Abstract
Background
Body-worn, inertial sensors can provide many objective measures of balance and gait. However, the objective measures that best reflect patient perception of mobility disability and clinician assessment of Parkinson disease (PD) are unknown.
Objective
The purposes of this study were: (1) to determine which objective measures of balance and gait are most related to patient perception of mobility disability and disease severity in people with PD and (2) to examine the effect of levodopa therapy on these correlates.
Design
This was an experimental correlation study.
Methods
One hundred four people with idiopathic PD performed 3 trials of the Instrumented Stand and Walk Test (ISAW) in the “on” and “off” medication states. The ISAW consists of quiet standing (30 seconds), gait initiation, straight walking (7 m), and turning (180°), yielding 34 objective measures of mobility from body-worn inertial sensors. Patient perception of mobility disability was assessed with the Activities-specific Balance Confidence (ABC) scale and the mobility subscale of the Parkinson's Disease Questionnaire (PDQ-39). Disease severity was assessed with the Unified Parkinson's Disease Rating Scale, part III (motor UPDRS). Spearman correlations were used to relate objective measures of mobility to patient perception and disease severity.
Results
Turning speed, gait speed, and stride length were most highly correlated to severity of disease and patient perception of mobility disability. The objective measures of mobility in the off-medication state were more indicative of patient perception of mobility disability and balance confidence compared with on-medication state measures.
Limitations
Causation is an inherent problem of correlation studies.
Conclusion
Physical therapists should evaluate mobility in people with PD in the off-medication state because the off-medication state is more related to disease severity and patient perception of mobility disability than the on-medication state mobility. Assessment and treatment of mobility in people with PD should target specific measures (ie, turning, gait speed, and stride length) because these measures best reflect patients' quality of life and balance confidence.
Gait and balance impairments are common manifestations of idiopathic Parkinson disease (PD). These motor symptoms are debilitating, leading to decline in balance confidence1 and self-perceived motor function.2 Reduced walking capacity and postural instability have consistently been identified as predictors of increased fear of falling and reduced mobility, with consequent reduced quality of life.3–6 Yet, it is not known which specific characteristics of gait and balance function contribute most strongly to patient perception of mobility disability (ie, balance confidence and perceived motor functioning) or whether patient perception is consistent with aspects that are associated with disease severity. For example, it is possible that balance impairments, as reflected by increased postural sway in stance or increased double support time in gait, are more reflective of perceived mobility disability than bradykinetic aspects of gait. Alternatively, bradykinetic aspects of gait may be more related to disease severity than balance because motor slowness is weighted heavily in the Unified Parkinson's Disease Rating Scale (UPDRS) evaluation.7
A recent exercise study showed that although measures of gait and balance change with exercise, patient perception of mobility disability and clinical ratings of disease severity can be insensitive to these changes.8 Canning et al9 showed that exercise can improve balance and gait, as well as balance confidence and quality of life. Therefore, it is important to understand which specific gait and balance impairments are best related to patient perception of mobility disability and disease severity.
Body-worn, inertial sensors can automatically provide many objective measures of balance and gait.10 We examined 34 mobility metrics in a large cohort of people with PD performing a defined locomotor sequence that was designed to capture key aspects of functional mobility: standing, gait initiation, walking, and turning.
The aim of this study was to determine which specific objective measures of balance and gait best correlate with balance confidence and perceived mobility function in people with PD. Motor function in people with PD can fluctuate greatly between the on- and off-medication states, and it is not known whether patients evaluate their past mobility disability based on their best or worst motor state. Understanding which specific aspects of functional mobility are representative of patient perception of mobility disability will help identify targets for rehabilitation interventions that improve quality of life. We hypothesized that postural instability and slowness of gait and turning (dynamic stability task) would best relate to perceived mobility disability and severity of disease because postural instability and bradykinesia are primary symptoms of PD. We also hypothesized that testing balance and gait in the on-levodopa state would be best related to patients' perceived mobility disability over the previous month, as the majority of their time is spent in the on-medication state.
Method
Participants
We recruited 104 participants with idiopathic PD (Tab. 1), who had been taking levodopa for at least 1 year. All participants were free of musculoskeletal and neurological impairments, other than PD, that could affect gait and balance. Participants gave their informed consent according to the Declaration of Helsinki.
Table 1.
