Abstract
Key points
In humans, excitation of peripheral chemoreceptors with systemic hypoxia causes hyperventilation, hypertension and tachycardia. However, the contribution of particular chemosensory areas (carotid vs. aortic bodies) to this response is unclear.
We showed that selective stimulation of the carotid body by the injection of adenosine into the carotid artery causes a dose‐dependent increase in minute ventilation and blood pressure with a concomitant decrease in heart rate in conscious humans. The ventilatory response was abolished and the haemodynamic response was diminished following carotid body ablation.
We found that the magnitude of adenosine evoked responses in minute ventilation and blood pressure was analogous to the responses evoked by hypoxia. By contrast, opposing heart rate responses were evoked by adenosine (bradycardia) vs. hypoxia (tachycardia).
Intra‐carotid adenosine administration may provide a novel method for perioperative assessment of the effectiveness of carotid body ablation, which has been recently proposed as a treatment strategy for sympathetically‐mediated diseases.
Abstract
Stimulation of peripheral chemoreceptors by acute hypoxia causes an increase in minute ventilation (VI), heart rate (HR) and arterial blood pressure (BP). However, the contribution of particular chemosensory areas, such as carotid (CB) vs. aortic bodies, to this response in humans remains unknown. We performed a blinded, randomized and placebo‐controlled study in 11 conscious patients (nine men, two women) undergoing common carotid artery angiography. Doses of adenosine ranging from 4 to 512 μg or placebo solution of a matching volume were administered in randomized order via a diagnostic catheter located in a common carotid artery. Separately, ventilatory and haemodynamic responses to systemic hypoxia were also assessed. Direct excitation of a CB with intra‐arterial adenosine increased VI, systolic BP, mean BP and decreased HR. No responses in these variables were seen after injections of placebo. The magnitude of the ventilatory and haemodynamic responses depended on both the dose of adenosine used and on the level of chemosensitivity as determined by the ventilatory response to hypoxia. Percutaneous radiofrequency ablation of the CB abolished the adenosine evoked respiratory response and partially depressed the cardiovascular response in one participant. The results of the present study confirm the excitatory role of purines in CB physiology in humans and suggest that adenosine may be used for selective stimulation and assessment of CB activity. The trial is registered at ClinicalTrials.gov NCT01939912.
Keywords: adenosine, carotid body, chemoreflex
Key points
In humans, excitation of peripheral chemoreceptors with systemic hypoxia causes hyperventilation, hypertension and tachycardia. However, the contribution of particular chemosensory areas (carotid vs. aortic bodies) to this response is unclear.
We showed that selective stimulation of the carotid body by the injection of adenosine into the carotid artery causes a dose‐dependent increase in minute ventilation and blood pressure with a concomitant decrease in heart rate in conscious humans. The ventilatory response was abolished and the haemodynamic response was diminished following carotid body ablation.
We found that the magnitude of adenosine evoked responses in minute ventilation and blood pressure was analogous to the responses evoked by hypoxia. By contrast, opposing heart rate responses were evoked by adenosine (bradycardia) vs. hypoxia (tachycardia).
Intra‐carotid adenosine administration may provide a novel method for perioperative assessment of the effectiveness of carotid body ablation, which has been recently proposed as a treatment strategy for sympathetically‐mediated diseases.
Abbreviations
- BP
arterial blood pressure
- BR
breathing rate
- CA
carotid angiography
- CAS
carotid artery stenting
- CB
carotid body
- DBP
diastolic arterial blood pressure
- HR
heart rate
- HRR
hypoxic heart rate response
- HVR
hypoxic ventilatory response
- MAP
mean arterial blood pressure
- PCh
peripheral chemoreceptors
- r2
coefficient of determination
- SBP
systolic arterial blood pressure
- SBPR
hypoxic systolic arterial blood pressure response
- SpO2
blood oxygen saturation
- VI
minute ventilation
- VT
tidal volume
Introduction
Transient hypoxia is known to cause increases in ventilation, blood pressure and heart rate in both human and animal models (O'Regan & Majcherczyk, 1982). This pattern of response is mediated by excitation of peripheral chemoreceptors (PCh), which activates a medullary cardiorespiratory reflex circuit driving autonomic and respiratory motor outputs (Timmers et al. 2003). The primary PCh response to hypoxia consisting of hyperpnoea, bradycardia and a pressor response is modulated by secondary activation of other autonomic reflexes such as the Hering–Breuer reflex, arterial baroreflex and by a direct action of hypoxia on the vasculature (Daly & Scott, 1963; Heistad & Abboud, 1980; Marshall, 1994) and includes hypopnoea, tachycardia and a reduction in vascular resistance.
PCh in humans are located mainly in the carotid (CBs) and aortic bodies. Although the contribution of the isolated carotid body to the cardiovascular‐respiratory response evoked by systemic hypoxia is well described in animals (Daly & Scott, 1958, 1963), not much is known about its function in humans. Previously, we showed that bilateral excision of the CBs, performed as a treatment for congestive heart failure, almost abolished the ventilatory response and reduced the pressor response to systemic hypoxia, whereas the tachycardia persisted (Niewinski et al. 2014 a). These data suggest that, in humans, excitation of various chemosensory areas evoke contrasting primary physiological responses.
Recent studies suggest that overactive PCh play a major role in the pathogenesis of sympathetically‐mediated diseases (Abdala et al. 2012; Ribeiro et al. 2013; Schultz et al. 2013). Unilateral or bilateral CB inactivation has been proposed as a treatment of hypertension, heart failure and diabetes (Del Rio et al. 2013; Niewinski et al. 2013 b; Paton et al. 2013; Ribeiro et al. 2013). Although the physiological effect of CB excision in humans with heart failure has been reported (Niewinski et al. 2013 b; Niewinski et al. 2014 a), the response to its selective activation in conscious humans with less severe comorbidities is unknown. Evoking such a response would have important clinical implications for patients under consideration for CB modulation therapy as it would: (1) provide a pre‐procedural assessment of the functional integrity of either left or right CB in isolation from the contralateral CB and the aortic bodies (Niewinski, 2014 b); (2) indicate whether there is a dominant CB on one side; and (3) demonstrate procedural efficacy of unilateral CB ablation postoperatively through an assessment of the magnitude of CB evoked reflex responses.
Recently, it has been reported that experimental blockade of adenosine receptors inhibited CB activity as measured by a reduction in carotid sinus nerve activity in response to hypoxia (Sacramento et al. 2015). Furthermore, adenosine‐mediated signalling has an excitatory impact on CB sensitivity to hypercapnia (Holmes et al. 2015), thus placing adenosine as a key player in chemotransduction. However, the relationship between the magnitude of the hypoxia‐induced and adenosine‐induced responses has not been tested so far. Adenosine has an ultra‐short plasma half‐time (Sollevi, 1986; Moser et al. 1989) and is unable to cross the blood–brain barrier (Isakovic et al. 2004). It is also safe and widely used in cardiology. These characteristics make it an ideal stimulatory molecule for transient, selective activation of the CB in humans.
Thus, we performed the single‐blinded, randomized, dose‐ranging study using adenosine injected directly into a common carotid artery (i.c.) to: (1) determine whether such an approach is safe in humans; (2) reveal the ventilatory and haemodynamic effects of unilateral, selective CB stimulation in conscious humans; and (3) assess the potential relationship between hypoxic PCh stimulation and CB excitation with adenosine. Moreover we tested the hypothesis that i.c. adenosine may be used for the perioperative assessment of the effectiveness of carotid body ablation.
Methods
Studied population
After obtaining approval from local Ethics Committee (Komisja Bioetyczna, Wroclaw Medical University) consecutive patients with significant unilateral internal carotid artery stenosis, referred for carotid artery angiography (CA) or carotid artery stenting (CAS) were invited to enter the study. The subjects who were excluded included those with significant bilateral lesions of common/internal carotid arteries, those with impaired left ventricular ejection fraction, those suffering from symptomatic pulmonary disease, post stroke/transient ischaemic attack in last 6 months or acute coronary syndrome in the last 3 months, and all those presenting contraindications to adenosine administration. From 107 patients screened, the study group finally consisted of 11 subjects (nine men, two women) (mean ± SD age: 66 ± 5 years). Within that group, 10 patients had hypertension, two had diabetes and eight had coronary artery disease. Detailed information about subjects’ demographic data and rest parameters are shown in Table 1. Additionally, four healthy male subjects (mean ± SD age: 30 ± 1 years) were invited to the study as a control group to test the effects of i.v. adenosine administered in maximal dose used in the study. All subjects provided their informed consent. The study was performed in accordance with the latest review of the Helsinki Declaration.
