 (88) | .640 | 70 (40) | 44 (51) | .110 |
| Cerebrovascular disease | 35 (29) | 17 (35) | .580 | 34 (20) | 23 (27) | .210 |
| Atrial fibrillation | 42 (35) | 23 (47) | .170 | 29 (17) | 20 (23) | .240 |
| Chronic lung disease* | 39 (33) | 20 (41) | .370 | 19 (11) | 20 (23) | .016 |
| Peripheral vascular disease | 80 (67) | 33 (67) | >.999 | 42 (24) | 29 (34) | .140 |
| Preoperative glomerular filtration rate (mL/min) | 63 ± 21 | 68 ± 38 | .390 | 76 ± 25 | 71 ± 27 | .130 |
| Chronic steroid use | 9 (8) | 12 (24) | .004 | 9 (5) | 6 (7) | .580 |
| Hemoglobin (g/dL) | 11.4 ± 1.5 | 11.1 ± 1.5 | .230 | 12.8 ± 1.9 | 11.9 ± 1.7 | <.001 |
| Albumin (g/dL) | 4.01 ± 0.4 | 4 ± 0.5 | .900 | 4.12 ± 0.4 | 4 ± 0.4 | .070 |
| Procedure time (min) | 150 ± 60 | 160 ± 53 | .370 | 251 ± 76 | 258 ± 66 | .480 |
| TAVR approach | .013 | |||||
| TAVR-transfemoral | 47 (39) | 10 (20) | ||||
| TAVR-transapical/transaortic | 72 (61) | 39 (80) | ||||
| Minimum alveolar concentration volatile anesthetic | 0.95 ± 0.13 | 0.95 ± 0.13 | .980 | 0.87 ± 0.11 | 0.85 ± 0.10 | .150 |
| Packed red blood cell transfusion | 42 (35) | 25 (51) | .080 | 120 (69) | 74 (86) | .004 |
Values are presented as mean ± standard deviation, n (%), or median (first quartile, third quartile).
TAVR, Transcatheter aortic valve replacement; SAVR, surgical aortic valve replacement.
Moderate/severe.
The independent predictors for the development of delirium are shown in Tables 5 and 6. Within the SAVR cohort, the independent predictors for the development of PD were age, and the presence of concomitant valvular heart disease, specifically mitral regurgitation. An intubation period <24 hours after SAVR was associated with less PD (Table 5). For TAVR, no specific comorbidities were identified as independent predictors for PD with only preoperative steroid use and the development of AKI postoperatively remaining statistically significant. The intraoperative associations for TAVR between transfusions (OR, 2.00; P = .08) and TAVR approach (OR, 2.00; P = .11) did not remain statistically significant within the multivariable model (Table 6).
TABLE 5.
Multivariate analysis for delirium in patients undergoing surgical aortic valve replacement.
| Variable | Odds ratio | 95% Confidence interval | P value |
|---|---|---|---|
| Age (per 1 year) | 1.043 | 1.010–1.078 | .011 |
| Society of Thoracic Surgeons predicted risk of mortality score (per 1 point) | 0.980 | 0.904–1.064 | .630 |
| Previous percutaneous coronary intervention (yes) | 1.531 | 0.761–3.078 | .230 |
| New York Heart Association functional class III–IV (yes) | 1.217 | 0.607–2.443 | .580 |
| Lung disease moderate/severe (yes) | 1.608 | 0.652–3.970 | .300 |
| Preoperative hemoglobin (per 1 unit) | 0.841 | 0.699–1.012 | .070 |
| Mitral regurgitation moderate/severe (yes) | 2.939 | 1.176–7.344 | .021 |
| Intraoperative packed red blood cells (yes) | 1.034 | 0.435–2.460 | .940 |
| Preoperative intra-aortic balloon pump (yes) | 3.509 | 0.878–14.027 | .080 |
| Intubation time < 24 h (yes) | 0.400 | 0.161–0.996 | .049 |
TABLE 6.
Multivariate Analysis for Delirium in patients undergoing transcatheter aortic valve replacement (TAVR)
| Variable | Odds ratio |
95% Confidence interval |
P value |
|---|---|---|---|
| Age (per 1 year) | 0.982 | 0.940–1.026 | .420 |
| Society of Thoracic Surgeons predicted risk of mortality score (per 1 unit) |
0.962 | 0.899–1.029 | .260 |
| Preoperative steroids (yes) | 3.944 | 1.371–11.376 | .011 |
| TAVR via transapical or transaortic approach |
2.002 | 0.851–4.710 | .110 |
| Intraoperative packed red blood cells (yes) |
2.000 | 0.919–4.352 | .080 |
| Acute kidney injury present (yes) | 3.473 | 1.191–10.129 | .023 |
TAVR, Transcatheter aortic valve replacement.
