Abstract
Objective:
Cannabis use, particularly at an early age, has been linked to suicidal thoughts and behavior, but minimal work has examined the association between cannabis use and lifetime nonsuicidal self-injury (NSSI). The current study aims to characterize the overlap between lifetime and early cannabis use and NSSI and to examine genetic and environmental mechanisms of this association.
Method:
Adult male and female twins from the Australian Twin Registry (N = 9,583) were used to examine the odds of NSSI associated with lifetime cannabis use and early cannabis use (i.e., <17 years of age). These associations were also examined within monozygotic (MZ) twins discordant for cannabis use and MZ twins discordant for early cannabis use. Analyses were replicated in an independent sample of female twins (n = 3,787) accounting for the age at onset of cannabis use and NSSI.
Results:
Lifetime cannabis use (odds ratio [OR] = 2.84, 95% CI [2.23, 3.61]) and early cannabis use were associated with increased odds of NSSI (OR = 2.15, 95% CI [1.75, 2.65]), and this association remained when accounting for covariates. The association was only significant, however, in MZ twin pairs discordant for early cannabis use (OR = 3.20, 95% CI [1.17, 8.73]). Replication analyses accounting for the temporal ordering of cannabis use and NSSI yielded similar findings of nominal significance.
Conclusions:
Results suggest that NSSI is associated with cannabis involvement via differing mechanisms. For lifetime cannabis use, the lack of association in discordant pairs suggests the role of shared genes and family environment. However, in addition to such shared familial influences, person-specific and putatively causal factors contribute to the relationship between early cannabis use and NSSI. Therefore, delaying the onset of cannabis use may reduce exposure to influences that exacerbate vulnerabilities to NSSI.
According to the 2013 U.S. National Survey on Drug Use and Health (Substance Abuse and Mental Health Services Administration, 2014), 7.1% of U.S. adolescents ages 12–17 years and 7.6% of adults (18 years or older) reported cannabis use in the past month. In Australia, 10.2% of individuals age 14 years or older reported using cannabis in the previous year (Australian Institute of Health and Welfare, 2014). Although the prevalence of cannabis use has not increased substantially over the past few years in either the United States or Australia, it remains high and has warranted increased research attention (Volkow et al., 2014). Moreover, the changing landscape of cannabis legalization, in addition to a decline in the perceived risk of occasional and regular cannabis use (Okaneku et al., 2015), raises concerns about future patterns of use and, as a consequence, related outcomes.
Most youth initiate cannabis use during late adolescence and early adulthood (Wagner & Anthony, 2002). Early onset of cannabis use has been linked to numerous adverse outcomes. For example, early initiation of cannabis has been robustly linked to the use of other illicit drugs, as well as drug abuse and dependence (Agrawal et al., 2004; Grant et al., 2010; Lessem et al., 2006; Lynskey et al., 2003, 2006). Early cannabis use has also been linked to lower educational attainment (Homel et al., 2014; Verweij et al., 2013) and to a variety of mental health problems, including anxiety and depression (Lynskey et al., 2004; Scholes-Balog et al., 2013), and psychosis (Bagot et al., 2015).
Growing evidence also suggests that cannabis users, including early-onset users, are more likely to report suicidal ideation and attempts (Delforterie et al., 2015; Lynskey et al., 2004). However, markedly less is known about the relationship between cannabis use and nonsuicidal self-injury (NSSI). NSSI is defined as the “direct, deliberate destruction of one’s own body tissue in the absence of suicidal intent” (Nock & Favazza, 2009, p. 9). Meta-analytic research (Swannell et al., 2014) has estimated that 17.2% of adolescents have engaged in NSSI, although estimates vary considerably because of methodological heterogeneity. Not only is NSSI associated with increased individual, societal, and economic cost, but it is a risk factor for later suicide attempt (Hamza et al., 2012). Although NSSI is a component of the diagnosis of borderline personality disorder, research documents that it occurs in a broader context of psychopathological vulnerability and is independently associated with functional impairment and suicidality (Selby et al., 2012). This dysfunction associated with NSSI, in part, stimulated the recent inclusion of NSSI disorder in Section III of the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5; American Psychiatric Association, 2013), to facilitate further research (Wilkinson & Goodyer, 2011).
NSSI and cannabis use co-occur, as a recent crossnational study (Giletta et al., 2012) found that increasing frequency of cannabis use was associated with increased odds of NSSI (odds ratios [ORs] = 1.02–3.13). Rossow et al. (2009) also found that adolescent cannabis users demonstrated elevated risk for NSSI. Although this initial evidence hints at an association, no study to date has examined escalation in NSSI vulnerability associated with early cannabis use. Furthermore, mechanisms underlying this association have not been examined, but may include (a) shared genetic influences, as genetic factors have been shown to influence both cannabis use (Verweij et al., 2010) and NSSI (Maciejewski et al., 2014); (b) shared environmental influences (e.g., familial environment); (c) shared individual-specific risk factors, such as emotion dysregulation (Dorard et al., 2008; Yurkowski et al., 2015) or childhood sexual abuse (Klonsky & Moyer, 2008; Sartor et al., 2013); or (d) causal mechanisms, whereby early initiation of cannabis directly increases the likelihood of NSSI or vice versa.
Although longitudinal data provide the most robust tests of causation, twin samples can facilitate efforts to disentangle correlated genetic (and familial) influences from those attributable to person-specific factors that may, putatively, be causal in nature. Monozygotic (MZ) twin pairs, on average, share all their segregating genes identical-by-descent as well as their early familial environment; therefore, any excess likelihood of NSSI in the cannabis-exposed member of a discordant MZ twin pair, relative to their genetically identical but unexposed cotwin, is evidence for the role of nongenetic (and potentially causal) links between cannabis involvement and NSSI. Importantly, the absence of differences in rates of NSSI within MZ pairs discordant for cannabis exposure rules out any possibility of causal influences.
