Abstract
Key points
The degree to which developmental programmes or environmental signals determine physiological phenotypes remains a major question in physiology.
Vertebrates change environments during development, confounding interpretation of the degree to which development (i.e. permanent processes) or phenotypic plasticity (i.e. reversible processes) produces phenotypes.
Tadpoles mainly breathe water for gas exchange and frogs may breathe water or air depending on their environment and are, therefore, exemplary models to differentiate the degree to which life‐stage vs. environmental context drives developmental phenotypes associated with neural control of lung breathing.
Using isolated brainstem preparations and patch clamp electrophysiology, we demonstrate that adult bullfrogs acclimatized to water‐breathing conditions do not exhibit CO2 and O2 chemosensitivity of lung breathing, similar to water‐breathing tadpoles.
Our results establish that phenotypes associated with developmental stage may arise from plasticity per se and suggest that a developmental trajectory coinciding with environmental change obscures origins of stage‐dependent physiological phenotypes by masking plasticity.
Abstract
An unanswered question in developmental physiology is to what extent does the environment vs. a genetic programme produce phenotypes? Developing animals inhabit different environments and switch from one to another. Thus a developmental time course overlapping with environmental change confounds interpretations as to whether development (i.e. permanent processes) or phenotypic plasticity (i.e. reversible processes) generates phenotypes. Tadpoles of the American bullfrog, Lithobates catesbeianus, breathe water at early life‐stages and minimally use lungs for gas exchange. As adults, bullfrogs rely on lungs for gas exchange, but spend months per year in ice‐covered ponds without lung breathing. Aquatic submergence, therefore, removes environmental pressures requiring lung breathing and enables separation of adulthood from environmental factors associated with adulthood that necessitate control of lung ventilation. To test the hypothesis that postmetamorphic respiratory control phenotypes arise through permanent developmental changes vs. reversible environmental signals, we measured respiratory‐related nerve discharge in isolated brainstem preparations and action potential firing from CO2‐sensitive neurons in bullfrogs acclimatized to semi‐terrestrial (air‐breathing) and aquatic‐overwintering (no air‐breathing) habitats. We found that aquatic overwintering significantly reduced neuroventilatory responses to CO2 and O2 involved in lung breathing. Strikingly, this gas sensitivity profile reflects that of water‐breathing tadpoles. We further demonstrated that aquatic overwintering reduced CO2‐induced firing responses of chemosensitive neurons. In contrast, respiratory rhythm generating processes remained adult‐like after submergence. Our results establish that phenotypes associated with life‐stage can arise from phenotypic plasticity per se. This provides evidence that developmental time courses coinciding with environmental changes obscure interpretations regarding origins of stage‐dependent physiological phenotypes by masking plasticity.
Keywords: development, electrophysiology, chemosensitivity, respiratory control, environmental plasticity
Key points
The degree to which developmental programmes or environmental signals determine physiological phenotypes remains a major question in physiology.
Vertebrates change environments during development, confounding interpretation of the degree to which development (i.e. permanent processes) or phenotypic plasticity (i.e. reversible processes) produces phenotypes.
Tadpoles mainly breathe water for gas exchange and frogs may breathe water or air depending on their environment and are, therefore, exemplary models to differentiate the degree to which life‐stage vs. environmental context drives developmental phenotypes associated with neural control of lung breathing.
Using isolated brainstem preparations and patch clamp electrophysiology, we demonstrate that adult bullfrogs acclimatized to water‐breathing conditions do not exhibit CO2 and O2 chemosensitivity of lung breathing, similar to water‐breathing tadpoles.
Our results establish that phenotypes associated with developmental stage may arise from plasticity per se and suggest that a developmental trajectory coinciding with environmental change obscures origins of stage‐dependent physiological phenotypes by masking plasticity.
Abbreviations
- CN
cranial nerve
- HVD
hypoxic ventilatory depression
- LC
locus coeruleus
Introduction
A major question in developmental physiology is to what extent does the environment vs. a genetic programme produce phenotypes (Burggren & Warburton, 2005)? Anatomical processes that determine body plan may be expected to follow strict genetic programming (Mallo et al. 2010), but plastic physiological functions could exhibit high sensitivity to environmental factors. Animals across vertebrate taxa inhabit drastically different environments (e.g. uterus, eggs, water, land) and abruptly switch from one to another at key developmental stages (e.g. birth, metamorphosis, hatching), during which time maturation of physiological processes from cells to whole systems occurs (Henning, 1981; Monyer et al. 1991; Hedrick, 2005; Imber & Putnam, 2012). Thus a developmental time course overlapping with irreversible shifts in the environment confounds interpretations as to whether particular phenotypes arise through genetically determined developmental processes (i.e. permanent changes during life‐history) or as plastic responses to extrinsic environmental factors (i.e. potentially reversible changes). Despite a well‐appreciated interaction between environmental influences and development (Burggren & Warburton, 2005; Gilbert, 2012), the possibility remains that phenotypic plasticity per se may generate phenotypes attributed to irreversible developmental processes (Ho et al. 2011). As potential treatments from certain human diseases assume genetically ‘hardwired’, developmental underpinnings (Köhler et al. 2014), deciphering whether development (irreversible) or phenotypic plasticity (potentially reversible) dictates certain phenotypes may have important ramifications for human health. Unfortunately, use of traditional mammalian model systems hinders disambiguating whether phenotypes observed at particular life stages occur due to development or to the environments in which the life‐stages occur because these two factors often change simultaneously and irreversibly.
Anuran amphibians (frogs and toads) experience profound environmental changes during development and throughout adult life. Therefore, anurans make unparalleled models to determine whether phenotypes observed at different developmental stages occur due to developmental trajectories or the environments in which the developmental stages occur. Larval anurans have a respiratory system adapted for water breathing, but they transition to air breathing during metamorphosis (Burggren & West, 1982; Burggren & Infantino, 1994). Along with maturation of lungs and loss of gills, a sensorimotor circuit in the brainstem controlling gill and lung ventilation changes to accommodate the switch from water to air. Neurobiological modifications to central control of ventilation during development from tadpole to frog involve (1) increased expression of a lung respiratory rhythm (Torgerson et al. 1997), (2) larger absolute lung breathing frequency responses to changes in CO2/pH (Torgerson et al. 1997; Taylor et al. 2003 a), and (3) enhanced inhibition of lung breathing frequency by hypoxia (Winmill et al. 2005; Fournier et al. 2007). As with other air‐breathing vertebrates such as mammals, these neural changes facilitate air breathing and blood gas homeostasis on land; however, many adult frog species from northern latitudes overwinter for several months in completely aquatic habitats without breathing air (Willis et al. 1956; Bradford, 1983; Stinner et al. 1994; Ultsch et al. 2004). Processes including cutaneous gas exchange and hypoxia tolerance promote survival of frogs in submerged overwintering environments (Tattersall & Ultsch, 2008). Intriguingly, overwintering in adult frogs recapitulates salient features of the juvenile physiological environment, namely, gas exchange in water without lung ventilation. Overwintering submergence, therefore, provides a unique opportunity to uncouple developmental stage (adulthood) from environmental factors associated with adulthood (terrestrial life) in the respiratory control system. Understanding the influence of overwintering submergence on respiratory control could provide insight into whether permanent developmental shifts or changes in the environment associated with terrestrial life generate mature respiratory phenotypes typically attributed to development of the breathing control system (Torgerson et al. 1997; Gdovin et al. 1999; Winmill et al. 2005; Gargaglioni & Milsom, 2007; Milsom, 2010).
After bullfrogs emerge from submerged overwintering conditions, breathing operates normally at rest, indicating functional respiratory rhythmogenic processes, but has diminished CO2 sensitivity (Santin & Hartzler, 2016 a). In response to increased brain and arterial CO2, air breathing vertebrates undergo hyperventilation primarily mediated by brainstem chemoreceptors (Torgerson et al. 1997; Taylor et al. 2003 a,b; Sundin et al. 2007; Milsom, 2010). Although early‐stage tadpoles appear to have central CO2 chemoreceptors (Taylor et al. 2003 b; Taylor & Brundage, 2013; Rousseau et al. 2016), the bulk of the evidence indicates that the ability for CO2/acidification to increase lung breathing frequency increases absolutely in magnitude with maturity (Torgerson et al. 1997; Taylor et al. 2003 a,b) in accordance with requirements for chemoreceptive control of lung ventilation (Gargaglioni & Milsom, 2007; Milsom, 2010). This trend may be conserved among air‐breathing vertebrates because increased ventilatory CO2 responsiveness also occurs during postnatal development in rats (Davis et al. 2006). Diminished ventilatory chemosensitivity after aquatic submergence, therefore, presents an intriguing case where an adult air‐breathing vertebrate has an environmentally induced loss of ventilatory CO2 responsiveness under conditions that otherwise result in large increases in ventilation. This suggests that stereotypical respiratory control phenotypes may be driven by environments associated with adulthood and immaturity (i.e. terrestrial and aquatic life) rather than maturation state per se. Using an in vitro neurophysiological approach, we tested the hypothesis that submerged overwintering in adult bullfrogs would reduce brainstem ‘fictive’ breathing frequency responses to respiratory gases CO2 and O2, so they are similar in magnitude to immature, water‐breathing tadpoles. In contrast, since we previously found that aquatic submergence does not affect resting ventilation, we hypothesized that respiratory rhythmogenic function would remain adult‐like.
Methods
Ethical approval
Experiments were approved by the Wright State University Institutional Animal Care and Use Committee.
Experimental animals
Three groups of bullfrogs were used in this study: (1) semi‐terrestrial controls at 22°C, (2) overwintered at 2°C with access to air, (3) overwintered at 2°C without access to air. The generation of the experimental animal groups has been described in detail (Santin & Hartzler, 2016 a). Briefly, semi‐terrestrial bullfrogs were maintained in 22°C aerated water, fed crickets twice per week, and had access to wet and dry areas. Both aquatic overwintered groups underwent an initial cooling phase from ∼22°C to 2°C over 6 weeks in aerated water. A plastic screen containing holes was placed at the surface of one tank containing aquatic overwintered bullfrogs and both tanks (one with air access and the other without) were maintained at 2°C for another 6 weeks. After 6–9 weeks of acclimation, experiments were performed on aquatic overwintered bullfrogs, alternating between the two experimental tanks.