Participant Characteristics (N=104)a
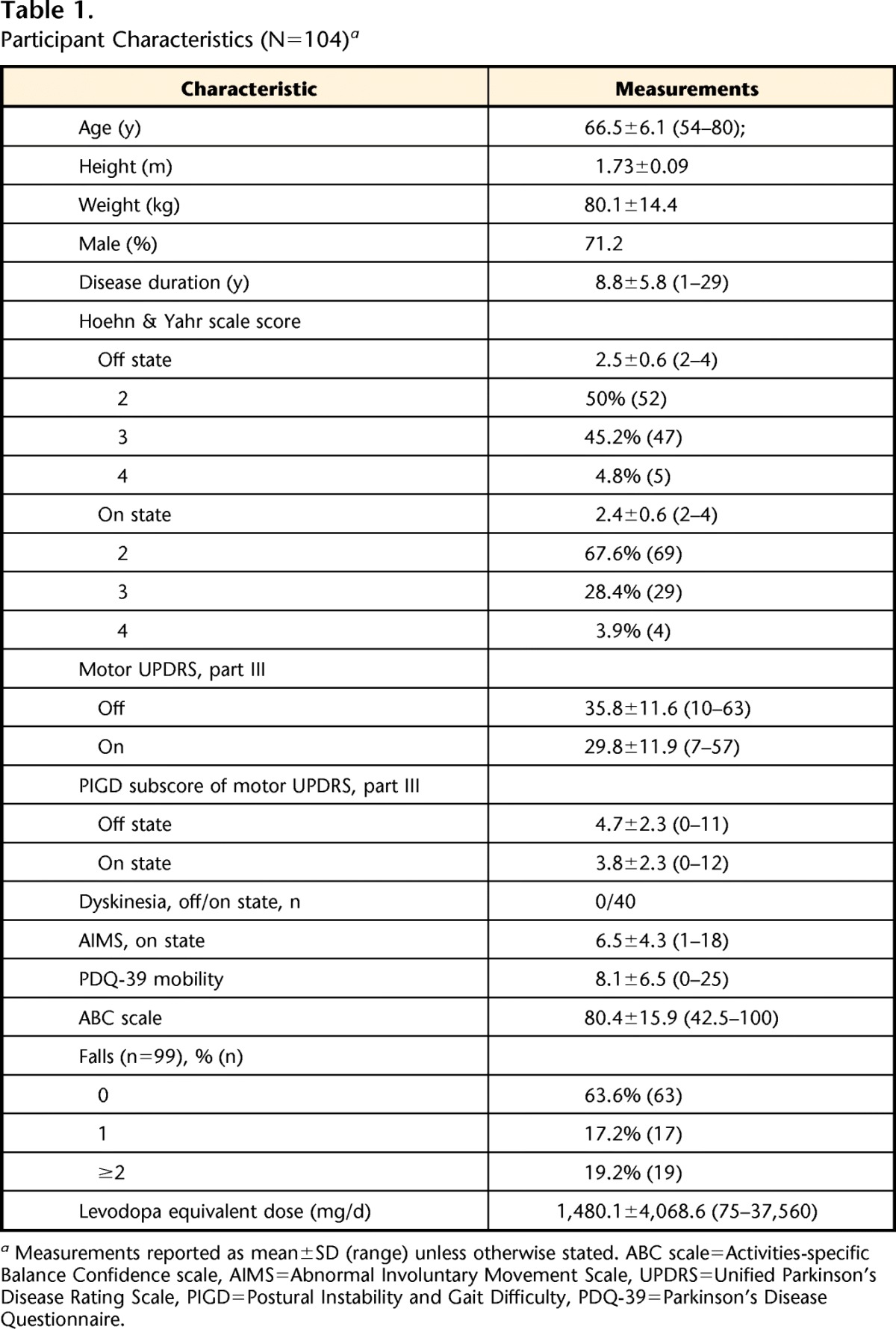
Measurements reported as mean±SD (range) unless otherwise stated. ABC scale=Activities-specific Balance Confidence scale, AIMS=Abnormal Involuntary Movement Scale, UPDRS=Unified Parkinson's Disease Rating Scale, PIGD=Postural Instability and Gait Difficulty, PDQ-39=Parkinson's Disease Questionnaire.
Procedure
The participants were tested in the morning in their practical off-medication state after withholding their antiparkinsonian medication for at least 12 hours. Subsequently, they were retested in their on-medication state 1 hour after taking a suprathreshold dose of levodopa (ie, 1.25 times their regular dose). A suprathreshold dose was administered to ensure an optimal on-medication state response that persisted through the assessment.
Objective Measures of Gait and Balance
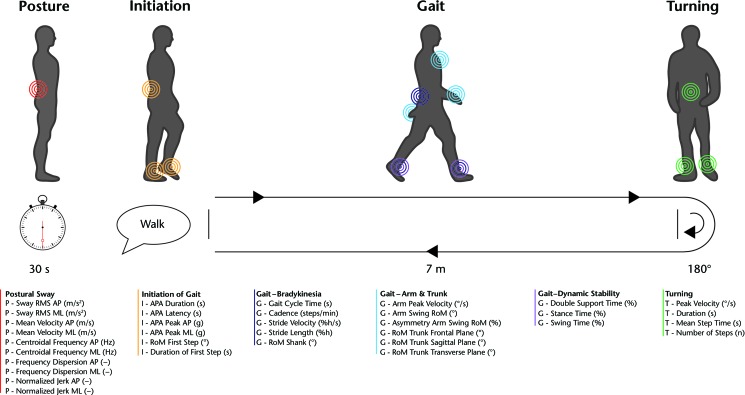
Gait and balance were assessed using the Instrumented Stand and Walk Test (ISAW) (Fig. 1).10 Participants were instructed as follows:
Start by standing with your arms at your side. Look straight ahead at a fixed object and remain still without talking or moving. You should stand naturally, not rigidly. When I say “Walk,” start walking forward at a natural and comfortable pace. When you cross the line at the end of the walkway, turn around, and start walking back. After you cross the line at the beginning of the walkway, stand with your arms by your side and look straight ahead until you hear the tone or are told the test is complete.