In the current study, we used a large sample (N = 9,583) of adult Australian twins (ages 24–40 years) to examine two aspects of cannabis involvement. First, using the full sample and 414 MZ pairs discordant for cannabis use, we examined whether a lifetime history of cannabis use (ever used cannabis) is associated with NSSI. Second, within those who reported a lifetime history of cannabis use (n = 6,009), we examined whether early-onset cannabis users were more likely to report NSSI than their later-onset counterparts in a sample of 180 MZ discordant pairs. By distinguishing between cannabis use and early initiation within users, our study attempted to disentangle contributions of predisposing and person-specific influences on two important yet somewhat distinct aspects of cannabis involvement and their relationship with NSSI. Finally, we leveraged a second sample of adult female twins (n = 3,788), which included data regarding the age at onset of NSSI, and used discordant twin models to further examine the temporal ordering of cannabis use and NSSI and whether their sequence of onset influenced results from discordant twin analyses.
Method
Participants
Sample 1.
The sample consisted of 9,583 adult twins from the Australian Twin Registry assessed in two separate cohorts. These data included 1,096 MZ female, 818 dizygotic (DZ) female, 667 MZ male, 513 DZ male, and 892 DZ opposite-sex twin pairs as well as 1,611 twins whose co-twin did not participate. Twins in the first cohort were born between 1964 and 1971 and were interviewed between 1996 and 2000. Twins in the second cohort were born between 1972 and 1979 and were interviewed between 2005 and 2009. More information on interview procedures and participant demographics are documented elsewhere for the first (Heath et al., 1997) and second (Lynskey et al., 2012) cohorts. All subjects provided written informed consent after receiving a complete description of the study.
Sample 2.
The Missouri Adolescent Female Twin Study (MOAFTS) represents a cohort of same-sex female twin pairs identified from birth records who were born between July 1, 1975, and June 30, 1985, to Missouri-born parents (Heath et al., 2002; Knopik et al., 2009). Further details regarding sample recruitment and characteristics of the baseline (adolescent) interview data (not used in this study) are given elsewhere (Knopik et al., 2009). Because a subset of the participants was 12–15 years of age at baseline, sensitive questions regarding illicit drug use were not administered. During 2002–2005, all eligible twins who were then adults, regardless of whether they had participated in the baseline assessments (and as long as they had not declined to participate in future interviews), were invited to participate in the first full-length adult follow-up interview. This sample, which represented 80% of all live twin births in the state, consists of 3,787 twins (including 964 MZ and 809 DZ pairs) ages 18–29 years, with 14.6% of African American ancestry.
Primary measures
All variables in Sample 1 were assessed via telephone interview using the Australian version of the Semi-Structured Assessment of the Genetics of Alcoholism (SSAGA-OZ; Bucholz et al., 1994). All variables in Sample 2 were assessed using a more recent version of the SSAGA that included a measure of age at onset of NSSI.
Lifetime cannabis use.
Lifetime cannabis use referred to ever using cannabis, even once.
Early cannabis use.
The age threshold for determining early cannabis use was based on the frequency distribution for self-reported age at first cannabis use and was operationalized in Sample 1 as using cannabis before age 17 years (28.6% prevalence; 26.8% female, 30.8% male). In Sample 2 (MOAFTS), early cannabis use was defined as using cannabis before age 16 years (30.2% prevalence). Within the early cannabis use discordant twin pairs in Samples 1 and 2, the mean difference in reported age at first use was 3.44 and 2.67 years, respectively.
Nonsuicidal self-injury.
In Sample 1, all participants were asked, “(Other than when you tried to take your own life), have you ever hurt yourself on purpose, for example, by cutting or burning yourself?” The question was the same in Sample 2 with the exception of “Other than” being replaced by “Apart from.” The parenthetical portion of the question was asked only if participants had previously reported a lifetime suicide attempt. If the respondent did not report a suicide attempt, the nonparenthetical portion of the question was presented. The prevalence of NSSI was 5.1% in both samples and slightly higher among those with a lifetime history of cannabis use (Sample 1: 6.7%; Sample 2: 7.8%). To account for temporal ordering, in Sample 2, females reporting the onset of NSSI before the onset of cannabis use were excluded from analyses.
Covariates
Several covariates were included in the analyses conducted in Sample 1 and were assessed using the SSAGA-OZ. For a covariate to be considered present, the age at onset had to occur before age 17 years (threshold for early cannabis use) both for those who reported ever using cannabis and for lifetime nonusers. Among those reporting cannabis use, the covariate was only considered present if occurring before the onset of cannabis use (i.e., time varying). Dichotomous covariates included the following:
(a) Depressed mood, assessed by asking participants if there has “ever been two weeks or more when you were depressed or down most of the day, nearly every day.”
(b) Childhood sexual abuse, assessed by asking participants if they were ever forced into sexual intercourse or sexual activity before age 18.
(c) Regular smoking, defined as smoking a cigarette at least 1 day a week for a period of 3 weeks or more (time frame assessed was 2 months or more in the second cohort).
(d) Regular alcohol use, defined as drinking alcohol at least once a month for a period of 6 months or more.
(e) Illicit drug use, defined as ever use of one of the following substances: sedatives, stimulants, opiates, hallucinogens, cocaine, solvents, inhalants, or phencyclidine.
(f) Conduct disorder, defined as the presence of three or more DSM-IV (American Psychiatric Association, 1994) conduct disorder symptoms within a 12-month period (e.g., physical fighting, hurting animals, shoplifting).
(g) Social anxiety, defined as the presence of one or more social fears (e.g., speaking in public) causing impairment in functioning.
Data analysis
Using SAS Version 9.2 (SAS Institute, Inc., Cary, NC), logistic regression analyses were used to examine the association between cannabis use and NSSI as well as between early cannabis use and NSSI, without and with covariates (including age, sex, and twin cohort). Survey procedures that use the Taylor method for adjustment of standard errors were used to analyze the full sample of twins. For discordant twin analyses (i.e., within-pair conditional logistic regression), two series of discordant twins were used: (a) those discordant for cannabis use (414 MZ pairs) and (b) within cannabis users, those discordant for early cannabis use (180 MZ pairs). An adjusted model including both MZ and DZ twins was tested if cannabis exposure was significantly associated with NSSI in the unadjusted model. All covariates significant in the full sample models were included in the discordant models, except age, sex, and cohort, which are matched in twin pairs. In addition, a zygosity interaction term was included to examine whether the association between cannabis exposure and NSSI differs for MZ and DZ twins. Similar analyses were conducted in Sample 2. Because of the substantially smaller sample size (in discordant pairs), only univariate conditional logistic regression models were tested in the combined MZ and DZ discordant pairs. An interaction term between MZ status and exposure to cannabis was used to account for zygosity differences.
