Abstract
Bacillus subtilis strain BIA was used for the production of bioactive lipopeptides. Different extraction and purification methods were assayed as liquid–liquid extraction, and acid and ammonium sulfate precipitation followed by TLC, SPE, and gel filtration. Active fractions were further purified using RP-HPLC. The molecular mass of the purified product from HPLC was determined through Tris-Tricine SDS-PAGE and MALDI–TOF-MS. The results revealed that Bacillus subtilis strain BIA produced surfactin and iturin like compounds. Coproduction of surfactin and iturin like compounds by this strain is a remarkable trait for a potential biocontrol agent. This paper also includeds techniques that have been developed for the optimal and convenient extraction of bioactive lipopeptides from microbial origin.
Keywords: Purification, Lipopeptides, TLC, Gel-filtration, RP-HPLC, MALDI–TOF-MS
Introduction
Several hundred wild-type Bacillus spp. have the potential to produce more than two dozen antibiotics with an amazing variety of structures [1]. Non-ribosomally generated amphipathic lipopeptide antibiotics with condensed β-hydroxyl or β-amino fatty acids are widespread in B. subtilis [2]. These are synthesized by large multienzyme systems that have a modular structure [3]. Lipopeptide antibiotics identified so far have been divided into three main groups according to their structure: surfactin group, iturin group, and fengycin group [4]. All these agents occur as families of closely related isoforms which differ in length and branching of the fatty acid side chains and in amino-acid substitutions in the peptide rings [5]. These agents are natural compounds with a high potential for biotechnological and pharmaceutical applications [6]. They are distinguished by excellent surface and membrane-active properties along with superior emulsifying and foaming properties [7], which can be utilized in food biotechnology and in the agricultural sector. Apart from this, bioactive lipopeptides act as antifungal agents [8], antiviral agents, antiameobocytic agents, and antimycoplasma agents [9]. Surfactin is well qualified to maintain virus and mycoplasma safety in biotechnological products [10]. Their mechanism of action is ongoing and has revealed even intercellular mechanisms [11]. Lipopeptides are often extracted from culture broth by classical methods, including acidic precipitation (HCl), recrystallization, and extraction by organic solvents. Unfortunately, impurities are coextracted and the extraction must be completed by the chromatographic procedures [12]. Therefore, to purify and concentrate lipopeptides, several authors have attempted one-step methods [13], including a two-phase extraction [14], an ultrafiltration method [15], and a solid-phase extraction [16]. The technique of foam fractionation is also of great interest, as it offers the double advantage of continuous in situ removal of surfactin from the fermentation broth and prevention of any possible feed-back inhibition [17]. A Bacillus strain, designated as BIA exhibiting antibacterial activity, was isolated from Pakistan with spectrum of activity mostly against Gram-positive bacteria. The strain was identified on the basis of 16S rRNA and biochemical characterization. Lipopeptide analysis was performed by Tris-Tricine SDS-PAGE and matrix-assisted laser desorption ionization–time of flight-mass spectrometry (MALDI–TOF-MS). The results from Tris-Tricine SDS-PAGE showed that B. subtilis BIA produces an antibacterial compound with molecular mass of about 3 kDa. The exact molecular mass was determined through MALDI–TOF-MS which is effective for peptides and proteins with molecular masses ranging from 0.5 to 30 kDa. Here, we report the co-production of surfactin and iturin like compounds from B. subtilis BIA based on their molecular masses through MALDI–TOF-MS.
Materials and Methods
Bacterial Strains and Culture Conditions
Twenty-four strains isolated from different soil samples were tested for their antibiotic production using the agar-well diffusion assay [18]. One strain was selected based upon the size and clarity of zone of inhibition. The test organisms are a collection of laboratory strains as well as environmental isolates. Bacillus fusiformis was routinely used for the sensitivity tests. All strains were routinely maintained on nutrient agar [19], however, for antibiotic production Landy medium [20] was used.
Antibiotic Assay
Samples of culture supernatant containing the antibiotic were assayed for activity using an agar-well diffusion assay [18]. Fifty microliter of Bacillus fusiformis liquid culture of 0.3 OD600 was spread onto the surface of Petri dish-containing l-agar. 50 μL antibiotic sample was transferred into the well made in media plates using a sterile cork borer. The sample was allowed to diffuse into the agar and the plate was inverted and incubated at 37 °C until a lawn of the indicator bacteria appeared on the plate (approximately 10–16 h).