Brainstem–spinal cord preparation and brainstem slices
To address whether aquatic overwintering alters the central control of breathing and cellular CO2 sensitivity in bullfrogs, we used the in vitro brainstem–spinal cord (Kinkead et al. 1994; Galante et al. 1996; Harris et al. 2002) and locus coeruleus (LC) brain slice preparations (Santin & Hartzler, 2013), respectively. For both preparations, the brainstem–spinal cord was dissected as previously described (Santin et al. 2013). Bullfrogs were killed by rapid decapitation. To generate the in vitro brainstem–spinal cord preparation, the dissected brainstem–spinal cord was transected rostral to the optic tectum and caudal to spinal nerve II and then pinned ventral side up in the recording chamber (6 ml) constructed from a petri dish coated with Sylgard (Dow Corning, Midland, MI, USA). To produce brain slices containing the LC, the whole‐brain was glued to an agar block and cut into ∼400 μm‐thick slices using a vibrating blade tissue slicer (Leica Microsystems, Buffalo Grove, IL, USA). Brain slices containing the LC were identified anatomically (González & Smeets, 1993), transferred to a 1 ml recording chamber, and stabilized with a nylon grid prior to cellular electrophysiology experiments. The brainstem–spinal cord preparation was superfused with control bullfrog artificial cerebral spinal fluid (aCSF) containing (in mm): 104 NaCl, 4 KCl, 1.4 MgCl2, 7.5 glucose, 40 NaHCO3, 2.5 CaCl2 and 1 NaH2PO4, and gassed with 90% O2, 1.3% CO2, balance N2, at 6 ml min–1 using a peristaltic pump (Rainin Instrument Co., Oakland, CA, USA). Brain slice preparations were superfused with the same aCSF, but bubbled with 80% O2, 1.3% CO2, balance N2, at 2 ml min–1 using gravity‐fed, stainless steel drip lines. Preparations were allowed to recover in their respective experimental chambers at 22°C for ∼1 h following dissection.
Whole‐nerve recordings from brainstem–spinal cord preparations
Cranial nerves V (trigeminal) and X (vagus) contain branches that innervate the respiratory muscles of amphibians; therefore, spontaneous rootlet activity correlates with respiratory‐related central nervous system activity that drives breathing in intact frogs (Sakakibara, 1983). CN V and CN X were drawn into borosilicate glass bipolar suction electrodes. Each glass electrode was pulled using a two‐stage micropipette puller (PC‐10; Narishige, East Meadow, NY, USA), broken to size to fit snugly around each nerve rootlet, and then fire polished. Activity of each nerve was amplified (×1000) using differential amplifiers (DP‐311; Warner Instruments, Hamden, CT, USA), filtered (100–1000 Hz), full‐wave rectified, integrated (time constant, 60 ms) and recorded using the Powerlab 8/35 data acquisition system (ADInstruments Inc., Colorado Springs, CO, USA) onto a personal computer.
Whole‐cell patch clamp electrophysiology
Whole‐cell current clamp recordings of spontaneous changes in membrane voltage (i.e. action potentials) in LC neurons were performed as previously described (Santin et al. 2013). Briefly, ∼5 MΩ borosilicate glass pipettes were back‐filled with artificial intracellular solution containing (in mm): 110 potassium gluconate, 2 MgCl2, 10 Hepes, 1 Na2‐ATP, 0.1 Na2‐GTP, 2.5 EGTA, pH 7.2 with KOH, and positioned over an AgCl2‐coated Ag wire. The chamber was located under a fixed‐stage microscope (Nikon, Elgin, IL, USA) where the slice was visualized at ×4 magnification to identify the LC. Individual neurons located in the area identified anatomically as the LC (González et al. 1994) were observed at ×60 magnification. The electrode was positioned near the neuron of interest using a Burleigh micromanipulator while applying positive pressure through the glass pipette (PCS 5000; Thorlabs, Newton, NJ, USA). When the pipette touched the neuron, positive pressure was removed and slight negative pressure was applied by mouth until the formation of a gigaohm seal. Rapid, but gentle, negative pressure was applied to break the gigaohm seal and gain whole‐cell electrochemical access. Membrane potential was determined in ‘current‐clamp mode’ using an Axopatch 200B integrating patch clamp amplifier (Molecular Devices, Sunnyvale, CA, USA) and collected using P10 Clampex software (Molecular Devices). Current‐clamp recordings were analysed off‐line using pCLAMP software (Molecular Devices).
Experimental procedures
Brainstem–spinal cord experiments
In semi‐terrestrial (n = 9), aquatic overwintered air access (n = 7), and aquatic overwintered submerged (n = 5) frogs, baseline burst activity from CN V and CN X was recorded from brainstem–spinal cord preparations. After acquiring stable baseline recordings (90% O2, 1.3% CO2, balance N2; pH 7.9), we applied hypercapnia (90% O2, 5% CO2, balance N2; pH 7.5) for 30 min or hypoxia (1.3% CO2, balance N2; pH 7.9) for 15 min sequentially, but in random order (i.e. the first application, either hypercapnia or hypoxia, was alternated). Two limitations must be acknowledged. This is a repeated exposures design and there is the possibility that previous exposure to hypercapnia influences the hypoxia responses and vice versa; however, by alternating the order of stimulus application this effect would be the same in each animal group. Within‐groups responses were consistent and preparations always recovered to near‐initial baseline activity when returned to control aCSF (Figs 3 and 6). Secondly, we are aware that transitioning from 90% O2 (hyperoxic control) to anoxic aCSF as a hypoxic stimulus is not physiological. Similar methods to ours (same flow rate and chamber volume) determined tissue to be 0 mmHg within 5 min (at any depth) using this aCSF anoxia as the stimulus (Winmill et al. 2005). Since intact frogs survive temporary anoxia (Rose & Drotman, 1967; Tattersall & Ultsch, 2008), interpretation errors from this experimental paradigm may stem from high control values and not the anoxic stimulus (Fournier & Kinkead, 2008).
Figure 3. Lung burst and buccal burst frequency lose sensitivity to hypercapnia after aquatic overwintering .
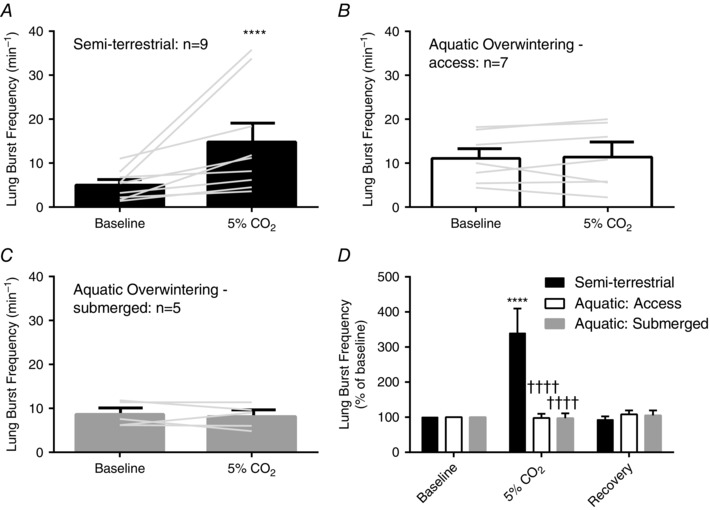
A–C, mean data and individual responses to hypercapnia in semi‐terrestrial and aquatic overwintered bullfrogs. In each panel, the grey lines connect baseline burst frequency to the response of that individual in hypercapnia. Sample sizes for each group are included in the figure. A, hypercapnia induces significant increases in lung burst frequency (P < 0.0001; repeated measures two‐way ANOVA with Holm–Sidak's multiple comparisons test); B and C, however, both groups of aquatic overwintering do not undergo increases in lung burst frequency after exposure to hypercapnia (P > 0.05; repeated measures two‐way ANOVA followed by Holm–Sidak's multiple comparisons test). D, mean responses from each normalized as a percentage of baseline burst frequency. The same sample sizes for groups in A–C apply to D. Only semi‐terrestrial controls increased lung burst frequency with exposure to hypercapnia compared to baseline (P < 0.0001; repeated measures two‐way ANOVA with Holm–Sidak's multiple comparisons test). Relative changes in lung burst frequency of semi‐terrestrial bullfrogs during hypercapnia were also greater compared to both groups following aquatic overwintering (P < 0.0001; repeated measures two‐way ANOVA with Holm–Sidak's multiple comparisons test *** P < 0.001 and **** P < 0.0001 for within group comparisons. †††† P < 0.0001 for between group comparisons. Error bars represent SEM.
Figure 6. CO2/pH sensitive neurons from a chemoreceptive nucleus, the locus coeruleus (LC), have reduced firing responses to hypercapnia after submerged overwintering .
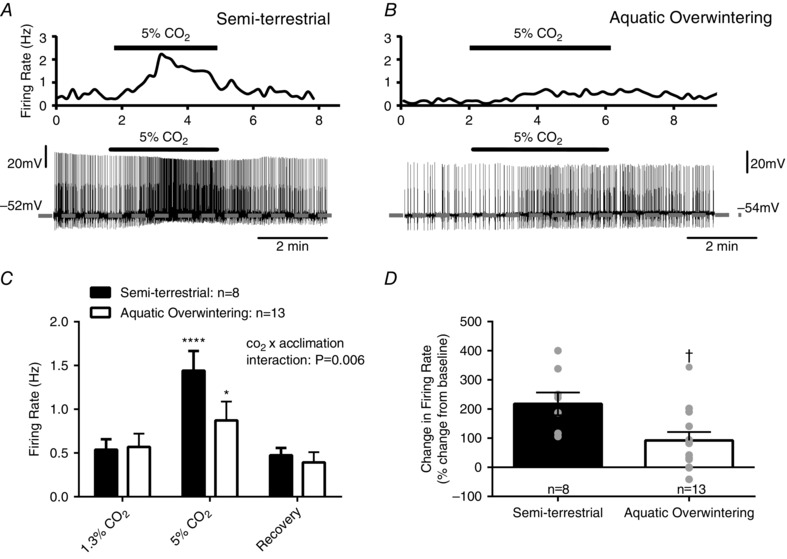
A, and B, representative integrated firing rate traces (top) and whole cell current‐clamp recordings of action potentials (bottom) in LC neurons from semi‐terrestrial and aquatic overwintered (air access only) bullfrogs. The dashed grey line indicates the interspike V m (i.e. V m between action potentials). A, upon transition from normocapnia (1.3% CO2) to hypercapnia (5% CO2), LC neurons from semi‐terrestrial bullfrogs increase action potential firing frequency and undergo slight membrane depolarization. This increase is reversed upon returning to normocapnia. B, in contrast, transition to hypercapnia in an LC neuron from an aquatic overwintered bullfrog results in a less robust increase in firing frequency with minimal membrane depolarization. C, mean firing frequencies before, during and after hypercapnia. Samples sizes are included in the figure. There is a significant interaction between CO2 and acclimation group indicating that aquatic overwintered bullfrogs have reduced firing responses to hypercapnia (temperature acclimation × CO2 interaction; P = 0.0062, two‐way ANOVA), even though LC neurons from both groups of bullfrogs undergo slight increases in firing frequency during hypercapnia (P < 0.05; Holm–Sidak's multiple comparisons test). D, firing rate in LC neurons from semi‐terrestrial bullfrogs expressed as percentage increase from baseline firing rate. Firing rate increases by ∼215% in semi‐terrestrial bullfrogs, while aquatic overwintered bullfrogs increase firing frequency by ∼90% (P = 0.01; two‐tailed unpaired t test). * P < 0.05, **** P < 0.0001 for within group comparisons. † P < 0.05 for between group comparisons. Error bars represent SEM.