Table 1.
Demographic data and rest values of measured parameters
| Subject number | Sex | Age (years) | Body mass index (kg m–2) | Office SBP/DBP (mmHg)a | Rest VI (l min−1)b | Rest HR (beats min–1)b | Rest SBP (mmHg)b | Rest DBP (mmHg)b | Rest MAP (mmHg)b |
|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 61 | 22.8 | 120/69 | 5.8 ± 1 | 76.9 ± 2 | 185 ± 6 | 102 ± 4 | 140 ± 4 |
| 2 | M | 73 | 24.9 | 132/65 | 7.8 ± 2 | 46.4 ± 3 | 138 ± 14 | 68 ± 5 | 87 ± 8 |
| 3 | F | 68 | 34.8 | 136/67 | 15.2 ± 4 | 79.1 ± 7 | 161 ± 11 | 71 ± 3 | 105 ± 6 |
| 4 | M | 65 | 26.8 | 113/62 | 5.2 ± 2 | 77 ± 4 | 184 ± 5 | 89 ± 3 | 127 ± 4 |
| 5 | M | 56 | 31.1 | 155/88 | 11.4 ± 2 | 52.1 ± 4 | 158 ± 16 | 77 ± 14 | 107 ± 12 |
| 6 | M | 72 | 27.6 | 160/86 | 17.3 ± 5 | 96.8 ± 2 | 167 ± 7 | 88 ± 3 | 117 ± 6 |
| 7 | M | 64 | 31.8 | 134/60 | 13.7 ± 1 | 58 ± 2 | 145 ± 4 | 63 ± 2 | 90 ± 3 |
| 8 | M | 71 | 24.2 | 126/71 | 9.8 ± 2 | 67.8 ± 3 | 167 ± 9 | 80 ± 4 | 107 ± 6 |
| 9 | M | 66 | 24.3 | 156/85 | 10.8 ± 3 | 82.8 ± 3 | 192 ± 7 | 98 ± 5 | 131 ± 1 |
| 10 | M | 65 | 21.1 | 138/67 | 10.1 ± 1 | 80 ± 1 | 146 ± 3 | 76 ± 2 | 104 ± 3 |
| 11 | M | 61 | 22 | 155/63 | 6.8 ± 1.1 | 58.1 ± 9 | 160 ± 8 | 82 ± 7 | 109 ± 7 |
Data are presented as the mean ± SD. M, male; F, female.
aOffice arterial blood pressure on admission to the hospital.
bMean values from the 4 min prior to the first adenosine injection.
Study protocol
Twenty‐four hours preceding the study, participants were asked to discontinue all cardiovascular drugs, including anti‐hypertensives and β‐blockers, and to avoid caffeine intake. All patients were fasted for 6 h prior to testing. On the first study day, all subjects underwent standard peripheral chemosensitivity testing using a transient hypoxia method (Chua & Coats, 1995). Unilateral, direct CB stimulation with adenosine was performed during the CA/CAS procedure on the next day. In controls, only the test with adenosine administered i.v. was performed.
Measurements
The same equipment was used during hypoxic chemosensitivity testing, assessment of the response to i.v. adenosine and direct unilateral CB stimulation with adenosine. Subjects were examined in the supine position using a one‐way open breathing circuit (Hans Rudolph, Inc., Shawnee, KS, USA). The inspiratory arm of the circuit was connected to a high‐pressure electric valve, which allowed switching between 100% nitrogen and room air in a silent manner. The expiratory arm was connected via a 1000 L min−1 flowhead (MLT3000L; ADInstruments, Sydney, Australia) to a differential pressure transducer (FE141 Spirometer; ADInstruments) for the measurement of breathing rate (BR), tidal volume (VT) and minute ventilation (VI). The haemodynamic parameters measured included heart rate (HR) and arterial blood pressure (BP), which were monitored non‐invasively, beat‐by‐beat, using a Nexfin device (BMEYE BV, Amsterdam, The Netherlands). Blood oxygen saturation (SpO2) was evaluated using a pulse oximeter (Radical‐7; Masimo Corporation Irvine, CA, USA) with an ear clip. All data were collected at a sampling rate of 1 kHz (16‐bit resolution) using PowerLab 16/30 (ADInstruments) and recorded on a laptop computer (Dell Inc., Round Rock, TX, USA).
Evaluation of the effects of direct unilateral carotid body stimulation with adenosine
Direct unilateral CB stimulation with adenosine was performed under normoxic conditions during CA/CAS. Before the procedure, all subjects underwent carotid ultrasound, which allowed for pre‐procedural selection of the investigated side. Adenosine was injected only on the side without a haemodynamically significant lesion. In all subjects, femoral access was used to perform the procedure. After carotid artery angiography, which confirmed lack of significant atheromas on pre‐procedurally selected side, and before stenting, in patients qualified for CAS, the tip of angiographic catheter was positioned 2 cm below the bifurcation of the common carotid artery. After a 5 min rest control period, 5 ml bolus injections containing various doses of adenosine or placebo (0.9% normal saline solution) warmed to 36o C were administered via the catheter in a single‐blinded order. Adenosine boluses (Adenocor; Sanofi‐Aventis, Paris, France; diluted with 0.9% normal saline solution) were prepared prior to the experiment in sterile conditions and comprised doses of: 4, 8, 16, 32, 64, 128, 256, 384 and 512 μg. After each bolus, subjects were allowed to rest until the measured parameters returned to baseline levels. For safety reasons, the total duration of the study did not exceed 30 min. To determine the effects of bolus injections of adenosine or placebo VI, VT, BR, HR, systolic BP (SBP), diastolic BP (DBP) and mean arterial blood pressure (MAP) were averaged from 80 s prior to each administration (i.e. baseline values). The response to adenosine was defined in two ways ‐ as the absolute change in measured parameters between baseline values and: either (1) the mean values from 20 s after adenosine injection (i.e. twice the half‐life time of adenosine in humans) or (2) the maximal or minimal values from 20 s following adenosine administration. Additionally, because of the biphasic character of the BP response (Biaggioni et al. 1987), we also analysed a subsequent 20 s epoch for mean changes in measured parameters.
The latency of the response to CB stimulation was defined as the time from the beginning of the injection to: (1) the peak of the first breath with VT greater than 125% of the preceding breath (ventilatory response onset) and (2) the point at the timeline when the trend to rise or fall in BP or HR appears, as determined independently by two researchers (BP response onset and HR response onset, respectively). Described latencies consist of: ‘methodological’ delay related to the dead space in the catheter lumen (estimated to be near 1 s) and ‘physiological’ delay related to the duration of the reflex arc.
In one of the study subjects, adenosine (in doses of 384 and 512 μg) was administered 5–10 min before and after unilateral carotid body ablation. Before the procedure standard CA was carried out to visualize the anatomy of the arteries. A bipolar, Y‐shape investigational ablation catheter system (CIBIEM, Los Altos, CA, USA; NCT02099851) was inserted into common carotid artery bifurcation via femoral access and tissues in the space between external and internal carotid arteries were destroyed using radiofrequency power (location of the CB in this area was confirmed by computed tomography prior to the procedure). Subsequent to the ablation, angiography was repeated to confirm the integrity of carotid vessels.
Assessment of individual peripheral chemosensitivity to adenosine
In each subject, three to seven doses of adenosine were administered; the exact number administered depended on individual tolerability and total duration of the responses and recovery. The ventilatory response to adenosine was calculated as the average of the three largest consecutive breaths following adenosine administration. A ventilatory response to adenosine was calculated as the slope of the linear regression between the doses of adenosine and the evoked VI responses. Only recordings with at least three successful adenosine administrations and coefficient of determination (r 2) ≥ 0.75 were used for further analysis.
