Abstract
Key points
In fetuses, chronic anaemia stimulates cardiac growth; simultaneously, blood flow to the heart muscle itself is increased, and reserve blood flow capacity of the coronary vascular bed is preserved.
Here we examined functional adaptations of the capillaries and small blood vessels responsible for delivering oxygen to the anaemic fetal heart muscle using contrast‐enhanced echocardiography.
We demonstrate that coronary microvascular flux rate doubled in anaemic fetuses compared to control fetuses, both at rest and during maximal flow, suggesting reduced microvascular resistance consistent with capillary widening.
Cardiac fractional microvascular blood volume was not greater in anaemic fetuses, suggesting that growth of new microvascular vessels does not contribute to the increased flow per volume of myocardium.
These unusual changes in microvascular function during anaemia may indicate novel adaptive strategies in the fetal heart.
Abstract
Fetal anaemia causes cardiac adaptations that have immediate and life‐long repercussions on heart function and health. It is known that resting and maximal coronary conductance both increase during chronic fetal anaemia, but the coronary microvascular changes responsible for the adaptive response are unknown. Until recently, technical limitations have prevented quantifying functional capillary‐level adaptations in the in vivo fetal heart. Our objective was to characterise functional microvascular adaptations in chronically anaemic fetal sheep. Chronically instrumented fetuses were randomized to a control group (n = 11) or were made anaemic by isovolumetric haemorrhage (n = 12) for 1 week prior to myocardial contrast echocardiography at 85% of gestation. Anaemia augmented cardiac mass by 23% without changing body weight. In anaemic fetuses, microvascular blood flow per volume of myocardium was twice that of control fetuses at rest, during vasodilatory hyperaemia, and during hyperaemia plus increased aortic pressure. The elevated blood flow was attributable almost entirely to an increase in microvascular blood flux rate whereas microvascular blood volumes were not different between groups at baseline, during hyperaemia, or with hyperaemia plus increased aortic pressure. Increased coronary microvascular flux rate in response to chronic fetal anaemia is consistent with expected reductions in capillary resistance from capillary diameter widening detected in earlier histological studies.
Keywords: coronary circulation, fetal anemia, fetal heart, microvascular imaging
Key points
In fetuses, chronic anaemia stimulates cardiac growth; simultaneously, blood flow to the heart muscle itself is increased, and reserve blood flow capacity of the coronary vascular bed is preserved.
Here we examined functional adaptations of the capillaries and small blood vessels responsible for delivering oxygen to the anaemic fetal heart muscle using contrast‐enhanced echocardiography.
We demonstrate that coronary microvascular flux rate doubled in anaemic fetuses compared to control fetuses, both at rest and during maximal flow, suggesting reduced microvascular resistance consistent with capillary widening.
Cardiac fractional microvascular blood volume was not greater in anaemic fetuses, suggesting that growth of new microvascular vessels does not contribute to the increased flow per volume of myocardium.
These unusual changes in microvascular function during anaemia may indicate novel adaptive strategies in the fetal heart.
Abbreviations
- A
plateau video intensity units
- β
microvascular flux rate
- IB
blood pool signal
- LV
left ventricle
- MBV
microvascular blood volume
- MCE
myocardial contrast echocardiography
Introduction
Since the advent of widespread use of Rh0(D) immune globulin for prevention of fetal Rh disease, fetal anaemia has become a relatively uncommon complication of pregnancy (Society for Maternal‐Fetal Medicine et al. 2015). However, fetal anaemia still occurs due to non‐Rh(D) maternal alloimmunization, infection (e.g. parvovirus), inherited disorders (e.g. α‐thalassaemia), and for other reasons such as fetomaternal haemorrhage. The treatment, serial fetal transfusion to restore haematocrit, is associated with considerable procedural risk (Nishie et al. 2012). Thus, to reduce procedure frequency, fetuses are often allowed to become moderately anaemic before repeating a transfusion. The anaemic fetus must increase cardiac output to maintain adequate tissue oxygen delivery (Davis & Hohimer, 1991); in response, the fetal heart grows rapidly (Jonker et al. 2010). How the vasculature adapts to support this myocardial growth is of interest due to the central role of the coronaries in adult cardiac disease.
Chronic anaemia stimulates structural coronary vascular remodelling, reducing vascular resistance in hearts of both adults (Scheel et al. 1976) and fetuses (Davis et al. 1999). In anaemic adult rats, this is achieved primarily through arterial growth (Rakusan et al. 2001). In the fetal and young postnatal heart, the more distal portions of the microcirculation are more adaptable (Flanagan et al. 1991; Huo & Kassab, 2012), manifest by increased capillary volume density and capillary diameter in the response to chronic anaemia (Martin et al. 1998; Mascio et al. 2005). Functional vascular changes persist into adulthood, long after correction of anaemia (Davis et al. 2003). That hearts of adults that were anaemic in utero are hypoxia‐resistant (Broberg et al. 2003), but paradoxically ischaemia‐sensitive (Yang et al. 2008), underscores the need to better understand the functional significance of prenatal anaemia‐related coronary vascular remodelling.
Anaemic fetuses have a steeper relationship between coronary perfusion pressure and arterial flow during maximal adenosine‐mediated hyperaemia, indicating greater maximal coronary conductance (Davis et al. 1999). In adult dogs, the fraction of total myocardial resistance residing in the capillaries shifts from 0.25 to 0.75 during hyperaemia (Jayaweera et al. 1999), suggesting the increased maximal conductance in hearts of anaemic fetuses probably involves capillary adaptations. Indeed, anatomical studies have determined that left ventricular (LV) minimal capillary diameter is 41% greater in anaemic fetuses (Martin et al. 1998). At the capillary level, blood flow is non‐Newtonian, and there are complex non‐linear relationships between capillary diameter, apparent viscosity and resistance (Pries et al. 1994). In vivo coronary microvasculature dynamics in the anaemic fetus, the relative contributions of microvascular flux rate and functional microvascular blood volume to total coronary flow are unknown.