CO2 chemosensitivity of LC neurons
Chemosensitive responses were determined in LC neurons from control (n = 8) and aquatic overwintered frogs (n = 13). After entry into the whole‐cell configuration, stable baseline firing in control aCSF (80% O2, 1.3% CO2, balance N2; pH 7.9) was recorded for 5 min. Neurons were then exposed to hypercapnia (80% O2, 5% CO2, balance N2; pH 7.5) for 5 min to elicit increases in firing frequency. We have shown previously that exposing neurons to 5% CO2 is sufficient to observe increases in firing frequency in ∼90% of LC neurons from bullfrogs (Santin et al. 2013). Neurons were then returned to control aCSF and firing rates recovered to near control values. We have previously reported that chemosensitive LC neurons from bullfrogs are paradoxically activated by cooling (Santin et al. 2013; Santin & Hartzler, 2015). We used cold‐activation to (1) determine if cold‐acclimation influences neuronal properties besides chemosensitivity, and (2) to provide a positive control for increases in firing frequency. Because the primary goal of this study was to understand if cold‐acclimation alters cellular chemosensitivity, cooling was always applied after the 5% CO2 exposure.
Data analysis and statistics
Classification of nerve activities
Fictive lung (large amplitude–low frequency) and buccal (small amplitude–high frequency) activities were analysed according to previously determined criteria (Taylor et al. 2003 b; Winmill et al. 2005). Since CN V and CN X must burst in phase for air to move in and out of the lungs of an intact frog (Sanders & Milsom, 2001), large amplitude bursts were classified as fictive lung activity only when CN V and CN X nerves fired in phase (Fournier et al. 2007; Fournier & Kinkead, 2008). In our hands, large amplitude CN V bursts almost always occurred simultaneously with CN X bursts. Similar to recent reports, CN V usually contained both fictive buccal and lung activities; however, as previously demonstrated (Baghdadwala et al. 2015), some postmetamorphic preparations exhibited inconsistent or ‘waxing and waning’ buccal activity (Taylor et al. 2003 b; Baghdadwala et al. 2015). Thus analysis of buccal motor activity only from preparations exhibiting a discernible ‘buccal’ rhythm during the control and hypercapnic sampling periods were used in analysis. Buccal activities from both aquatic overwintered groups were pooled. CN X only contained large amplitude bursts that occurred in phase with large amplitude bursts of CN V.
Brainstem–spinal cord preparation analysis
Five minutes of baseline properties (fictive lung burst frequency CN X duration and normalized CN X rise time) were compared among control, cold‐acclimation air access, and aquatic overwintered submerged preparations before switching to hypercapnia or hypoxia. For hypercapnia experiments, burst properties (fictive lung burst frequency, fictive buccal frequency, burst duration, normalized rise time, burst amplitude and burst integral) were analysed 5 min before treatment and in the last 5 min of each treatment. Results for burst frequency are expressed both absolutely and as a percentage of baseline. For hypoxia experiments, burst frequency was analysed in the 5 min before transitioning to hypoxia and in the last 5 min of hypoxia. Data were analysed using Labchart peak analysis (ADInstruments Inc.). Burst frequencies obtained in hypercapnia and hypoxia are expressed absolutely and as percentages of baseline.
Chemosensitivity of LC neurons analysis
Firing frequency of LC neurons was integrated into 10 s bins using Clampfit (Molecular Devices). Integrated firing rate of LC neurons from semi‐terrestrial and aquatic overwintered bullfrogs was analysed in the 2 min preceding the switch to hypercapnia and in the last 2 min of the hypercapnic treatment. Recovery firing rates were recorded ∼5 min after the return to control aCSF. The recovery firing rate was then used as the control value for acute cooling experiments. Neurons (n = 7 for semi‐terrestrial and n = 6 aquatic overwintered) were then cooled from 20°C to 10°C over ∼4 min. One minute of steady‐state firing was measured at 10°C. Firing rate increases during hypercapnia and cooling are expressed absolutely and as a percentage increase from baseline. Interspike membrane potential was determined by measuring membrane potential in between action potentials, following the after‐hyperpolarization and before the next action potential threshold ramp. Voltages were corrected for a +12 mV liquid junction potential between the pipette filling solution and the aCSF calculated by the Henderson equation using the ‘junction potential calculator’ function in P10 Clampex (Molecular Devices).
Statistics
Whole‐nerve recordings
Each baseline characteristic was analysed using a one‐way ANOVA. For gas exposure experiments, absolute and normalized data were analysed using a two‐way ANOVA. Since we randomized the order of hypercapnia and hypoxia, each parameter was treated as independent. When a main effect (temperature acclimation or gas treatment) or interaction (temperature acclimation × gas treatment) was detected, Holm–Sidak's multiple comparisons test was used to determine differences between means.
Chemosensitivity of LC neurons
Absolute firing frequencies of LC neurons in normo‐ and hypercapnia were compared between semi‐terrestrial and aquatic overwintered bullfrogs using a two‐way ANOVA. When a main effect (temperature acclimation or gas treatment) or interaction (temperature acclimation × gas treatment) was detected, Holm–Sidak's multiple comparisons test was used to determine differences between means. Differences in normalized increases in firing increases of LC neurons between semi‐terrestrial and aquatic overwintered bullfrogs due hypercapnia or cold were analysed during Student's two‐tailed unpaired t test. If standard deviations were different between groups as determined by the F test, a Welch's correction was applied to the t test to correct for unequal variances.
Results
In vitro brainstem preparation
To assess central processes controlling breathing we employed in vitro brainstem preparations (Baghdadwala et al. 2015). In vitro brainstem preparations from bullfrogs produce motor patterns similar to the ventilatory pattern observed in intact bullfrogs (Santin & Hartzler, 2016 b). This preparation generates two distinct motor patterns: (1) low frequency, large amplitude, near‐synchronous bursts on cranial nerve (CN) V and X and (2) high frequency, small amplitude bursts that occur mainly on CN V (Fig. 1 A). Near‐synchronous, large amplitude bursts from CN V and X correspond with neural activity driving ventilation of the lungs, while small amplitude bursts correspond with motor activity that ventilates the oropharyngeal (buccal) cavity (Kogo et al. 1994; Sanders & Milsom, 2001).
Figure 1. Aquatic overwintering increases lung burst frequency, but does not fundamentally change adult motor behaviour .
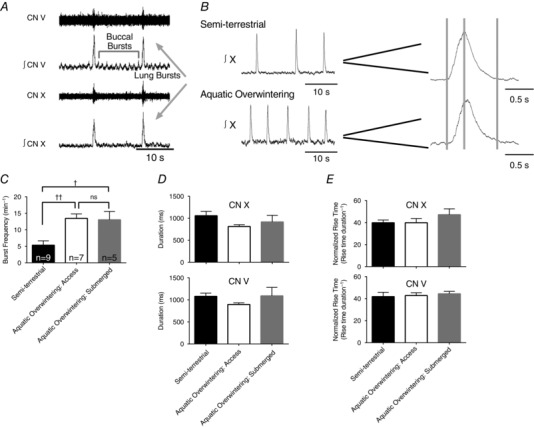
A, representative raw and integrated neurograms of cranial nerve (CN) V and X, illustrating the typical respiratory motor pattern of adult bullfrogs. Small amplitude, high frequency bursts observed on CN V represent neural correlates of buccal ventilation (i.e. pumping of air in and out of the oropharyngeal cavity that does not contribute to breathing). Near‐synchronous, large amplitude bursts on CN V and X represent neural correlates of lung ventilation, as this motor output would result in air flow in or out of the lung. B, representative integrated CN X neurograms from semi‐terrestrial (top) and aquatic overwintered (bottom) bullfrogs. Sample sizes are presented in the figure and apply to C and D. The left panel shows CN X bursts at a condensed time scale, illustrating a faster frequency in aquatic overwintered bullfrogs. The right panel shows individual bursts at an expanded time scale to demonstrate that duration (time from first to third grey bar) and normalized rise time (time from the first to the second grey bar divided by the burst duration) do not differ between semi‐terrestrial and overwintered aquatic bullfrogs. C, mean lung burst frequency data from semi‐terrestrial and both groups of aquatic overwintered bullfrogs demonstrating that lung burst frequency increases following aquatic overwintering. D and E, mean data for lung burst duration and normalized rise time for semi‐terrestrial and overwintered aquatic bullfrogs from CN X (top) and V (bottom). Lung burst morphology did not change after aquatic overwintering. † P < 0.05 and †† P < 0.01. Error bars represent standard error of the mean (SEM).
Burst characteristics of the brainstem–spinal cord preparation under control conditions
We used two aquatic overwintered groups: one that had access to air and one that was submerged. Overwintered aquatic bullfrogs with air access rarely surfaced, but rather remained voluntarily submerged in their tank (Santin & Hartzler, 2016 a). Since these animals were housed in separate tanks, we analysed these groups separately. To determine if disuse of the respiratory control system affects respiratory rhythm generation and pattern formation, we compared burst frequency, duration and rise time among semi‐terrestrial and both aquatic groups of bullfrogs under baseline conditions. Figure 1 B shows example CN X neurograms at compressed (left panel) and expanded (right panel) time scales from semi‐terrestrial and aquatic overwintered bullfrogs. Preparations from aquatic frogs with and without air access had burst frequencies greater than semi‐terrestrial frogs (Fig. 1 C; P = 0.0021; F (2,18) = 8.860; one‐way ANOVA; both aquatic overwintered groups greater than control, but not different from each other; P < 0.05 and P > 0.05, respectively; Holm–Sidak's multiple comparisons test). Although the burst frequency was elevated, buccal frequency did not differ between groups (semi‐terrestrial: 41.1 ± 5.2 vs. 40.1 ± 4.2 bursts min–1; P = 0.9767; t 16 = 0.0296; two‐tailed unpaired t test).