Figure 1.
Instrumented Stand and Walk Test (ISAW). Color-coded circles indicate the placement of inertial sensors used for the determination of the various measures of balance and gait.18 See Appendix for description of measures.
Participants performed 3 trials of the ISAW, consisting of standing quietly for 30 seconds, initiating gait with the most affected leg (participant specified), walking 7 m, turning 180 degrees, and walking back 7 m. Initial foot placement before each trial was standardized to 10 cm between heels and 30-degree outward rotation of feet, with participants' arms at their sides.11 Participants wore 6 inertial sensors (MTX, Xsens, Enschede, the Netherlands, or Opals, APDM Inc, Portland, Oregon) attached to ankles, wrists, sternum, and lumbar (L5) region. A total of 34 measures of gait and balance function were computed using Mobility Lab's ISAW test (APDM Inc). These measures were categorized into 6 domains: (1) postural sway, (2) initiation of gait, (3) gait–pace, (4) gait–arm and trunk movement, (5) gait–dynamic stability, and (6) turning (Fig. 1, Appendix).
Disease Severity
The Unified Parkinson's Disease Rating Scale, part III (motor UPDRS) was administered by a UPDRS-trained researcher. The motor UPDRS was used as a clinician's rating of PD severity and includes ratings of tremor, bradykinesia, rigidity, and postural instability and gait difficulties. The motor UPDRS has a score range of 0 to 108; higher scores indicate greater severity. Ratings of disease severity are sensitive to medication state (time since and size of last dose of levodopa); therefore, the off-medication state was chosen as the reference for disease severity.
The 4-item Postural Instability and Gait Difficulty (PIGD) subscale score was calculated from the motor UPDRS (arising from a chair, standing posture, gait, and postural stability). In addition, participants were assessed for dyskinesia using the modified Abnormal Involuntary Movement Scale (AIMS)12 and for severity level with the Hoehn & Yahr scale.13 Video recordings of the assessment were rated by an experienced clinician who was blinded to the on- and off-medication states (except for rigidity, which was assessed in person).
Patient Perceptions of Mobility Disability
Self-reported confidence in performing various ambulatory activities without falling was assessed using the Activities-specific Balance Confidence (ABC) scale.14 The ABC scale score arises from the average confidence in avoiding a fall while performing 16 items of everyday life. A score of 0 represents no confidence, and a score of 100 represents complete confidence in performing the various ambulatory activities without falling or sense of unsteadiness. Perceived motor functioning was assessed using the mobility domain of the Parkinson's Disease Questionnaire (PDQ-39).15 The 10-item mobility domain of the PDQ-39 is a health-related quality-of-life instrument that measures the frequency with which respondents have difficulty doing functional activities in the previous month. The PDQ-39 mobility section has a possible score range of 0 to 40; higher scores are associated with greater impairment. Participants also were asked about falls in the 12 months preceding the evaluation.
Data Analysis
We calculated the median of 3 ISAW trials for each balance and gait metric in the on- and off-medication states. The absolute strength of correlation of these metrics with the ABC scale, PDQ-39 mobility, and motor UPDRS (off-medication state) was assessed using Spearman rho correlations and plotted as polar plots for comparisons. Statistical significance was set at P<.05. Multiple comparisons were accounted for by using a false discovery rate (FDR) adjustment set at .05.16 Given the large number of associations under question, the FDR was considered more appropriate over the more conservative, family-wise error rate corrections such as Bonferroni.17 This approach allowed for the false positive rate to be based on the number of significant associations observed instead of the total number of tests.
Role of the Funding Source
This publication was made possible with support from a grant from the National Institute on Aging (AG006457); a Challenge Grant from the National Institute of Neurological Disorders and Stroke (RC1 NS068678) and the Oregon Clinical and Translational Research Institute (OCTRI) at OHSU; and grant number UL1 RR024140 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research.