We hypothesized that remodelling during fetal anaemia would reduce functional resistance at the capillary level and increase microvascular volume. The main limitation to testing this hypothesis in the past had been a lack of methods that can image adaptations at the capillary level. We applied myocardial contrast echocardiography (MCE) perfusion imaging, which can parametrically assess microvascular blood flux rate and microvascular blood volume (MBV) in the capillary compartment, to reveal changes of conductance at the capillary level in response to anaemia in the fetal sheep.
Methods
Ethical approval
Animal experiments were approved by the Institutional Animal Care and Use Committee of Oregon Health and Science University, which is accredited by The Association for Assessment and Accreditation of Laboratory Animal Care International.
Animal surgery
Timed‐bred pregnant ewes of mixed western breed were obtained from a local vendor. Thirteen pregnant ewes bearing twins underwent sterile surgery at 118 gestational days (term delivery is at 147 days) for fetal instrumentation as previously described (Jonker et al. 2010). After ewes were given intramuscular atropine (7.5 mg) to control secretions, they were anaesthetized with an intravenous injection of ketamine (400 mg) and diazepam (10 mg), intubated and ventilated with a mix of oxygen (2 l min−1), nitrous oxide (0.7 l min−1) and isoflurane (1.5–2.0%). A midline abdominal incision was made and the uterus was opened to expose first one fetal head, and then the other. All instrumented fetuses had saline‐filled catheters placed in a jugular vein and carotid artery. Twelve fetuses also had a left lateral thoracotomy to place a left atrial catheter, and, of these, four fetuses had inflatable silastic occluders placed on their descending thoracic aorta below the level of the ductus arteriosus. All catheters, including an amniotic catheter, were anchored to the fetal skin and exteriorised to a pouch on the flank of the ewe. Fetal and ewe incisions were closed in layers. Ciprofloxacin (2 mg) and penicillin G (1 million units) were injected into each fetal amniotic sac. Ewes received subcutaneous buprenorphine (0.33 mg) immediately after surgery and twice daily for 2 days thereafter for analgesia.
Experimental model
Following surgical recovery, but prior to study, ewes were acclimatized to metabolic crate housing in which food and water were continuously available. While in metabolic crate housing, fetal catheters were continuously flushed (Minipuls 3, Gilson, Middleton, WI, USA) with a very small volume of heparinized (3000 U l−1) lactated Ringer solution to keep them open (<1 ml h−1 per catheter). Pressures were measured from in‐line transducers (Transpac, Abbott, Abbott Park, IL, USA) with a bridge amplifier and recorder (PowerLab, ADInstruments Inc., Colorado Springs, CO, USA). Vascular pressures were normalized to intra‐amniotic pressure and corrected daily for transducer voltage drift. Heart rate was determined from the arterial waveform. Haemodynamics were recorded continuously and sampled from a quiet period of at least 1 h. A daily sample for arterial blood parameters was taken and analysed on a Radiometer ABL 825 (Radiometer America Inc., Cleveland, OH, USA).
One twin from each pregnancy was randomized to the control group (n = 11) and the other to the anaemic group (n = 12) if they had blood gas values within the normal range (day 0, 3.3 ± 0.1 days after surgery, mean ± SEM). Two fetuses were excluded on the basis of spontaneous hypoxia (including one with a left atrial catheter). Fetal sheep in the anaemic group were made profoundly anaemic (target oxygen content 1.8–2.5 ml dl−1 for one or more study days) by daily phlebotomy with equal volume non‐heparinized normal saline replacement as previously described (Jonker et al. 2010). One fetus was excluded for not reaching the target oxygen content. More blood was removed early (for example, day 0: 88 ± 3 ml) than late (day 6: 27 ± 7 ml) in the experiment (total: 422 ± 8 ml).
MCE perfusion imaging
On day 7 (at 128 days gestational age), ewes were anaesthetized with an intravenous injection of ketamine (600 mg), intubated and ventilated with oxygen (2 l min−1) and isoflurane (1.5–2.0%). Staples and sutures were removed to re‐enter the midline abdominal incision, and a Mercedes incision was continued across the base of the udder to prevent uterine blood flow restriction during exteriorization. Fetal vascular pressures were recorded as described for daily monitoring. Fetal study order was random. Each uterine horn was serially exteriorised and the fetus manipulated through the closed uterus to allow parasternal long‐axis view imaging of the fetal left ventricle. Time under anaesthesia prior to study initiation was similar between groups (control, 44 ± 8 min; anaemic, 37 ± 5 min) as was imaging study length (control, 10 ± 1 min; anaemic, 12 ± 2 min).
MCE was performed using a linear‐array transducer at a centreline frequency of 7 MHz (Sequoia 512, Siemens Medical Systems, Malvern, PA, USA) as previously described (Wu et al. 2015). The non‐linear fundamental signal component for microbubbles was detected using multi‐pulse phase‐ and amplitude‐modulation at a mechanical index of 0.18 and a dynamic range of 55 dB. Gain settings were optimized and held constant. A decafluorobutane gas‐saturated aqueous suspension of 2 mg ml−1 distearoylphosphatidylcholine and 1 mg ml−1 polyoxyethylene‐40‐stearate was sonicated to produce lipid‐shelled microbubbles. Size distribution and concentration of microbubbles were measured by electrozone sensing (Multisizer III, Beckman Coulter, Brea, CA, USA). Concentration was adjusted to 107 ml−1.
