Abstract
Key points
Recent evidence indicates that metaboreflex regulates heart rate recovery after exercise (HRR).
An increased metaboreflex activity during the post‐exercise period might help to explain the reduced HRR observed in hypertensive subjects.
Using lower limb circulatory occlusion, the present study showed that metaboreflex activation during the post‐exercise period delayed HRR in never‐treated hypertensive men compared to normotensives.
These findings may be relevant for understanding the physiological mechanisms associated with autonomic dysfunction in hypertensive men.
Abstract
Muscle metaboreflex influences heart rate (HR) regulation after aerobic exercise. Therefore, increased metaboreflex sensitivity may help to explain the delayed HR recovery (HRR) reported in hypertension. The present study assessed and compared the effect of metaboreflex activation after exercise on HRR, cardiac baroreflex sensitivity (cBRS) and heart rate variability (HRV) in normotensive (NT) and hypertensive (HT) men. Twenty‐three never‐treated HT and 25 NT men randomly underwent two‐cycle ergometer exercise sessions (30 min, 70% ) followed by 5 min of inactive recovery performed with (occlusion) or without (control) leg circulatory occlusion (bilateral thigh cuffs inflated to a suprasystolic pressure). HRR was assessed via HR reduction after 30, 60 and 300 s of recovery (HRR30s, HRR60s and HRR300s), as well as by the analysis of short‐ and long‐term time constants of HRR. cBRS was assessed by sequence technique and HRV by the root mean square residual and the root mean square of successive differences between adjacent RR intervals on subsequent 30 s segments. Data were analysed using two‐ and three‐way ANOVA. HRR60s and cBRS were significant and similarly reduced in both groups in the occlusion compared to the control session (combined values: 20 ± 10 vs. 26 ± 9 beats min–1 and 2.1 ± 1.2 vs. 3.2 ± 2.4 ms mmHg−1, respectively, P < 0.05). HRR300s and HRV were also reduced in the occlusion session, although these reductions were significantly greater in HT compared to NT (−16 ± 11 vs. −8 ± 15 beats min–1 for HRR300s, P < 0.05). The results support the role of metaboreflex in HRR and suggest that increased metaboreflex sensitivity may partially explain the delayed HRR observed in HT men.
Keywords: baroreflex sensitivity, exercise pressor reflex, hypertension
Key points
Recent evidence indicates that metaboreflex regulates heart rate recovery after exercise (HRR).
An increased metaboreflex activity during the post‐exercise period might help to explain the reduced HRR observed in hypertensive subjects.
Using lower limb circulatory occlusion, the present study showed that metaboreflex activation during the post‐exercise period delayed HRR in never‐treated hypertensive men compared to normotensives.
These findings may be relevant for understanding the physiological mechanisms associated with autonomic dysfunction in hypertensive men.
Abbreviations
- BLC
blood lactate concentration
- BP
blood pressure
- cBRS
cardiac baroreflex sensitivity
- DBP
diastolic blood pressure
- HFRR
high frequency spectral band of heart rate variability
- HR
heart rate
- HRR
heart rate recovery
- HRpeak
peak heart rate
- HRR30s
heart rate reduction after 30 s of recovery
- HRR60s
heart rate reduction after 60 s of recovery
- HRR300s
heart rate reduction after 300 s of recovery
- HRRt
long‐term time constant of heart rate recovery
- HRV
heart rate variability
- HT
hypertensive group
- LFRR
low frequency spectral band of heart rate variability
- NT
normotensive group
- RMS
root mean square residual of RR intervals
- RMSSD
square root of the mean of the sum of the squares of differences between adjacent normal RR intervals
- RRi
RR intervals
- SBP
systolic blood pressure
- T30
short‐term time constant of heart rate recovery
peak oxygen consumption
minute ventilation
Introduction
Heart rate (HR) responses during exercise are tightly regulated by central (i.e. central command) and peripheral (i.e. mechano‐ and metaboreflex) neural mechanisms (Coote et al. 1971; Goodwin et al. 1972; McCloskey & Mitchell, 1972; Eldridge et al. 1981) and are modulated by arterial baroreflex (Potts, 2006; Fadel & Raven, 2012). Among these mechanisms, muscle metaboreflex arises from the contracting muscle in response to the accumulation of metabolites within the muscle fibre and interstitium (Kaufman et al. 1983; Boushel, 2010). Therefore, during exercise, the increase in muscle metabolites (i.e. H+, ADP, Pi, K+, lactate) activates these metabolically sensitive muscle afferents, which resets the cardiac arterial baroreflex (Ichinose et al. 2008; Fadel & Raven, 2012) and reduces its sensitivity (cBRS) (Hartwich et al. 2011). These co‐ordinated responses contribute to the decreased cardiac parasympathetic and increased cardiac sympathetic activities, resulting in the exercise‐induced increase in HR (White & Raven, 2014).