Results
Association between NSSI, covariates, and early cannabis use
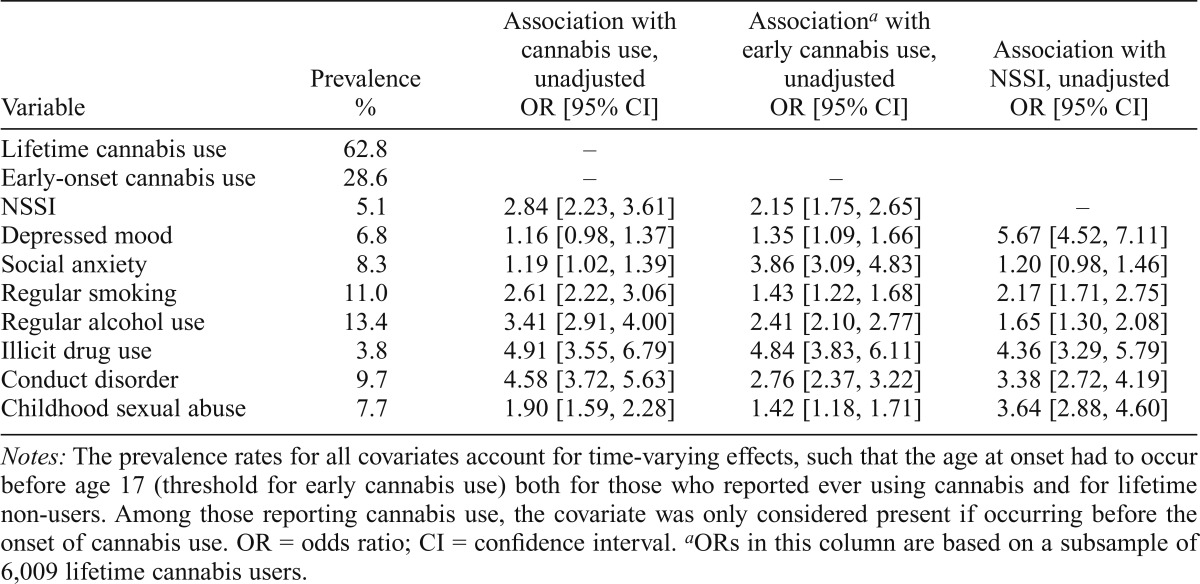
The association between cannabis use, early cannabis use, and NSSI as well as individual covariates is presented in Table 1. Both cannabis use (N = 9,583; OR = 2.84, 95% CI [2.23, 3.61], p < .0001) and early cannabis use within the subgroup of lifetime cannabis users (n = 6,009; OR = 2.15, 95% CI [1.75, 2.65], p < .0001) were associated with NSSI. Moreover, cannabis use, early cannabis use, and NSSI were also significantly associated with all covariates, with the exception of depressed mood, which was not significantly associated with cannabis use. In addition, social anxiety was not significantly associated with NSSI and was, therefore, not included in the adjusted models. Even after adjustment for covariates, the association between cannabis use and NSSI remained significant (OR = 2.07, 95% CI [1.60, 2.67], p < .0001). Similarly, within the subgroup of lifetime cannabis users, early cannabis use remained associated with NSSI after covariate inclusion (OR = 1.42, 95% CI [1.13, 1.78], p < .0001). In both models, all covariates other than sex, age, and early regular alcohol use were significantly associated with NSSI. Given that age, sex, and cohort are matched in twin pairs, these covariates were excluded in discordant twin analyses. Early regular alcohol use was also excluded given the lack of association with NSSI in the adjusted model.
Table 1.
Prevalence of and associations between covariates used in the study and lifetime and early cannabis use (<17 years of age) and nonsuicidal self-injury (NSSI) in 9,583 male and female Australian twins
| Variable | Prevalence % | Association with cannabis use, unadjusted OR [95% CI] | Associationa with early cannabis use, unadjusted OR [95% CI] | Association with NSSI, unadjusted OR [95% CI] |
| Lifetime cannabis use | 62.8 | – | ||
| Early-onset cannabis use | 28.6 | – | – | |
| NSSI | 5.1 | 2.84 [2.23, 3.61] | 2.15 [1.75, 2.65] | – |
| Depressed mood | 6.8 | 1.16 [0.98, 1.37] | 1.35 [1.09, 1.66] | 5.67 [4.52, 7.11] |
| Social anxiety | 8.3 | 1.19 [1.02, 1.39] | 3.86 [3.09, 4.83] | 1.20 [0.98, 1.46] |
| Regular smoking | 11.0 | 2.61 [2.22, 3.06] | 1.43 [1.22, 1.68] | 2.17 [1.71, 2.75] |
| Regular alcohol use | 13.4 | 3.41 [2.91, 4.00] | 2.41 [2.10, 2.77] | 1.65 [1.30, 2.08] |
| Illicit drug use | 3.8 | 4.91 [3.55, 6.79] | 4.84 [3.83, 6.11] | 4.36 [3.29, 5.79] |
| Conduct disorder | 9.7 | 4.58 [3.72, 5.63] | 2.76 [2.37, 3.22] | 3.38 [2.72, 4.19] |
| Childhood sexual abuse | 7.7 | 1.90 [1.59, 2.28] | 1.42 [1.18, 1.71] | 3.64 [2.88, 4.60] |
Notes: The prevalence rates for all covariates account for time-varying effects, such that the age at onset had to occur before age 17 (threshold for early cannabis use) both for those who reported ever using cannabis and for lifetime non-users. Among those reporting cannabis use, the covariate was only considered present if occurring before the onset of cannabis use. OR = odds ratio; CI = confidence interval.