To gain an understanding of whether or not aquatic overwintering influences pattern formation of the network and/or motor function, we assessed total burst duration (Shao & Feldman, 2005) and rise time normalized to the total duration of each burst. In contrast to the frequency, duration and rise time of individual fictive breaths recorded from cranial nerves V and X were similar among all semi‐terrestrial and overwintered aquatic frogs (Fig. 1 D and E; P > 0.05 for both nerves; one‐way ANOVA). In summary, rhythmogenic processes producing lung breaths persist, albeit at a greater frequency, and maintain the normal burst morphology of the motor output after aquatic overwintering.
Aquatic overwintering reduces sensitivity to hypercapnia of brainstem–spinal cord preparations
We next took advantage of the brainstem–spinal cord preparation to understand whether the reduction in CO2 sensitivity that we previously observed in the intact animal (Santin & Hartzler, 2016 a) is reflected in the hypercapnic sensitivity of the brainstem. The in vitro brainstem preparation from adult bullfrogs undergoes increased fictive lung breathing during hypercapnia (Harris et al. 2002; Morales & Hedrick, 2002), while the brain of premetamorphic tadpoles undergoes little to no absolute change (Torgerson et al. 1997; Taylor et al. 2003 b). Figure 2 illustrates examples of raw and integrated nerve discharge from CN V and X from semi‐terrestrial and aquatic overwintered bullfrogs. The left panel shows resting burst frequency under control (1.3% CO2) and the right panel shows burst frequency during hypercapnia (5% CO2). Aquatic overwintering reduced the sensitivity of the brainstem–spinal cord preparation to hypercapnia (Figs 2 and 3; temperature acclimation × CO2 interaction; P = 0.0024; F (4,36) = 5.056; two‐way ANOVA). Figure 3 A–C shows the individual and mean neuroventilatory response to hypercapnia in semi‐terrestrial and aquatic overwintered bullfrogs. Preparations from semi‐terrestrial bullfrogs underwent significant increases in fictive lung‐related discharge (P < 0.0001; Holm–Sidak's multiple comparisons test), but preparations from both groups of aquatic overwintered bullfrogs did not (P > 0.05; Holm–Sidak's multiple comparisons test). Figure 3 D shows mean data expressed as a percentage increase from baseline (temperature acclimation × CO2 interaction; P < 0.0001; F (4,36) = 8.299; two‐way ANOVA). This highlights that preparations from semi‐terrestrial bullfrogs undergo an ∼3.5‐fold increase in fictive lung‐burst frequency during exposure to hypercapnia (P < 0.0001; Holm–Sidak's multiple comparisons test), but preparations from aquatic overwintered bullfrogs are not influenced by hypercapnia (P > 0.05; Holm–Sidak's multiple comparisons test).
Figure 2. Hypercapnia does not increase lung burst frequency following aquatic overwintering .
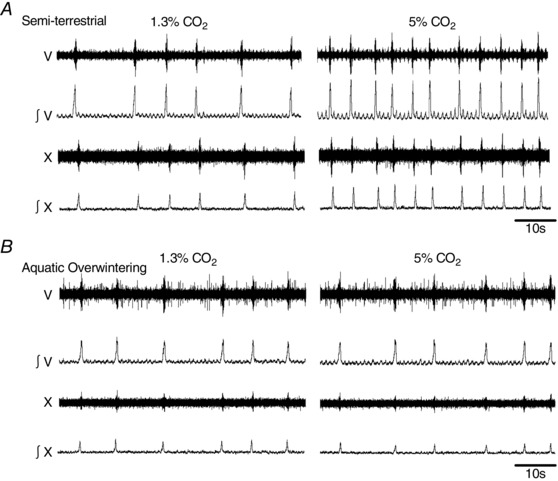
Example raw and integrated CN V and X neurograms from semi‐terrestrial and aquatic overwintered bullfrogs before and after 30 min exposure to hypercapnia (5% CO2). Notice that lung‐related nerve discharge from the semi‐terrestrial bullfrog (A) increases after exposure to hypercapnia (right panel) compared to baseline (left panel). In contrast, the lung discharge from the aquatic overwintered bullfrog (B) maintains a similar frequency in baseline conditions (left) and hypercapnia (right).
Additionally, lack of increases in lung burst frequency in overwintered aquatic bullfrogs do not appear to occur because of a ‘ceiling effect’ (i.e. no change due to a higher starting frequency) since initial burst frequency does not correlate with the change in burst frequency induced by hypercapnia (Fig. 4). If lack of responsiveness to hypercapnia occurred due to a ceiling effect, we would have expected to observe an inverse relationship between initial burst frequency and change induced by CO2, but this did not occur in either group of bullfrogs.
Figure 4. Change in burst frequency by hypercapnia does not correlate with initial burst frequency .
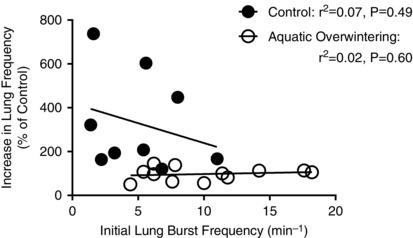
A possible explanation for lack of CO2 sensitivity of lung‐related nerve discharge in aquatic overwintered bullfrogs is that the higher initial burst frequency creates a ‘ceiling effect’, preventing further increases in burst discharge independent of reduced CO2 chemosensitivity. If higher burst frequencies accounted for lack of CO2 chemosensitivity, we would expect preparations with low starting burst frequencies to have large changes in lung bursting during hypercapnia and preparations with high starting frequencies to have small changes in lung bursting during hypercapnia. In both semi‐terrestrial and aquatic overwintered (pooled) preparations, there was no relationship initial burst frequency and change in burst frequency. If a ‘ceiling effect’ caused elimination of CO2 sensitivity, we would have expected an inverse linear correlation between initial burst frequency and change in burst frequency in aquatic overwintered bullfrogs. P‐values and r 2 values are presented in the figure.
In contrast to altered frequency responses to hypercapnia, burst properties including duration, rise time and integral have been shown to be unaffected by hypercarbia in in vitro preparations from adult bullfrogs (Morales & Hedrick, 2002). We reasoned that these properties could be altered as a compensatory response to reduced hypercapnic frequency stimulation in overwintered bullfrogs. Similar to a previous report (Morales & Hedrick, 2002), hypercapnia did not alter burst shape properties in semi‐terrestrial and overwintered aquatic groups (Fig. 5 A–C; no effect of temperature acclimation, CO2, or interaction; P > 0.05; two‐way ANOVA). These results indicate that aquatic overwintering conditions reduce aspects of the central respiratory control network that dictate the frequency responses to CO2, but do not induce compensatory changes that lead to changes in burst properties during hypercapnia.
Figure 5. Aquatic overwintering does not alter burst morphology during hypercapnia .
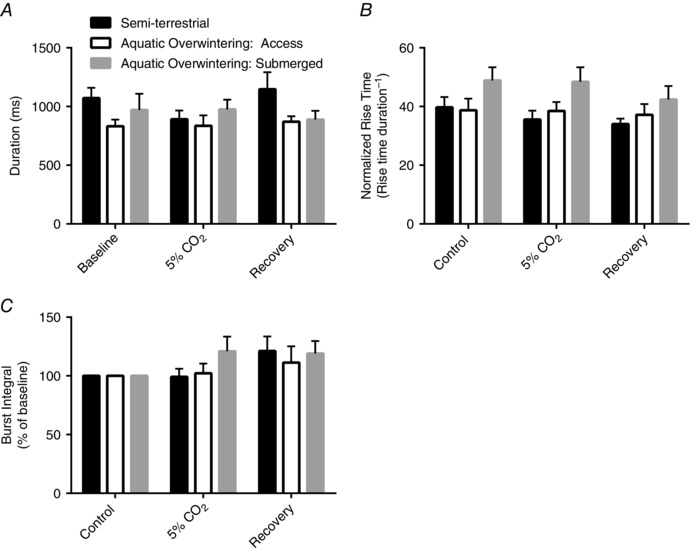
Hypercapnia did not affect lung burst duration (A), normalized rise time (B), and integral (C) in any acclimation group (no main effects of interactions). Given that there was no frequency response to hypercapnia after aquatic overwintering, there could have been a compensatory increase in burst characteristics; however, this did not occur, further confirming that CO2 chemosensitivity of the respiratory control system is eliminated after overwintering.
Aquatic overwintering reduces firing responses to CO2/pH challenge in putative respiratory chemoreceptive neurons
The locus coeruleus (LC) is an important CO2 chemoreceptive brain region that regulates lung ventilation in amphibians (Noronha‐de‐Souza et al. 2006). This area also contains neurons that are stimulated by high CO2/acidification in in vitro brain slice preparations (Santin & Hartzler, 2013). Since the LC is an important chemosensory brain region involved in increasing breathing, we performed whole‐cell patch clamp electrophysiology experiments in brain slices to further determine whether aquatic overwintering leads to alterations in cellular processes determining CO2/pH sensitivity of lung breathing. Figure 6 A and B show example integrated firing rate traces and whole‐cell action potential recordings before, during and after exposure to hypercapnia (5% CO2) in LC neurons from semi‐terrestrial and aquatic overwintered bullfrogs, respectively. LC neurons from aquatic overwintered frogs had reduced firing responses to hypercapnia (Fig. 4 C; temperature acclimation × CO2 interaction; P = 0.0062; F (2,38) = 5.830; two‐way ANOVA), but both groups underwent statistically significant increases in firing frequency during hypercapnia (semi‐terrestrial; P < 0.0001; Holm–Sidak's multiple comparisons test, aquatic overwintered; P < 0.05; Holm–Sidak's multiple comparisons test). The reduced firing responses to hypercapnia of LC neurons after aquatic overwintering is also apparent when expressed as an increase relative to baseline (Fig. 6 D; ∼215% increase vs. ∼90% increase; P = 0.0175; t 19 = 2.603; two‐tailed unpaired t test) and also when examining changes in membrane potential induced by 5% CO2 (semi‐terrestrial: +4.3 ± 0.7 mV vs. +1.5 ± 0.4 mV; P = 0.0012; t 19 = 3.810). Resting, interspike membrane potential did not differ in LC neurons from semi‐terrestrial and cold‐aquatic bullfrogs (semi‐terrestrial: −54.1 ± 1.6 mV vs. aquatic overwintered: −51.8 ± 1.1 mV; P = 0.2472; t 19 = 1. Given that a large fraction of CO2/pH sensitivity of the respiratory control system of amphibians emanates from the LC (Noronha‐de‐Souza et al. 2006), these results suggest that reduced central sensitivity to hypercapnia is, in part, mediated by reduced firing responses of individual LC neurons to CO2 challenge.