DISCUSSION
We found that PD is common after TAVR and SAVR, occurring in nearly one-third of patients. Importantly, patients undergoing TAVR-TF had the lowest incidence of PD when compared with SAVR or TAVR performed by any other access route. PD was associated with increased morbidity and resource use after both TAVR and SAVR and a 3-fold increase in mortality during the first year after valve replacement. The predictors of PD after TAVR or SAVR were different and based primarily upon preexisting comorbidities rather than intraoperative characteristics.
This is the largest study of PD performed specifically among AS patients undergoing valve replacement. It also examines a cohort of patients at higher risk for PD in that it includes a substantial number of patients undergoing TAVR who are older and have more comorbidities than patients typically offered traditional cardiac surgery. Our findings from the patients treated with SAVR are consistent with the literature available regarding PD after cardiac surgery with respect to incidence and inciting risk factors. And although the list of possible risk factors for PD after SAVR remains long, the variables identified in this study such as age, a history of coronary artery disease, a need for an intra-aortic balloon pump preoperatively, and greater PRBC transfusions have all previously been cited as candidate risk factors for PD with on-pump surgery.3,6,8,10,19 And although there are only a few studies available describing PD after TAVR, the data available thus far are consistent with those from this investigation suggesting that PD is an equally prevalent complication for TAVR, particularly when performed using alternative access techniques,20,21 It is possible that despite the fact that TAVR may be a less-invasive procedure than SAVR, given the high-risk patient population for whom TAVR is currently restricted to as a procedural option, the incidence of PD following TAVR will remain high. The results from propensity-matched patients within this study suggest that a typical patient undergoing TAVR, with numerous comorbidities, would likely have an even higher likelihood of PD if offered an SAVR approach. The predictors of PD after TAVR remain poorly defined due to the small data sets currently available. What does appear clear based on the results of this investigation is that the association of PD with perioperative morbidity and mortality after TAVR are consistent with those reported after SAVR affecting patient outcomes within 30 days and extending out to 1 year.4,22
The implications of this study are several and highlight that increased effort may need to be directed toward the identification of PD after any cardiac surgery, including specifically TAVR procedures. This report suggests that diagnosing PD after TAVR procedures may also be difficult given the frequency of the hypoactive phenotype seen in this investigation, which can be easily overlooked but yet associated with even worse outcomes.9 This study reinforces that patients with PD represent a subgroup at higher risk for death up to 1 year postoperatively.8,22 By identifying these patients in hospital, an opportunity may be provided to increase the awareness of patients and caregivers of delirium and its potential complications. Resources could be targeted to prevent hospital readmissions, and approaches could be explored to mitigate larger issues such as increased mortality and cognitive decline.4,22 From a pragmatic standpoint, this may require that delirium assessments be performed even after patients have left the ICU with different instruments such as the 3 dimensional CAM, which may be better suited than the CAM-ICU toward diagnosing PD in the stepdown setting.23
This study does suggest that maintaining a TF first approach for TAVR has the greatest potential to further decrease the incidence of PD after AVR. Because TAVR delivery systems decrease in size, patients eligible for TAVR will more likely be TF candidates, with only few patients requiring TA or TAo procedures. And although the results of this investigation and those of Eide and colleagues12,21 suggest that TAVR-TF has the lowest incidence of PD among all AVR procedures currently performed, smaller TAVR devices now allow for TF procedures to be performed with a totally percutaneous approach, without the need for any incision or general anesthesia. Despite anesthetic use not having been shown to be a factor associated with PD in this study, the general premise that less postoperative pain and providing a lighter depth of anesthesia in older patients will reduce postoperative delirium23 has implications for all AVR procedures and is currently the focus of an ongoing prospective trial (NCT02241655). These efforts for a minimalist approach to AVR, while not specifically addressing the predisposition or vulnerability of older patients susceptible to developing PD, focus on minimizing the precipitating factors for PD as much as possible.24
Limitations
This study has several limitations. The patients studied in both the SAVR and TAVR cohorts for the development of PD were quite different with respect to the severity and frequency of their comorbidities, potentially biasing the frequency of PD within each cohort. Assessments for PD were done for patients while in the ICU only, and by the CAM-ICU only. Although the CAM-ICU is highly specific, its sensitivity is less, predisposing to underreporting of PD for both the TAVR and SAVR groups.
The relatively small number of patients with PD, particularly in the TAVR, cohort limited the ability to identify clinically significant, independent predictors for delirium after TAVR. Greater numbers of patients will be required to better delineate independent risk factors for PD for TAVR and these types of patients. Given the association between PD and later cognitive impairment, future studies should incorporate cognitive and frailty assessments with PD because these metrics are particularly valuable to this older population.
Lastly, because this was a retrospective analysis for PD, it is impossible to account for the many confounding variables that exist for the assessment of PD and future prospective studies will be required to further explore the findings of this investigation.