ORs in this column are based on a subsample of 6,009 lifetime cannabis users.
Prevalence of NSSI in concordant and discordant twin pairs
NSSI was most frequently reported by members of twin pairs in which both twins reported cannabis use (7.1%, 95% CI [6.1%, 8.0%]), and rates of NSSI were lowest among twins concordant for no cannabis use (2.3%, 95% CI [1.5%, 3.0%]). The cannabis use member of discordant pairs was also more likely to report NSSI (5.2%, 95% CI [3.7%, 6.7%]) than their co-twin who never used cannabis (2.9%, 95% CI [1.8%, 4.0%]).
Excluding never-users of cannabis, 10.1% (95% CI [7.5%, 12.7%]) of twin pairs concordant for early cannabis use (before age 17 years) reported a lifetime history of NSSI compared with 5.3% (95% CI [4.2%, 6.4%]) of concordant twin pairs who reported cannabis use after age 16 years. In twin pairs discordant for early cannabis use, the early-using twin was more than twice as likely to report NSSI (12.1%, 95% CI [8.9%, 15.4%]) as their later-using co-twin (5.3%, 95% CI [3.0%, 7.5%]).
Discordant twin analyses
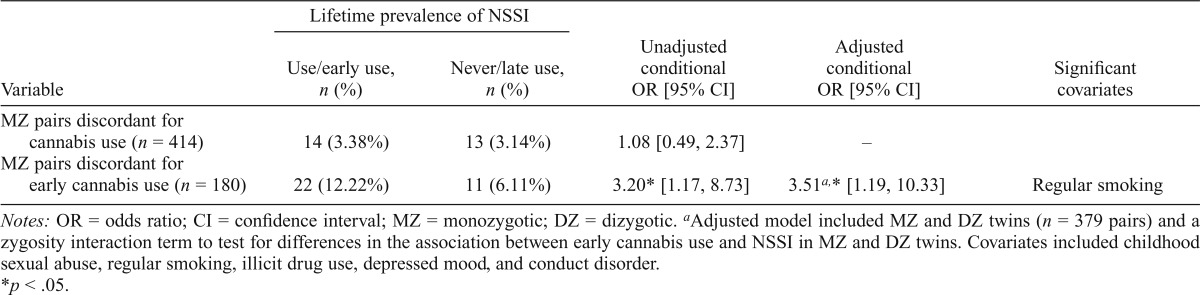
As shown in Table 2, within MZ twin pairs discordant for cannabis use (npairs = 414), the cannabis-using twin was no different from their genetically identical but lifetime nonuser co-twin to report NSSI (OR = 1.08, p = .84). However, within cannabis users, early cannabis use was associated with an elevated likelihood of NSSI (npairs = 180; OR = 3.20, p < .05), indicating that even after we controlled for familial environmental and genetic influences shared identical-bydescent, the twin who used cannabis before age 17 years was considerably more likely to also report NSSI when compared with his or her genetically identical co-twin who used cannabis at a later age. Therefore, an adjusted model including covariates was tested in MZ and DZ twins combined (npairs = 379). A zygosity interaction term (i.e., MZ Status × Early Cannabis Use Exposure) was included to test for differences in the association between early cannabis use and NSSI in MZ and DZ twins. Adjustment for covariates did not modify the results, such that the association between early cannabis use and NSSI remained significant (OR = 3.51, 95% CI [1.19, 10.33], p = .02). Importantly, the zygosity interaction term was nonsignificant (p = .76), suggesting that the OR for MZs and DZs is equivalent.
Table 2.
Prevalence and odds of lifetime nonsuicidal self-injury (NSSI) among lifetime and early cannabis users (<17 years old) compared with their co-twin who never used cannabis or did not use cannabis early
| Lifetime prevalence of NSSI |
|||||
| Variable | Use/early use, n (%) | Never/late use, n (%) | Unadjusted conditional OR [95% CI] | Adjusted conditional OR [95% CI] | Significant covariates |
| MZ pairs discordant for cannabis use (n = 414) | 14 (3.38%) | 13 (3.14%) | 1.08 [0.49, 2.37] | – | |
| MZ pairs discordant for early cannabis use (n = 180) | 22 (12.22%) | 11 (6.11%) | 3.20* [1.17, 8.73] | 3.51a,* [1.19, 10.33] | Regular smoking |
Notes: OR = odds ratio; CI = confidence interval; MZ = monozygotic; DZ = dizygotic.
Adjusted model included MZ and DZ twins (n = 379 pairs) and a zygosity interaction term to test for differences in the association between early cannabis use and NSSI in MZ and DZ twins. Covariates included childhood sexual abuse, regular smoking, illicit drug use, depressed mood, and conduct disorder.
p < .05.
Replication in Sample 2
The median age at onset for initiation of cannabis was 16 years, and for NSSI, age 15 years. There were no significant racial differences in the prevalence of cannabis use (White: 42.6%; African American: 46.6%) or NSSI (White: 5.1%; African American: 5.2%). Of the 128 women reporting both cannabis use and NSSI, 27% reported the same age at onset, and 33% reported the onset of NSSI after initiation of cannabis. Approximately 40% (n = 51) of women reported the onset of NSSI before the onset of cannabis use—these respondents were excluded from analyses. Twin individuals with a lifetime history of cannabis use had significantly increased odds of NSSI (OR = 1.62, 95% CI [1.13, 2.31], p < .01), and early cannabis use was associated with even greater odds of NSSI (OR = 3.66, 95% CI [2.51, 5.33], p < .01). Among twin pairs discordant for cannabis use (n = 441) and early cannabis use (n = 137), the interaction between zygosity and cannabis was not significant (p = .38 for cannabis use and .89 for early cannabis use), indicating a similar magnitude of association in discordant MZ and DZ pairs. Cannabis-using twins were no more likely to report same-year or subsequent NSSI than their non–cannabis-using co-twin (OR = 2.0, 95% CI [0.86, 4.67]); however, among lifetime cannabis users, there was a nominally significant (p = .054) association between early cannabis use and NSSI, suggesting that the twin who used cannabis before age 16 years was at 4.5 times greater odds (95% CI [0.97, 20.83]) of reporting same-year or subsequent NSSI compared with their later-onset cannabis-using co-twin. Despite the reduced significance, all point estimates were consistent with findings from the larger Australian cohort, indicating that the lack of temporal ordering did not significantly bias findings in that sample.