We sought to gain a clearer understanding of whether aquatic overwintering altered the ability for CO2 to stimulate firing or resulted in a change in LC neuron excitability which might account for reduced firing responses to hypercapnia. We previously showed that LC neurons from bullfrogs are paradoxically activated by acute exposure to cold temperatures (Santin et al. 2013; Santin & Hartzler, 2015). Thus, the next series of experiments was performed to determine whether firing responses to stimulation by another modality are disrupted following aquatic overwintering. Figure 7 A and B shows example whole‐cell action potentials recorded from LC neurons before and after a 4 min cooling ramp from 20°C to 10°C in semi‐terrestrial and aquatic overwintered bullfrogs. As expected, acute cooling increased firing frequencies of LC neurons, but unlike hypercapnic stimulation, there was no interaction between acclimation group and response to acute cooling (Fig. 7 C; P > 0.05; two‐way ANOVA) indicating that cold sensitivity of LC neurons does not depend on temperature acclimation. Additionally, unlike hypercapnic stimulation, acute cooling results in relative increases in firing frequency (Fig. 7 D; P = 0.6608; t 11 = 0.4409; two‐tailed unpaired t test) and membrane depolarization (semi‐terrestrial: +5.5 ± 0.4 mV vs. aquatic overwintered: 5.7 ± 0.9 mV; P = 0.8419; t 11 = 0.2042) that do not differ between LC neurons from semi‐terrestrial and aquatic overwintered bullfrogs. Lastly, when comparing the percentage increase in firing rate induced by CO2 and cooling in aquatic overwintered bullfrogs, cooling induces a significantly larger increase in firing frequency (92.3 ± 29.2% increase by CO2 vs. 704.6 ± 217.8% increase by cooling; P = 0.0371; t 5.18 = 2.787; two‐tailed unpaired t test with Welch's correction). Collectively, these findings imply that reduced firing responses to hypercapnia occur by altering mechanisms underlying the ability of CO2/pH to manipulate firing (be they intrinsic or extrinsic to the neurons) and are not a consequence of generally decreasing cellular excitability or extra‐CO2 sensory processing. This further confirms that changes in respiratory gas sensitivity in the absence of air breathing are induced by phenotypic plasticity acting on chemosensory mechanisms and not general dysfunction of the respiratory network.
Figure 7. Aquatic overwintering does not disrupt firing responses to another modality of stimulation, acute cooling .
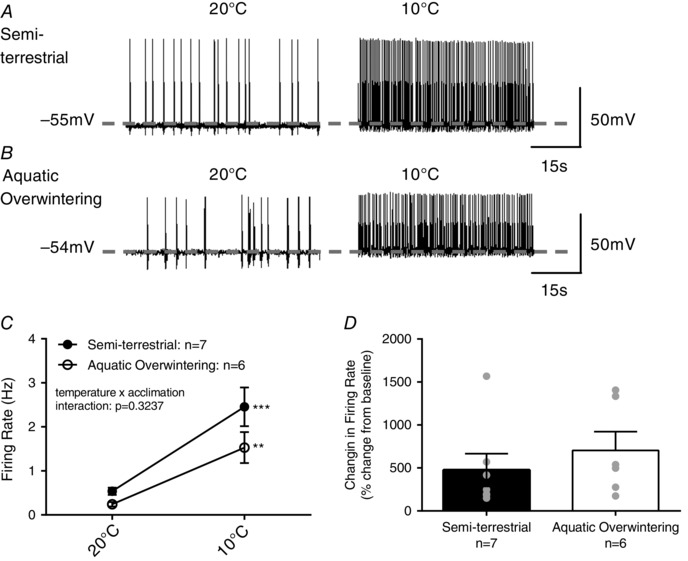
Paradoxically, LC neurons are stimulated by acute decreases in temperature. This provides a convenient way to assess whether reduced firing responses to CO2 reflect a general decrease in responsiveness to stimulation vs. decreases in CO2/pH sensitivity. A and B, representative whole cell current‐clamp recordings of action potentials in LC neurons from semi‐terrestrial and aquatic overwintered (air access only) bullfrogs before (left panel) and after (right panel) acute transition from 20°C to 10°C. The dashed grey line indicates the interspike V m (i.e. V m between action potentials). Both A and B illustrate that firing rate stimulation by acute cooling is similar between semi‐terrestrial and aquatic overwintered bullfrogs. C, mean data indicating that both semi‐terrestrial and aquatic overwintered bullfrogs increase firing frequency during acute cooling (P = 0.3237 for temperature × acclimation group interaction; P = 0.0003 for main effect of temperature). Sample sizes are included in the figure. D, firing rate increases induced by acute cooling as a percentage of baseline firing frequency. Relative firing rate increases by acute cooling do not differ between semi‐terrestrial and aquatic overwintered bullfrogs indicating that reduced firing responses to CO2 result from decreases CO2/pH sensitivity per se. ** P < 0.01 and *** P < 0.001 for within group comparisons. Error bars represent SEM.
Aquatic overwintering reduces central sensitivity to hypoxia
In addition to understanding how aquatic overwintering influences aspects of the CO2 sensitivity of the respiratory network, we also assessed the sensitivity of the central respiratory control system to acute hypoxia (aCSF bubbled with control CO2 and balanced N2). Acute hypoxia suppresses respiratory discharge in the central respiratory control system of adults, while premetamorphic tadpoles maintain bursting during hypoxia (Winmill et al. 2005; Fournier et al. 2007). Figure 8 A shows examples of integrated CN V and X discharge before, during and after 15 min exposure to hypoxia in semi‐terrestrial and aquatic overwintered bullfrogs. As illustrated in Fig. 8 A (left panel), semi‐terrestrial bullfrogs underwent stereotypical reductions in nerve discharge. In 7/9 preparations, bursting was completely and reversibly silenced by acute hypoxia in semi‐terrestrial frogs. As represented in Fig. 8 A (right panel), hypoxia did not silence rhythmic bursting in preparations from aquatic overwintered bullfrogs (access: 0/6; submerged: 0/5). The proportion of preparations silenced by hypoxia did not occur by chance (i.e. preparations from aquatic overwintered bullfrogs were more likely to continue bursting during hypoxia) (P = 0.0014; χ² test). Mean data are shown in Fig. 8 B. There was a significant effect of hypoxia on lung burst frequency (hypoxia effect; P = 0.0031; F (1,17) = 11.85; two‐way ANOVA) because of decreases in burst frequency in semi‐terrestrial preparations during hypoxia (P < 0.05 for semi‐terrestrial bullfrogs; Holm–Sidak's multiple comparisons test). Both groups of aquatic overwintered bullfrogs did not undergo significant decreases in lung bursting during hypoxia (Fig. 6 C and D; P > 0.05; Holm–Sidak's multiple comparisons test). When expressed as a percentage change from baseline, it was apparent that aquatic overwintering reduced the hypoxia sensitivity of the fictive‐lung rhythm (Fig. 8 E; temperature acclimation × hypoxia interaction; P = 0.0037; F (4,34) = 4.749; two‐way ANOVA). Aquatic overwintering, therefore, decreases hypoxia sensitivity of the respiratory rhythm in adult bullfrogs.
Figure 8. Aquatic overwintering eliminates hypoxic ventilatory depression characteristic of the mature central breathing control system .
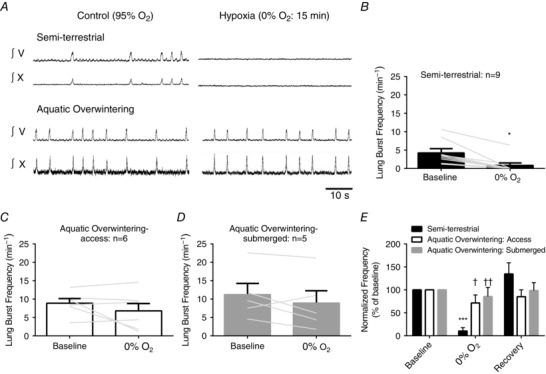
A, integrated CN V neurograms from semi‐terrestrial (top) and aquatic overwintered (bottom) bullfrogs. Upon transition to hypoxic aCSF (0% O2) (and presumably tissue anoxia) burst activity recorded through CN V underwent complete inhibition in semi‐terrestrial bullfrogs. In contrast to the normal adult response to hypoxia, brainstems from aquatic overwintered bullfrogs did not stop bursting during hypoxia. B–D, mean data and individual lung burst frequency responses to hypoxia. Sample sizes are included in each figure. In each figure, the grey lines connect baseline burst frequency to the response of that individual after 15 min of hypoxia. There was a main effect of hypoxia (P = 0.0031; two‐way ANOVA) because of decreases in burst frequency in semi‐terrestrial bullfrogs (B; P < 0.05; Holm–Sidak's multiple comparisons test). Both groups of aquatic overwintered bullfrogs did not change burst frequency during hypoxia (C and D; P > 0.05 Holm–Sidak's multiple comparisons test). E, the response to hypoxia as a percentage of baseline. There was a significant interaction between acclimation group and hypoxia (P = 0.0037; two‐way ANOVA). Hypoxia depressed lung bursting in semi‐terrestrial bullfrogs (P < 0.001; Holm–Sidak's multiple comparisons test), but not in both group of aquatic overwintered bullfrogs. Bursting during hypoxia decreased more in semi‐terrestrial bullfrogs compared to both overwintered groups. * P < 0.05, *** P < 0.001 for within group comparisons. † P < 0.05, †† P < 0.01 for between group comparisons. Error bars represent SEM.