DNA Extraction PCR and Ribotyping
Genomic DNA was extracted from overnight incubated bacterial culture in Luria–Bertani LB-broth [19] at 37 °C with 120 rpm. The extraction was carried out using gene extraction kit (Biorad, UK). PCR amplification of 16S rDNA was performed successfully following the method described by [21] with forward primer 27f (5-AGAGTTTGATCCTGGCTCAG) and reverse primer 1522r (5-AAGGAGGTGATCCA(AG)CCGCA) [22]. To 0.5–0.1 ng of chromosomal template DNA, 0.25 μM each primer, 200 μM deoxynucleoside triphosphate, and 1 unit of Taq polymerase were added. Solution was heated to 94 °C for 5 min and passed through 29 cycles as follows: denaturation for 20 s at 94 °C, primer annealation for 20 s at 50 °C, and extension at 72 °C for 2 min. Final extension was at 72 °C for 5 min. The product was purified using Aqua pure extraction kit (Fermantas, UK) and sequenced using both 27f and 1522r primers by automatic sequencer (Applied Biosystems, USA).
Solvent Extraction and Thin-Layer Chromatography Analysis
Bacterial supernatant, recovered by 15,000×g centrifugation for 20 min of 36-h old shaken culture, was screened for the presence of active compounds. Supernatants were extracted with same amount ethyl acetate (Fishers, USA) and vacuum dried. Residues were dissolved in minimal amount of ethyl acetate and subjected to thin-layer chromatography (TLC) sheet (Merck, Germany) and developed with 1:1:1 v/v/v of n-hexane, chloroform, and methanol as mobile phase. The spots were detected under UV light; by spraying with water for the detection of hydrophilic compounds; with ninhydrin for detection of compound with free amino groups [23, 24]. However, active fractions were detected using a narrow strip of developed sheets by a bioassay method with a sensitive test organism [13]. The active fraction was scrapped from the TLC plate and extracted with eluent A (0.1 % (vol/vol) trifluoroacetic acid and 20 % (vol/vol) acetonitrile).
Acid Precipitation and Solid-Phase Extraction
Lipopeptides (LPS) were precipitated with 12-N HCl (pH 2.0) and the precipitate was extracted with minimal amount of methanol. The sample was dried and dissolved in 20 % (vol/vol) acetonitrile. This sample was fractionated through solid-phase extraction (SPE) columns (CHROMABOND C18ec) purchased from Macherey–Nagel, Germany. First columns were preconditioned with methanol then water and samples were applied under low pressure. The columns were washed with water and seven fractions were eluted by consecutive seven solutions 5, 15, 25, 35, 50, 75, and 100 % acetonitrile. The active fractions were monitored through the spot-plate method using test organism.
Ammonium Sulfate Precipitation (ASP) and Gel Filtration Chromatography
Ammonium sulfate (Sigma) was gently added to the cell-free supernatant (maintained at 4 °C) to obtain 80 % saturation, and stirred for 4 h. After centrifugation (1 h at 20,000×g, 4 °C), the pellet was resuspended in 50-mM Tris–HCl, pH 7.5, and loaded on Econo-Pac 10DG column (Biorad, UK) equilibrated in 50 mM Tris–HCl, pH 7.5. The lipopeptides were eluted from the column using a flow rate of 0.5 mL/min and fractions were collected every 1 mL. The similar fractions evaluated by TLC were combined and tested for their antimicrobial activity by agar-well diffusion assay.
Purification by Reverse Phase HPLC
Active fractions from TLC, SPE, and gel filtration were further purified by reversed-phase HPLC using Thermo Hypersil-Keystone ODS (particle size, 5 μm; column dimensions, 250 by 4.6 mm, Thermo Hypersil, USA). The sample was applied along with eluent A (Milli-Q HPLC grad water) and eluted with segmented gradients of eluent B (0.1 % vol/vol trifluoroacetic acid and acetonitrile). The system used was 40 % eluent B for 30 min and 40–100 % eluent B for 10 min.