HR responses after exercise are also under a complex regulation (Coote, 2010; Pecanha et al. 2014). HR recovery (HRR) after exercise presents a monoexponential behaviour with an initial rapid decay, mainly determined by cardiac parasympathetic reactivation, followed by a slow decay that is attributed to a sum of parasympathetic reactivation and sympathetic withdrawal (Imai et al. 1994). Despite such evidence regarding the autonomic determinants of HRR, the role of the metaboreflex in HRR is still unclear. The classic hypothesis is that the progressive removal of metabolites from the muscles during the post‐exercise period would gradually diminish metaboreflex activation, leading to a progressive restoration of baroreflex activity, which could then contribute to increased parasympathetic and decreased sympathetic activity to the heart. This could result in the progressive decrease of HR during recovery (Pecanha et al. 2014). Nevertheless, previous studies employing the circulatory occlusion manoeuvre after exercise to trap metabolites within the muscle did not report delayed HRR despite the maintenance of metaboreflex activation (Iellamo et al. 1999; Ichinose et al. 2002; Fisher et al. 2008), suggesting no metaboreflex involvement in HRR. However, most of these studies were conducted with static or dynamic handgrip exercises (Ichinose et al. 2002; Rondon et al. 2006; Fisher et al. 2008; Sausen et al. 2009; Delaney et al. 2010; McNulty et al. 2014) that involved only a small amount of muscle mass, limiting the production of metabolites. More recent investigations, employing large muscle mass dynamic aerobic exercises, have suggested that the metaboreflex may actively regulate both cBRS and HRR after exercise (Hartwich et al. 2011; Fisher et al. 2013).
This new perspective linking metaboreflex activation with cBRS and HRR provides a potential explanation for the delay in HRR observed in some cardiovascular diseases characterized by autonomic dysfunction and increased metaboreflex sensitivity. Among these diseases, hypertension is a prevalent condition that has been shown to present increased sympathetic nerve activity (Grassi et al. 1998), decreased heart rate variability (HRV) (Pagani & Lucini, 2001), reduced cBRS (Watkins et al. 1996), delayed HRR (Polonia et al. 2006; Erdogan et al. 2011; Aneni et al. 2014; Best et al. 2014) and increased metaboreflex sensitivity (Sausen et al. 2009; Greaney et al. 2014). The delayed HRR in hypertension is already evident in pre‐hypertensives (Erdogan et al. 2011) and is further aggravated by progressive hypertensive levels (Aneni et al. 2014), ultimately contributing to the increased risk of cardiovascular morbidity and mortality in this population (Cole et al. 1999). However, despite the fact that reduced HRR in hypertension has been well documented (Polonia et al. 2006; Erdogan et al. 2011; Aneni et al. 2014; Best et al. 2014), the neural mechanisms responsible for this reduction are poorly understood.
Therefore, in accordance with the previous explanation, it is possible to hypothesize that the delayed HRR in hypertensives may, at least in part, be related to increased metaboreflex activation during recovery, leading to decreased cBRS during the post‐exercise period. To test this hypothesis, the present study was designed to assess and compare, in normotensive (NT) and never‐treated hypertensive (HT) men, the influence of metaboreflex activation after exercise on HRR, cBRS and cardiac autonomic modulation, as assessed after a typical aerobic exercise involving a large muscle mass.
Methods
Ethical approval
After a detailed explanation of the experimental procedures, subjects provided their written informed consent to participate. This study was conducted in accordance with the Declaration of Helsinki, and was approved by the Institutional Ethic Committee (281.905/2013) and registered at the Brazilian Clinical Trials (http://www.ensaiosclinicos.gov.br/rg/RBR-5nw65z).
Subjects
Subjects were recruited through advertisements on the university campus, internet announcements, and in hypertension awareness campaigns. Given the potential effects of menstrual cycle and menopause status on cardiovascular autonomic modulation (Minson et al. 2000) and metaboreflex control (Choi et al. 2012), we decided not to include women in the present study. To participate in the study, subjects needed to be men, aged between 30 and 60 years, and present no diagnosed heart or metabolic disease. In addition, the presence of smoking, a body mass index equal to or greater than 35 kg m–2, medications that could affect cardiovascular responses to exercise, abnormal resting or exercise electrocardiograms and performance of physical exercise more than twice a week were reasons for exclusion from the study.
Subjects were included in the NT or HT groups if their systolic/diastolic blood pressures (SBP/DBP) were, respectively, below 120/80 mmHg or between 140/90 and 159/99 mmHg. In addition, HT subjects should have been newly diagnosed, and never treated with anti‐hypertensive drugs. In addition, they could not have target organ damage or secondary hypertension. Accomplishment with respect to meeting the study criteria was checked by preliminary examinations.
Preliminary evaluation
Before taking part in the experimental sessions, subjects underwent a set of preliminary exams. A subject's health status was investigated through a detailed interview with a physician. Body weight and height were measured using a calibrated scale (Filizola S.A, Personal, Campo Grande, Brazil) and body mass index was calculated. Auscultatory resting blood pressure was assessed using a mercury sphygmomanometer (Uniteq, São Paulo, Brazil), three times after 5 min of seated rest in two distinct visits to the laboratory (Chobanian et al. 2003). HT subjects also underwent a detailed screening in the Hypertension Unit of the General Hospital of the São Paulo University, which included a clinical evaluation and blood and urine analysis to detect target organ damage, secondary hypertension and any other clinical condition that could preclude exercise practice.