Evaluation of the effects of i.v. adenosine injections
In four healthy subjects breathing room air, an additional test was performed to assess the potential systemic effects of the maximal adenosine dose used in the study. Via the cannula placed into basilic vein, a series of adenosine bolus injections (Adenocor diluted with 0.9% normal saline solution, dose 512 μg, volume 5ml) or placebo (0.9% normal saline solution, volume 5ml) was administered in randomized order and flushed immediately with 10 ml of normal saline solution to reduce the circulatory delay. Subsequent administrations were separated by 3–5 min of rest recording. To determine the effects of i.v. adenosine, VI, VT, BR, HR, SBP, DBP and MAP were averaged from 80 s prior to each administration (i.e. baseline values). As Biaggioni et al. (1987) observed that onset of the response to i.v. adenosine occurs 20–30 s after the administration, we decided to compare baseline values with mean values of measured parameters from three consecutive 20 s time periods after adenosine injection: (1) immediately after the adenosine injection; (2) between 20 and 40 s after adenosine injection; and (3) between 40 and 60 s after adenosine injection. The absolute change in measured parameters between baseline values and 20 s time period was defined as the response to i.v. adenosine.
Assessment of individual peripheral chemosensitivity to hypoxia (HVR)
We employed an established method for assessing the sensitivity of PCh to intermittent hypoxia (i.e. the hypoxic HVR; Niewinski et al. 2013 a). Subjects resting in supine position, breathing room air, were silently switched to breathing with 100% nitrogen gas for 10–35 s, which resulted in a decrease in SpO2 to 90–65%. Hypoxic exposures of randomized lengths were repeated five to eight times per test. After each administration of nitrogen, subjects were allowed to rest until measured parameters had returned to baseline levels. Each ventilatory response was calculated as an average of the three largest consecutive breaths following nitrogen administration. HVR was expressed as the slope of the linear regression describing the relationship between the single ventilatory responses and the associated nadirs of SpO2, including the baseline values of VI and SpO2. Slopes with a coefficient of determination (r 2) < 0.75 were considered inaccurate and excluded from further analysis.
Assessment of individual haemodynamic response to hypoxia
The HR and SBP responses to acute hypoxia were assessed simultaneously with the HVR by associating the peak HR and peak SBP with the nadir of SpO2 following each hypoxic exposure with the use of a linear regression similar to the HVR calculation (Niewinski et al. 2013 a). Prior to the slope assessment, haemodynamic data were smoothed by taking a moving average based on a 3 s moving window and then a weighted average of a 200 ms window centered on each data point. The second technique was achieved by convolving the original signal with a Gaussian filter built from 201 sample points with a variance of 100. Linear regression of HR and SBP slopes (i.e. HRR and SBPR, respectively) reflected the magnitude of the haemodynamic responses to acute hypoxia.
Evaluation of the effects of systemic hypoxia
The ventilatory and haemodynamic responses to non‐selective peripheral chemoreceptors stimulation with systemic hypoxia were assessed during HVR testing. Briefly, the absolute change in measured parameters between baseline values (80 s period prior to each hypoxic exposure) and (1) the mean values from 20 s after the nadir SpO2 or (2) mean values from a subsequent 20 s epoch (20–40 s post nadir SpO2) were calculated for each hypoxic exposure.
Statistical analysis
Statistica, version 12 (StatSoft Inc., Tulsa, OK, USA), LabChart 7 Pro (ADInstruments) and MATLAB (MathWorks, Natick, MA, USA) were used to analyse the data. The distribution of the variables was tested using Shapiro–Wilk's W test. The statistical comparisons were evaluated using Wilcoxon matched pairs test for non‐normally distributed variables (HVR; HRR; SBPR; latency of onset of HR response; individual peripheral chemosensitivity to adenosine; the following components of the response to i.c. adenosine: mean VI, mean VT, mean SBP, peak VI, peak MAP, peak SBP; the following components of the response to i.v. adenosine: VI, VT, SBP, DBP and MAP; the following components of the response to hypoxia: VT, HR, MAP, DBP) and with Student's t test for normally distributed variables (latency of onset of VI response; latency of onset of SBP response; the following components of the response to i.c. adenosine: mean BR, mean HR, mean MAP, mean DBP, peak HR; the following components of the response to i.v. adenosine: BR, HR; the following components of the response to hypoxia: VI, BR, SBP). Data are reported as the mean ± SEM. The correlations were calculated with Spearman's rank. P < 0.05 was considered statistically significant.
Results
Adverse effects of adenosine injections
No serious adverse events were observed with intra‐common carotid artery injections of adenosine. One subject with high individual peripheral chemosensitivity to adenosine and high HVR reported dyspnoea after a dose of 128 μg (no higher doses were administered), which resolved within 30 s. Another patient reported a headache, which was not time‐related to the injection of adenosine and abated spontaneously after the end of the procedure; it remains equivocal as to whether this was related to the adenosine injections. No other complaints were reported by the participants during the present study.
Effects of intra‐common carotid artery adenosine bolus injection
In total, 56 bolus injections containing placebo (n = 8) or adenosine (n = 48) in fixed doses of 4, 8, 16, 32, 64, 128, 256 or 512 μg were administered. Six administrations were excluded from the analysis as a result of artefacts (caused by cough, speaking or Nexfin cuff oscillations) during the pre‐ or post‐injection period. A typical response to i.c. adenosine bolus injection is shown in Fig. 1.
Figure 1. Typical ventilatory and haemodynamic responses to the stimuli .
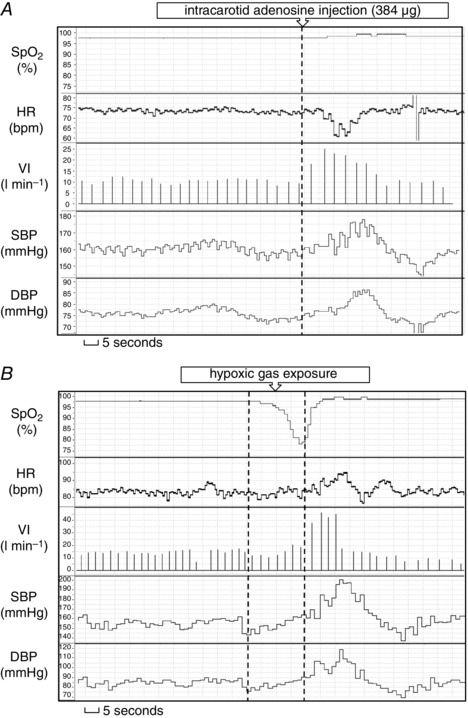
Typical ventilatory and haemodynamic responses to intra‐carotid adenosine injection (A) and transient systemic hypoxia (B).
Figure 2 illustrates the mean values from analysed time periods. Administration of adenosine induced significant increase in VI (6.4 ± 0.6 l min−1; P < 0.01) relative to the baseline, which was not seen with placebo (0.18 ± 0.15 l min−1; P = 0.33). Augmented VI was the result of raised VT (0.47 ± 0.05 l; P < 0.01) with a paradoxically diminished BR (–0.96 ± 0.34 breaths min−1; P < 0.01). Concomitantly, there was a transient decrease in HR after adenosine injection (–2.03 ± 0.44 beats min–1; P < 0.01), which was not seen following placebo (0.64 ± 0.37 beats min–1; P = 0.13). The administration of adenosine also caused an increase in MAP (2.68 ± 1 mmHg; P = 0.01) and SBP (3.81 ± 1.2 mmHg; P < 0.01). Such an effect was not observed after placebo (–0.24 ± 1 mmHg; P = 0.72 and 0.48 ± 1 mmHg; P = 0.78 for MAP and SBP, respectively). DBP was influenced neither by adenosine (1.07 ± 0.69 mmHg; P = 0.13), nor placebo (–1.55 ± 1.6 mmHg; P = 0.37).
Figure 2. Ventilatory and haemodynamic parameters across analysed time periods .