Tris-Tricine SDS-PAGE
Gel electrophoresis was performed according to the method of Schagger [25]. Tris-Tricine SDS-PAGE gels were made and run using a Mini Protean II slab gel electrophoresis unit (Biorad). One millimeter spacers were used to set the thickness of the gels. Each gel consisted of two portions: a 2-cm stacking gel, and a 5.0-cm separating gel. Culture supernatant samples were lyophilized and dissolved in loading buffer prior to PAGE. The purified antibiotic was dissolved directly in loading buffer. Gels were run at 40 V until the tracking dye passed stacking gel, and then, a constant voltage of 140 V was applied until the dye reached the bottom of the gel. Gels were visualized by staining with coomassie brilliant blue. Antibiotic activity was specifically located on the gel using a direct activity assay [26].
MALDI–TOF-Mass Spectrometry Analysis
Purified compound from RP-HPLC was analyzed through matrix assistant laser desorption ionization time of flight-mass spectroscopy (MALDI–TOF-MS) to find the exact molecular mass. 2 µL of sample mixed with 2-µL matrix solution (2 mg of alpha-cyano-4-hydroxycinnaminic acid per ml in acetonitrile-methanol–water (1:1:1) on the target plate). MALDI–TOF-MS spectra were recorded using a 337-nm nitrogen laser for desorption and ionization. The mass spectrometer was operated in the linear mode at an accelerating voltage of 18 kV with an ion flight path that was 0.7-m long. The delay time was 375 ns. Matrix suppression was also used, and the mass spectra were averaged over 50–100 individual laser shots. The laser intensity was set just above the threshold for ion production. External calibration was performed using the [M + H]+ signals of renin, adrenocorticotropic hormone, insulin oxidized B, and bovine insulin (Sigma-Aldrich Co.).
Results
16S rDNA Sequence Analysis and Phylogeny
Bacillus subtilis strain BIA was identified on the basis of the 16S rDNA sequence analysis. For that purpose, nearly complete 16S rDNA sequence was determined and analyzed using NCBI Website. Strain BIA showed more than 97 % homology with B. subtilis strain B-FS01 (DQ520955).
Thin-Layer Chromatography
Eight prominent spots were visible under UV having R f values of 0.08, 0.12, 0.21, 0.37, 0.49, 0.53, and 0.57. However, only one active fraction was observed upon bioassay with R f value of 0.49. The spot was ninhydrin negative, indicating the absence of free amino groups and presence of peptide bonds in the compound. A white spot formed with same R f value when the plate was sprayed with water, indicating that the compound is lipophilic. In addition, spots with R f values of 0.08 and 0.53 could not be stained with ninhydrin, while all other spots were ninhydrin positive.
Solid-Phase Extraction
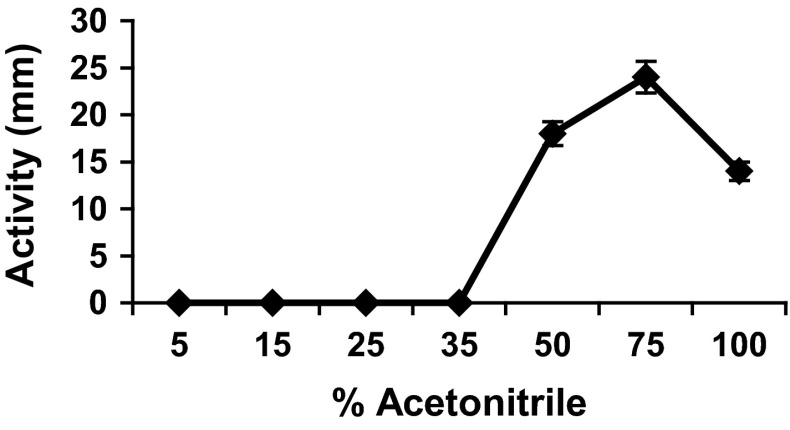
Seven fractions were collected by decreasing the polarity with acetonitrile until 100 % and tested for activity. Fractions eluted with 5, 15, 25, and 35 % acetonitrile did not show any activity. However, three active fractions were eluted with 50, 75, and 100 % acetonitrile with peak activity at 75 % (Fig. 1), showing that maximum product is eluted when the polarity is decreased until 3/4 to that of start.
Fig. 1.