Results
One hundred four participants with idiopathic PD were tested (Table 1). Participants had a mean age of 66.5 years (SD=6.1) and a mean disease duration of 8.8 years (SD=5.8). They were treated with a mean of 1,480.1 levodopa equivalents (SD=4,068.6) daily. For measurements of disease severity, we found a mean Hoehn & Yahr scale score of 2.5 (SD=0.6, range=2–4), a mean motor UPDRS score in the off-medication state of 35.8 (SD=11.6), and a mean motor UPDRS score in the on-medication state of 29.8 (SD=15.9). The average ABC scale score was 80.4 (SD=15.9), and mean PDQ-39 mobility score was 8.1 (SD=6.5). Objective measurements of mobility were obtained from all 104 participants. The effect of levodopa on balance and gait is described elsewhere.18
Balance Confidence
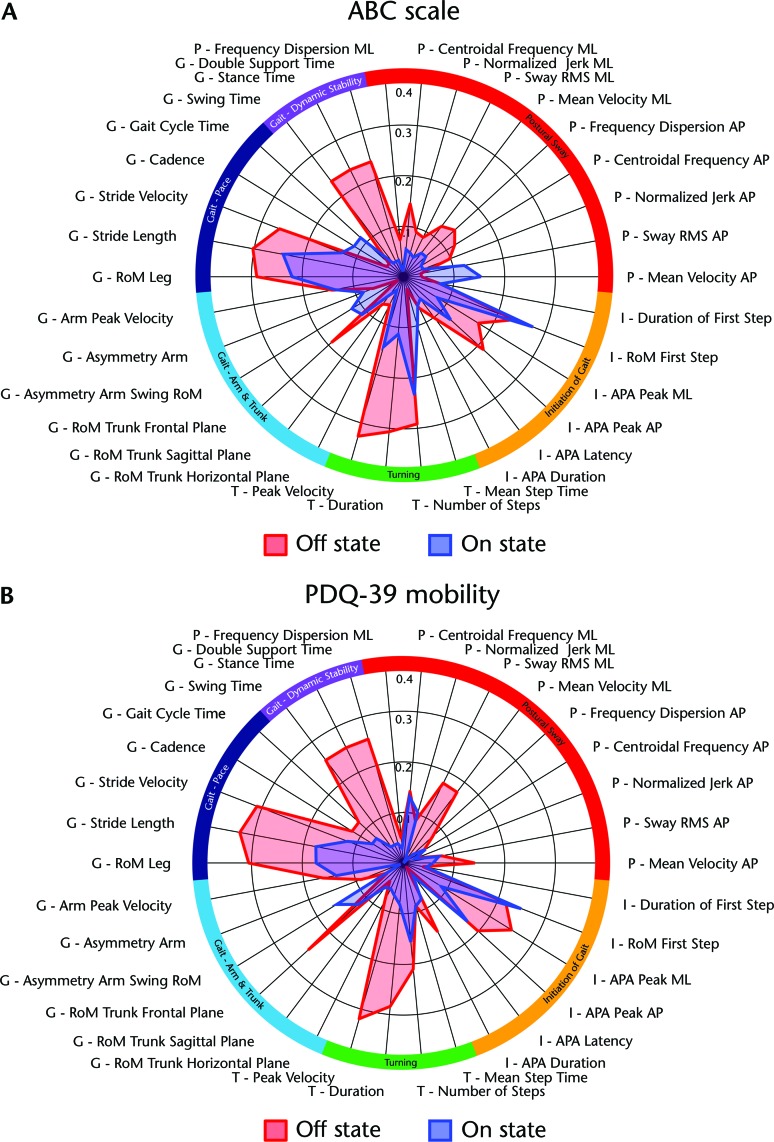
The ABC scale correlated strongest with turning, pace-related measures of gait, and dynamic stability during gait in the off-medication state (Tab. 2). Interestingly, we found only weak correlations of the ABC scale with postural sway in a standing position. For measurements taken in the on-medication state, the correlations with the ABC scale were overall weaker than in the off-medication state. Correlations of the ABC scale with objective measures of balance and gait function in the off- and on-medication states are shown in the Figure 2A. Note that for comparison among metrics, absolute values of the correlation coefficients are given (0=no relationship; 1=perfect relationship). Correlations with a Spearman rho >.2 were significant at P<.05. Objective measures of gait and balance function are grouped into 6 domains, indicated by different colors on the outer radial of the polar plot.
Table 2.