Discussion
Our aim was to gain insight into whether permanent developmental changes or environmental factors associated with terrestrial life produce the respiratory control system adapted for breathing air. Therefore, we examined function of central control of lung breathing in adult bullfrogs following an absence of air breathing in conditions that mimic aquatic overwintering. Lack of air breathing is a common respiratory scenario in both larval and overwintering‐adult bullfrogs as lungs are minimally used for gas exchange, leading to little need for chemical control of lung breathing. After aquatic overwintering, motor output driving lung breathing was similar to semi‐terrestrial controls suggesting that permanent neural changes convert gill control to lung control during development. However, we identified that central control of lung breathing lost sensitivity to respiratory gases CO2 and O2. This is intriguing because until now, simultaneous absence (or small absolute levels) of centrally mediated CO2‐ and O2‐induced breathing responses has mainly been restricted to immature vertebrates and water breathing fish. Therefore, our findings suggest that a developmental time course overlapping with major environmental changes can convolute interpretations regarding developmental origins of certain phenotypes by masking phenotypic plasticity.
Aquatic overwintering eliminates hypercapnic and hypoxia sensitivity characteristic of adult, air breathing vertebrates
Larval bullfrogs have little necessity for chemical control of lung ventilation because the majority of metabolically produced CO2 is lost across the gills and skin. Although extrapulmonary gas exchange dominates in premetamorphic tadpoles, there is controversy as to whether the central respiratory control system of premetamorphic tadpoles stimulates lung breathing during hypercapnia. Specifically, some reports demonstrate relative increases during hypercapnia (Taylor et al. 2003 a,b; Rousseau et al. 2016), while others do not (Walker et al. 1990; Infantino, 1992; Torgerson et al. 1997). Despite these discrepancies, hypercapnia always results in small absolute (Taylor et al. 2003 b; Taylor & Brundage, 2013; Rousseau et al. 2016) or no significant (Walker et al. 1990; Infantino, 1992; Torgerson et al. 1997; Taylor et al. 2003 a) increases in lung breathing compared with consistent significant and larger absolute increases in postmetamorphic tadpoles and adult bullfrogs. On these grounds, it has been interpreted that CO2 chemoreceptors, although present in the brainstem at early stages of development, increase in importance in ventilatory control of acid–base balance in accordance with the transition to air‐breathing (Gargaglioni & Milsom, 2007; Milsom, 2010). In addition to more uniform and robust CO2 responsiveness, postmetamorphic tadpoles exhibit a central hypoxic ventilatory depression (HVD) that is absent in early‐stage tadpoles (Winmill et al. 2005; Fournier et al. 2007). Similar trends seem to exist in rats and mice since ventilatory responses to CO2 challenge and central HVD increase throughout pre‐ and postnatal development (Saiki & Mortola, 1996; Ramirez et al. 1997; Viemari et al. 2003; Davis et al. 2006; Huang et al. 2010; Hempleman & Pilarski, 2011; Ramanantsoa et al. 2011).
Appearance of ventilatory control through central chemosensing during development seems to have adaptive advantages for controlling breathing during acid–base disturbances, providing a drive to breathe, and maintaining energy homeostasis in air‐breathing vertebrates (Milsom, 2002; Winmill et al. 2005; Milsom, 2010; Guyenet & Bayliss, 2015). This is presumably the case because after central CO2 (Milsom, 2010) and O2 (Ramirez et al. 1997; Winmill et al. 2005) chemoreceptors appear during development, they generally produce predictable and reproducible effects on ventilation. Therefore we found it intriguing that an air‐breather, the adult bullfrog, exposed to fully aquatic overwintering conditions lacks stereotypical increases and decreases in respiratory output during hypercapnia and hypoxia, respectively.
Several lines of evidence suggest that mechanisms underlying CO2 chemosensitivity of lung breathing are reduced following overwintering. First, ventilatory responses to CO2 challenge are reduced following aquatic overwintering in vivo through inability to increase breathing frequency (Santin & Hartzler, 2016 a). Second, although we showed that aquatic overwintered bullfrogs had a higher baseline lung frequency, reduced brainstem chemosensitivity was unlikely to have occurred as a result of a ‘ceiling effect’. CO2/pH sensitivity did not inversely correlate with burst frequency under control conditions (Fig. 4) as would be expected if a ceiling effect prevented further increases in burst frequency during hypercapnia. Third, altered burst properties did not compensate for decreases in frequency sensitivity to hypercapnia (Fig. 5). Fourth, we showed that individual CO2/pH‐sensitive neurons in the locus coeruleus, a brain region involved in increasing lung ventilation during hypercapnia (Noronha‐de‐Souza et al. 2006), have reduced firing responses to CO2 in aquatic overwintered bullfrogs (Fig. 6). Finally, we demonstrated that reduced ability to increase firing during CO2/pH challenge occurs specifically through altering CO2/pH‐dependent processes, since acute cooling (a paradoxical known stimulus of LC neurons) elicits large increases in firing after aquatic overwintering (Fig. 7). This strongly implies that phenotypic plasticity decreases CO2/pH sensitivity in response to aquatic overwintering by acting on chemosensory mechanisms (be they intrinsic or extrinsic to the neuron) and not general dysfunction of neurons in the respiratory control network. Collectively, these data indicate that aquatic overwintering conditions lead to a (at least temporary) reduction of ventilatory (Santin & Hartzler, 2016 a) and central (Figs 2, 3, 4) CO2 chemosensitivity, at least in part, by decreasing CO2‐induced firing responses of presumed respiratory control neurons (Figs 6 and 7).
As with sensitivity to CO2, we provide evidence that aquatic overwintering reduces O2 sensitivity of the respiratory control system. In hypoxia‐intolerant vertebrates including frogs, rats, and mice, a central HVD appears during development and dampens breathing frequency during sustained hypoxia. Unlike the typical HVD of hypoxia‐intolerant adult vertebrates, we observed that bullfrogs emerging from conditions mimicking aquatic overwintering did not contain a central HVD (Fig. 8). Specifically, hypoxia (tissue anoxia) completely and reversibly suppressed respiratory‐related discharge in 7/9 brainstem preparations from semi‐terrestrial adult bullfrogs, while lung bursting persisted in aquatic overwintered bullfrogs. Similar to CO2 sensitivity, continued bursting during hypoxia in adult bullfrogs after overwintering resembles that of immature vertebrates and water breathing fish (Hedrick et al. 1991; Ramirez et al. 1997; Viemari et al. 2003; Neubauer & Sunderram, 2004; Winmill et al. 2005; Côté et al. 2014). Based on evidence that pharmacological inhibition of anaerobic metabolism during hypoxia did not decrease respiratory motor output faster than hypoxia alone, Winmill et al. (2005) suggested that a brainstem oxygen/energy sensor mediates the central HVD in postmetamorphic bullfrogs. Although we did not determine mechanisms here, our results imply that persistent respiratory motor output during hypoxia in overwintered bullfrogs may result from decreasing hypoxia sensitivity of the noradrenergic mechanism(s) responsible for depressing respiratory motor output (Fournier et al. 2007; Fournier & Kinkead, 2008). Alternatively or additionally, aquatic overwintering may improve hypoxia tolerance of the central respiratory control system of adult bullfrogs through enhancement of anaerobically generated ATP as occurs in premetamorphic tadpoles (Winmill et al. 2005) and anoxia‐tolerant turtles (Johnson et al. 1998). Regardless of mechanism, our data demonstrate that unlike most other hypoxia‐intolerant adult vertebrates, bullfrogs that recently emerge from aquatic overwintering conditions do not contain central HVD and therefore resemble juveniles.
Development of respiratory rhythm generation in American bullfrogs
The neurobiological basis for a developmental transition from water to air breathing involves a switch from control of gill to lung ventilation. In premetamorphic bullfrog tadpoles, gill ventilation dominates respiratory motor output, with occasional lung‐related nerve activity (Hedrick, 2005). Activity of the gill/buccal rhythm generator phasically inhibits the lung rhythm generator to suppress the occurrence of lung breaths through GABAB‐dependent mechanisms (Straus et al. 2000). A greater occurrence of lung breaths during metamorphosis corresponds with decreased inhibition of the lung rhythm generator by the gill/buccal rhythm generator and also a switch from a pacemaker‐driven to a network‐driven rhythm generator (Broch et al. 2002; Winmill & Hedrick, 2003; Duchcherer et al. 2013).
Unlike respiratory gas sensitivity of the breathing control system, our results do not suggest that aquatic overwintering results in neuroplasticity that drastically alters mature rhythmogenic processes for lung breathing. Specifically, semi‐terrestrial and aquatic overwintered frogs had a relatively high number of fictive lung breaths and relatively long burst durations as is typical of motor output from mature bullfrogs. Premetamorphic tadpoles typically produce lung bursts at a rate of ≤ 2 min–1, while adult preparations typically produce ∼5–15 lung bursts min–1 (Broch et al. 2002; Winmill et al. 2005) (Fig. 1 A). In fact, brainstem preparations from aquatic overwintered bullfrogs produced lung bursts at a greater frequency compared to controls (Fig. 1 C), although these values fall within the range determined by other studies (Broch et al. 2002; Winmill et al. 2005). Possible mechanisms that could underlie the elevated lung frequency in overwintered frogs may include further disinhibition from the buccal generator on the lung generator (Duchcherer et al. 2013), less tonic inhibition by hypoxia through reductions in O2 sensitivity (Fig. 8), increased excitability of the lung rhythm generator through changes in intrinsic and neuromodulatory processes, or increased excitability of motor neurons involved in lung bursts. In addition to adult‐like burst frequency, burst duration and relative rise time of both cranial nerves remained unchanged (Fig. 1 D and E). Burst duration of respiratory‐related nerve activity tends to increase throughout vertebrate development (Viemari et al. 2003; Hedrick, 2005), presumably, since lung breathing requires increased neural drive to produce more forceful contractions of respiratory muscles. How the respiratory motor system of bullfrogs defends function despite probable inactivity during aquatic overwintering remains unclear; nonetheless, these results suggest that the mature motor pattern dominates throughout adulthood after environmental challenges that diminish air breathing.
Developmental change or phenotypic plasticity coinciding with a developmental time course?