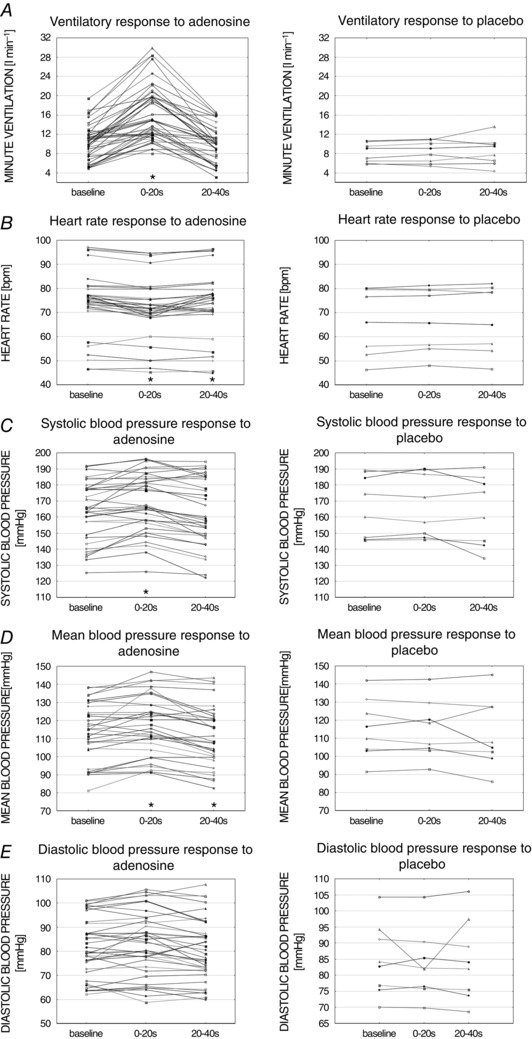
Mean values of ventilatory (A) and haemodynamic (B–E) parameters across time periods for all intra‐carotid adenosine (left) or placebo (right) administration. * P < 0.05 vs. baseline.
Between 20 and 40 s after adenosine injection (Fig. 2), a small but statistically significant fall in MAP (–2.17 ± 1 mmHg; P = 0.04) and HR (–0.78 ± 0.3 beats min–1; P = 0.01) was found compared to baseline values. There was no significant change compared to baseline for described time period in either VI (0.07 ± 0.29 l min−1; P = 0.81), SBP (–1.46 ± 1.5 mmHg; P = 0.27) and DBP (–1.09 ± 0.76 mmHg; P = 0.16).
When peak responses were analysed, adenosine administration increased VI, MAP and SBP, and decreased HR, compared to baseline. Minimal and maximal values of measured parameters after adenosine injections were significantly different from the placebo evoked responses (Fig. 3) (all P < 0.05).
Figure 3. Absolute changes between baseline values and maximal/minimal values following adenosine and placebo injections .
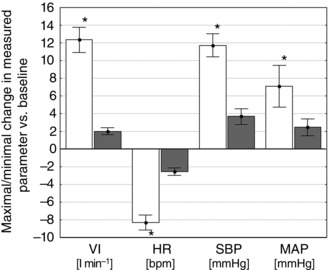
Comparison of the absolute changes between baseline values and maximal/minimal values from 20 s after adenosine (open columns) and placebo (shaded columns) injections (across all patient and doses). Data are presented as the mean ± SEM. * P < 0.05 vs. placebo.
Latency of onset of the response to adenosine stimulation of a carotid body
The latency of onset of the ventilatory response to adenosine was 5.69 ± 0.3 s. For haemodynamic variables, the latency was 7.15 ± 0.48 s and 4.4 ± 0.31 s for SBP and HR, respectively.
Dose‐dependence of the response to adenosine
Figure 4 shows the magnitude of VI and HR responses correlated linearly with the dose of adenosine used (r = 0.7; P < 0.01 and r = 0.61; P < 0.01 for mean and maximal VI, respectively; r = −0.41; P < 0.01 and r = −0.57; P < 0.01 for mean and minimal HR, respectively). SBP and MAP responses were also dose‐dependent. However, the correlations reached statistical significance for maximal but not for mean BP values (r = 0.26; P = 0.09 and r = 0.39; P = 0.01 for mean and maximal SBP, respectively; r = 0.2; P = 0.18 and r = 0.33; P = 0.03 for mean and maximal MAP, respectively). Generally, at all doses, the patterns of the respiratory (VT and BR) and haemodynamic (HR and SBP) responses were qualitatively similar.
Figure 4. Dose dependence of the ventilatory and haemodynamic responses evoked by adenosine injections administered into a common carotid artery .
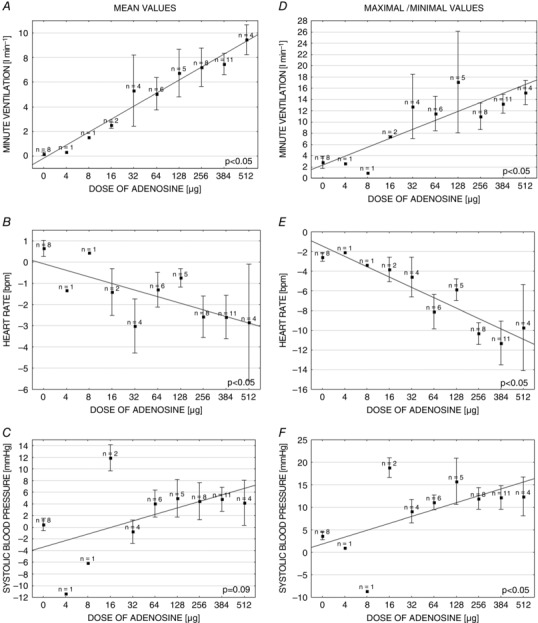
Left: absolute changes in measured parameters between baseline values and mean values from 20 s after adenosine injection in relation to the adenosine doses (A–C). Right: absolute changes in measured parameters between baseline values and maximal/minimal values from 20 s after adenosine injection in relation to the adenosine doses (D–F). Data are presented as the mean ± SEM.
Individual variability in the response to adenosine
The magnitude of the response to particular doses of adenosine differed between patients and depended on the individuals’ sensitivity to hypoxia. Generally, the mean increase in VI following adenosine administration was more exaggerated in individuals with higher HVR (r = 0.47; P < 0.01). Similarly, higher SBPR predicted greater mean increase in SBP following adenosine boluses (r = 0.34; P = 0.04). Interestingly, in subjects with high HRR (more exaggerated increase in HR following hypoxic exposure), the mean decrease in HR after adenosine injections was less pronounced (r = 0.39; P = 0.02). There was no correlation between HVR and either HR or SBP responses to adenosine (r = −0.22; P = 0.19 and r = 0.11; P = 0.5, respectively).
Individual peripheral chemosensitivity to adenosine was calculated in eighth of 11 subjects. In two cases, the assessment was not possible as a result of a low number of successful adenosine administrations and, in one case, r 2 was less than 0.75. Individual peripheral chemosensitivity to adenosine across this group was 0.078 ± 0.03 l min−1 μg−1 and correlated linearly with HVR (r = 0.81; P = 0.01). Detailed information about the ventilatory response to adenosine in individual subjects is provided in Table 2.
Table 2.
Individual responses to hypoxia and to intra‐carotid adenosine administration
| Subject number | Peripheral chemosensitivity to hypoxia (l min−1 SpO2 −1) | Heart rate response to hypoxia (beats min–1 SpO2 −1) | Systolic blood pressure response to hypoxia (mmHg SpO2 −1) | Peripheral chemosensitivity to adenosine (l min−1 μg−1) |
|---|---|---|---|---|
| 1 | 0.470 | 0.170 | 1.699 | 0.021 |
| 2 | 0.312 | 0.038 | 0.779 | –b |
| 3 | 0.760 | 1.781 | 0.663 | 0.045 |
| 4 | 0.572 | 0.620 | 0.397 | 0.029 |
| 5 | 0.655 | 0.171 | 0.879 | 0.107 |
| 6 | 2.329 | 0.317 | 1.502 | 0.276 |
| 7 | –a | –a | –a | –b |
| 8 | 0.478 | 0.480 | 0.641 | 0.030 |
| 9 | 0.943 | 0.712 | 2.302 | 0.067 |
| 10 | 0.963 | 0.007 | 1.182 | 0.047 |
| 11 | 1.249 | 0.736 | 1.537 | –a |
aNot calculated due to low r 2 (<0.75).
bNot calculated due to low number of successful administrations of adenosine.