Solid-phase extraction from the acid precipitation of culture supernatant. Seven fractions were eluted with decreasing polarity and activity observed using sensitive test organism
Gel Filtration Chromatography
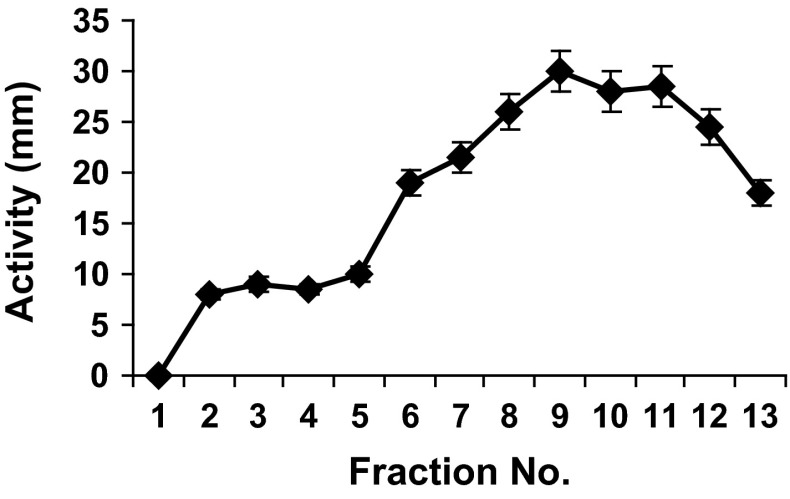
The sample eluted from the column at two distinct peaks (Fig. 2). Initially high-molecular weight components were eluted which are above 6 kDa and not of our interest. Fractions corresponding to molecular weight of about 1.5 kDa were further purified using RP-HPLC. As the initial fractions also showed activity, these may represent the aggregated form of lipopeptide present in the sample.
Fig. 2.
Elution pattern of peptide antibiotic from a Bio-Gel P-10 column equilibrated in 50-mM Tris buffer, pH 7.5. Fractions were collected and measured for activity (zone of inhibition)
Reverse Phase HPLC
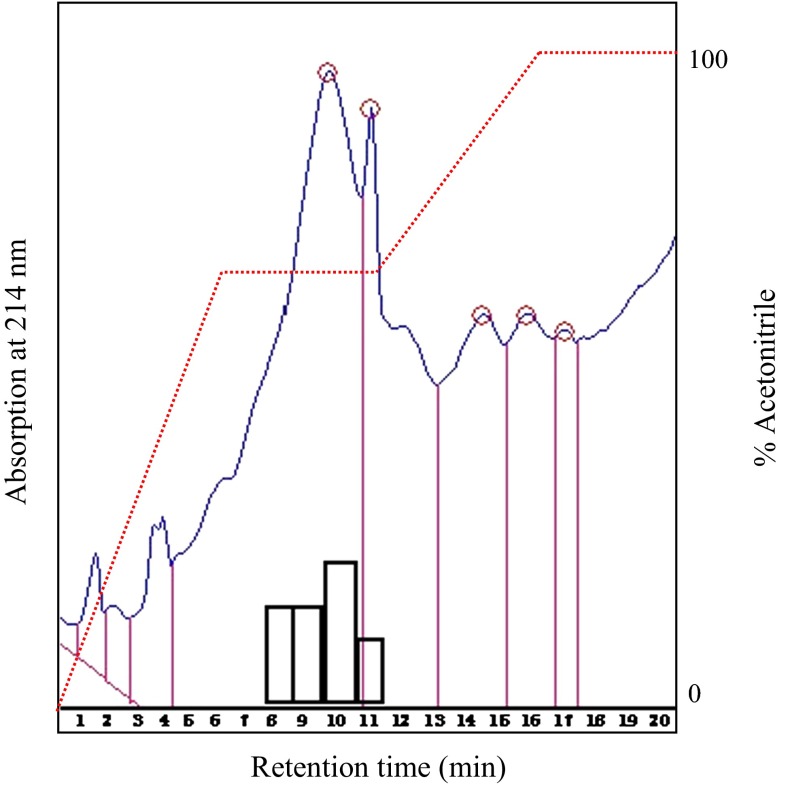
Figure 3 shows the chromatogram of the HPLC purification of samples obtained from TLC, SPE, and gel filtration. Four active fractions were observed at 75 % acetonitrile with retention time ranging from 8 to 11 min. However, maximum activity was observed in fraction corresponding to retention time 10 min. This fraction was further analyzed through Tricin PAGE and MALDI–TOF-MS for their molecular mass determination.