Subjects also performed a maximal cardiopulmonary exercise test with assessment of resting and exercise electrocardiograms. The test was conducted on a cycle ergometer (Computrainer TM Pro 3D; RacerMate, Seattle, WA, USA) with an initial workload of 50 W for 3 min followed by increments of 20 W every 3 min until the subjects were unable to keep pedalling at a frequency of 60 rpm. Immediately after the exercise, workload was reduced to 50 W and subjects pedalled for 5 min in recovery. During the test, ventilatory variables were continuously measured using a metabolic cart (CPX Ultima; Medical Graphics Corporation, St Paul, MN, USA). Peak oxygen consumption () and peak HR (HRpeak) were determined as the maximal values attained during the effort (average of 30 s of data), and HRR after the maximal test was assessed through the calculation of the HRR60s index (i.e. HRpeak – HR at 60 s of recovery).
Experimental protocol
All subjects randomly performed two experimental sessions (control and occlusion), which were conducted in a random order and with an interval of at least 48 h between them. The sessions were performed in a temperature‐controlled laboratory (20–22°C) during the morning period (between 07.00 and 11.00 h). The subjects were instructed to arrive in a fasting state and to avoid caffeinated and alcoholic beverages for the previous 24 h. In each session, they received a standardized meal (two 25 g cereal bars and 50 ml of juice), and the experiments began 30 min after this meal.
The experiments started with 10 min of resting in the seated position (pre‐exercise period). Then, the subjects underwent 30 min of exercise on the cycle ergometer (Computrainer TM Pro 3D; RacerMate) at 70% of and 60 rpm. After the exercise, subjects stopped pedalling and remained seated on the cycle ergometer for 5 min (recovery period). In the occlusion session, in the last 30 s of the exercise, bilateral thigh cuffs were inflated to 20 mmHg above the exercise SBP (absolute occlusion pressure: HT = 202 ± 21 mmHg and NT = 180 ± 10 mmHg; P < 0.01) and were kept inflated throughout the recovery period. In the control session, the recovery was conducted without any circulatory occlusion.
Experimental measurements
During the experiments, HR was continuously measured using a three‐lead electrocardiogram (EMG System, São Paulo, Brazil), beat‐by‐beat blood pressure (BP) was obtained via finger photoplethysmography (Finometer; Finapres Medical System, Arnhem, The Netherlands), and respiratory movements were monitored using an elastic thoracic belt (Pneumotrace; UFI, Morro Bay, CA, USA). These signals were continuously acquired from the pre‐exercise until the end of recovery period, via a data acquisition system (Windaq, Dataq Instruments, Akron, OH, USA) and with a sampling rate of 500 Hz per channel. Ventilatory variables (i.e. minute ventilation, , and oxygen consumption, ) were measured breath by breath using a metabolic cart (CPX Ultima; Medical Graphics Corporation). Auscultatory SBP and DBP were assessed in triplicate in the pre‐exercise resting period and in a single measurement at the last minute of exercise using a mercury sphygmomanometer (Uniteq). Capillary blood samples (25 μl) were drawn from the earlobe during the pre‐exercise period, the end of the exercise, and 1 min after the period of circulatory occlusion in the occlusion session, and in a similar moment (6 min after the exercise) during the control session. The individual's rating of perceived exertion was assessed at the end of exercise using Borg's 6–20 scale (3). Finally, subjects were asked to report their thigh pain sensation, using the 0–10 Numerical Pain Rating Scale (17) at the end of recovery.
Data analysis
Cardiovascular data
Data treatment
HR, finger BP and respiratory movement signals were exported into Heart Scope, version 1.3.0.1 (AMPS‐LLC, New York, NY, USA) for the generation of the RR intervals (RRi), beat‐by‐beat BP and breath‐by‐breath respiration time series. All time series were visually inspected and occasional misdetections were manually corrected. Ectopic beats were identified and replaced by interpolated RRi values (less than 2% of the total signal). Pre‐exercise and exercise HR were calculated, respectively, as the average HR measured in the last 5 min of pre‐exercise period and from 15 to 25 min of exercise.
Pre‐exercise HRV
In stationary segments of 250–300 beats of the pre‐exercise HR series, HRV was assessed via spectral analysis using Heart Scope, version 1.3.0.1 (AMPS‐LLC, New York, NY, USA), employing the autoregressive method, and in accordance with the recommendations of the Task Force (Task‐Force, 1996). For this purpose, the spectral components were calculated via the Levinson–Durbin recursion and the order of the model was chosen according to Akaike's criterion (Malliani et al. 1991). Low‐frequency (LFRR) (0.04–0.15 Hz) and high‐frequency (HFRR) (0.15–0.4Hz) components of HRV were expressed in normalized units (nu), which represent the relative contribution of each component for the total power minus the very low frequency component (Task‐Force, 1996). LF/HF ratio was also calculated.