Microbubbles were infused into the right atrium via a catheter previously advanced through a jugular vein, from whence they crossed the foramen ovale into the left heart to enter the coronary circulation. Several frames were measured from the left ventricular cavity at end‐diastole during a microbubble infusion rate of 0.5 ml min−1 to determine the blood pool signal (I B). Myocardial imaging was performed during a microbubble infusion rate of 1.5 ml min−1. End‐systolic images were acquired for a minimum of 8 s after a high‐power (mechanical index 0.9–1.0) five‐frame destructive pulse sequence. MCE was performed in all fetuses under basal conditions (11 control, 12 anaemic). MCE was also performed during vasodilatory hyperaemia (5 control, 6 anaemic). Adenosine (147 μg kg−1 min−1) was infused via the left atrial catheter for 2 min prior to and during image acquisition. This dose of adenosine has been shown to maximally dilate fetal sheep coronary arteries without systemic effects (Davis et al. 1999). Finally, coronary perfusion was studied during hyperaemia plus increased perfusion pressure (control, n = 2; anaemic, n = 2). Pressure was elevated by transient maximal inflation of an aortic occluder cuff for approximately 5 s during image acquisition. Changes in cardiac perfusion for such a brief pressure increase are attributed not to increased metabolic demand but rather to increased perfusion pressure in conjunction with adenosine‐mediated abrogation of coronary autoregulation. A rest period was permitted between each occlusion. All MCE image data were acquired in triplicate.
For MCE analysis, signal from non‐capillary microvessels was eliminated by background subtraction of a frame obtained 0.5 s after destruction from all subsequent frames. Time‐intensity data after the destructive pulse sequence were fitted to the function:
where y is intensity at time t, A is the plateau intensity reflecting relative microvascular blood volume, and the rate constant β represents the microvascular flux rate (Wei et al. 1998; Dawson et al. 2002; Le et al. 2002). Left ventricular microvascular blood volume was quantified by scaled comparison of plateau intensity with I B:
where 1.06 is tissue density (g cm−1), F is the scaling factor to correct for different infusion rates during determination of I B and myocardial perfusion to avoid dynamic range saturation, and C is a coefficient to correct for tissue attenuation. Myocardial blood flow was calculated as the product of A and β.
At the conclusion of the MCE study, animals were humanely killed with an intravenous overdose of a pentobarbital euthanasia solution. Fetal sex, heart and body weights were recorded.
Statistical analysis
Daily haemodynamics and blood parameters were analysed by mixed measures analysis of variance (ANOVA). If indicated by the ANOVA F statistic, further analysis was performed within groups by Dunnett's multiple comparisons test, and/or between groups by Sidak's multiple comparisons test. Comparisons of weights and myocardial perfusion values were performed by Student's unpaired t test. t tests were one‐sided when clearly indicated by hypotheses (e.g. anaemia will increase MBV, flux rate and flow), otherwise they were two‐sided. Linear regression was performed on microvascular flow during hyperaemia at normal and elevated perfusion pressure. Values are shown as mean ± standard error of the mean (SEM) or, for data obtained during elevated perfusion pressure, as individual values. Differences were considered statistically significant at P < 0.05.
Results
Daily physiological parameters
Isovolumetric haemorrhage significantly reduced total haemoglobin and haematocrit over 2–3 days, after which these parameters remained stable at half‐normal values (Table 1). Because partial pressure of oxygen () also declined slightly after the first removal of blood, oxygen content levels in anaemic fetuses declined to half of control levels by day 2, and further to one‐third of control values by the end of the study.
Table 1.
Daily haemodynamics and arterial blood parameters of anaemic and control fetuses
| Day 0 | Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | Day 6 | Day 7 | |
|---|---|---|---|---|---|---|---|---|
| Arterial pressure (mmHg) | ||||||||
| Control | 41 ± 1 | 41 ± 1 | 41 ± 1 | 41 ± 1 | 40 ± 1 | 41 ± 1 | 40 ± 1 | 42 ± 1 |
| Anaemic | 41 ± 1 | 38 ± 1† | 36 ± 1†* | 37 ± 1†* | 36 ± 1†* | 36 ± 1†* | 36 ± 1†* | 37 ± 1†* |
| Right atrial pressure (mmHg) | ||||||||