Fig. 3.
Purification of lipopeptides by reversed-phase HPLC on a Thermo Hypersil-Keystone ODS column. A segmented gradient was used and product eluted at 75 % of acetonitrile. Flow rate was maintained at 1 mL/min
Tris-Tricine SD-PAGE
Tris-Tricine SDS was unable to discriminate between the lipopeptides produced by the strain BIA; however, it confirmed the purity of sample preparation. The purified product was considerably smaller than the marker band with lowest molecular weight and is free of any contaminating material as evidenced by the lack of any other bands. As a comparison, the culture supernatant produced many bands of stained contaminated material. A direct activity assay confirmed that the purified product is about 3 kDa, as revealed by the zone of inhibition on test organism plate.
MALDI–TOF Mass Spectrometry
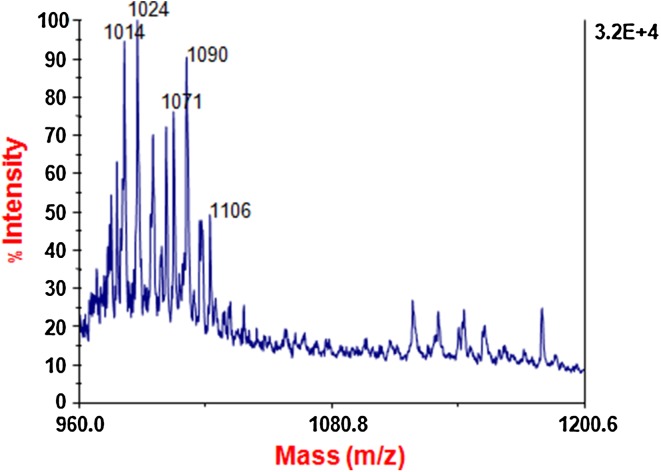
Purified product from RP-HPLC and whole bacterial cells were selected for analysis with MALDI–TOF-MS. When whole bacterial cells were used, these did not produce a good spectrum (data not shown). In the linear mode, we observed two clusters of peaks with different mass/charge (m/z) ratios for purified product (Fig. 4). One cluster was observed with m/z ratios between 1022 and 1036 which may be regarded as surfactin isoforms. The peak with a m/z ratio 1022.7 corresponds to the mass of [M + H]+ ion of surfactin with a fatty acid chain length of 14 carbon atoms. Other cluster was detected with mass/charge (m/z) ratios between 1070 and 1112.6, which could be attributed to protonated iturin isoforms. For example, the peak with a m/z of 1070.6 corresponds to the mass of [M + H]+ ion of iturin with a fatty acid chain length of 16 carbon atoms. Mass spectrometric analysis revealed that surfactin and iturin like compounds are produced by B. subtilis strain BIA.
Fig. 4.