CONCLUSIONS
PD occurs commonly after SAVR and TAVR and is associated with increased perioperative morbidity and resource use. Moreover, the development of PD is associated with increased mortality at both 30 days and over the first year after procedure. Although further studies are needed to confirm and extend the findings of this investigation, addressing PD and any potentially modifiable risk factors for its development has the potential to reduce this specific postoperative complication and may also influence other associated adverse outcomes after AVR therapy.
Supplementary Material
Central Message.
Delirium after surgical and transcatheter aortic valve replacement is associated with increased mortality.
Perspective.
Postoperative delirium was assessed in patients undergoing surgical and transcatheter aortic valve replacement. The development of postoperative delirium was associated with increased perioperative morbidity as well as both early and 1-year mortality.
Acknowledgments
Supported by grant BJHF#7937-77 from the Barnes-Jewish Hospital Foundation. BRL is supported by grant No. K23 HL116660 from the National Institutes of Health.
Dr Damiano reports consulting fees from Atricure, lecture fees and grant support from Edwards and On-X Lifesciences, and grant support from Thrasos Inc. Dr Lindman reports consulting fees from Roche Diagnostics. Dr Lasala reports consulting fees from Boston Scientific; equity ownership in Abiomed and DirectFlow; and lecture fees from Abiomed, St. Jude, Boston Scientific, and Eli Lilly. Dr Melby reports consulting fees from On-X and lecture fees from ClearFlow.
Abbreviations and Acronyms
- AKI
acute kidney injury
- AS
aortic stenosis
- AVR
aortic valve replacement
- CAM
confusion assessment method
- ICU
intensive care unit
- NYHA
New York Heart Association
- PRBC
packed red blood cell
- PD
postoperative delirium
- PROM
predicted risk of mortality
- RASS
Richmond Agitation and Sedation Scale
- SAVR
surgical aortic valve replacement
- STS
Society of Thoracic Surgeons
- TAVR
transcatheter aortic valve replacement
- TA
transapical
- Tao
transaortic
- TF
transfemoral
Footnotes
Read at the 95th Annual Meeting of The American Association for Thoracic Surgery, Seattle, Washington, April 25–29, 2015.
Supplemental material is available online.
Conflict of Interest Statement All other authors have nothing to disclose with regard to commercial support.
References
- 1.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th. Arlington, VA: American Psychiatric Association; 2013. [Google Scholar]
- 2.Avidan MS, Fritz BA, Maybrier HR, Muench MR, Escallier KE, Chen Y, et al. The Prevention of Delirium and Complications Associated with Surgical Treatments (PODCAST) study: protocol for an international multicenter randomized controlled trial. BMP Open. 2014;4:e005651. doi: 10.1136/bmjopen-2014-005651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jung P, Pereira MA, Hiebert B, Song X, Rockwood K, Tangri N, et al. The impact of frailty on postoperative delirium in cardiac surgery patients. J Thorac Cardiovasc Surg. 2015;149:869–875. doi: 10.1016/j.jtcvs.2014.10.118. [DOI] [PubMed] [Google Scholar]
- 4.Rudolph JL, Inouye SK, Jones RN, Yang FM, Fong TG, Levkoff SE, et al. Delirium: an independent predictor of functional decline after cardiac surgery. J Am Geriatr Soc. 2010;58:643–649. doi: 10.1111/j.1532-5415.2010.02762.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zaal IJ, Devlin JW, Peelen LM, Slooter AJ. A systematic review of risk factors for delirium in the ICU. Crit Care Med. 2015;43:40–47. doi: 10.1097/CCM.0000000000000625. [DOI] [PubMed] [Google Scholar]
- 6.Gottesman RF, Grega MA, Bailey MM, Pham LD, Zeger SL, Baumgartner WA, et al. Delirium after coronary artery bypass graft surgery and late mortality. Ann Neurol. 2010;67:338–344. doi: 10.1002/ana.21899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ely EW, Shintani A, Truman B, Speroff T, Gordon SM, Harrell FE, Jr, et al. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA. 2004;291:1753–1764. doi: 10.1001/jama.291.14.1753. [DOI] [PubMed] [Google Scholar]
- 8.Whiltlock EL, Torres BA, Lin N, Helsten DL, Nadelshon MR, Mashour GA, et al. Postoperative delirium in a substudy of cardiothoracic surgical patients in the BAG-RECALL clinical trial. Anesth Analg. 2014;118:809–817. doi: 10.1213/ANE.0000000000000028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Postoperative delirium in older adults: best practice statement from the American Geriatrics Society. J Am Coll Surg. 2015;2:136–148. doi: 10.1016/j.jamcollsurg.2014.10.019. [DOI] [PubMed] [Google Scholar]