| Control | 2.3 ± 0.2 | 2.2 ± 0.2 | 2.1 ± 0.3 | 2.2 ± 0.1 | 2.5 ± 0.2 | 2.7 ± 0.3 | 2.3 ± 0.2 | 2.3 ± 0.3 |
| Anaemic | 2.1 ± 0.3 | 2.1 ± 0.2 | 2.3 ± 0.3 | 2.9 ± 0.2 | 2.8 ± 0.3 | 3.4 ± 0.3† | 3.9 ± 0.2†* | 4.1 ± 0.3†* |
| Heart rate (beats min−1) | ||||||||
| Control | 177 ± 4 | 182 ± 4 | 180 ± 4 | 178 ± 4 | 167 ± 4 | 168 ± 3 | 164 ± 4† | 159 ± 4† |
| Anaemic | 182 ± 2 | 190 ± 3 | 193 ± 3† | 185 ± 4 | 184 ± 4* | 184 ± 3* | 182 ± 3* | 180 ± 3* |
| pH | ||||||||
| Control | 7.352 ± 0.005 | 7.349 ± 0.007 | 7.348 ± 0.008 | 7.340 ± 0.008 | 7.340 ± 0.006 | 7.345 ± 0.005 | 7.335 ± 0.006 | 7.347 ± 0.003 |
| Anaemic | 7.353 ± 0.004 | 7.338 ± 0.005 | 7.328 ± 0.005† | 7.339 ± 0.005 | 7.323 ± 0.008† | 7.330 ± 0.008† | 7.333 ± 0.004† | 7.336 ± 0.005† |
| (mmHg) | ||||||||
| Control | 50 ± 1 | 51 ± 1 | 50 ± 0 | 50 ± 0 | 50 ± 1 | 51 ± 0 | 51 ± 1 | 49 ± 0 |
| Anaemic | 50 ± 1 | 52 ± 1† | 53 ± 1†* | 51 ± 1 | 52 ± 1 | 52 ± 1 | 53 ± 1† | 52 ± 1* |
| (mmHg) | ||||||||
| Control | 21 ± 1 | 20 ± 1 | 20 ± 1 | 20 ± 1 | 21 ± 1 | 20 ± 1 | 20 ± 1 | 21 ± 1 |
| Anaemic | 21 ± 1 | 19 ± 1† | 18 ± 1† | 19 ± 1† | 19 ± 1† | 18 ± 1† | 18 ± 1† | 19 ± 1† |
| (%) | ||||||||
| Control | 55.5 ± 2.7 | 49.0 ± 4.1 | 50.8 ± 3.6 | 51.1 ± 3.6 | 54.2 ± 2.9 | 51.0 ± 1.9 | 49.3 ± 3.4 | 54.4 ± 2.7 |
| Anaemic | 57.5 ± 2.0 | 45.2 ± 3.0† | 38.2 ± 2.3†* | 43.7 ± 1.9† | 42.2 ± 2.5†* | 40.9 ± 2.7† | 39.4 ± 2.9† | 40.7 ± 1.9†* |
| Content O2 (ml dl−1) | ||||||||
| Control | 8.0 ± 0.4 | 6.9 ± 0.5 | 7.1 ± 0.4 | 7.4 ± 0.6 | 7.8 ± 0.4 | 7.7 ± 0.4 | 7.3 ± 0.4 | 8.1 ± 0.4 |
| Anaemic | 8.1 ± 0.3 | 5.1 ± 0.4†* | 3.3 ± 0.2†* | 3.3 ± 0.3†* | 3.0 ± 0.3†* | 2.6 ± 0.1†* | 2.4 ± 0.2†* | 2.6 ± 0.2†* |
| Haemoglobin (g dl−1) | ||||||||
| Control | 10.8 ± 0.6 | 10.7 ± 0.8 | 10.7 ± 0.7 | 10.8 ± 0.7 | 10.9 ± 0.7 | 11.2 ± 0.7 | 11.2 ± 0.6 | 11.2 ± 0.6 |
| Anaemic | 10.6 ± 0.4 | 8.2 ± 0.5†* | 6.4 ± 0.4†* | 5.6 ± 0.4†* | 5.3 ± 0.5†* | 4.9 ± 0.4†* | 4.7 ± 0.3†* | 4.7 ± 0.3†* |
| Haematocrit (fraction) | ||||||||
| Control | 0.36 ± 0.02 | 0.36 ± 0.02 | 0.37 ± 0.02 | 0.37 ± 0.02 | 0.38 ± 0.02 | 0.38 ± 0.02 | 0.38 ± 0.02 | 0.38 ± 0.02 |
| Anaemic | 0.36 ± 0.01 | 0.28 ± 0.01†* | 0.22 ± 0.01†* | 0.20 ± 0.01†* | 0.20 ± 0.02†* | 0.19 ± 0.01†* | 0.18 ± 0.01†* | 0.18 ± 0.01†* |
is partial pressure of CO2; is partial pressure of O2; is O2 saturation. †Different within‐group from day 0, P < 0.05. *Different from same‐day control, P < 0.05. Values shown as mean ± SEM.
Following the first isovolumetric haemorrhage in anaemic fetuses, arterial pressure declined by ∼10% and remained about 5 mmHg lower than in controls throughout the study (Table 1). In anaemic fetuses, right atrial pressure increased to twice normal levels by the end of the study. Anaemia prevented the age‐associated decline in heart rate observed in the control group.
Experimental groups at necropsy
Terminal gestational age was the same in the two groups (128 days). Control and anaemic fetuses had similar body weights (kg: 3.4 ± 0.2 vs. 3.6 ± 0.1) but heavier hearts (g: 21.7 ± 1.0 vs. 26.6 ± 1.4, P < 0.01), resulting in higher heart/body weight ratios (g kg−1: 6.4 ± 0.2 vs. 7.5 ± 0.3, P < 0.02). At necropsy, the sex ratio (male/female) of both groups was 1.2. All fetuses were twins.
Myocardial perfusion
Parametric analysis of MCE perfusion imaging data indicated that neither the A value (video intensity units: control, 61.9 ± 7.8; anaemic, 63.5 ± 6.6) nor the calculated MBV (Fig. 1 A) were significantly different between groups at baseline. In contrast, the microvascular flux rate (β) of anaemic fetuses was more than twice that of control fetuses (Fig. 1 B), resulting in a higher basal microvascular blood flow (Fig. 1 C). Cardiac blood flow regulation is responsive to cardiac work and oxygen delivery, therefore we looked at flow in relationship to these parameters. The product of heart rate and blood pressure (double product), an index of work, was not different under baseline conditions between anaesthetized control fetuses (6531 ± 495 mmHg × beats min−1) and anaemic fetuses (6946 ± 815 mmHg × beats min−1). Thus, microvascular flow per work in anaemic fetuses was twofold higher than in control fetuses (Fig. 1 D). Arterial oxygen content was low in anaemic compared to control fetuses at the time of MCE (g dl−1: 2.3 ± 0.3 vs. 5.2 ± 0.9, P < 0.002). Microvascular blood flow per work was noted to vary with arterial oxygen content (Fig. 1 E), resulting in a similar oxygen delivery per work between groups (Fig. 1 F).
Figure 1. Baseline LV microvascular perfusion .