Post‐exercise heart rate recovery analysis
Post‐exercise RRi time series were exported as .txt files into Matlab, version 6.0 (MathWorks, Natick, MA, USA) for the assessment of HRR. The indices calculated were: (i) HRR30s; (ii) HRR60s; (iii) HRR300s [i.e. the absolute difference between peak HR (mean HR obtained in the last 60 s of exercise) and the HR values measured at 30, 60 and 300s of recovery, respectively]; (iv) T30 index [i.e. short‐term time constant of HRR, obtained from the negative reciprocal of the linear regression line between HR and the time in the first 30 s of recovery (25)]; and (v) HRRt (i.e. long‐term time‐constant of HRR obtained after exponential fitting of the HR during the entire 300s of recovery). For the exponential fitting analysis, the non‐linear least squares fitting was performed using the Levenberg–Marquardt algorithm. This algorithm returns a vector of estimated coefficients by the non‐linear fit of the HR responses as a function of time, using the model specified by the equation: HR(t) = HR0 + HRamp.e−t/HRRt + εi, where HR0 is the asymptotic value of HR as t = ∞, HRamp is the difference between peak HR and HR0, HRRt is the time constant, and εi is the residual.
Post‐exercise heart rate variability
Because of the non‐stationary behaviour of RRi during the post‐exercise period, the assessment of HRV during this period was performed using the time‐varying approach proposed by Goldberger et al. (2006). First, the RRi time series was processed using a median filter operation. Then, HRV was assessed through the calculation of the square root of the mean of the sum of the squares of differences between adjacent normal RRi (RMSSD) and the root mean square residual of RRi (RMS) indices on successive non‐overlapped 30 s segments during the entire 5 min of recovery. These analyses were performed using a previously implemented routine in Matlab, version 6.0 (MathWorks) (Peçanha et al. 2015).
Beat‐by‐beat systolic blood pressure
Beat‐by‐beat SBP was analysed in Heart Scope, version 1.3.0.1 (AMPS‐LLC). SBP was determined at rest by the average of the last 5 min of the pre‐exercise period and, during recovery, by the average of each successive 30 s segment during the entire 5 min of recovery.
Spontaneous cardiac baroreflex sensitivity
Spontaneous cBRS was assessed during the pre‐exercise period and during recovery using the sequence technique (Parati et al. 1988). Briefly, Heart Scope, version 1.3.0.1 (AMPS‐LLC) identified sequences of three or more consecutive beats in which SBP and RRi changed in the same direction (at least 1 mmHg for SBP and 4 ms for RRi). In each sequence, the slope of the linear regression line between SBP and RRi was determined (only sequences with r 2 > 0.8 were used) and the mean of the slopes was determined as the mean cBRS.
Ventilatory data
during exercise was determined using the average of the values measured from 15 to 25 min of exercise and was expressed in absolute units (mL min–1) or as percentage of the . was determined during the recovery period by the average of the absolute values (L min–1) obtained for every successive 30 s segment during the entire 5 min of recovery.
Lactate
For the blood lactate concentration (BLC) analysis, 25 μl capillary blood samples were first transferred to microtubes containing 25 μl of 1% sodium fluoride solution. Then, the samples were centrifuged at 2348 g for 5 min at 4°C and stored at −80°C. Plasma BLC was then determined in duplicate using spectrophotometry (wavelength 546 nm; EON, BioTek Inc., Winooski, VT, USA).
Statistical analysis
Based on the results of a pilot study demonstrating a medium effect size (Cohen's f = 0.25) of metaboreflex activation on HRR300s (Peçanha et al. 2015), the required sample size to obtain a power of 0.80 and α of 0.05 was 34 subjects (i.e. 17 subjects per group) (G*Power, version 3.1.9.2; Universität Kiel, Kiel, Germany) (13). To account for possible dropouts, a total sample of 50 subjects (i.e. 25 subjects per group) represented the study goal. Normal distribution was checked using the Shapiro–Wilk test and was rejected for RMS and RMSSD. Thus, these variables were natural log‐transformed (ln) and normality was achieved. A t‐test was employed for comparing baseline characteristics between the groups. Two‐ (session vs. group) or three‐way (session vs. time vs. group) ANOVAs were employed for comparing behaviours between the groups and the sessions. When a main effect or an interaction was significant, post hoc comparisons were made using the Newman–Keuls test. For all analyses, P ≤ 0.05 was considered statistically significant.
Results
Sixty‐seven subjects volunteered for the study and provided their written consent. However, 14 were excluded for not fulfilling the study criteria, and five subjects dropped out during the experiments for various reasons (three because of a lack of time, one as a result of presenting complex arrhythmias and one for initiating a pharmacological treatment). Thus, 48 subjects completed the study protocol. Among them, 23 were HT and 25 were NT. Groups were similar with respect to age, body mass index, HRrest, and HRpeak, whereas SBP and DBP were significantly higher, and HRR60s after the maximal test was significantly lower in HT compared to NT (Table 1).