Discussion
To our knowledge, this is the first genetically informed study of the relationship between cannabis use (ever used cannabis), early cannabis use, and NSSI. In both samples, cannabis use and early cannabis use were associated with a lifetime history of NSSI, but the contributors to this association were somewhat distinct. According to comparisons within pairs of discordant twins, the correlation between cannabis use and NSSI could be attributed to a shared predisposition (genetic and/or familial environment), whereas person-specific factors that may or may not be of a causal nature contributed to the correlation between early cannabis use and NSSI. These findings suggest that delaying the onset of cannabis use may be a promising pathway toward reducing the risk for NSSI.
Even within pairs of genetically identical individuals, the twin who used cannabis at an early age was considerably more likely (12.2%) to report NSSI than their co-twin who used cannabis but at a later age (6.1%). Remarkably, a prior study by Lynskey and colleagues (2004) that used a subset of these data (i.e., only the first twin cohort) noted that twins who used cannabis before age 17 years were at 3.5 times increased odds of reporting suicide attempts when compared with their co-twins who used cannabis at a later age. Such a within-pair elevation was not noted when major depressive disorder or suicidal ideation was examined, suggesting that factors shared by members of twin pairs, including genetic background and shared environmental influences, were largely responsible for these associations. We noted a similar finding for the relationship between cannabis use and NSSI. Such shared genetic pathways may include a general liability to impulsive or disinhibited behaviors, which are related to both cannabis use (Felton et al., 2015) and NSSI (Glenn & Klonsky, 2010).
Importantly, there is substantial overlap between NSSI and suicidal behavior. In the current study (Sample 1), those reporting NSSI had substantially greater odds of lifetime suicidal ideation (OR = 8.41) and suicide attempt (OR = 9.75). Of the 491 participants who endorsed a lifetime history of NSSI, 72% also reported a lifetime history of suicidal ideation and 24% reported a history of suicide attempt. Only 14% of those endorsing suicidal ideation reported NSSI, whereas 29% of those endorsing a previous suicide attempt reported NSSI. This degree of overlap is consistent with data from other community samples (e.g., Bebbington et al., 2010). Therefore, it is possible that the association between early cannabis use and suicide attempt within twin pairs that was observed in the study by Lynskey et al. (2004) could, in part, reflect the association with NSSI noted in the present study (and vice versa). Although we did not have sufficient power to examine NSSI in isolation from other suicidal behaviors, future studies should examine whether the association between cannabis involvement and NSSI within discordant twin pairs remains when excluding those individuals who report other forms of suicidal behavior.
The association between early cannabis use and NSSI within twin pairs, which remained significant after the inclusion of covariates, suggests that factors other than shared genes or family environment, including potential unmeasured (third) variables, might be involved. These person-specific factors that make an early-onset cannabis user more likely to also report NSSI might also exert causal effects. The similar findings in Sample 2, which accounted for the temporality between onset of cannabis exposure and NSSI, further support the plausibility of this assertion. For instance, affiliation with deviant peers is strongly related to problematic cannabis use (Tarter et al., 2011), and emerging evidence also finds a role for peer socialization in the development of NSSI (Hasking et al., 2013).
Similarly, childhood adversity increases vulnerability to both early cannabis use (Sartor et al., 2013) and NSSI (Klonsky & Moyer, 2008) and might do so directly or via the induction of deficits in emotion regulation and/or development of other forms of psychopathology (e.g., posttraumatic stress disorder; Klonsky & Moyer, 2008; Shenk et al., 2010). We controlled for the confounding effects of childhood sexual abuse but not for other traumas. An alternative, more causal pathway may relate to pain processing, which can be modified in individuals exposed to early adversity (Ballard et al., 2010). Furthermore, because Δ9-tetrahydrocannabinol (THC) has analgesic properties (Borgelt et al., 2013), as do the endocannabinoids (Woodhams et al., 2015), which interface with THC during neurotransmitter signaling, it is theoretically possible that early exposure to THC modifies pain perception and contributes directly to the risk for NSSI, although given the typically low frequency of cannabis use before age 17 years in this sample, we consider this improbable. Functional neuroimaging research also provides evidence for alteration of neural pathways associated with pain processing among individuals reporting NSSI and borderline personality disorder, of which NSSI is a prominent feature (Kluetsch et al., 2012; Osuch et al., 2014).
However, we are skeptical about positing potential causal effects with these cross-sectional data. It is worth noting that we did not have data on age at onset of NSSI in Sample 1, and thus any putatively causal pathways may extend from NSSI to early cannabis use. The typical age at onset of NSSI is 16 years (Klonsky, 2011), which coincides with the most commonly reported age for initiation of cannabis use in early-onset users (49%). Therefore, it is possible in Sample 1 that NSSI preceded onset of cannabis use and, in fact, contributed to its early onset via pathways related to selfmedication or general impulsivity. The results from Sample 2 provide some initial evidence that this is not the case given that individuals reporting an onset of NSSI before initiation of cannabis use were excluded. However, twin individuals reporting the same age at onset for cannabis use and NSSI were included, and thus a third variable could give rise to initiation of these behaviors simultaneously.
Overall, our findings from two large samples of twins suggest that nongenetic mechanisms are at play in the relationship between NSSI and cannabis use that occurs at earlier but not at later ages. Given its substantial prevalence in adolescence and associated dysfunction, NSSI has recently been included in DSM-5 Section III as a potential standalone disorder (Wilkinson & Goodyer, 2011). Therefore, any putative risk factors that are modifiable should be isolated. In particular, prospective studies of commonly occurring correlates, such as early cannabis use, should be prioritized. NSSI can rapidly progress to suicidal intent and attempts (Hamza et al., 2012), and because early cannabis use has previously been linked to suicidal thoughts and behaviors (Swahn et al., 2012), understanding the links between early cannabis use and NSSI may provide clues regarding these subsequent relationships with suicide as well.