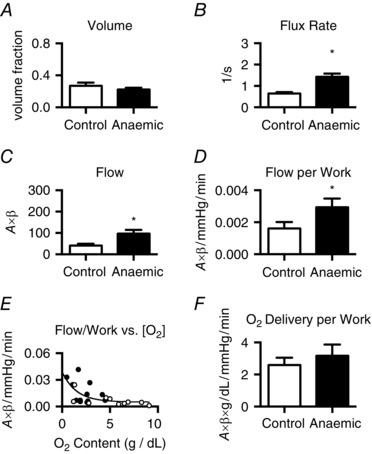
Baseline microvascular volume (A) was not different between groups. Microvascular blood flux rate (B) in anaemic fetuses was twice that of control fetuses, thus LV microvascular flow (C) was also increased. As the rate–pressure product was not different between groups, microvascular flow per work (D) was almost doubled in anaemic fetuses. At low arterial oxygen contents, flow per work was elevated (E; open circles, control; filled circles, anaemic), thus oxygen delivery per work (F) was not different between groups. *Different from control (P < 0.05). Values shown as mean ± SEM (control, n = 10–11; anaemic, n = 12).
Intracoronary adenosine vasodilates fetal coronary conductance vessels and abolishes autoregulatory control linking arterial oxygen content, cardiac oxygen requirement and myocardial blood flow. MCE perfusion imaging was performed during adenosine infusion so that any differences in microvascular blood flow, in particular blood flux rate, would reflect differences in resistance at the capillary level. Adenosine infusion did not change fetal arterial pressure or heart rate. For both groups, microvascular flow was doubled by adenosine‐mediated hyperaemia compared to baseline (P < 0.0001), while MBV was increased by about 30% (P < 0.01). There were no significant differences between the anaemic and control groups with respect to A value (control, 80.5 ± 9.9; anaemic, 70.5 ± 5.3) or MBV (Fig. 2 A) during adenosine. In contrast, mean microvascular flux rate (β) and flow were doubled in anaemic fetuses compared to control fetuses (Fig. 2 B and C) during adenosine. In the absence of autoregulation, coronary flow is collinear with coronary perfusion pressure (aortic minus right atrial pressure) which was similar between control (31.9 ± 2.8 mmHg) and anaemic (31.8 ± 4.0 mmHg) animals. Yet, microvascular blood flow normalized to perfusion pressure was twofold higher in anaemic fetuses compared to controls (Fig. 2 D).
Figure 2. LV microvascular perfusion during hyperaemia .
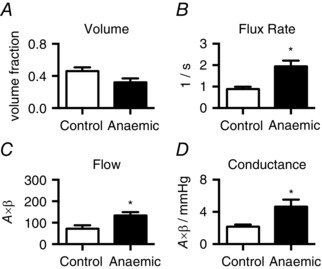
During adenosine‐mediated coronary vasodilatation, microvascular volume (A) was not different between groups. Microvascular blood flux rate (B) in anaemic fetuses was twice that of control fetuses. Hence, microvascular flow (C) and conductance (D) were elevated. *Different from control (P < 0.05). Values shown as mean ± SEM (control, n = 5; anaemic, n = 6).
Not all capillaries are continuously perfused (Krogh, 1919), and capillary recruitment can increase effective MBV (Le et al. 2002). Thus, to recruit putative closed capillaries via perfusion pressure‐mediated mechanisms, fetuses undergoing adenosine‐induced hyperaemia were studied during transient increases in coronary perfusion pressure. Occluder inflation further elevated microvascular flow 50% over hyperaemic values (P < 0.02), while MBV was increased 19% over hyperaemic values (P < 0.03). Perfusion pressure during occluder inflation was similar between the anaemic (35.2 ± 0.3 mmHg) and control (37.0 ± 3.9 mmHg) groups. The A value was not different between groups during elevated perfusion pressure (control, 97.8 ± 3.3; anaemic, 107.6 ± 15.5), thus MBV was also similar between anaemic and control fetuses (Fig. 3 A). Microvascular pressure–flow relationships in control and anaemic fetuses are demonstrated by flow measurements at two pressures during adenosine‐mediated hyperaemia (Fig. 3 E).
Figure 3. LV microvascular perfusion during hyperaemia and elevated perfusion pressure .
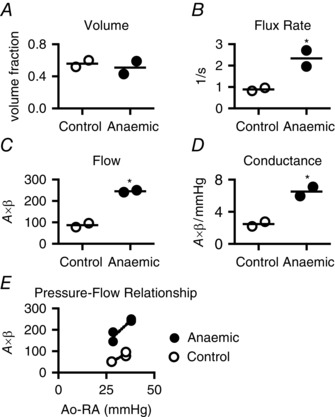
During occluder‐mediated elevation of perfusion pressure with simultaneous adenosine‐mediated coronary vasodilatation, LV microvascular volume (A) remained similar between control and anaemic fetuses. Both microvascular blood flux rate (B) and flow (C) in anaemic fetuses were more than twice that in control fetuses. Perfusion pressure was not different between groups, thus LV microvascular conductance (D) doubled in anaemic fetuses. E, demonstration of the relationship between pressure (aortic (Ao) minus right atrial (RA)) and microvascular hyperaemic flow in anaemic and control fetuses. Perfusion pressures were similar between groups at the two different points. *Different from control (P < 0.05). Mean and raw data shown (control, n = 2; anaemic, n = 2).
Discussion
Coronary neovascularization is thought to be required to support an adaptive myocardial growth response to increased haemodynamic load (Shiojima & Walsh, 2006; Sano et al. 2007). In chronic fetal anaemia, cardiac output and mass increase (Davis & Hohimer, 1991; Martin et al. 1998; Jonker et al. 2010). Not only is total coronary flow elevated (with preserved flow reserve), but also flow per gram of myocardium is greater to maintain oxygen supply (Davis & Hohimer, 1991; Davis et al. 1999). In this study we found that the elevated cardiac microvascular flow in anaemic fetuses was achieved by faster average microvascular blood flux rate, but that MBV fraction at rest was not altered by chronic fetal anaemia. This finding is unexpected as MBV typically increases when growth or remodelling increases coronary flow in adults (Di Bello et al. 2003; Indermuhle et al. 2006; Ikonomidis et al. 2010; Santos et al. 2010; Wang et al. 2012).