MALDI–TOF-MS analysis of surfactin and iturin like compounds produced by B. subtilis strain BIA
Discussion
Lipopeptides produced by Bacillus subtilis are members of a particular antibiotic class that included surfactin, iturin, and fengycin families [27]. These are non-ribosomally generated peptides and the synthesis is directed by large multienzyme complexes that have a modular structural organization and are thought to orderly link the amino-acid residues of the final peptide [28]. These represent potent biosurfactants and show efficient antimicrobial and antiviral properties, and, thus, are valuable, industrially important compounds. Studying the HPLC analysis of the culture supernatants of seven Bacillus subtilis strains, Ahimou et al. [29] showed that lipopeptide profile varied greatly according to the strain. Coproduction of surfactin and iturin or plipastatin B1, an inhibitor of phospholipase A2 [30], has also been reported in some B. subtilis strains [31–33]. This paper describes the extraction and purification of lipopeptide antibiotics from a newly isolated B. subtilis BIA strain. Table 1 outlines the effectiveness of each purification protocol. On the basis of these results, acid precipitation of the culture supernatant followed by SPE, ethyl acetate extraction followed by TLC, and ammonium sulfate precipitation followed by gel filtration actually increased antibiotic activity relative to supernatant. SPE proved to be the optimum method of isolation, as it accomplished 20 % recovery of the initial antibiotic activity and increases specific activity to 7.3 fold over that of original sample. Gel filtration indicated that lipopeptides were eluted from the column in two different forms, that is, as a monomer (MW ≤1200 Da) and as an aggregate (MW >6000 Da). Lipopeptides like surfactins have the ability to form micelle structures that form high-molecular weight aggregates. Mulligan and Gibbs [15] took advantage of this property in a one-step method to purify and concentrate surfactin from the culture supernatant by ultrafiltration. The ability of surfactant molecules to form high-molecular aggregates allows them to be retained by relatively high-molecular weight cut-off membranes. HPLC purification provided a highly purified sample for molecular mass determination with an increase of specific activity to 10.69 fold. As previously reported [34], using whole-cell MALDI–TOF, mass spectrometry cellular products can be detected which either are attached to the cell surface or are integrated into the outer cell membrane. In this way, information on the secondary metabolites produced by a microorganism can be obtained in minutes with high precision and sensitivity with no need to fractionate and purify the detected compounds. However, in this study, we were not able to produce a good spectrum from the whole-cell analysis. The purified product from RP-HPLC revealed that B. subtilis strain BIA produced a C14 surfactin and C16 iturin like compounds based on their molecular masses. The co-production by this strain is an interesting characteristic with potential practical applications. Further characterization of these lipopeptides would have relevance to minimize the use of synthetic fungicides and surfactants.
Table 1.
Purification of lipopeptides from B. subtilis BIA
| Sample | Sample volume (mL) | Total activity (AU) | Total absorbance (A 280 nm) | Specific activity (AU/A280) | Activity recovered (%) | Fold purification |
|---|---|---|---|---|---|---|
| Supernatant | 120 | 18,720 | 680 | 28 | 100 | 1 |
| Solvent extraction | 3 | 17,244 | 253 | 68 | 92 | 2.42 |
| TLC | 0.5 | 2808 | 19 | 148 | 15 | 5.29 |
| HCl ppt. | 5 | 15,724 | 171 | 92 | 84 | 3.28 |
| SPE | 1 | 3744 | 18 | 208 | 20 | 7.43 |
| ASP | 5 | 13,104 | 260 | 50 | 70 | 1.8 |
| Gel filtration | 1 | 2246 | 21 | 107 | 12 | 3.82 |
| HPLC peak | 1 | 1497 | 5 | 300 | 8 | 10.69 |