Footnotes
This research was funded by National Institute on Alcohol Abuse and Alcoholism (NIAAA) Grant AA023693 (to Lauren R. Few); NIAAA Grants AA11998, AA07728, and AA13221 (to Andrew C. Heath); and National Institute on Drug Abuse Grants DA040411 (to Arpana Agrawal and Richard A. Grucza), DA23668 (to Arpana Agrawal), DA031288 (to Richard A. Grucza), DA32573 (to Arpana Agrawal), and DA18267 (to Michael T. Lynskey) facilitated through access to the Australian Twin Registry, a national resource supported by an Enabling Grant (ID 628911) from the National Health &Medical Research Council (NHMRC). Nicholas G. Martin acknowledges support from the Australian NHMRC Centre for Research Excellence on Suicide Prevention (CRESP, principal investigator Helen Christensen). Karin J. H. Verweij is supported by a 2014 NARSADYoung Investigator Grant from the Brain & Behavior Research Foundation.
References
- Agrawal A., Neale M. C., Prescott C. A., Kendler K. S. A twin study of early cannabis use and subsequent use and abuse/dependence of other illicit drugs. Psychological Medicine. 2004;34:1227–1237. doi: 10.1017/s0033291704002545. doi:10.1017/S0033291704002545. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. Washington, DC: Author; 1994. [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed. Arlington, VA: Author; 2013. [Google Scholar]
- Australian Institute of Health and Welfare. Canberra, Australia: Author; 2014. National Drug Strategy Household Survey detailed report 2013. Retrieved from http://www.aihw.gov.au/alcohol-and-other-drugs/ndshs-2013/ [Google Scholar]
- Bagot K. S., Milin R., Kaminer Y. Adolescent initiation of cannabis use and early-onset psychosis. Substance Abuse. 2015;36:524–533. doi: 10.1080/08897077.2014.995332. doi:10.1080/08897077.2014.995332. [DOI] [PubMed] [Google Scholar]
- Ballard E., Bosk A., Pao M. Invited commentary: Understanding brain mechanisms of pain processing in adolescents’ non-suicidal self-injury. Journal of Youth and Adolescence. 2010;39:327–334. doi: 10.1007/s10964-009-9457-1. doi:10.1007/s10964-009-9457-1. [DOI] [PubMed] [Google Scholar]
- Bebbington P. E., Minot S., Cooper C., Dennis M., Meltzer H., Jenkins R., Brugha T. Suicidal ideation, self-harm and attempted suicide: Results from the British psychiatric morbidity survey 2000. European Psychiatry. 2010;25:427–431. doi: 10.1016/j.eurpsy.2009.12.004. doi:10.1016/j.eurpsy.2009.12.004. [DOI] [PubMed] [Google Scholar]
- Borgelt L. M., Franson K. L., Nussbaum A. M., Wang G. S. The pharmacologic and clinical effects of medical cannabis. Pharmacotherapy. 2013;33:195–209. doi: 10.1002/phar.1187. doi:10.1002/phar.1187. [DOI] [PubMed] [Google Scholar]
- Bucholz K. K., Cadoret R., Cloninger C. R., Dinwiddie S. H., Hesselbrock V. M., Nurnberger J. I., Jr., Schuckit M. A. A new, semi-structured psychiatric interview for use in genetic linkage studies: A report on the reliability of the SSAGA. Journal of Studies on Alcohol. 1994;55:149–158. doi: 10.15288/jsa.1994.55.149. doi:10.15288/jsa.1994.55.149. [DOI] [PubMed] [Google Scholar]
- Delforterie M. J., Lynskey M. T., Huizink A. C., Creemers H. E., Grant J. D., Few L. R., Agrawal A. The relationship between cannabis involvement and suicidal thoughts and behaviors. Drug and Alcohol Dependence. 2015;150:98–104. doi: 10.1016/j.drugalcdep.2015.02.019. doi:10.1016/j.drugalcdep.2015.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorard G., Berthoz S., Phan O., Corcos M., Bungener C. Affect dysregulation in cannabis abusers: A study in adolescents and young adults. European Child & Adolescent Psychiatry. 2008;17:274–282. doi: 10.1007/s00787-007-0663-7. doi:10.1007/s00787-007-0663-7. [DOI] [PubMed] [Google Scholar]
- Felton J. W., Collado A., Shadur J. M., Lejuez C. W., MacPherson L. Sex differences in self-report and behavioral measures of disinhibition predicting marijuana use across adolescence. Experimental and Clinical Psychopharmacology. 2015;23:265–274. doi: 10.1037/pha0000031. doi:10.1037/pha0000031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giletta M., Scholte R. H., Engels R. C., Ciairano S., Prinstein M. J. Adolescent non-suicidal self-injury: A cross-national study of community samples from Italy, the Netherlands and the United States. Psychiatry Research. 2012;197:66–72. doi: 10.1016/j.psychres.2012.02.009. doi:10.1016/j.psychres.2012.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenn C. R., Klonsky E. D. A multimethod analysis of impulsivity in nonsuicidal self-injury. Personality Disorders. 2010;1:67–75. doi: 10.1037/a0017427. doi:10.1037/a0017427. [DOI] [PubMed] [Google Scholar]
- Grant J. D., Lynskey M. T., Scherrer J. F., Agrawal A., Heath A. C., Bucholz K. K. A cotwin-control analysis of drug use and abuse/dependence risk associated with early-onset cannabis use. Addictive Behaviors. 2010;35:35–41. doi: 10.1016/j.addbeh.2009.08.006. doi:10.1016/j.addbeh.2009.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamza C. A., Stewart S. L., Willoughby T. Examining the link between nonsuicidal self-injury and suicidal behavior: A review of the literature and an integrated model. Clinical Psychology Review. 2012;32:482–495. doi: 10.1016/j.cpr.2012.05.003. doi:10.1016/j.cpr.2012.05.003. [DOI] [PubMed] [Google Scholar]