Spearman Correlations of Gait and Balance Function (Off- and On-Medication States), Patient Perception, and Clinical Ratinga
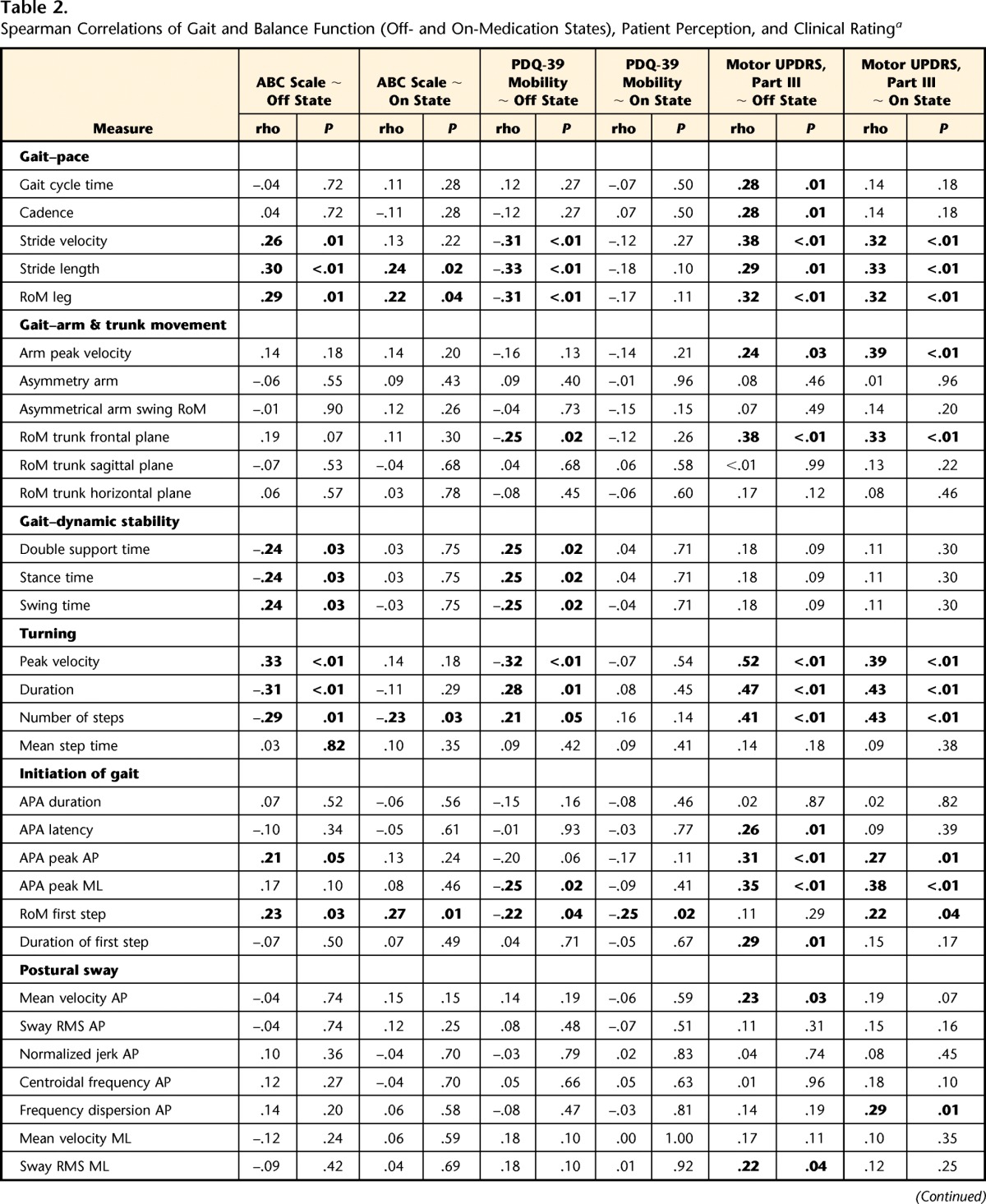
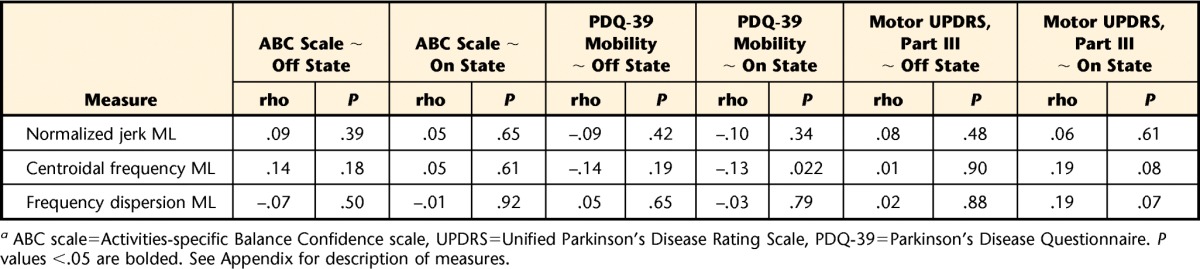
ABC scale=Activities-specific Balance Confidence scale, UPDRS=Unified Parkinson's Disease Rating Scale, PDQ-39=Parkinson's Disease Questionnaire. P values <.05 are bolded. See Appendix for description of measures.
Figure 2.
Polar plot comparing Spearman correlation of (A) Activities-specific Balance Confidence scale (ABC scale) and (B) mobility domain of the Parkinson's Disease Questionnaire (PDQ-39 mobility) with measures of gait and balance function in the off- and on-medication states. Absolute values given. See Appendix for description of measures.
Perceived Mobility Function
The PDQ-39 mobility score showed correlation patterns with the objective measures similar to those of the ABC scale (Fig. 2B). The PDQ-39 mobility score was strongly correlated with turning, pace-related gait measures, dynamic stability during gait, range of motion of the trunk in the frontal plane during gait, and anticipatory postural adjustment peak amplitude in the medial-lateral direction in the off-medication state. Gait and balance in the on-medication state were less predictive of perceived motor function than in the off-medication state (smaller surface area in the polar plot).