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Cochrane SA, Vederas JC. Lipopeptides from Bacillus and Paenibacillus spp.: a gold mine of antibiotic candidates. Med Res Rev. 2016;36(1):4–31. doi: 10.1002/med.21321. [DOI] [PubMed] [Google Scholar]
- 2.Stein T. Bacillus subtilis antibiotics: structures, syntheses and specific functions. Mol Microbiol. 2005;56:845–857. doi: 10.1111/j.1365-2958.2005.04587.x. [DOI] [PubMed] [Google Scholar]
- 3.Von Doehren H, Keller U, Vater J, Zocher R. Multifunctional peptide synthetases. Chem Rev. 1997;97:2675–2705. doi: 10.1021/cr9600262. [DOI] [PubMed] [Google Scholar]
- 4.Tsuge K, Inoue S, Ano T, Itaya M, Shoda M. Horizontal transfer of iturin A operon, itu, to Bacillus subtilis 168 and conversion into an iturin A producer. Antimicrob Agents Chemother. 2005;49:4641–4648. doi: 10.1128/AAC.49.11.4641-4648.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vater J, Kablitz B, Wilde C, Franke P, Mehta N, Cameotra SS. Matrix-assisted laser desorption ionization—time of flight mass spectrometry of lipopeptide biosurfactants in whole cells and culture filtrates of Bacillus subtilis C-1 isolated from petroleum sludge. Appl Environ Microbiol. 2002;68:6210–6219. doi: 10.1128/AEM.68.12.6210-6219.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Steller S, Vollenbroich D, Leenders F, Stein T, Conrad B, Hofemeister J, Jacques P, Thonart P, Vater J. Structural and functional organization of the fengycin synthetase multienzyme system from Bacillus subtilis b213 and A1/3. Chem Biol. 1998;6(1):31–41. doi: 10.1016/S1074-5521(99)80018-0. [DOI] [PubMed] [Google Scholar]
- 7.Deleu M, Razafindralambo H, Popineau Y, Jacques P, Thonart P, Paquot M. Interfacial and emulsifying properties of lipopeptides from Bacillus subtilis. Colloid Surf A. 1999;152:3–10. doi: 10.1016/S0927-7757(98)00627-X. [DOI] [Google Scholar]
- 8.Loeffler W, Tschen S-M, Vanittanakom N, Kugler M, Knorpp E, Hsieh T-F, Wu T-G. Antifungal effects of bacilysin and fengymycin from Bacillus subtilis F29–3: a comparison with activities of other Bacillus antibiotics. J Phytopathology. 1986;115:204–213. doi: 10.1111/j.1439-0434.1986.tb00878.x. [DOI] [Google Scholar]
- 9.Yilmaz M, Soran H, Beyatli Y. Antimicrobial activities of some Bacillus spp. strains isolated from the soil. Microbiol Res. 2005;161:127–131. doi: 10.1016/j.micres.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 10.Vollenbroich D, Ozel M, Vater J, Kamp R, Pauli G. Mechanism of inactivation of enveloped viruses by the biosurfactant surfactin from Bacillus subtilis. Biologicals. 1997;25:289–297. doi: 10.1006/biol.1997.0099. [DOI] [PubMed] [Google Scholar]
- 11.McCoy LS, Roberts KD, Nation RL, Thompson PE, Velkov T, Li J, Tor Y. Polymyxins and analogues bind to ribosomal RNA and interfere with eukaryotic translation in vitro. ChemBioChem. 2013;14(16):2083–2086. doi: 10.1002/cbic.201300496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peypoux F, Bonmatin J, Wallach J. Recent trends in the biochemistry of surfactin. Appl Microbiol Biotechnol. 1999;51:553–563. doi: 10.1007/s002530051432. [DOI] [PubMed] [Google Scholar]
- 13.Al-Ajlani MM, Hasnain S. Simple and rapid method for isolation of novel antibiotic from Bacillus subtilis Mz-7. J Liq Chrom Relat Tech. 2006;29(5):639–647. doi: 10.1080/10826070500509132. [DOI] [Google Scholar]
- 14.Drouin CM, Cooper DG. Biosurfactants and aqueous two-phase fermentation. Biotechnol Bioeng. 1992;40:86–90. doi: 10.1002/bit.260400113. [DOI] [PubMed] [Google Scholar]
- 15.Mulligan CN, Gibbs BF. Recovery of biosurfactants by ultrafiltration. J Chem Technol Biotechnol. 1990;47:23–29. doi: 10.1002/jctb.280470104. [DOI] [PubMed] [Google Scholar]
- 16.Razafindralambo H, Paquot M, Hbid C, Jacques P, Destain J, Thonart P. Purification of antifungal lipopeptides by reversed-phase high performance liquid chromatography. J Chromatogr. 1993;639:81–85. doi: 10.1016/0021-9673(93)83091-6. [DOI] [PubMed] [Google Scholar]