- Hasking P., Andrews T., Martin G. The role of exposure to self-injury among peers in predicting later self-injury. Journal of Youth and Adolescence. 2013;42:1543–1556. doi: 10.1007/s10964-013-9931-7. doi:10.1007/s10964-013-9931-7. [DOI] [PubMed] [Google Scholar]
- Heath A. C., Bucholz K. K., Madden P. A. F., Dinwiddie S. H., Slutske W. S., Bierut L. J., Martin N. G. Genetic and environmental contributions to alcohol dependence risk in a national twin sample: Consistency of findings in women and men. Psychological Medicine. 1997;27:1381–1396. doi: 10.1017/s0033291797005643. doi:10.1017/S0033291797005643. [DOI] [PubMed] [Google Scholar]
- Heath A. C., Howells W., Bucholz K. K., Glowinski A. L., Nelson E. I., Madden P. A. Ascertainment of a mid-western US female adolescent twin cohort for alcohol studies: Assessment of sample representativeness using birth record data. Twin Research. 2002;5:107–112. doi: 10.1375/1369052022974. doi:10.1375/1369052022974. [DOI] [PubMed] [Google Scholar]
- Homel J., Thompson K., Leadbeater B. Trajectories of marijuana use in youth ages 15–25: Implications for postsecondary education experiences. Journal of Studies on Alcohol and Drugs. 2014;75:674–683. doi: 10.15288/jsad.2014.75.674. doi:10.15288/jsad.2014.75.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kluetsch R. C., Schmahl C., Niedtfeld I., Densmore M., Calhoun V. A., Daniels J., Lanius R. A. Alterations in default mode network connectivity during pain processing in borderline personality disorder. Archives of General Psychiatry. 2012;69:993–1002. doi: 10.1001/archgenpsychiatry.2012.476. doi:10.1001/archgenpsychiatry.2012.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klonsky E. D. Non-suicidal self-injury in United States adults: Prevalence, sociodemographics, topography and functions. Psychological Medicine. 2011;41:1981–1986. doi: 10.1017/S0033291710002497. doi:10.1017/S0033291710002497. [DOI] [PubMed] [Google Scholar]
- Klonsky E. D., Moyer A. Childhood sexual abuse and nonsuicidal self-injury: Meta-analysis. British Journal of Psychiatry. 2008;192:166–170. doi: 10.1192/bjp.bp.106.030650. doi:10.1192/bjp.bp.106.030650. [DOI] [PubMed] [Google Scholar]
- Knopik V. S., Heath A. C., Bucholz K. K., Madden P. A., Waldron M. Genetic and environmental influences on externalizing behavior and alcohol problems in adolescence: A female twin study. Pharmacology, Biochemistry, and Behavior. 2009;93:313–321. doi: 10.1016/j.pbb.2009.03.011. doi:10.1016/j.pbb.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lessem J. M., Hopfer C. J., Haberstick B. C., Timberlake D., Ehringer M. A., Smolen A., Hewitt J. K. Relationship between adolescent marijuana use and young adult illicit drug use. Behavior Genetics. 2006;36:498–506. doi: 10.1007/s10519-006-9064-9. doi:10.1007/s10519-006-9064-9. [DOI] [PubMed] [Google Scholar]
- Lynskey M. T., Agrawal A., Henders A., Nelson E. C., Madden P. A., Martin N. G. An Australian twin study of cannabis and other illicit drug use and misuse, and other psychopathology. Twin Research and Human Genetics. 2012;15:631–641. doi: 10.1017/thg.2012.41. doi:10.1017/thg.2012.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynskey M. T., Glowinski A. L., Todorov A. A., Bucholz K. K., Madden P. A. F., Nelson E. C., Heath A. C. Major depressive disorder, suicidal ideation, and suicide attempt in twins discordant for cannabis dependence and early-onset cannabis use. Archives of General Psychiatry. 2004;61:1026–1032. doi: 10.1001/archpsyc.61.10.1026. doi:10.1001/archpsyc.61.10.1026. [DOI] [PubMed] [Google Scholar]
- Lynskey M. T., Heath A. C., Bucholz K. K., Slutske W. S., Madden P. A. F., Nelson E. C., Martin N. G. Escalation of drug use in early-onset cannabis users vs co-twin controls. JAMA. 2003;289:427–433. doi: 10.1001/jama.289.4.427. doi:10.1001/jama.289.4.427. [DOI] [PubMed] [Google Scholar]
- Lynskey M. T., Vink J. M., Boomsma D. I. Early onset cannabis use and progression to other drug use in a sample of Dutch twins. Behavior Genetics. 2006;36:195–200. doi: 10.1007/s10519-005-9023-x. doi:10.1007/s10519-005-9023-x. [DOI] [PubMed] [Google Scholar]
- Maciejewski D. F., Creemers H. E., Lynskey M. T., Madden P. A. F., Heath A. C., Statham D. J., Verweij K. J. H. Overlapping genetic and environmental influences on nonsuicidal self-injury and suicidal ideation: Different outcomes, same etiology? JAMA Psychiatry. 2014;71:699–705. doi: 10.1001/jamapsychiatry.2014.89. doi:10.1001/jamapsychiatry.2014.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nock M. K., Favazza A. Non-suicidal self-injury: Definition and classification. In: Nock M. K., editor. Understanding non-suicidal self-injury: Origins, assessment, and treatment. Washington, DC: American Psychological Association; 2009. pp. 9–18. [Google Scholar]
- Okaneku J., Vearrier D., McKeever R. G., LaSala G. S., Greenberg M. I. Change in perceived risk associated with marijuana use in the United States from 2002 to 2012. Clinical Toxicology. 2015;53:151–155. doi: 10.3109/15563650.2015.1004581. doi:10.3109/15563650.2015.1004581. [DOI] [PubMed] [Google Scholar]