Disease Severity
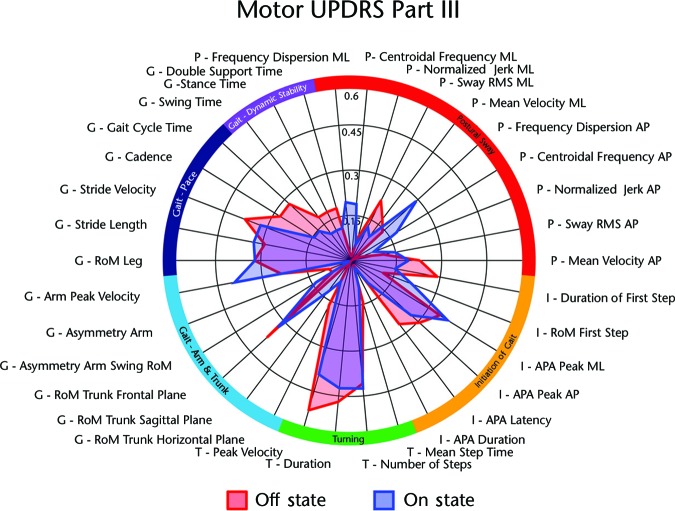
The UPDRS motor score was most related to measures of turning, pace-related gait measures, range of motion of the trunk in the frontal plane, and initiation of gait. Clinical ratings of disease severity show a pattern of correlation similar to that of the ABC scale and the PDQ-39 mobility scale (Fig. 3; note difference in axis scaling). However, unlike the self-perceived measures, we found no prominent difference in correlation between the UPDRS motor score taken in the off-medication state and measures of balance and gait in the off- and on-medication states.
Figure 3.
Polar plot comparing Spearman correlation of motor Unified Parkinson's Disease Rating Scale (UPDRS), part III (off medication state) with measures of gait and balance function in the off- and on-medication states. Absolute values given. See Appendix for description of measures.
False Discovery Rate
A total of 204 correlations were performed, of which 56 were detected as significant (Spearman rho >.2, P<.05). After FDR correction, the 56 significant tests revealed 30 significant associations.
Discussion
The advent of wireless inertial sensors has generated considerable interest because of the potential to quickly and automatically provide many objective measures of mobility to quantify patient status. With the proliferation of such measures a need arises to understand the relationship between measures of mobility and perceived balance confidence, perceived motor function, and disease severity.
Turning and Gait–Pace Are Most Indicative of Patient Status
Our study revealed striking coherence in correlation patterns (ie, both disease severity and patient perception of mobility disability were related to the same objective measures of mobility). Specifically, turning peak velocity, gait speed, and stride length were the most highly related to clinical ratings of severity of disease, patient perception of balance confidence, and perceived motor function.
Consistent with our findings, Bryant et al3 reported marked differences in walking speed and stride length during backward and forward walking and 360-degree turning between people with PD who had high and low fear of falling. In a survey study, Nilsson et al4 identified self-reported walking difficulties and turning hesitations as also being associated with fear of falling in people with PD.
Turning measurements were the strongest correlates of disease severity, as assessed with the UPDRS motor score. This finding is interesting, as the UPDRS motor section includes assessment of postural instability and gait difficulty, but not turning difficulty. It appears that turning difficulty strongly reflects patient status, agreeing with our previous findings on continuous monitoring of turning during daily activities.19 In that study, we found a strong association between the UPDRS motor score in the on-medication state and turning velocity during a week of continuous monitoring in the home.19 Turning, compared with straight-ahead walking, is more demanding for dynamic postural stability, as it requires complex sequencing of body reorientation toward a new travel direction with small stability margins20 and, therefore, might be more vulnerable to neurological impairments.
Postural sway during quiet standing was an overall low predictor of UPDRS motor score, which agrees with findings by Adkin et al.1 They found only low associations of the UPDRS PIGD (on-medication state) with various standing balance tasks and one-leg stance duration correlated better with the UPDRS PIGD.1 In contrast to the ABC scale and PDQ-39 mobility, the motor UPDRS also was not correlated with gait dynamic stability in our study.
Effect of Levodopa State
Although participants evaluated their balance confidence and perceived motor function over the last month, the strongest relationships with mobility metrics occurred for measurements taken in the off-medication state, suggesting that patients evaluate function in their worst state. This finding was surprising because patients taking levodopa spend less time during the day in their off-medication state than their on-medication state. Furthermore, the instructions of the ABC scale and the PDQ-39 do not ask the patient to imagine a particular medication state but the general past perception. We are uncertain why this prominent off-on difference in correlations between perceived mobility and gait/balance metrics was not observed for the clinical severity ratings. Although the motor UPDRS was measured only in the off-medication state, it was equally related to both the on- and off-medication state balance and gait metrics, suggesting little change in these functions or little contribution of balance and gait to the motor UPDRS score in the off-medication state.