Table 1.
Subject characteristics
| NT | HT | P | |
|---|---|---|---|
| (n = 25) | (n = 23) | ||
| Age (years) | 45 ± 8 | 43 ± 8 | 0.29 |
| Body mass index (kg m–2) | 28.6 ± 2.7 | 29.4 ± 3.6 | 0.37 |
| HRrest (beats min–1) | 63 ± 7 | 68 ± 10 | 0.06 |
| SBP (mmHg) | 115 ± 4 | 142 ± 9* | <0.01 |
| DBP (mmHg) | 77 ± 2 | 96 ± 3* | <0.01 |
| (ml kg−1 min−1) | 26.6 ± 4.2 | 25.1 ± 4.4 | 0.24 |
| HRpeak (beats min–1) | 169 ± 11 | 169 ± 16 | 0.93 |
| HRR60s after maximal test (beats min−1) | 23 ± 10 | 18 ± 6* | 0.04 |
Values are presented as the mean ± SD.
* P < 0.05 vs. NT.
In both groups, variables measured pre‐exercise and during the exercise were similar in the control and the occlusion sessions (Table 2). In both sessions and groups, exercise intensity corresponded to approximately 70% of and 15 on Borg's 6–20 scale. On the other hand, although similar between the sessions, pre‐exercise HR, SBP, DBP, LFRR and LF/HF, as well as exercise SBP and DBP, were significantly higher in the HT compared to the NT, whereas pre‐exercise HFRR was significantly lower in the HT compared to the NT.
Table 2.
Cardiovascular and metabolic variables measured before and during the exercise in the occlusion and the control sessions in the NT and the HT groups
| NT | HT | ||||||
|---|---|---|---|---|---|---|---|
| Control | Occlusion | Control | Occlusion | P (session) | P (group) | P (interaction) | |
| Pre‐exercise | |||||||
| HR (beats min–1) | 64 ± 8 | 64 ± 8 | 71 ± 8* | 70 ± 8* | 0.45 | <0.01 | 0.56 |
| SBP (mmHg) | 114 ± 6 | 114 ± 6 | 142 ± 11* | 141 ± 8* | 0.47 | <0.01 | 0.96 |
| DBP (mmHg) | 76 ± 4 | 75 ± 5 | 91 ± 5* | 95 ± 5* | 0.19 | <0.01 | 0.20 |
| LFRR (nu) | 46 ± 23 | 45 ± 22 | 63 ± 18* | 63 ± 22* | 0.99 | <0.01 | 0.81 |
| HFRR (nu) | 53 ± 23 | 54 ± 22 | 35 ± 17* | 36 ± 22* | 0.79 | <0.01 | 0.96 |
| LF/HF | 1.6 ± 2.5 | 1.5 ± 2.0 | 3.0 ± 2.9* | 3.0 ± 2.7* | 0.93 | 0.04 | 0.82 |
| Exercise | |||||||
| HR (beats min–1) | 128 ± 13 | 129 ± 12 | 130 ± 13 | 128 ± 14 | 0.70 | 0.94 | 0.18 |
| SBP (mmHg) | 159 ± 17 | 159 ± 10 | 178 ± 19* | 182 ± 21* | 0.49 | <0.01 | 0.17 |
| DBP (mmHg) | 69 ± 8 | 73 ± 7 | 83 ± 7* | 83 ± 9* | 0.18 | <0.01 | 0.10 |
| (mL min–1) | 1478 ± 183 | 1478 ± 193 | 1483 ± 252 | 1446 ± 208 | 0.31 | 0.77 | 0.49 |
| (% peak) | 71 ± 4 | 71 ± 6 | 69 ± 5 | 70 ± 7 | 0.31 | 0.77 | 0.49 |
| RPE (6–20) | 15 ± 2 | 15 ± 2 | 14 ± 2 | 14 ± 2 | 0.21 | 0.30 | 0.87 |
Values are presented as the mean ± SD. RPE, rating of perceived exertion.
* P < 0.05 vs. NT.
At the end of the recovery period, subjects from both groups reported significantly more pain in the occlusion than in the control session (NT: 6 ± 2 vs. 1 ± 2 and HT: 5 ± 2 vs. 1 ± 2 on a 0–10 scale, P < 0.05, respectively). Pain sensation was similar between the NT and HT groups.
HRR indices are presented in Fig. 1. The HT group presented reduced HRR30s and HRR60s compared to NT in both sessions (P = 0.02 and 0.05 for the group main effect, respectively). In addition, HRR60s was reduced in the occlusion compared to the control session, regardless of the group (P < 0.01 for the session main effect). In both groups, the HRR300s index was reduced in the occlusion session compared to the control session, although this reduction was greater in the HT group (P = 0.05 for the session vs. group interaction). T30 and HRRt were similar between the groups and the sessions.