There was no relationship between adenosine induced VI, HR and SBP responses and baseline VI, HR and SBP values (all P > 0.05).
Response to adenosine after carotid body ablation
Unilateral CB ablation in a single patient abolished the ventilatory response to adenosine. Adenosine injected i.c. prior to the procedure caused an averaged increase in mean VI of 7.5 l min−1 (54.6% of baseline ventilation), which was suppressed dramatically after the ablation (0.72 l min−1 or 6.3% of baseline ventilation) (Fig. 5). The responses of haemodynamic parameters to adenosine were also smaller following CB ablation (mean change in MAP 5.71 mmHg vs. 0.37 mmHg; SBP 3.82 mmHg vs. −0.74 mmHg; HR 3.16 beats min–1 vs. 1.3 beats min–1, for pre‐ and post‐procedural administrations, respectively). However, a relatively low number of adenosine injections and periprocedural usage of propofol and fentanyl should be taken into account when haemodynamic data are considered.
Figure 5. Carotid body ablation and the ventilatory response to adenosine .
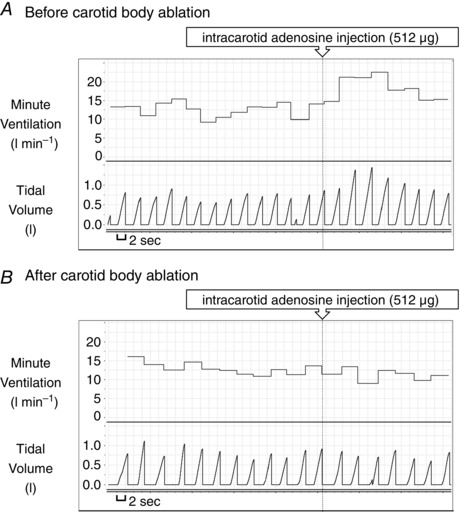
Ventilatory response to intra‐carotid injection of adenosine before (A) and after (B) carotid body ablation in the same patient.
Effects of i.v. adenosine injections
In total, 24 i.v. injections of adenosine and 18 injections of placebo were administered. i.v. adenosine caused no significant change across measured parameters in the 20 s immediately after the bolus injection, between 20 and 40 s and between 40 and 60 s following the administration comparing to baseline values (all P > 0.05). Similarly, placebo injections had no influence on measured parameters (all P > 0.05). Mean values of measured parameters are shown in Table 3.
Table 3.
Mean values of measured parameters during baseline and subsequent 20 s periods following i.v. adenosine (512 μg) and placebo bolus injections
| Measured parameter | Baseline values | 20 s after bolus injection | Between 20 and 40 s | Between 40 and 60 s |
|---|---|---|---|---|
| Adenosine (n = 24) | ||||
| VI (l min−1) | 7.6 ± 0.5 | 7.5 ± 0.5 | 7.5 ± 0.5 | 7.7 ± 0.4 |
| VT (l) | 0.58 ± 0.03 | 0.56 ± 0.03 | 0.57 ± 0.03 | 0.57 ± 0.03 |
| BR (breaths min−1) | 13.3 ± 0.7 | 13.5 ± 0.8 | 13.5 ± 0.7 | 13.7 ± 0.7 |
| HR (beats min–1) | 64.8 ± 0.8 | 64 ± 0.8 | 64.1 ± 0.8 | 63.8 ± 0.8 |
| SBP (mmHg) | 144.9 ± 3.4 | 144.8 ± 3.2 | 145.6 ± 3.3 | 145.4 ± 3.6 |
| DBP (mmHg) | 83.1 ± 3 | 82.8 ± 2.9 | 83.1 ± 3 | 83.1 ± 3.1 |
| MAP (mmHg) | 105.7 ± 3.4 | 105.3 ± 3.2 | 105.7 ± 3.3 | 105.8 ± 3.5 |
| Placebo (n = 18) | ||||
| VI (l min−1) | 8.4 ± 0.5 | 8.2 ± 0.4 | 8.2 ± 0.5 | 8.1 ± 0.5 |
| VT (l) | 0.66 ± 0.05 | 0.63 ± 0.05 | 0.62 ± 0.05 | 0.65 ± 0.06 |
| BR (breaths min−1) | 13.5 ± 0.7 | 13.6 ± 0.8 | 13.7 ± 0.8 | 13.4 ± 0.9 |
| HR (beats min–1) | 65 ± 1.3 | 64.2 ± 1.2 | 64.5 ± 1.1 | 64.9 ± 1.1 |
| SBP (mmHg) | 145.6 ± 4.6 | 145.8 ± 4.6 | 146.9 ± 4.5 | 147.4 ± 4.9 |
| DBP (mmHg) | 84.2 ± 3.9 | 84.3 ± 3.8 | 85 ± 3.8 | 85 ± 4 |
| MAP (mmHg) | 106.4 ± 4.4 | 106.3 ± 4.4 | 107.4 ± 4.3 | 107.8 ± 4.6 |
Data are presented as the mean ± SEM.
All P > 0.05 vs. baseline.
Effects of systemic hypoxia and individual variability in the response to hypoxia
In total, 60 hypoxic gas exposures were performed of which seven were excluded from the analysis because of a lack of target desaturation or artefacts occurring during pre‐ or post‐hypoxia period. A typical response to systemic hypoxia is shown in Fig. 1.
Hypoxic gas exposures caused mean drop in SpO2 of −13.6 ± 0.74% and were followed by a significant increase in VI (8.1 ± 0.8 l min−1; P < 0.01), HR (3.19 ± 0.53 beats min–1; P < 0.01), SBP (11.3 ± 1.2 mmHg; P < 0.01), DBP (5.03 ± 0.77 mmHg; P < 0.01) and MAP (7.26 ± 0.9 mmHg; P < 0.01). Hyperventilation was the result of an increase in VT (0.56 ± 0.05 l; P < 0.01) with no significant change in BR (0.2 ± 0.35 breaths min−1; P = 0.58).
Analysis of mean values of measured parameters from the subsequent 20 s epoch (from 20 to 40 s following the lowest SpO2 level) revealed significantly lower SBP (−2.8 ± 1.1 mmHg; P = 0.01), DBP (−2.3 ± 0.5 mmHg; P < 0.01) and MAP (−2.7 ± 0.65 mmHg; P < 0.01) and elevated HR (0.98 ± 0.4 beats min–1; P < 0.01) compared to baseline values. Mean values of VI, VT, BR were comparable to baseline values (all P > 0.05).
Assessment of ventilatory (HVR) and haemodynamic responses (HRR, SBPR) to hypoxia was performed in all individuals but one, in whom r 2 was less than 0.75. In the study group, mean HVR was 0.87 ± 0.18 l min−1 SpO2 −1, mean HRR was 0.5 ± 0.17 beats min–1 SpO2 −1 and mean SBPR was 1.16 ± 0.19 mmHg SpO2 −1. Detailed information about the hypoxic responses in individual subjects is provided in Table 2.
There was no correlation between rest VI, rest HR, rest SBP and HVR, HRR or SBPR (all P > 0.05).
Discussion
For the first time, we have described the respiratory and cardiovascular responses to selective unilateral CB stimulation in conscious humans. There are several novel findings of the present study: (1) CB function testing employing intra‐carotid artery adenosine bolus injections is safe and well tolerated in conscious humans; (2) selective stimulation of the CB in conscious humans leads to an increase in VI, SBP and MAP, and decrease in HR; (3) the response to adenosine is dose‐dependent; and (4) the magnitude of individual chemosensitivity to adenosine is directly related to the level of chemosensitivity to hypoxia. Also, for the first time, we used i.c. adenosine injections for the periprocedural CB activity testing, which may provide a novel method for the acute assessment of the effectiveness of carotid body deactivation.