Clinical Implications
Our study provides a better understanding of the relationship between objective balance and gait metrics and patients' and neurologists' perceptions of mobility disability. It justifies the physical therapy focus on improving gait speed, turning, and postural stability in patients with PD. A detailed understanding of how neurological disorders result in balance and gait impairments that affect patients' lives and are related to severity of disease will be useful to target rehabilitation efforts. That is, improvements in turning speed, gait speed, and stride length will more likely be recognized by both patients and their neurologists, as improvements in function and disease severity compared with other measures of mobility, such as changes in postural sway, double support time, trunk displacement, or arm swing during gait. In a randomized controlled trial, it has been shown that exercise can not only improve physical outcomes but also reduce fear of falling and quality of life.9 However, changing both physical and perceived outcomes with exercise depends on identifying specific physical outcomes that are highly relevant to patient perceptions of mobility disability.
Considering that people with PD associated their functional balance and gait performance with their worse state and not their most common state, the on-medication state also has important clinical implications. Therapists seldom measure balance and gait in patients with PD in their off-levodopa state, although these measures best reflect self-perceived quality-of-life mobility function and balance confidence. Our study suggests it could be useful for therapists to evaluate patients with PD in the off-medication state (ie, after withholding antiparkinson medication for 12 hours), when mobility is most vulnerable and most related to patients' perception of balance confidence and quality of life. Providing safe strategies for mobility for patients with PD in those off-medication state periods may be helpful to improve quality of life and functional mobility and reduce falls in patients with PD. Efficacy of balance and gait training should include objective measures of mobility for optimal responsiveness to change.8 Individuals with PD recruited for our study had mild-to-moderate disability. Recent efforts have promoted starting exercise programs early in PD to prevent motor complications and falls, rather than to react when they have already occurred.21,22
Limitations of the Study
There are 2 main limitations to this study. First, this correlational study cannot determine whether impaired mobility leads to fear of falling and mobility disability, or vice versa. Only the strength of the relationships can be established. Second, the large number of correlations could result in spurious relationships. Multiple comparisons were accounted for by using FDR corrections; after FDR correction, 30 associations were significant.
In this study, we identified a marked congruence between patient-reported outcome measures and specific objective measures of mobility. Gait–pace and turning were most indicative of patient-reported outcome measures and clinical measures. These results suggest that turning velocity in the off-medication state would be a valuable objective outcome of mobility that reflects both physician and patient judgments of mobility for patients with PD.
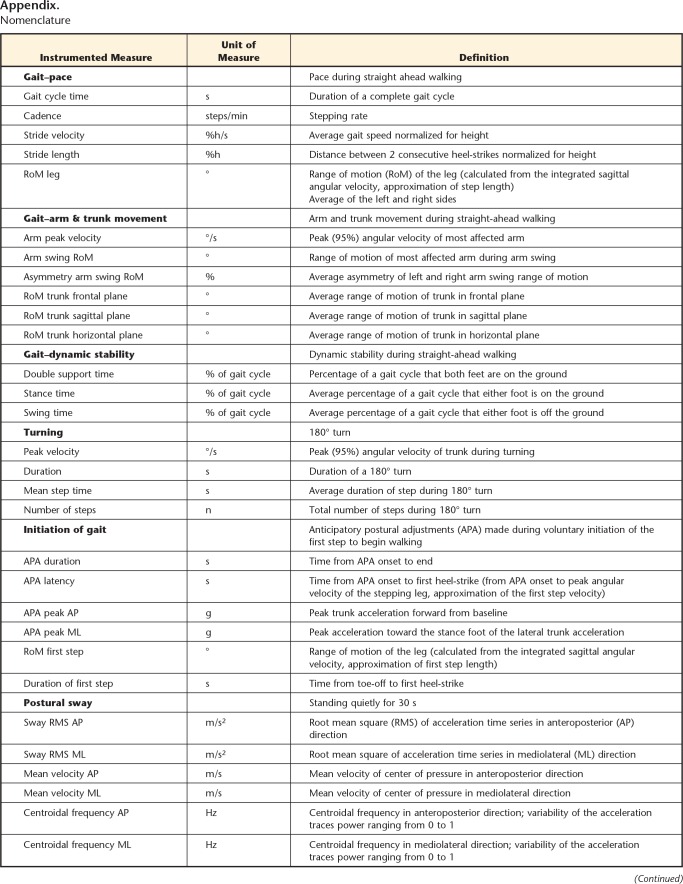
Appendix.
Appendix.
Nomenclature
a °=degree, g=acceleration of gravity, %h=percentage of patient's height, n=number, m/s2=acceleration, s=seconds, -=dimensionless.
Footnotes
All authors provided concept/idea/research design. Dr Curtze, Dr Nutt, Dr Carlson-Kuhta, and Dr Horak provided writing. Dr Carlson-Kuhta, Dr Mancini, and Dr Horak provided data collection. Dr Curtze and Dr Mancini provided data analysis. Dr Carlson-Kuhta and Dr Horak provided project management. Dr Horak provided fund procurement and facilities/equipment. Dr Nutt provided participants. Dr Nutt, Dr Carlson-Kuhta, Dr Mancini, and Dr Horak provided consultation (including review of manuscript before submission).
The study protocol was approved by the Oregon Health & Science University Institutional Review Board.