Although the mechanisms by which the environment manipulates development are well appreciated (Gilbert, 2012), a key question remains: is a physiological phenotype observed at a particular developmental stage driven by a genetically determined processes (that may or may not be regulated by the environment) or by environmental factors specific to that stage independent of a developmental trajectory? Our results demonstrate that removal of air breathing in adults through aquatic overwintering, surprisingly, results in specific neuroventilatory responses to CO2 and hypoxia combinatorially observed typically in immature air‐breathers and adult water‐breathing teleost fishes (Milsom, 2010; Côté et al. 2014). Our intriguing findings imply that a developmental trajectory coinciding with major environmental shifts may lead to incorrect conclusions about developmental causes underlying phenotypes by masking what is truly phenotypic plasticity. We acknowledge that a contradictory argument could be made because the proportion of CO2‐stimulated neurons in the respiratory control system from rats has been shown to increase over a developmental time course in cell culture (i.e. independent of native environmental factors), which correlates with similar developmental changes observed in acute brain slices (i.e. native environmental factors present during development) (Wang & Richerson, 1999). However, the magnitude of excitatory and inhibitory firing responses to CO2 in brain slices over development was not reported, making comparisons to results obtained from cell culture difficult to draw. Furthermore, differences in the proportion of CO2‐inhibited neurons were observed between cell culture and brain slices at early time points suggesting that the native developmental environment influences normal cellular chemosensitivity in rats.
Our findings have important implications for identifying proximate causes of phenotypes observed at different stages of development. First, although we performed these experiments in an adult amphibian, our speculation that developmental time course masks phenotypic plasticity likely applies to developing organisms in general. All animals experience internal (e.g. developing organ systems with interacting hormones) (Mueller et al. 2014) and external (e.g. transition from inside to outside of the uterus or egg) environmental changes during development. Therefore, phenotypes at different developmental stages have the potential to be driven by plasticity resulting from the developmental environment (Ho et al. 2011) in parallel with permanent changes associated with a genetic developmental program. Second, it would be difficult, if not impossible, to determine whether phenotypes at specific stages of development result from environmentally induced plasticity (as central chemoreception appears to be) or developmentally directed change (as lung rhythm generation appears to be) with exclusive use of gene manipulation approaches. Although genetic tools are common for deducing generation of phenotypes (Amsterdam & Hopkins, 2006; Guo et al. 2011; Sauvageau et al. 2013), our results imply that exclusive use of these approaches has the potential to dramatically underestimate the role of phenotypic plasticity per se in producing specific phenotypes. In the framework of our study, if a particular genetic manipulation resulted in disruption of the ‘development’ of respiratory chemosensitivity in bullfrogs, then the function of this gene would likely be involved in transducing an environmental signal required for modulating chemosensitivity, but not regulating its development. In contrast, if a genetic manipulation inhibited maturation of the lung rhythm generator, then the interpretation would be that the gene in question is involved in development of lung breathing. Without a comparative environmental physiology approach, this non‐trivial distinction would not have been obvious because conventional laboratory animals develop while irreversibly shifting to novel environments.
In conclusion, when we exposed adult bullfrogs to entirely aquatic environments without lung breathing, the respiratory gas sensitivity of the breathing control system was reduced in magnitude to appear more similar to that of predominately water breathing tadpoles. However, baseline ‘fictive’ breathing frequency remained similar to that of adults. Further studies should determine how mechanisms resulting in the reduced ability for respiratory gases to modulate breathing frequency and maintained basal respiratory motor output after overwintering compare to those of immature tadpoles and semi‐terrestrial adult bullfrogs. Given that our findings suggest that respiratory responses to gas stimuli may be determined by phenotypic plasticity independent of development, future work must acknowledge that overlap of developmental time course with co‐occurring environmental changes may result in an inability to differentiate genetically programmed phenotypes from those generated by phenotypic plasticity per se.
Additional information
Author contributions
J.S. conceived and designed the research; J.S. performed the experiments; J.S. analysed the data; J.S. and L.H. interpreted the results; J.S. wrote the manuscript; J.S. and L.H. edited and revised the manuscript. Both authors have approved the final version of the manuscript and agree to be accountable for all aspects of the work. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Funding
We would like to thank the National Science Foundation (IOS‐1257338; LH) for funding and the Biomedical Sciences PhD Program at Wright State University (J.S.) for stipend support.
Acknowledgements
We appreciate comments on the contents of this manuscript from two anonymous reviewers.
References
- Amsterdam A & Hopkins N (2006). Mutagenesis strategies in zebrafish for identifying genes involved in development and disease. Trends Genet 22, 473–478. [DOI] [PubMed] [Google Scholar]
- Baghdadwala MI, Duchcherer M, Paramonov J & Wilson RJ (2015). Three brainstem areas involved in respiratory rhythm generation in bullfrogs. J Physiol 519, 2941–2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford DF (1983). Winterkill, oxygen relations, and energy metabolism of a submerged dormant amphibian, Rana muscosa . Ecology 1171–1183. [Google Scholar]
- Broch L, Morales RD, Sandoval AV & Hedrick MS (2002). Regulation of the respiratory central pattern generator by chloride‐dependent inhibition during development in the bullfrog (Rana catesbeiana). J Exp Biol 205, 1161–1169. [DOI] [PubMed] [Google Scholar]
- Burggren WW & Infantino RL (1994). The respiratory transition from water to air breathing during amphibian metamorphosis. Am Zool 34, 238–246. [Google Scholar]
- Burggren W & Warburton S (2005). Comparative developmental physiology: an interdisciplinary convergence. Annu Rev Physiol 67, 203–223. [DOI] [PubMed] [Google Scholar]
- Burggren WW & West NH (1982). Changing respiratory importance of gills, lungs and skin during metamorphosis in the bullfrog Rana catesbeiana. Respir Physiol 47, 151–164. [DOI] [PubMed] [Google Scholar]
- Côté É, Rousseau J‐P, Fournier S & Kinkead R (2014). Control of breathing in in vitro brain stem preparation from goldfish (Carassius auratus; Linnaeus). Physiol Biochem Zool 87, 464–474. [DOI] [PubMed] [Google Scholar]
- Davis SE, Solhied G, Castillo M, Dwinell M, Brozoski D & Forster HV (2006). Postnatal developmental changes in CO2 sensitivity in rats. J Appl Physiol 101, 1097–1103. [DOI] [PubMed] [Google Scholar]
- Duchcherer M, Baghdadwala MI, Paramonov J & Wilson RJ (2013). Localization of essential rhombomeres for respiratory rhythm generation in bullfrog tadpoles using a binary search algorithm: rhombomere 7 is essential for the gill rhythm and suppresses lung bursts before metamorphosis. Dev Neurobiol 73, 888–898. [DOI] [PubMed] [Google Scholar]
- Fournier S, Allard M, Roussin S & Kinkead R (2007). Developmental changes in central O2 chemoreflex in Rana catesbeiana: the role of noradrenergic modulation. J Exp Biol 210, 3015–3026. [DOI] [PubMed] [Google Scholar]
- Fournier S & Kinkead R (2008). Role of pontine neurons in central O2 chemoreflex during development in bullfrogs Lithobates catesbeiana . Neurosci 155, 983–996. [DOI] [PubMed] [Google Scholar]
- Galante R, Kubin L, Fishman A & Pack A (1996). Role of chloride‐mediated inhibition in respiratory rhythmogenesis in an in vitro brainstem of tadpole, Rana catesbeiana . J Physiol 492, 545–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gargaglioni LH & Milsom WK (2007). Control of breathing in anuran amphibians. Comp Biochem Physiol A Mol Integr Physiol 147, 665–684. [DOI] [PubMed] [Google Scholar]
- Gdovin M, Torgerson C & Remmers J (1999). The fictively breathing tadpole brainstem preparation as a model for the development of respiratory pattern generation and central chemoreception. Comp Biochem Physiol A Mol Int Physiol 124, 275–286. [DOI] [PubMed] [Google Scholar]
- Gilbert SF (2012). Ecological developmental biology: environmental signals for normal animal development. Evol Dev 14, 20–28. [DOI] [PubMed] [Google Scholar]
- González A, Marin O, Tuinhof R & Smeets WJ (1994). Ontogeny of catecholamine systems in the central nervous system of anuran amphibians: an immunohistochemical study with antibodies against tyrosine hydroxylase and dopamine. J Comp Neurol 346, 63–79. [DOI] [PubMed] [Google Scholar]
- González A & Smeets WJ (1993). Noradrenaline in the brain of the south african clawed frog Xenopus laevis: A study with antibodies against noradrenaline and dopamine‐β‐hydroxylase. J Comp Neurol 331, 363–374. [DOI] [PubMed] [Google Scholar]
- Guo T, Mandai K, Condie BG, Wickramasinghe SR, Capecchi MR & Ginty DD (2011). An evolving NGF‐Hoxd1 signalling pathway mediates development of divergent neural circuits in vertebrates. Nat Neurosci 14, 31–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyenet PG & Bayliss DA (2015). Neural control of breathing and CO2 homeostasis. Neuron 87, 946–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris MB, Wilson RJ, Vasilakos K, Taylor BE & Remmers JE (2002). Central respiratory activity of the tadpole in vitro brain stem is modulated diversely by nitric oxide. Am J Physiol Regul Integr Comp Physiol 283, R417–R428. [DOI] [PubMed] [Google Scholar]
- Hedrick MS ( 2005). Development of respiratory rhythm generation in ectothermic vertebrates. Respir Physiol Neurobiol 149, 29–41. [DOI] [PubMed] [Google Scholar]
- Hedrick MS, Burleson ML, Jones DR & Milsom WK (1991). An examination of central chemosensitivity in an air‐breathing fish (Amia calva). J Exp Biol 155, 165–174. [Google Scholar]
- Hempleman SC & Pilarski JQ (2011). Prenatal development of respiratory chemoreceptors in endothermic vertebrates. Respir Phys Neurobiol 178, 156–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henning SJ (1981). Postnatal development: coordination of feeding, digestion, and metabolism. Am J Physiol Gastrointest Liver Physiol 241, G199–G214. [DOI] [PubMed] [Google Scholar]
- Ho DH, Reed WL & Burggren WW (2011). Egg yolk environment differentially influences physiological and morphological development of broiler and layer chicken embryos. J Exp Biol 214, 619–628. [DOI] [PubMed] [Google Scholar]
- Huang Y‐H, Brown AR, Cross SJ, Cruz J, Rice A, Jaiswal S & Fregosi RF (2010). Influence of prenatal nicotine exposure on development of the ventilatory response to hypoxia and hypercapnia in neonatal rats. J Appl Physiol 109, 149–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imber AN & Putnam RW (2012). Postnatal development and activation of L‐type Ca2+ currents in locus ceruleus neurons: implications for a role for Ca2+ in central chemosensitivity. J Appl Physiol 112, 1715–1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Infantino RL Jr (1992). Ontogeny of ventilatory regulation in the bullfrog Rana catesbeiana. PhD Thesis, University of Massechusets, Amherst.