- 10.Koster S, Hensens AG, Scuurmans MJ, van der Palen J. Consequences of delirium after cardiac operations. Ann Thorac Surg. 2012;93:705–711. doi: 10.1016/j.athoracsur.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 11.Smith CR, Leon MB, Mack MJ, Miller DC, Moses JW, Svensson LG, et al. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med. 2011;23:2187–2198. doi: 10.1056/NEJMoa1103510. [DOI] [PubMed] [Google Scholar]
- 12.Comparison of transfemoral transcatheter aortic valve replacement performed in the catheterization laboratory (minimalist approach) versus hybrid operating room (standard approach): outcomes and cost analysis. J Am Coll Cardiol Cardiovasc Interv. 2014;7:898–904. doi: 10.1016/j.jcin.2014.04.005. [DOI] [PubMed] [Google Scholar]
- 13.Nickalls RW, Mapleson WW. Age-related iso-MAC charts for isoflurane, sevoflurane and desflurane in man. Br J Anaesth. 2003;91:170–174. doi: 10.1093/bja/aeg132. [DOI] [PubMed] [Google Scholar]
- 14.Ely EW, Inouye SK, Bernard GR, Gordon S, Francis J, May L, et al. Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU) JAMA. 2001;286:2703–2710. doi: 10.1001/jama.286.21.2703. [DOI] [PubMed] [Google Scholar]
- 15.McNicoll L, Pisani MA, Ely EW, Gifford D, Inouye SK. Detection of delirium in the intensive care unit: comparison of confusion assessment method for the intensive care unit with confusion assessment method ratings. J Am Geriatr Soc. 2005;53:495–500. doi: 10.1111/j.1532-5415.2005.53171.x. [DOI] [PubMed] [Google Scholar]
- 16.Peterson JF, Pun BT, Dittus RS, Thomason JW, Jackson JC, Shintani AK, et al. Delirium and its motoric subtypes: a study of 614 critically ill patients. J Am Geriatr Soc. 2006;54:479–484. doi: 10.1111/j.1532-5415.2005.00621.x. [DOI] [PubMed] [Google Scholar]
- 17.Pandharipande P, Cotton BA, Shintani A, Thompson J, Costabile S, Truman Pun B, et al. Motoric subtypes of delirium in mechanically ventilated surgical and trauma intensive care unit patients. Intens Care Med. 2007;33:1726–1731. doi: 10.1007/s00134-007-0687-y. [DOI] [PubMed] [Google Scholar]
- 18.Kappetein AP, Head SJ, Genereux P, Piazza N, van Mieghem NM, Blackstone EH, et al. Updated standardized endpoint definitions for transcatheter aortic valve implantation: the Valve Academic Research Consortioum-2 consensus document. J Am Coll Cardiol. 2012;60:1438–1454. doi: 10.1016/j.jacc.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 19.Trabold B, Metterlein T. Posoperative delirium: risk factors, prevention and treatment. J Cardiothorac Vasc Anesth. 2014;28:1352–1360. doi: 10.1053/j.jvca.2014.03.017. [DOI] [PubMed] [Google Scholar]
- 20.Tse L, Bowering JB, Schwarz SKW, Moore RL, Burnds KD, Barr AM, et al. Postoperative delirium following transcatheter aortic valve implantation: a historical cohort study. Can J Anesth. 2015;62:22–30. doi: 10.1007/s12630-014-0254-2. [DOI] [PubMed] [Google Scholar]
- 21.Eide LSP, Ranhoff AH, Fridlund B, Haaverstad R, Hufthammer KO, Kuiper KK, et al. comparison of frequency, risk factors, and time course of postoperative delirium in octogenarians after transcatheter aortic valve implantation versus surgical aortic valve replacement. Am J Cardiol. 2015;115:802–809. doi: 10.1016/j.amjcard.2014.12.043. [DOI] [PubMed] [Google Scholar]
- 22.Wilbring M, Tugtekin SM, Alexiou K, Simonis G, Matschke K, Kappert U. Transapical transcatheter aortic valve implantation vs conventional aortic valve replacement in high-risk patients with previous cardiac surgery: a propensity-score analysis. Eur J Cardiothorac Surg. 2013;44:42–47. doi: 10.1093/ejcts/ezs680. [DOI] [PubMed] [Google Scholar]
- 23.Marcantonio ER, Ngo LH, O’Connor M, Jones RN, Crane PK, Metzger ED, et al. 3D-CAM: derivation and validation of a 3 minute diagnostic interview for CAM-defined delirium: a cross-sectional diagnostic test study. Ann Intern Med. 2014;161:554–561. doi: 10.7326/M14-0865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chan MT, Cheng BC, Lee TM, Gin T CODATrial Group. BIS-guided anesthesia decreases postoperative delirium and cognitive decline. J Neurosurg Anesthesiol. 2013;25:33–42. doi: 10.1097/ANA.0b013e3182712fba. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.