- Osuch E., Ford K., Wrath A., Bartha R., Neufeld R. Functional MRI of pain application in youth who engaged in repetitive non-suicidal self-injury vs. psychiatric controls. Psychiatry Research. 2014;223:104–112. doi: 10.1016/j.pscychresns.2014.05.003. doi:10.1016/j.pscychresns.2014.05.003. [DOI] [PubMed] [Google Scholar]
- Rossow I., Hawton K., Ystgaard M. Cannabis use and deliberate self-harm in adolescence: A comparative analysis of associations in England and Norway. Archives of Suicide Research. 2009;13:340–348. doi: 10.1080/13811110903266475. doi:10.1080/13811110903266475. [DOI] [PubMed] [Google Scholar]
- Sartor C. E., Waldron M., Duncan A. E., Grant J. D., McCutcheon V. V., Nelson E. C., Heath A. C. Childhood sexual abuse and early substance use in adolescent girls: The role of familial influences. Addiction. 2013;108:993–1000. doi: 10.1111/add.12115. doi:10.1111/add.12115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholes-Balog K. E., Hemphill S. A., Patton G. C., Toumbourou J. W. Cannabis use and related harms in the transition to young adulthood: A longitudinal study of Australian secondary school students. Journal of Adolescence. 2013;36:519–527. doi: 10.1016/j.adolescence.2013.03.001. doi:10.1016/j.adolescence.2013.03.001. [DOI] [PubMed] [Google Scholar]
- Selby E. A., Bender T. W., Gordon K. H., Nock M. K., Joiner T. E., Jr. Non-suicidal self-injury (NSSI) disorder: A preliminary study. Personality Disorders. 2012;3:167–175. doi: 10.1037/a0024405. doi:10.1037/a0024405. [DOI] [PubMed] [Google Scholar]
- Shenk C. E., Noll J. G., Cassarly J. A. A multiple mediational test of the relationship between childhood maltreatment and non-suicidal self-injury. Journal of Youth and Adolescence. 2010;39:335–342. doi: 10.1007/s10964-009-9456-2. doi:10.1007/s10964-009-9456-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. The NSDUH Report: Substance Use and Mental Health Estimates from the 2013 National Survey on Drug Use and Health: Overview of findings. Rockville, MD: Author; 2014. Retrieved from http://www.samhsa.gov/data/sites/default/files/NSDUH-SR200-RecoveryMonth-2014/NSDUH-SR200-RecoveryMonth-2014.htm. [PubMed] [Google Scholar]
- Swahn M. H., Bossarte R. M., Choquet M., Hassler C., Falissard B., Chau N. Early substance use initiation and suicide ideation and attempts among students in France and the United States. International Journal of Public Health. 2012;57:95–105. doi: 10.1007/s00038-011-0255-7. doi:10.1007/s00038-011-0255-7. [DOI] [PubMed] [Google Scholar]
- Swannell S. V., Martin G. E., Page A., Hasking P., St John N. J. Prevalence of nonsuicidal self-injury in nonclinical samples: Systematic review, meta-analysis and meta-regression. Suicide & Life-Threatening Behavior. 2014;44:273–303. doi: 10.1111/sltb.12070. doi:10.1111/sltb.12070. [DOI] [PubMed] [Google Scholar]
- Tarter R. E., Fishbein D., Kirisci L., Mezzich A., Ridenour T., Vanyukov M. Deviant socialization mediates transmissible and contextual risk on cannabis use disorder development: A prospective study. Addiction. 2011;106:1301–1308. doi: 10.1111/j.1360-0443.2011.03401.x. doi:10.1111/j.1360-0443.2011.03401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verweij K. J., Huizink A. C., Agrawal A., Martin N. G., Lynskey M. T. Is the relationship between early-onset cannabis use and educational attainment causal or due to common liability? Drug and Alcohol Dependence. 2013;133:580–586. doi: 10.1016/j.drugalcdep.2013.07.034. doi:10.1016/j.drugalcdep.2013.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verweij K. J. H., Zietsch B. P., Lynskey M. T., Medland S. E., Neale M. C., Martin N. G., Vink J. M. Genetic and environmental influences on cannabis use initiation and problematic use: A meta-analysis of twin studies. Addiction. 2010;105:417–430. doi: 10.1111/j.1360-0443.2009.02831.x. doi:10.1111/j.1360-0443.2009.02831.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow N. D., Baler R. D., Compton W. M., Weiss S. R. Adverse health effects of marijuana use. The New England Journal of Medicine. 2014;370:2219–2227. doi: 10.1056/NEJMra1402309. doi:10.1056/NEJMra1402309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner F. A., Anthony J. C. From first drug use to drug dependence: Developmental periods of risk for dependence upon marijuana, cocaine, and alcohol. Neuropsychopharmacology. 2002;26:479–488. doi: 10.1016/S0893-133X(01)00367-0. doi:10.1016/S0893-133X(01)00367-0. [DOI] [PubMed] [Google Scholar]
- Wilkinson P., Goodyer I. Non-suicidal self-injury. European Child & Adolescent Psychiatry. 2011;20:103–108. doi: 10.1007/s00787-010-0156-y. doi:10.1007/s00787-010-0156-y. [DOI] [PubMed] [Google Scholar]
- Woodhams S. G., Sagar D. R., Burston J. J, Chapman V. The role of the endocannabinoid system in pain. Handbook of Experimental Pharmacology. 2015;227:119–143. doi: 10.1007/978-3-662-46450-2_7. doi:10.1007/978-3-662-46450-2_7. [DOI] [PubMed] [Google Scholar]
- Yurkowski K., Martin J., Levesque C., Bureau J. F., Lafontaine M. F., Cloutier P. Emotion dysregulation mediates the influence of relationship difficulties on non-suicidal self-injury behavior in young adults. Psychiatry Research. 2015;228:871–878. doi: 10.1016/j.psychres.2015.05.006. doi:10.1016/j.psychres.2015.05.006. [DOI] [PubMed] [Google Scholar]