- Johnson SM, Johnson RA & Mitchell GS (1998). Hypoxia, temperature, and pH/CO2 effects on respiratory discharge from a turtle brain stem preparation. J Appl Physiol 84, 649–660. [DOI] [PubMed] [Google Scholar]
- Kinkead R, Filmyer W, Mitchell G & Milsom W (1994). Vagal input enhances responsiveness of respiratory discharge to central changes in pH/CO2 in bullfrogs. J Appl Physiol 77, 2048–2051. [DOI] [PubMed] [Google Scholar]
- Kogo N, Perry SF & Remmers JE (1994). Neural organization of the ventilatory activity in the frog, Rana catesbeiana. I. J Neurobiol 25, 1067–1079. [DOI] [PubMed] [Google Scholar]
- Köhler S, Doelken SC, Mungall CJ, Bauer S, Firth HV, Bailleul‐Forestier I, Black GC, Brown DL, Brudno M & Campbell J (2014). The Human Phenotype Ontology project: linking molecular biology and disease through phenotype data. Nucleic Acids Res 42, D966–D974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallo M, Wellik DM & Deschamps J (2010). Hox genes and regional patterning of the vertebrate body plan. Dev Biol 344, 7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milsom W (2002). Phylogeny of CO2/H+ chemoreception in vertebrates. Resp Physiol Neurobiol 131, 29–41. [DOI] [PubMed] [Google Scholar]
- Milsom W (2010). The phylogeny of central chemoreception. Respir Physiol Neurobiol 173, 195–200. [DOI] [PubMed] [Google Scholar]
- Monyer H, Seeburg PH & Wisden W (1991). Glutamate‐operated channels: developmentally early and mature forms arise by alternative splicing. Neuron 6, 799–810. [DOI] [PubMed] [Google Scholar]
- Morales RD & Hedrick MS (2002). Temperature and pH/CO2 modulate respiratory activity in the isolated brainstem of the bullfrog Rana catesbeiana . Comp Biochem Physiol A Mol Int Physiol 132, 477–487. [DOI] [PubMed] [Google Scholar]
- Mueller CA, Crossley DA & Burggren WW (2014). The actions of the renin–angiotensin system on cardiovascular and osmoregulatory function in embryonic chickens (Gallus gallus domesticus). Comp Biochem Physiol A Mol Int Physiol 178, 37–45. [DOI] [PubMed] [Google Scholar]
- Neubauer JA & Sunderram J (2004). Oxygen‐sensing neurons in the central nervous system. J Appl Physiol 96, 367–374. [DOI] [PubMed] [Google Scholar]
- Noronha‐de‐Souza CR, Bícego KC, Michel G, Glass ML, Branco LG & Gargaglioni LH (2006). Locus coeruleus is a central chemoreceptive site in toads. Am J Physiol Regul Integr Comp Physiol 291, R997–R1006. [DOI] [PubMed] [Google Scholar]
- Ramanantsoa N, Hirsch M‐R, Thoby‐Brisson M, Dubreuil V, Bouvier J, Ruffault P‐L, Matrot B, Fortin G, Brunet J‐F & Gallego J (2011). Breathing without CO2 chemosensitivity in conditional Phox2b mutants. J Neurosci 31, 12880–12888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez J, Quellmalz U & Wilken B (1997). Developmental changes in the hypoxic response of the hypoglossus respiratory motor output in vitro . J Neurophysiol 78, 383–392. [DOI] [PubMed] [Google Scholar]
- Rose FL & Drotman RB (1967). Anaerobiosis in a frog, Rana pipiens . J Exp Zool 166, 427–431. [DOI] [PubMed] [Google Scholar]
- Rousseau J‐P, Bairam A & Kinkead R (2016). Aldosterone, corticosterone, and thyroid hormone and their influence on respiratory control development in Lithobates catesbeianus: An in vitro study. Resp Physiol Neurobiol 224, 104–113. [DOI] [PubMed] [Google Scholar]
- Saiki C & Mortola JP (1996). Effect of CO2 on the metabolic and ventilatory responses to ambient temperature in conscious adult and newborn rats. J Physiol 491, 261–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakakibara Y ( 1983). The pattern of respiratory nerve activity in the bullfrog. Jpn J Physiol 34, 269–282. [DOI] [PubMed] [Google Scholar]
- Sanders CE & Milsom WK (2001). The effects of tonic lung inflation on ventilation in the American bullfrog Rana catesbeiana Shaw. J Exp Biol 204, 2647–2656. [DOI] [PubMed] [Google Scholar]
- Santin J & Hartzler L (2013). Respiratory signalling of locus coeruleus neurons during hypercapnic acidosis in the bullfrog, Lithobates catesbeianus . Respir Physiol Neurobiol 185, 553–561. [DOI] [PubMed] [Google Scholar]
- Santin J & Hartzler L (2015). Activation state of the hyperpolarization‐activated current (Ih) modulates temperature‐sensitivity of firing in locus coeruleus neurons from bullfrogs. Am J Physiol Regul Integr Comp Physiol 308, R1045–R1061. [DOI] [PubMed] [Google Scholar]
- Santin JM & Hartzler LK (2016. a). Control of lung ventilation following overwintering conditions in bullfrogs, Lithobates catesbeianus . J Exp Biol 219, 2003–2014. [DOI] [PubMed] [Google Scholar]
- Santin JM & Hartzler LK (2016. b). Reassessment of chemical control of breathing in undisturbed bullfrogs, Lithobates catesbeianus, using measurements of pulmonary ventilation. Respir Physiol Neurobiol 224, 80–89. [DOI] [PubMed] [Google Scholar]
- Santin JM, Watters KC, Putnam RW & Hartzler LK (2013). Temperature influences neuronal activity and CO2/pH sensitivity of locus coeruleus neurons in the bullfrog, Lithobates catesbeianus . Am J Physiol Regul Integr Comp Physiol 305, R1451–R1464. [DOI] [PubMed] [Google Scholar]
- Sauvageau M, Goff LA, Lodato S, Bonev B, Groff AF, Gerhardinger C, Sanchez‐Gomez DB, Hacisuleyman E, Li E & Spence M (2013). Multiple knockout mouse models reveal lincRNAs are required for life and brain development. Elife 2, e01749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao X & Feldman J (2005). Cholinergic neurotransmission in the preBötzinger complex modulates excitability of inspiratory neurons and regulates respiratory rhythm. Neurosci 130, 1069–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinner J, Zarlinga N & Orcutt S (1994). Overwintering behaviour of adult bullfrogs, Rana catesbeiana, in northeastern Ohio. Ohio J Sci 94, 8–13. [Google Scholar]
- Straus C, Wilson RJ & Remmers JE (2000). Developmental disinhibition: turning off inhibition turns on breathing in vertebrates. J Neurobiol 45, 75–83. [DOI] [PubMed] [Google Scholar]
- Sundin L, Burleson ML, Sanchez AP, Amin‐Naves J, Kinkead R, Gargaglioni LH, Hartzler LK, Wiemann M, Kumar P & Glass ML (2007). Respiratory chemoreceptor function in vertebrates—comparative and evolutionary aspects. Int Comp Biol 47, 592–600. [DOI] [PubMed] [Google Scholar]
- Tattersall GJ & Ultsch GR (2008). Physiological ecology of aquatic overwintering in ranid frogs. Biol Rev 83, 119–140. [DOI] [PubMed] [Google Scholar]
- Taylor BE & Brundage CM (2013). Chronic, but not acute, ethanol exposure impairs central hypercapnic ventilatory drive in bullfrog tadpoles. Respir Physiol Neurobiol 185, 533–542. [DOI] [PubMed] [Google Scholar]
- Taylor BE, Harris MB, Coates EL, Gdovin MJ & Leiter J (2003. a). Central CO2 chemoreception in developing bullfrogs: anomalous response to acetazolamide. J Appl Physiol 94, 1204–1212. [DOI] [PubMed] [Google Scholar]
- Taylor BE, Harris MB, Leiter J & Gdovin MJ (2003. b). Ontogeny of central CO2 chemoreception: chemosensitivity in the ventral medulla of developing bullfrogs. Am J Physiol Regul Integr Comp Physiol 285, R1461–R1472. [DOI] [PubMed] [Google Scholar]
- Torgerson C, Gdovin M & Remmers J (1997). Ontogeny of central chemoreception during fictive gill and lung ventilation in an in vitro brainstem preparation of Rana catesbeiana . J Exp Biol 200, 2063–2072. [DOI] [PubMed] [Google Scholar]
- Ultsch GR, Reese SA & Stewart E (2004). Physiology of hibernation in Rana pipiens: metabolic rate, critical oxygen tension, and the effects of hypoxia on several plasma variables. J Exp Zool A Comp Exp Biol 301, 169–176. [DOI] [PubMed] [Google Scholar]
- Viemari JC, Burnet H, Bévengut M & Hilaire G (2003). Perinatal maturation of the mouse respiratory rhythm‐generator: in vivo and in vitro studies. Eur J Neurosci 17, 1233–1244. [DOI] [PubMed] [Google Scholar]
- Walker R, Galante R, Fishman A & Pack A (1990). Effect of GABA on gill and lung ventilation in an in vitro isolated brain stem preparation in the tadpole. Physiologist 33, A35. [Google Scholar]
- Wang W & Richerson G (1999). Development of chemosensitivity of rat medullary raphe neurons. Neuroscience 90, 1001–1011. [DOI] [PubMed] [Google Scholar]
- Willis YL, Moyle DL & Baskett TS (1956). Emergence, breeding, hibernation, movements and transformation of the bullfrog, Rana catesbeiana, in Missouri. Copeia, 30–41. [Google Scholar]
- Winmill RE, Chen AK & Hedrick MS (2005). Development of the respiratory response to hypoxia in the isolated brainstem of the bullfrog Rana catesbeiana . J Exp Biol 208, 213–222. [DOI] [PubMed] [Google Scholar]
- Winmill RE & Hedrick MS (2003). Developmental changes in the modulation of respiratory rhythm generation by extracellular K+ in the isolated bullfrog brainstem. J Neurobiol 55, 278–287. [DOI] [PubMed] [Google Scholar]