Figure 1. Heart rate recovery indices .
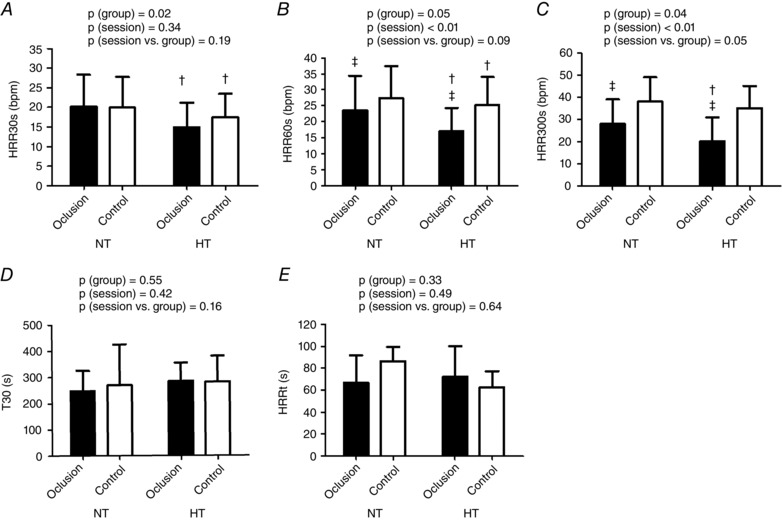
Heart rate recovery indices assessed after the control and the occlusion sessions in the normotensive (NT) and hypertensive (HT) groups. A, heart rate recovery at 30 s of recovery (HRR30s). B, heart rate recovery at 60 s of recovery (HRR60s). C, heart rate recovery at 300 s of recovery (HRR300s). D, short‐term time‐constant of heart rate recovery (T30). E, long‐term time‐constant of heart rate recovery (HRRt). † P ≤ 0.05 vs. NT group. ‡ P ≤ 0.05 vs. control session.
Figure 2 shows RMSSD and RMS behaviours along the recovery in both groups after both sessions. In both groups, RMSSD and RMS were significantly reduced in the occlusion compared to the control session from 120 to 300 s (excluding 180 s for RMS) of recovery (P < 0.01 for the session vs. time interaction). In addition, RMSSD and RMS were further reduced in the occlusion session in the HT compared to the NT (P = 0.04 and 0.03 for the session vs. group interaction, respectively).
Figure 2. Post‐exercise heart rate variability indices .
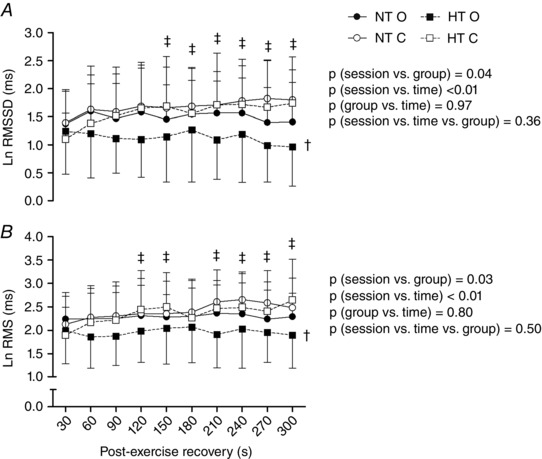
Heart rate variability indices assessed in the first 300s of recovery after the control and the occlusion sessions in the normotensive (NT) and hypertensive (HT) groups. A, square root of the mean of the sum of the squares of differences between adjacent RR intervals in segments of 30s (RMSSD). B, root mean square of residuals of RR intervals in segments of 30s (RMS). NT O, NT group + occlusion session; NT C, NT group + control session; HT O, HT group + occlusion session; HT C, HT group + control session. † P ≤ 0.05 vs. NT. ‡ P ≤ 0.05 vs. control session.
Regardless of the group, in the occlusion session, SBP was increased from 30 to 210 s of recovery (P < 0.01 for the session vs. time interaction) (Fig. 3) and cBRS was reduced during the recovery (P = 0.04 for the session vs. time interaction) (Fig. 3) compared to the control session. Additionally, the HT group presented lower pre‐exercise cBRS compared to the NT group, regardless of the session (P = 0.04 for the group vs. time interaction). Finally, regardless of the group, and BLC during the recovery period were significantly increased in the occlusion compared to the control session (P < 0.01 and P = 0.03 for the session vs. time interaction, respectively) (Fig. 4).
Figure 3. Systolic blood pressure and cardiac baroreflex sensitivity responses .