Adenosine as a carotid body stimulant in humans
In animals, adenosine is a neurotransmitter released from type I cells of PCh in response to hypoxia (Prabhakar, 2000; Conde et al. 2012). Exogenous adenosine has been shown to stimulate CBs in animals (McQueen & Ribeiro, 1983; Monteiro & Ribeiro, 1987). Biaggioni et al. (1987) and Watt et al. (1987) suggested a similar role for adenosine in PCh physiology in humans. They infused adenosine directly into the aorta and observed various ventilatory and haemodynamic changes, the direction of which were dependent on the location of the tip of the catheter. When the catheter tip was placed in the ascending aorta, proximally to the branches of the aortic arch, adenosine injections caused hyperventilation but, when it was located in the descending aorta, distally to carotid arteries, no change in VI was recorded. The present study confirms the excitatory effect of adenosine on the CB in several ways: (1) the effects of CB stimulation were dose‐dependent; (2) the response occurred a few seconds after the injection, whereas the onset of the response to i.v. injection is observed after 20–30 s (Watt & Routledge, 1985; Biaggioni et al. 1987); (3) the individual peripheral chemosensitivity to adenosine correlated with the HVR; and (4) the reflex responses to adenosine were abolished following ipsilateral CB ablation in one patient.
In conscious humans, adenosine administered i.v. or into the ascending aorta causes hyperventilation; however, the changes in haemodynamics are not clear. Bradycardia was reported when adenosine was administered i.v. and tachycardia was found with no change in BP during intra‐aortic administration (Watt & Routledge, 1985; Watt et al. 1987). By contrast, Biaggioni et al. (1987) showed increases in HR and BP in both cases. The differences in these cardiovascular responses may result from the direct vasodilatatory (Collis, 1989) and/or direct negative chronotropic effects (Belardinelli et al. 1989) of systematically administered adenosine; or from activation of modulatory mechanisms secondary to hyperventilation (e.g. the Hering–Breuer reflex) (Daly & Scott, 1963). Furthermore, aortic body co‐activation should be taken into consideration with systemic administrations because adenosine was found to increase activity of these PCh in the cat (Runold et al. 1990).
To evaluate the response of the CB selectively, we injected low‐doses of adenosine directly into the common carotid artery, using the angiographic catheter located 2 cm below its bifurcation to prevent potential backflow of the chemical into the aortic arch. This, together with ultra‐short plasma half‐time of adenosine (reported to be less than 10 s) (Klabunde, 1983; Sollevi, 1986), suggests that concurrent aortic bodies stimulation and direct cardiovascular actions are unlikely. Selectivity of the CB stimulation was further confirmed by i.v. injections of the maximal dose of adenosine used in the present study, which had no effect on any of the measured parameters. This experiment rules out the possibility that changes observed following intra‐carotid adenosine administration are the result of the stimulation of other pathways, in particular those originating from the pulmonary afferents. Central nervous system effects of adenosine are also unlikely because it does not cross brain–blood barrier (Isakovic et al. 2004). Enzymes located within endothelial cells that form an enzymatic brain–blood barrier metabolize almost 90% of adenosine infused into the internal carotid artery; this would further prohibit potential central nervous system actions and possible recirculation of adenosine injected into a common carotid artery (Pardridge et al. 1994). Moreover, almost 50% of the drug administered into the common carotid artery will flow into the external carotid artery and never reach the cerebral circulation.
Selective carotid body stimulation
Heart rate response
The cardiovascular response to hypoxia or adenosine depends on an interplay between the: (1) primary response of PCh excitation; (2) secondary modulatory mechanisms of pulmonary stretch receptors and the arterial baroreflex (recruited by the primary response), which can diminish or even reverse the primary response (Daly & Scott, 1963; Heistad & Abboud, 1980; O'Regan & Majcherczyk, 1982; Marshall, 1994); and (3) direct actions of the stimuli on the vasculature, heart and central nervous system (e.g. pressor effect of hypoxia, negative chronotropic effects of adenosine on sinus node).
To our knowledge, there are no published data avalable regarding the effects of selective CB stimulation in humans; however, such investigations have been conducted in animals. Isolated stimulation of CB employing perfusion of carotid arteries with hypoxic blood in anaesthetized, artificially ventilated dogs resulted in an increase in BP and a decrease in HR (Daly & Scott, 1958, 1963). Artificial ventilation under conditions of neuromuscular blockade allowed investigators to disentangle one of the secondary mechanisms: the pulmonary stretch receptor reflex, which decreases vascular resistance (Daly & Scott, 1963) and increases HR via central vagal inhibition in response to increased VT (Paintal, 1973), thereby reducing the primary PCh reflex haemodynamic response. In spontaneously breathing dogs, hyperventilation was observed; however, HR and BP responses varied between animals and probably depended on the degree of pulmonary stretch receptor activation and thus the magnitude of the hyperventilation (Daly & Scott, 1958, 1963).
In the present study in conscious, spontaneously breathing humans, a significant decrease in HR was observed after selective CB stimulation with adenosine. Nevertheless, the magnitude of the HR response may be underestimated because of concurrent activation of the secondary modulatory mechanisms described above. Despite opposing effects of the pulmonary stretch receptors on the bradycardia evoked by selective CB stimulation, we observed higher magnitude bradycardia (the lower nadir HR) that was associated with higher VI responses (peak VI) to adenosine (r = −0.34; P = 0.04). This association was evident for peak VT (r = −0.35; P = 0.03) but not for peak BR (r = −0.06; P = 0.7), consistent with the increase in VT but not BR during unilateral CB stimulation. The negative chronotropic effect of selective CB stimulation predominated over any positive chronotropic effect evoked from pulmonary stretch receptors; based on subject HVR values, this was most probably assisted by the CB hyperreflexia observed in these patients.
By contrast to the effects of selective CB activation, systemic hypoxia or i.v. adenosine infusion causes an increase in HR, which may not be attributed to Hering–Breuer reflex activation only. As reported previously, bilateral excision of CBs almost abolished VI and BP responses to hypoxia; however, despite a lack of pulmonary stretch receptors stimulation (secondary to the CB evoked hyperventilation), the increase in HR following hypoxic exposure remained unchanged (Niewinski et al. 2014 a). Thus, we suggest that this tachycardic response is, at least partially, related to activation of other primary chemosensory areas (e.g. aortic bodies). Moreover, the present study revealed a low magnitude bradycardia to selective CB stimulation in subjects with enhanced hypoxia‐induced tachycardia (HRR). This observation may be explained by a predominating aortic bodies evoked tachycardia during simultaneous CBs and aortic bodies stimulation with hypoxia. It also suggests the existence of a central interaction between CBs and aortic bodies. The differences in HR response to CBs and aortic bodies activation might be substantiated from an evolutionary point of view. In lower vertebrates, chemoreceptors located mainly on the first gill arch (which evolve to form mammalian CBs) are responsible for sensing environmental oxygen partial pressure, rather than blood oxygen levels (Milsom & Burleson, 2007). The responses mediated by these receptors serve to minimize the effects of environmental hypoxia by (1) hyperventilation (increased oxygen uptake) and (2) bradycardia (improved oxygen‐conservation). On the other hand, arterial chemoreceptors (located on other gill arches), which evolve to form the aortic bodies, monitor blood oxygen content (SpO2) and respond functionally to ensure an adequate oxygen delivery to the tissues, which may explain the tachycardia following their stimulation (Milsom & Burleson, 2007).
The reported adenosine‐induced HR response may be attributed to co‐activation of carotid sinus baroreceptors or direct actions of adenosine on cardiac cells (Watt & Routledge, 1986). There are two hypothetical mechanisms of potential baroreceptors activation: (1) a compensatory reaction to the adenosine‐related increase in SBP and (2) a direct volume‐effect of adenosine bolus injection. However, the onset time of the HR response precedes the BP response and hence precludes the former scenario. The latter also appears unlikely due to the lack of a bradycardia after equivalent injections of placebo. Observed bradycardia may also be the result of direct action of the chemical on cell surface receptors in the sinus and atrioventricular nodes. Nevertheless, the maximal dose of adenosine used in the present study (512 μg) administered i.v. had no effect on the heart rate. Moreover, there were no atrioventricular conduction disturbances observed after bolus injections of adenosine; hence, this mechanism of bradycardia also has a low probability.