Fetal capillary widening in concert with decreased haematocrit can explain the increased coronary microvascular flow observed by MCE. Viscosity is a major component of resistance in microvessels (Jayaweera et al. 1999), and, at the level of the capillary, both vessel diameter and haematocrit alter effective blood viscosity (Pries et al. 1994). Davis and colleagues (1999) transfused anaemic fetal sheep, increasing haematocrit from 0.15 to 0.30, and found that 57% of the anaemia‐induced increase in conductance was due to changed haematocrit; the remaining 43% of conductance change was attributed to structural changes. Coronary adaptation in the anaemic fetus has been described histologically to include increased capillary diameter (Martin et al. 1998; Mascio et al. 2005). Simulations by Pries and colleagues (1994) show that relevant changes in capillary diameter and haematocrit would decrease relative effective viscosity (vis‐à‐vis resistance) by about half. The magnitude of this predicted change is close to the change in flux rate seen in this study, supporting our conclusion that elevated coronary microvascular flow results from capillary widening in fetal anaemia.
Baseline MBV between control and anaemic fetuses was similar, and increased by a similar magnitude with hyperaemia and aortic occlusion in both groups. In adult dogs, work‐induced increases in myocardial oxygen consumption and coronary blood flow are accompanied by increased MBV, suggesting that capillary recruitment is a mechanism in acute regulation of coronary blood flow (Le et al. 2002). We ruled out the possibility that anaemic fetuses possess greater recruitable capillary reserves by showing that MBV remains similar between control and anaemic fetuses during hyperaemia and at increased perfusion pressures. That MBV increased during these conditions suggests that, although not changed by anaemia, capillary recruitment is a mechanism active in the fetal heart.
As greater fractional MBV typically results from more perfused microvascular units per volume of myocardium, the similarity in MBV between groups suggests that the capillarity of the anaemic hearts was not greater. The histological record is unclear as to whether angiogenesis increases myocardial capillarity in the anaemic fetal heart. Anatomical studies have not shown increased capillary length density (Mascio et al. 2005), numeric density (Martin et al. 1998) or fractional area (Jonker et al. 2010), although they have shown increased volume density (Martin et al. 1998). Our in vivo study supports the previous findings of increased capillary diameter (Martin et al. 1998; Mascio et al. 2005), and may thus help with interpretation of the histological record. An alternative explanation for the discrepancy between anatomical studies and this functional study is that MCE may not detect specific changes in capillary diameter as differences in MBV. We are unaware of any studies investigating this specific relationship. However, the increases in MBV following adenosine administration and aortic occlusion suggest that the disagreement between anatomical and functional studies is not attributable to a fundamental inability of MCE to detect differences in fetal MBV.
In addition to the microvascular changes described here, coronary conductance vessels adaptation is also likely to have contributed to total coronary conductance changes observed in chronically anaemic fetal sheep. In the adult dog heart, the fraction of total myocardial resistance residing in the capillaries is 0.25 at rest (Jayaweera et al. 1999). In anaemic fetuses at rest, microvascular flow was doubled compared to controls in order to normalize oxygen delivery per work (Davis et al. 1999). The magnitude of this difference in flow at rest implicates involvement of coronary resistance arterioles as well as capillaries, either by active feedback causing arteriolar dilatation or by remodelling of arterioles to increase flow. In pigs with cardiac hypertrophy, the normalization of coronary flow to the expanded myocardium has been shown to be mediated by an increase in the number of vascular orders as determined by diameter‐defined Strahler analysis (Huo & Kassab, 2012). A similar increase in the coronary arteriolar network may have occurred in the anaemic hearts in this study, which cannot be directly measured by MCE but would also be manifest by an increase in microvascular flux rate (Pascotto et al. 2007).
The changes made by the fetal heart during chronic anaemia can be explained as adaptations necessary to ensure adequate tissue oxygenation and enable survival to birth (Davis & Hohimer, 1991). This stress‐induced process disrupts normal myocardial growth and maturation, and transfusion to restore fetal haematocrit does not completely return these trajectories to normal (Carter et al. 1990; Jonker et al. 2011). Further, it is clear that long‐term cardiac outcomes for adults that were anaemic in utero are not normal (Broberg et al. 2003; Yang et al. 2008; Wallace et al. 2011). Although cardiac capillary number, luminal area and length density are not abnormal in adults that were anaemic in utero, these hearts have both improved contractility in response to acute hypoxaemia and increased infarct size following coronary occlusion (Broberg et al. 2003; Yang et al. 2008). As increased coronary blood flow can improve function with hypoxaemia but may increase washout of cardioprotective interstitial adenosine, changed cardiac function and risk may result from increased coronary conductance and maximal coronary blood flow persistent in adulthood (Davis et al. 2003). Thus, fetal programming of the coronary vasculature has different consequences for the adult that are either beneficial or deleterious depending upon the physiological circumstances. We conclude that the microvascular changes found in this study may play a role in long‐term cardiac outcomes.
A limitation of this study is that ewe and fetus were anaesthetized during MCE measurements. This approach was chosen to reduce the impact of maternal movement and to control fetal thoracic orientation in this initial effort. General anaesthesia is not a requirement of contrast enhanced ultrasound, and indeed maternal measurements of placental intervillous blood flow have been made in humans (Roberts et al. 2016). Our study shows that ultrasonographic evaluation with MCE is a valuable research methodology with the potential to be less invasive than other conventional techniques such as microspheres. MCE also offers the unique possibility of targeted molecular imaging and delivery in the fetal heart (Inaba & Lindner, 2012).