- 17.Cooper DG, MacDonald CR, Duff SB, Kosaric N. Enhanced production of surfactin from Bacillus subtilis by continuous product removal and metal cation additions. Appl Environ Microbiol. 1981;42:408–412. doi: 10.1128/aem.42.3.408-412.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tagg JR, McGiven AR. Assay system for bacteriocins. Appl Microbiol. 1971;21:943–948. doi: 10.1128/am.21.5.943-943.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gerhardt P, Murray RGE, Wood WA, Krieg NR. Methods for general and molecular bacteriology. Washington, D.C.: American Society for Microbiology; 1994. [Google Scholar]
- 20.Landy M, Warren GH, Roseman SB, Golio LG. Bacillomycin, an antibiotic from Bacillus subtilis active against pathogenic fungi. Proc Soc Exp Biol Med. 1948;67:539–541. doi: 10.3181/00379727-67-16367. [DOI] [PubMed] [Google Scholar]
- 21.Hasnain S, Thomas CM. Two related rolling circle replication plasmids from salt-tolerant bacteria. Plasmid. 1996;36(3):191–199. doi: 10.1006/plas.1996.0046. [DOI] [PubMed] [Google Scholar]
- 22.Johnson JL. Similarity analysis of rRNAs. In: Gerhardt P, Murray RGE, Wood WA, Krieg NR, editors. Methods for general and molecular bacteriology. Washington, D.C.: American Society for Microbiology; 1994. pp. 683–700. [Google Scholar]
- 23.Brueckner H, Przybylski M. Methods for the rapid detection, isolation and sequence determination of peptaibols and other aib-containing peptides of fungal origin. J Chromatogr. 1984;19(1):188–199. doi: 10.1007/BF02687737. [DOI] [Google Scholar]
- 24.Winkelmann G, Allgaier H, Jung G. Iturin AL—a new long chain iturin A possessing an unusual high content of C16-β-amino acids. J Antibiot. 1983;36:1451–1457. doi: 10.7164/antibiotics.36.1451. [DOI] [PubMed] [Google Scholar]
- 25.Schagger H. Tricine-SDS-PAGE. Nat. Protocols. 2006;1(1):16–22. doi: 10.1038/nprot.2006.4. [DOI] [PubMed] [Google Scholar]
- 26.Bhunia K, Johnson C, Ray B. Direct detection of an antimicrobial peptide of Pediococcus acidilactici in sodium dodecyl polyacrylamide gel electrophoresis. J Ind Microbiol. 1987;2(5):319–322. doi: 10.1007/BF01569434. [DOI] [Google Scholar]
- 27.Lang S. Biological amphiphiles (microbial biosurfactants) Curr Opin Colloid Interface Sci. 2002;7:12–20. doi: 10.1016/S1359-0294(02)00007-9. [DOI] [Google Scholar]
- 28.Challis G, Ravel J, Townsend C. Predictive structure-based model of amino acid recognition by nonribosomal peptide synthetase adenylation domains. Chem Biol. 2000;7(3):211–224. doi: 10.1016/S1074-5521(00)00091-0. [DOI] [PubMed] [Google Scholar]
- 29.Ahimou F, Jacques P, Deleu M. Surfactin and iturin A effects on Bacillus subtilis surface hydrophobicity. Enzyme Microb Technol. 2000;27(10):749–754. doi: 10.1016/S0141-0229(00)00295-7. [DOI] [PubMed] [Google Scholar]
- 30.Umezawa H, Aoyagi T, Nishikiori T, Okuyama A, Yamagishi Y, Hamada M, Takeuchi T. Plipastatins: new inhibitors of phospholipase A2 produced by Bacillus cereus BMG 302-fF67. I. Taxonomy, production, isolation and preliminary characterization. J Antibiot. 1986;39:737–744. doi: 10.7164/antibiotics.39.737. [DOI] [PubMed] [Google Scholar]
- 31.Hiraoka H, Asaka O, Ano T, Shoda M. Characterization of Bacillus subtilis RB14, coproducer of peptide antibiotics iturin A and surfactin. J Gen Appl Microbiol. 1992;38:635–640. doi: 10.2323/jgam.38.635. [DOI] [Google Scholar]
- 32.Sandrin C, Peypoux F, Michel G. Coproduction of surfactin and iturin A, lipopeptides with surfactant and antifungal properties by Bacillus subtilis. Biotechnol Appl Biochem. 1990;12:370–375. [PubMed] [Google Scholar]
- 33.Tsuge K, Ano T, Shoda M. Characterization of Bacillus subtilis YB8, coproducer of lipopeptides surfactin and plipastatin B1. J Gen Appl Microbiol. 1995;41:541–545. doi: 10.2323/jgam.41.541. [DOI] [Google Scholar]
- 34.Leenders F, Stein T, Kablitz B, Franke P, Vater J. Rapid typing of Bacillus subtilis strains by their secondary metabolites using matrix assisted laser desorption/ionization mass spectrometry of intact cells. Rapid Commun Mass Spectrom. 1999;13:943–949. doi: 10.1002/(SICI)1097-0231(19990530)13:10<943::AID-RCM591>3.0.CO;2-0. [DOI] [Google Scholar]