Blood pressure response
The analysis of BP following bolus injections of adenosine revealed a significant increase in SBP and MAP during the first 20 s and a decrease in MAP compared to baseline levels during the subsequent 20 s. This biphasic BP response was also observed after hypoxic exposures, as well as by Biaggioni et al. (1987) after i.v. adenosine bolus injections. The first phase of the BP response is associated with the primary PCh evoked sympathoexcitation (Daly & Scott, 1963; Lugliani et al. 1973; Guyenet, 2000; Niewinski et al. 2014 a), whereas the genesis of the second phase is unclear. We hypothesize that this phase may be attributed to activation of arterial baroreceptors or pulmonary stretch receptors.
Minute ventilation response
Stimulation of PCh leads to hyperventilation, which, in humans, is mainly dependent on the increase in VT, rather than BR (Caruana‐Montaldo et al. 2000). The contribution of particular chemosensory areas to the magnitude of the ventilatory response in mammals is estimated to be 90% for CBs and 10% for aortic bodies (Caruana‐Montaldo et al. 2000); this ratio was also found in our previous studies in humans (Niewinski et al. 2014 a). Whether there is any difference between CBs and aortic bodies in the pattern of the response (rise in VT vs. BR) is not known. However, a study by Smith and Mills suggested that CBs drive mostly an increase in VT, whereas aortic bodies increase in BR (Smith & Mills, 1980).
Among previous studies in humans employing i.v. adenosine as a stimulant of PCh, variability in the pattern of the ventilatory response was found. Biaggioni et al. (1987) reported an increase in VT with no change in BR following administration of adenosine i.v., whereas another study observed an increase in both VT and BR (Watt & Routledge, 1985). This difference may reflect contributions from the aortic bodies (increasing BR) and CBs responsible for the increase in VT. According to our results, selective CB stimulation led to hyperventilation caused by an increase in VT with a paradoxical decrease in BR. This suggests that, in humans, CBs are predominantly responsible for regulation of VT, although we cannot rule out a different pattern of response in humans with PCh hyperreflexia and cardiovascular disease.
The magnitude of the ventilatory response to selective carotid body stimulation, expressed as the individual ventilatory response to adenosine, depends on the HVR assessed with the transient hypoxia method. Our data confirm the excitatory role of adenosine on CB in conscious humans and its possible role in chemotransduction. Furthermore, we propose that intra‐common carotid artery injections of adenosine may be used as a test for the assessment of CB function, such as before and after CB ablation as a procedural efficacy test (Fig. 5); however, this method requires further validation.
Endovascular carotid body ablation
The ventilatory response to intra‐common carotid artery injection of adenosine was lower in the subject who underwent subsequent CB ablation compared to other subjects. This difference may be explained by concomitant administration of anaesthetic medications (fentanyl and propofol) during the procedure. Both drugs are known to blunt ventilatory and haemodynamic responses to CB stimulation (Weil et al. 1975; Mayer et al. 1989; Jonsson et al. 2005). However, the changes in VI and BP responses to adenosine following the ablation were evident despite sedation. Similarly, the lack of a bradycardia in the pre‐procedural assessment may be attributed to drug‐related inhibition of parasympathetic cardiac tone (Kanaya et al. 2003; Sato et al. 2005) causing tachycardia, secondary to hyperpnoea, to become dominant. Nevertheless, all described components of the response to adenosine were diminished following carotid body ablation, which confirms the contribution of this structure in its generation.
Study limitations
Despite selective stimulation of CBs, we were unable to deactivate all secondary modulatory mechanisms recruited by the primary response, such as reflexes mediated by pulmonary stretch receptors and baroreceptors. Thus, the magnitude of changes in recorded parameters may be underestimated. Also, there are data suggesting a vasodilatatory influence of systematically infused adenosine on brain vasculature (Sollevi et al. 1987), which may lead to increased CO2 washout and a rise in local pH leading to desensitization of central chemoreceptors. However, such a side effect of adenosine, if it exists, would not affect the initial response. Furthermore, the study design, assuming unilateral adenosine administration (only on the non‐stenosed side), together with the small sample of studied patients, made the assessment of the potential lateralization of the response from carotid bodies impossible. This will be investigated in specifically designed future studies. Finally, we did not record sympathetic activity, which would provide a more comprehensive explanation for the observed haemodynamic changes.
Conclusions
In the study, we present novel insights into the physiology of selective unilateral CB stimulation in conscious humans. We found that bolus administration of adenosine given in close proximity to a CB leads to a decrease in HR, which is different from systemic activation of PCh (e.g. when hypoxia is used), most probably as a result of the elimination of the concurrent stimulation of aortic bodies. Moreover, we noted that the response evoked by adenosine injection into the common carotid artery is closely related to the ventilatory response to hypoxia, which further suggests a possible role of adenosinergic signalling in physiological chemotransduction in humans. Adenosine given into the common carotid artery constitutes a novel approach for the study of the physiology and sensitivity of an isolated CB. This method may also be used for perioperative assessment of the effectiveness of carotid body deactivation.
Additional information
Conflict of interest
PP and KR have received consultancy contracts from CIBIEM, Inc., CA, USA. PN and ST have received research support from CIBIEM, Inc., CA, USA. ZJE is employed by CIBIEM, Inc., CA, USA. The other authors declare that they have no competing interests.
Funding
ST and PN received a scientific grant from Cibiem Inc. JFRP is funded by the British Heart Foundation.
Author contributions
The experiments were performed in the Laboratory for Applied Cardiovascular Research at Department of Cardiology, 4th Military Hospital, Wroclaw, Poland. ST, PN, KR, DJ, ZJE, WB, JFRP and PP were responsible for conception and design of the experiments. ST, KR and BP were responsible for collection of data. ST, PN, AR, BP, ZJE, WB, JFRP and PP were responsible for analysis and interpretation of data. ST was responsible for drafting the article. PN, KR, DJ, AR, BP, ZJE, WB, JFRP and PP were responsible for revision of the manuscript. All authors approved the final version of the manuscript. All persons designated as authors qualify for authorship. All those who qualify for authorship are listed.