Conclusion
Chronic fetal anaemia caused coronary microvascular remodelling to increase flux rate, but not microvascular fractional volume, as measured by myocardial contrast echocardiography. These changes are consistent with wider capillary diameter rather than angiogenesis as the basis for increased microvascular flow per myocardial volume. The MBV and microvascular flux relationships between control and anaemic fetuses were preserved during hyperaemia, as both experienced similar increases in these values. Remodelling to increase capillary flux rate enables necessary oxygen delivery to the anaemic fetal heart while preserving coronary reserve, but may contribute to changed cardiovascular risk in adulthood. Further studies are needed to determine if remodelling to increase flux rate without increased MBV is a common mechanism in fetal hearts requiring increased microvascular blood flow.
Additional information
Competing interests
None declared.
Author contributions
S.S.J., L.D., G.D.G. and J.R.L. designed the experiments, which were carried out in the laboratories of S.S.J. and L.D. at the Oregon Health and Science University. S.S.J., D.S. and S.L. performed the surgeries. S.S.J., D.S. and A.W. performed the daily experiments to produce the model. All authors participated in the MCE experiments; J.T.B., B.P.D. and J.R.L. performed the echocardiography, while J.T.B. and T.M.A. obtained the MCE data. S.S.J. drafted the manuscript; all authors participated in critical revision. All authors approved the final version of the manuscript, agree to be accountable for all aspects of the work, and declare that all persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Funding
Research reported in this manuscript was supported by the NIH Eunice Kennedy Shriver National Institute of Child Health and Human Development under award numbers P01HD034430 and R01HD071068. D.S. and T.M.A. were supported by the NIH National Heart, Lung, and Blood Institute under award number T32HL094294. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Acknowledgements
We thank Loni Socha and Daniel Kamna for technical assistance.
This is an Editor's Choice article from the 1 November 2016 issue.
References
- Broberg CS, Giraud GD, Schultz JM, Thornburg KL, Hohimer AR & Davis LE (2003). Fetal anemia leads to augmented contractile response to hypoxic stress in adulthood. Am J Physiol Regul Integr Comp Physiol 285, R649–R655. [DOI] [PubMed] [Google Scholar]
- Carter BS, DiGiacomo JE, Balderston SM, Wiggins JW & Merenstein GB (1990). Disproportionate septal hypertrophy associated with erythroblastosis fetalis. Am J Dis Child 144, 1225–1228. [DOI] [PubMed] [Google Scholar]
- Davis L, Roullet JB, Thornburg KL, Shokry M, Hohimer AR & Giraud GD (2003). Augmentation of coronary conductance in adult sheep made anaemic during fetal life. J Physiol 547, 53–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis LE & Hohimer AR (1991). Hemodynamics and organ blood flow in fetal sheep subjected to chronic anemia. Am J Physiol 261, R1542–R1548. [DOI] [PubMed] [Google Scholar]
- Davis LE, Hohimer AR & Morton MJ (1999). Myocardial blood flow and coronary reserve in chronically anemic fetal lambs. Am J Physiol 277, R306–R313. [DOI] [PubMed] [Google Scholar]
- Dawson D, Vincent MA, Barrett EJ, Kaul S, Clark A, Leong‐Poi H & Lindner JR (2002). Vascular recruitment in skeletal muscle during exercise and hyperinsulinemia assessed by contrast ultrasound. Am J Physiol Endocrinol Metab 282, E714–E720. [DOI] [PubMed] [Google Scholar]
- Di Bello V, Giorgi D, Pedrinelli R, Talini E, Palagi C, Nardi C, Dell'Omo G, Delle Donne MG, Paterni M & Mariani M (2003). Coronary microcirculation into different models of left ventricular hypertrophy – hypertensive and athlete's heart: a contrast echocardiographic study. J Hum Hypertens 17, 253–263. [DOI] [PubMed] [Google Scholar]
- Flanagan MF, Fujii AM, Colan SD, Flanagan RG & Lock JE (1991). Myocardial angiogenesis and coronary perfusion in left ventricular pressure‐overload hypertrophy in the young lamb. Evidence for inhibition with chronic protamine administration. Circ Res 68, 1458–1470. [DOI] [PubMed] [Google Scholar]
- Huo Y & Kassab GS (2012). Compensatory remodelling of coronary microvasculature maintains shear stress in porcine left‐ventricular hypertrophy. J Hypertens 30, 608–616. [DOI] [PubMed] [Google Scholar]
- Ikonomidis I, Iliodromitis EK, Tzortzis S, Antoniadis A, Paraskevaidis I, Andreadou I, Fountoulaki K, Farmakis D, Kremastinos DT & Anastasiou‐Nana M (2010). Staccato reperfusion improves myocardial microcirculatory function and long‐term left ventricular remodelling: a randomised contrast echocardiography study. Heart 96, 1898–1903. [DOI] [PubMed] [Google Scholar]
- Inaba Y & Lindner JR (2012). Molecular imaging of disease with targeted contrast ultrasound imaging. Transl Res 159, 140–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indermuhle A, Vogel R, Meier P, Wirth S, Stoop R, Mohaupt MG & Seiler C (2006). The relative myocardial blood volume differentiates between hypertensive heart disease and athlete's heart in humans. Eur Heart J 27, 1571–1578. [DOI] [PubMed] [Google Scholar]
- Jayaweera AR, Wei K, Coggins M, Bin JP, Goodman C & Kaul S (1999). Role of capillaries in determining CBF reserve: new insights using myocardial contrast echocardiography. Am J Physiol 277, H2363–H2372. [DOI] [PubMed] [Google Scholar]