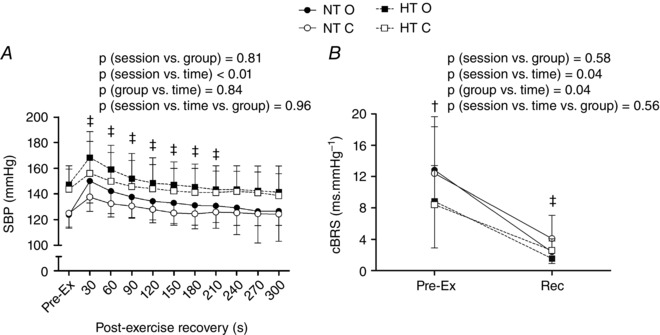
Systolic blood pressure (SBP, A) and cardiac spontaneous baroreflex sensitivity (cBRS, B), measured pre‐ and post‐exercise in the control and the occlusion sessions in the normotensive (NT) and the hypertensive (HT) groups. NT O, NT group + occlusion session; NT C, NT group + control session; HT O, HT group + occlusion session; HT C, HT group + control session. † P ≤ 0.05 vs. NT group. ‡ P ≤ 0.05 vs. control session.
Figure 4. Ventilation and lactate responses .
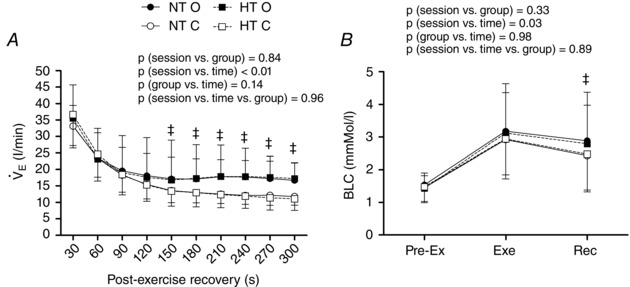
Ventilation (, A) measured during recovery and lactate (BLC, B) measured pre‐exercise, during exercise and at recovery in the control and the occlusion sessions in the normotensive (NT) and the hypertensive (HT) groups. NT O, NT group + occlusion session; NT C, NT group + control session; HT O, HT group + occlusion session; HT C, HT group + control session. ‡ P ≤ 0.05 vs. control session.
Discussion
The main findings of the present study are that: (1) in both NT and HT men, circulatory occlusion reduced HRR60, HRR300, RMSSD, RMS and cBRS and increased SBP, VE and BLC during recovery, indicating that metaboreflex activation delayed HRR and influenced its regulation after a typical aerobic exercise session; (2) occlusion‐induced reductions in HRR300, RMSSD and RMS were greater in HT than in NT men, suggesting a link between greater metaboreflex sensitivity in hypertension and delayed HRR; and (3) occlusion‐induced reductions in cBRS, as well as increases in SBP, VE and BLC, were not different between HT and NT men, suggesting that the greater metaboreflex‐induced delay in HRR for the HT group cannot be attributed to greater changes in baroreflex sensitivity, blood pressure, metabolite accumulation or ventilation.
HRR is controlled via several neural mechanisms and, among them, the role of the muscle metaboreflex is still controversial (Iellamo et al. 1999; Ichinose et al. 2002; Delaney et al. 2010). In the present study, the metaboreflex activation after exercise delayed HRR, as demonstrated by the reduction in HRR60s and HRR300s in the occlusion session compared to the control session. The present study employed a classic method to assess metaboreflex influence, namely the promotion of supra‐systolic circulatory occlusion to trap the metabolites produced during exercise within the muscles, sustaining metaboreflex activation during the recovery period (Bonde‐Petersen et al. 1978). Previous studies using this technique have mainly demonstrated a complete return of HR to pre‐exercise levels during the recovery regardless of the occlusion (Iellamo et al. 1999; Ichinose et al. 2002; Rondon et al. 2006; Fisher et al. 2008; Sausen et al. 2009; Delaney et al. 2010; McNulty et al. 2014), suggesting no role of metaboreflex in HRR. However, most of these studies employed isometric handgrip exercises (Ichinose et al. 2002; Rondon et al. 2006; Fisher et al. 2008; Sausen et al. 2009; Delaney et al. 2010; McNulty et al. 2014). The present study employed a dynamic cycle ergometer exercise, performed at moderate‐to‐high intensity (70% of ) for a long period (30 min), which may have produced more metabolites and stimulated more metabolically sensitive muscle afferent fibres (Rybicki et al. 1984), increasing the metaboreflex activation. Other recent studies, also employing dynamic exercises involving large muscle masses, reported a role of metaboreflex in HRR (Hartwich et al. 2011; Fisher et al. 2013). Hartwich et al. (2011) observed a reduced cBRS and an increased HR in the post‐exercise occlusion period after the leg cycling exercise. Thus, the present study supports a role of metaboreflex in HRR regulation, at least, after dynamic exercise involving large muscle masses.