References
- Abdala AP, McBryde FD, Marina N, Hendy EB, Engelman ZJ, Fudim M, Sobotka PA, Gourine AV & Paton JF (2012). Hypertension is critically dependent on the carotid body input in the spontaneously hypertensive rat. J Physiol 590, 4269–4277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belardinelli L, Linden J & Berne RM (1989). The cardiac effects of adenosine. Prog Cardiovasc Dis 32, 73–97. [DOI] [PubMed] [Google Scholar]
- Biaggioni I, Olafsson B, Robertson R, Hollister A & Robertson D (1987). Cardiovascular and respiratory effects of adenosine in conscious man. Evidence for chemoreceptor activation. Circ Res 61, 779–786. [DOI] [PubMed] [Google Scholar]
- Caruana‐Montaldo B, Gleeson K & Zwillich CW (2000). The control of breathing in clinical practice. CHEST Journal 117, 205–225. [DOI] [PubMed] [Google Scholar]
- Chua T & Coats A (1995). The reproducibility and comparability of tests of the peripheral chemoreflex: comparing the transient hypoxic ventilatory drive test and the single‐breath carbon dioxide response test in healthy subjects. Eur J Clin Invest 25, 887–892. [DOI] [PubMed] [Google Scholar]
- Collis MG (1989). The vasodilator role of adenosine. Pharmacol Ther 41, 143–162. [DOI] [PubMed] [Google Scholar]
- Conde SV, Monteiro EC, Rigual R, Obeso A & Gonzalez C (2012). Hypoxic intensity: a determinant for the contribution of ATP and adenosine to the genesis of carotid body chemosensory activity. J Appl Physiol 112, 2002–2010. [DOI] [PubMed] [Google Scholar]
- Daly M & Scott MJ (1958). The effects of stimulation of the carotid body chemoreceptors on heart rate in the dog. J Physiol 144, 148–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly M & Scott MJ (1963). The cardiovascular responses to stimulation of the carotid body chemoreceptors in the dog. J Physiol 165, 179–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Rio R, Marcus NJ & Schultz HD (2013). Carotid chemoreceptor ablation improves survival in heart failure: rescuing autonomic control of cardiorespiratory function. J Am Coll Cardiol 62, 2422–2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyenet PG (2000). Neural structures that mediate sympathoexcitation during hypoxia. Respir Physiol 121, 147–162. [DOI] [PubMed] [Google Scholar]
- Heistad D & Abboud F (1980). Dickinson W. Richards Lecture: Circulatory adjustments to hypoxia. Circulation 61, 463–470. [DOI] [PubMed] [Google Scholar]
- Holmes AP, Nunes AR, Cann MJ & Kumar P (2015). Ecto‐5′‐nucleotidase, adenosine and transmembrane adenylyl cyclase signalling regulate basal carotid body chemoafferent outflow and establish the sensitivity to hypercapnia. Adv Exp Med Biol 860, 279–289. [DOI] [PubMed] [Google Scholar]
- Isakovic AJ, Abbott NJ & Redzic ZB (2004). Brain to blood efflux transport of adenosine: blood–brain barrier studies in the rat. J Neurochem 90, 272–286. [DOI] [PubMed] [Google Scholar]
- Jonsson MM, Lindahl SG & Eriksson LI (2005). Effect of propofol on carotid body chemosensitivity and cholinergic chemotransduction. Anesthesiology 102, 110–116. [DOI] [PubMed] [Google Scholar]
- Kanaya N, Hirata N, Kurosawa S, Nakayama M & Namiki A (2003). Differential effects of propofol and sevoflurane on heart rate variability. Anesthesiology 98, 34–40. [DOI] [PubMed] [Google Scholar]
- Klabunde RE (1983). Dipyridamole inhibition of adenosine metabolism in human blood. Eur J Pharmacol 93, 21–26. [DOI] [PubMed] [Google Scholar]
- Lugliani R, Whipp BJ & Wasserman K (1973). A role for the carotid body in cardiovascular control in man. Chest 63, 744–750. [DOI] [PubMed] [Google Scholar]
- Marshall JM (1994). Peripheral chemoreceptors and cardiovascular regulation. Physiol Rev 74, 543–594. [DOI] [PubMed] [Google Scholar]
- Mayer N, Zimpfer M, Raberger G & Beck A (1989). Fentanyl inhibits the canine carotid chemoreceptor reflex. Anesth Analg 69, 756–762. [PubMed] [Google Scholar]
- McQueen D & Ribeiro J (1983). On the specificity and type of receptor involved in carotid body chemoreceptor activation by adenosine in the cat. Br J Pharmacol 80, 347–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milsom WK & Burleson ML (2007). Peripheral arterial chemoreceptors and the evolution of the carotid body. Respir Physiol Neurobiol 157, 4–11. [DOI] [PubMed] [Google Scholar]
- Monteiro E & Ribeiro J (1987). Ventilatory effects of adenosine mediated by carotid body chemoreceptors in the rat. Naunyn‐Schmiedeberg's Arch Pharmacol 335, 143–148. [DOI] [PubMed] [Google Scholar]
- Moser G, Schrader J & Deussen A (1989). Turnover of adenosine in plasma of human and dog blood. Am J Physiol Cell Physiol 256, C799–C806. [DOI] [PubMed] [Google Scholar]
- Niewinski P (2014. b). Pathophysiology and potential clinical applications for testing of peripheral chemosensitivity in heart failure. Curr Heart Fail Rep 11, 126–133. [DOI] [PubMed] [Google Scholar]
- Niewinski P, Engelman ZJ, Fudim M, Tubek S, Paleczny B, Jankowska EA, Banasiak W, Sobotka PA & Ponikowski P (2013. a). Clinical predictors and hemodynamic consequences of elevated peripheral chemosensitivity in optimally treated men with chronic systolic heart failure. J Card Fail 19, 408–415. [DOI] [PubMed] [Google Scholar]
- Niewinski P, Janczak D, Rucinski A, Jazwiec P, Sobotka PA, Engelman ZJ, Fudim M, Tubek S, Jankowska EA, Banasiak W, Hart EC, Paton JF & Ponikowski P (2013. b). Carotid body removal for treatment of chronic systolic heart failure. Int J Cardiol 168, 2506–2509. [DOI] [PubMed] [Google Scholar]
- Niewinski P, Janczak D, Rucinski A, Tubek S, Engelman ZJ, Jazwiec P, Banasiak W, Sobotka PA, Hart EC & Paton JF (2014. a). Dissociation between blood pressure and heart rate response to hypoxia after bilateral carotid body removal in men with systolic heart failure. Exp Physiol 99, 552–561. [DOI] [PubMed] [Google Scholar]
- O'Regan R & Majcherczyk S (1982). Role of peripheral chemoreceptors and central chemosensitivity in the regulation of respiration and circulation. J Exp Biol 100, 23–40. [DOI] [PubMed] [Google Scholar]
- Paintal A (1973). Vagal sensory receptors and their reflex effects. Physiol Rev 53, 159–227. [DOI] [PubMed] [Google Scholar]
- Pardridge WM, Yoshikawa T, Kang Y‐S & Miller LP (1994). Blood‐brain barrier transport and brain metabolism of adenosine and adenosine analogs. J Pharmacol Exp Ther 268, 14–18. [PubMed] [Google Scholar]
- Paton JF, Sobotka PA, Fudim M, Engelman ZJ, Hart EC, McBryde FD, Abdala AP, Marina N, Gourine AV & Lobo M (2013). The carotid body as a therapeutic target for the treatment of sympathetically mediated diseases. Hypertension 61, 5–13. [DOI] [PubMed] [Google Scholar]
- Prabhakar NR (2000). Oxygen sensing by the carotid body chemoreceptors. J Appl Physiol 88, 2287–2295. [DOI] [PubMed] [Google Scholar]
- Ribeiro MJ, Sacramento JF, Gonzalez C, Guarino MP, Monteiro EC & Conde SV (2013). Carotid body denervation prevents the development of insulin resistance and hypertension induced by hypercaloric diets. Diabetes 62, 2905–2916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runold M, Cherniack NS & Prabhakar NR (1990). Effect of adenosine on chemosensory activity of the cat aortic body. Respir Physiol 80, 299–306. [DOI] [PubMed] [Google Scholar]
- Sacramento JF, Gonzalez C, Gonzalez‐Martin MC & Conde SV (2015). Adenosine receptor blockade by caffeine inhibits carotid sinus nerve chemosensory activity in chronic intermittent hypoxic animals. Adv Exp Med Biol 860, 133–137. [DOI] [PubMed] [Google Scholar]
- Sato M, Tanaka M, Umehara S & Nishikawa T (2005). Baroreflex control of heart rate during and after propofol infusion in humans. Br J Anaesth 94, 577–581. [DOI] [PubMed] [Google Scholar]
- Schultz HD, Marcus NJ & Del Rio R (2013). Role of the carotid body in the pathophysiology of heart failure. Curr Hypertens Rep 15, 356–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith P & Mills E (1980). Restoration of reflex ventilatory response to hypoxia after removal of carotid bodies in the cat. Neuroscience 5, 573–580. [DOI] [PubMed] [Google Scholar]
- Sollevi A (1986). Cardiovascular effects of adenosine in man; possible clinical implications. Prog Neurobiol 27, 319–349. [DOI] [PubMed] [Google Scholar]
- Sollevi A, Ericson K, Eriksson L, Lindqvist C, Lagerkranser M & Stone‐Elander S (1987). Effect of adenosine on human cerebral blood flow as determined by positron emission tomography. J Cereb Blood Flow Metab 7, 673–678. [DOI] [PubMed] [Google Scholar]
- Timmers HJ, Wieling W, Karemaker JM & Lenders JW (2003). Denervation of carotid baro‐and chemoreceptors in humans. J Physiol 553, 3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt A, Reid P, Stephens M & Routledge P (1987). Adenosine‐induced respiratory stimulation in man depends on site of infusion. Evidence for an action on the carotid body? Br J Clin Pharmacol 23, 486–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt A & Routledge P (1985). Adenosine stimulates respiration in man. Br J Clin Pharmacol 20, 503–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt A & Routledge P (1986). Transient bradycardia and subsequent sinus tachycardia produced by intravenous adenosine in healthy adult subjects. Br J Clin Pharmacol 21, 533–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weil JV, McCullough RE, Kline J & Sodal IE (1975). Diminished ventilatory response to hypoxia and hypercapnia after morphine in normal man. N Engl J Med 292, 1103–1106. [DOI] [PubMed] [Google Scholar]