- Jonker SS, Giraud MK, Giraud GD, Chattergoon NN, Louey S, Davis LE, Faber JJ & Thornburg KL (2010). Cardiomyocyte enlargement, proliferation and maturation during chronic fetal anaemia in sheep. Exp Physiol 95, 131–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonker SS, Scholz TD & Segar JL (2011). Transfusion effects on cardiomyocyte growth and proliferation in fetal sheep after chronic anemia. Pediatr Res 69, 485–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krogh A (1919). The supply of oxygen to the tissues and the regulation of the capillary circulation. J Physiol 52, 457–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le DE, Bin JP, Coggins MP, Wei K, Lindner JR & Kaul S (2002). Relation between myocardial oxygen consumption and myocardial blood volume: a study using myocardial contrast echocardiography. J Am Soc Echocardiogr 15, 857–863. [DOI] [PubMed] [Google Scholar]
- Martin C, Yu AY, Jiang BH, Davis L, Kimberly D, Hohimer AR & Semenza GL (1998). Cardiac hypertrophy in chronically anemic fetal sheep: Increased vascularization is associated with increased myocardial expression of vascular endothelial growth factor and hypoxia‐inducible factor 1. Am J Obstet Gynecol 178, 527–534. [DOI] [PubMed] [Google Scholar]
- Mascio CE, Olison AK, Ralphe JC, Tomanek RJ, Scholz TD & Segar JL (2005). Myocardial vascular and metabolic adaptations in chronically anemic fetal sheep. Am J Physiol Regul Integr Comp Physiol 289, R1736–R1745. [DOI] [PubMed] [Google Scholar]
- Nishie EN, Liao AW, Brizot Mde L, Assunção RA & Zugaib M (2012). Prediction of the rate of decline in fetal hemoglobin levels between first and second transfusions in red cell alloimmune disease. Prenat Diagn 32, 1123–1126. [DOI] [PubMed] [Google Scholar]
- Pascotto M, Leong‐Poi H, Kaufmann B, Allrogen A, Charalampidis D, Kerut EK, Kaul S & Lindner JR (2007). Assessment of ischemia‐induced microvascular remodelling using contrast‐enhanced ultrasound vascular anatomic mapping. J Am Soc Echocardiogr 20, 1100–1108. [DOI] [PubMed] [Google Scholar]
- Pries AR, Secomb TW, Gessner T, Sperandio MB, Gross JF & Gaehtgens P (1994). Resistance to blood flow in microvessels in vivo. Circ Res 75, 904–915. [DOI] [PubMed] [Google Scholar]
- Rakusan K, Cicutti N & Kolar F (2001). Effect of anemia on cardiac function, microvascular structure, and capillary hematocrit in rat hearts. Am J Physiol Heart Circ Physiol 280, H1407–H1414. [DOI] [PubMed] [Google Scholar]
- Roberts VH, Lo JO, Salati JA, Lewandowski KS, Lindner JR, Morgan TK & Frias AE (2016). Quantitative assessment of placental perfusion by contrast‐enhanced ultrasound in macaques and human subjects. Am J Obstet Gynecol 214, 369.e1–369.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sano M, Minamino T, Toko H, Miyauchi H, Orimo M, Qin Y, Akazawa H, Tateno K, Kayama Y, Harada M, Shimizu I, Asahara T, Hamada H, Tomita S, Molkentin JD, Zou Y & Komuro I (2007). p53‐induced inhibition of Hif‐1 causes cardiac dysfunction during pressure overload. Nature 446, 444–448. [DOI] [PubMed] [Google Scholar]
- Santos JM, Kowatsch I, Tsutsui JM, Negrao CE, Canavesi N, Carvalho Frimm C, Mady C, Ramires JA & Mathias W Jr (2010). Effects of exercise training on myocardial blood flow reserve in patients with heart failure and left ventricular systolic dysfunction. Am J Cardiol 105, 243–248. [DOI] [PubMed] [Google Scholar]
- Scheel KW, Brody DA, Ingram LA & Keller F (1976). Effects of chronic anemia on the coronary and coronary collateral vasculature in dogs. Circ Res 38, 553–559. [DOI] [PubMed] [Google Scholar]
- Shiojima I & Walsh K (2006). Regulation of cardiac growth and coronary angiogenesis by the Akt/PKB signalling pathway. Genes Dev 20, 3347–3365. [DOI] [PubMed] [Google Scholar]
- Society for Maternal‐Fetal Medicine (SMFM) , Norton ME, Chauhan SP & Dashe JS (2015). Society for maternal‐fetal medicine (SMFM) clinical guideline #7: nonimmune hydrops fetalis. Am J Obstet Gynecol 212, 127–139. [DOI] [PubMed] [Google Scholar]
- Wallace AH, Dalziel SR, Thornburg KL, Broberg CS, Jerosh‐Herold M, Coelho‐Filho OR & Harding JE (2011). Cardiovascular risk in adulthood after in‐utero transfusion for fetal anaemia. J Dev Orig Health Dis 2, S15. [Google Scholar]
- Wang J, Chen YD, Zhi G, Xu Y, Chen L, Liu HB, Zhou X & Tian F (2012). Beneficial effect of adenosine on myocardial perfusion in patients treated with primary percutaneous coronary intervention for acute myocardial infarction. Clin Exp Pharmacol Physiol 39, 247–252. [DOI] [PubMed] [Google Scholar]
- Wei K, Jayaweera AR, Firoozan S, Linka A, Skyba DM & Kaul S (1998). Quantification of myocardial blood flow with ultrasound‐induced destruction of microbubbles administered as a constant venous infusion. Circulation 97, 473–483. [DOI] [PubMed] [Google Scholar]
- Wu MD, Belcik JT, Qi Y, Zhao Y, Benner C, Pei H, Linden J & Lindner JR (2015). Abnormal regulation of microvascular tone in a murine model of sickle cell disease assessed by contrast ultrasound. J Am Soc Echocardiogr 28, 1122–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Q, Hohimer AR, Giraud GD, Van Winkle DM, Underwood MJ, He GW & Davis LE (2008). Effect of fetal anaemia on myocardial ischaemia‐reperfusion injury and coronary vasoreactivity in adult sheep. Acta Physiol (Oxf) 194, 325–334. [DOI] [PMC free article] [PubMed] [Google Scholar]