This publication was made possible with support from a grant from the National Institute on Aging (AG006457); a Challenge Grant from the National Institute of Neurological Disorders and Stroke (RC1 NS068678) and the Oregon Clinical and Translational Research Institute (OCTRI) at OHSU; and grant number UL1 RR024140 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research.
The OHSU and Dr Horak have a significant financial interest in APDM Inc, a company that may have a commercial interest in the results of this research and technology. This potential institutional and individual conflict has been reviewed and managed by OHSU.
References
- 1. Adkin AL, Frank JS, Jog MS. Fear of falling and postural control in Parkinson's disease. Mov Disord. 2003;18:496–502. [DOI] [PubMed] [Google Scholar]
- 2. Schrag A, Jahanshahi M, Quinn N. What contributes to quality of life in patients with Parkinson's disease? J Neurol Neurosurg Psychiatry. 2000;69:308–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bryant MS, Rintala DH, Hou JG, Protas EJ. Influence of fear of falling on gait and balance in Parkinson's disease. Disabil Rehabil. 2014;36:744–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nilsson MH, Hariz GM, Iwarsson S, Hagell P. Walking ability is a major contributor to fear of falling in people with Parkinson's disease: implications for rehabilitation. Parkinsons Dis. 2012;2012:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mak MK, Pang MY, Mok V. Gait difficulty, postural instability, and muscle weakness are associated with fear of falling in people with Parkinson's disease. Parkinsons Dis. 2012;2012:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ellis T, Cavanaugh JT, Earhart GM, et al. Which measures of physical function and motor impairment best predict quality of life in Parkinson's disease? Parkinsonism Relat Disord. 2011;17:693–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fahn S, Elton R; Members of the UPDRS Development Committee. Unified Parkinson's Disease Rating Scale. In: Fahn S, Marsden CD, Calne DB, Goldstein M, eds. Recent Developments in Parkinson's Disease. Vol 2 Florham Park, NJ: Macmillan Health Care Information; 1987;153–163, 293–304. [Google Scholar]
- 8. King LA, Salarian A, Mancini M, et al. Exploring outcome measures for exercise intervention in people with Parkinson's disease. Parkinsons Dis. 2013;2013:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Canning CG, Sherrington C, Lord SR, et al. Exercise for falls prevention in Parkinson disease: a randomized controlled trial. Neurology. 2015;84:304–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mancini M, King L, Salarian A, et al. Mobility lab to assess balance and gait with synchronized body-worn sensors. J Bioeng Biomed Sci. 2011;suppl 1:007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. McIlroy WE, Maki BE. Preferred placement of the feet during quiet stance: development of a standardized foot placement for balance testing. Clin Biomech (Bristol, Avon). 1997;12:66–70. [DOI] [PubMed] [Google Scholar]
- 12. Guy W. ECDEU Assessment Manual for Psychopharmacology. Rockville, MD: US Dept of Health, Education, and Welfare; 1976:533–537. [Google Scholar]
- 13. Hoehn MM, Yahr MD. Parkinsonism: onset, progression and mortality. Neurology. 1967;17:427–442. [DOI] [PubMed] [Google Scholar]
- 14. Powell LE, Myers AM. The Activities-specific Balance Confidence (ABC) Scale. J Gerontol A Biol Sci Med Sci. 1995;50:M28–M34. [DOI] [PubMed] [Google Scholar]
- 15. Jenkinson C, Fitzpatrick R, Peto V, et al. The Parkinson's Disease Questionnaire (PDQ-39): development and validation of a Parkinson's disease summary index score. Age Ageing. 1997;26:353–357. [DOI] [PubMed] [Google Scholar]
- 16. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Royal Statistical Society Series B (Methodological). 1995;57:289–300. [Google Scholar]
- 17. Glickman ME, Rao SR, Schultz MR. False discovery rate control is a recommended alternative to Bonferroni-type adjustments in health studies. J Clin Epidemiol. 2014;67:850–857. [DOI] [PubMed] [Google Scholar]
- 18. Curtze C, Nutt JG, Carlson-Kuhta P, et al. Levodopa is a double-edged sword for balance and gait in people with Parkinson's disease. Mov Disord. 2015;30:1361–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mancini M, El-Gohary M, Pearson S, et al. Continuous monitoring of turning in Parkinson's disease: rehabilitation potential. NeuroRehabilitation. 2015;37:3–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Patla AE, Adkin A, Ballard T. Online steering: coordination and control of body center of mass, head and body reorientation. Exp Brain Res. 1999;129:629–634. [DOI] [PubMed] [Google Scholar]
- 21. Schmidt P, Cubillos F, Simuni T, et al. Identifying potential best practices for treating Parkinson's disease: a mixed methods approach. Mov Disord. 2015;S119–S120. [Google Scholar]
- 22. King LA, Horak FB. Delaying mobility disability in people with Parkinson disease using a sensorimotor agility exercise program. Phys Ther. 2009;89:384–393. [DOI] [PMC free article] [PubMed] [Google Scholar]