Mechanisms by which the metaboreflex may influence HRR have yet to be investigated. Interestingly, in the present study, the delay in HRR induced by metaboreflex activation occurred despite the increased SBP during recovery in the occlusion session, and this was expected to accelerate HRR via baroreflex activation (O'Leary, 1993; Iellamo et al. 1999). However, cBRS was similarly reduced in the occlusion session, which probably prevented baroreflex‐induced reduction in HR, suggesting that the influence of metaboreflex on HRR occurs, at least in part, by reducing baroreflex sensitivity. Regarding the autonomic determinants of this response, the present study showed that HRR60, HRR300, RMS and RMSSD were reduced in the occlusion session compared to the control session. Because HRR60s quantifies HRR in the first seconds of recovery and RMSSD measures beat‐to‐beat variability in HR, they are considered as markers of parasympathetic reactivation (Kannankeril et al. 2004; Ng et al. 2009). On the other hand, because HRR300s accounts for the HRR during the entire recovery period and RMS quantifies overall HR fluctuation around a linear trend, they are considered as markers of both parasympathetic reactivation and sympathetic withdrawal (Ng et al. 2009; Pecanha et al. 2014). Thus, because occlusion reduced all of these indices, the present results suggest that metaboreflex delays HRR by slowing both cardiac parasympathetic reactivation and cardiac sympathetic withdrawal during the recovery period.
The evidence suggesting that the muscle metaboreflex may have an influence on HRR after dynamic exercise opens up a new perspective for explaining the potential mechanisms of delayed HRR in cardiovascular diseases characterized by autonomic dysfunction, such as hypertension (Mancia & Grassi, 2014). Indeed, the increased pre‐exercise HR, LFRR and LF/HF, along with the decreased HFRR and cBRS observed in the HT compared to the NT, confirm the presence of autonomic dysfunction in the HT group, which supports one of the basic assumptions of the present study. This autonomic dysfunction in HT culminates with the delay of HRR in this group, as demonstrated by the reduced HRR60s after exercise test and by the reduced HRR30s, HRR60s and HRR300s after the experimental sessions compared to the NT group. These differences in HRR between HT and NT have already been reported in previous studies (Erdogan et al. 2011; Aneni et al. 2014; Best et al. 2014). Erdogan et al. (2011) observed a reduction of 6 beats min–1 in HRR60s in never‐treated HT compared to NT, which is similar to the results of the present study (i.e. a reduction of 5 beats min–1 in HRR60s after the maximal exercise test). Slightly greater differences were reported by Best et al. (2014), who observed a reduction of 10 beats min–1 in HRR60s in washed‐out HT compared to NT. Differences in subject characteristics may account for the different results because the present study, as well as that of Erdogan et al. (2011), investigated middle‐aged never‐treated HTs, whereas Best et al. (2014) studied elderly long‐term HTs. The longer period of hypertension summed with age effects may explain a greater autonomic impairment in the HT of the later study (Gribbin et al. 1971).
The novelty of the present study, however, was to demonstrate the involvement of altered metaboreflex on the delayed HRR response. To the best of our knowledge, this is the first study to show that occlusion‐induced delay on HRR and its regulatory mechanisms were greater in HT (i.e. HRR300s, RMSSD and RMS), which suggests that metaboreflex activation in HT men promotes a greater delay in HRR by slowing post‐exercise cardiac parasympathetic activation and sympathetic withdrawal. The greater metaboreflex‐induced reduction in HRR and cardiac autonomic modulation in the HT group could be explained by a greater metaboreflex stimulus and/or by a greater metaboreflex sensitivity in the HT group. Regarding a greater stimulus, BLC was higher in the occlusion session compared to the control session, showing that the occlusion manoeuvre successfully arrested metabolites and stimulated metaboreflex. However, there was no difference between the NT and HT groups regarding BLC in the occlusion session, despite the delayed HRR in the HT group. This finding suggests that, greater metaboreflex sensitivity, and not a greater stimulus, may have accounted for the delayed HRR observed in the HT group.
Previous studies have already reported increased metaboreflex sensitivity in subjects with hypertension (Sausen et al. 2009; Delaney et al. 2010; Greaney et al. 2014) and pre‐hypertension (Choi et al. 2013; Kim et al. 2015) compared to NT subjects. However, these studies reported an increased response of BP but not HR. Surprisingly, in the present study, BP increases induced by occlusion were not different between the groups. Differences in the exercise and metaboreflex activation protocols may explain the disparities. Accordingly, an increased BP response to forearm metaboreflex activation is mainly attributed to sympathetic vasoconstriction produced in other vascular beds, such as splanchnic, renal and lower limbs (Wallin et al. 1989; Boushel, 2010), and this effect is particularly exacerbated in HT (Delaney et al. 2010). However, both splanchnic and renal beds are already constricted during moderate‐to‐high intensity dynamic exercise (Perko et al. 1998). In addition, with lower limb metaboreflex activation, the legs, which account for a substantial part of the vasculature, are already under a complete mechanical‐induced occlusion that is similar between NT and HT. Thus, only a few vascular beds remain available for further increases in vasoconstriction in HT. Taken together, these methodological aspects may explain why BP increase during lower limb metaboreflex activation was not different between NT and HT.
The differences between HT and NT men in the metaboreflex‐induced reduction in HRR appear to be independent of cBRS responses because there was no difference in cBRS response to the occlusion session between groups. Thus, other mechanisms might be involved. According to Potts et al. (2006), neurogenic inputs from skeletal muscles can directly activate sympathetic premotor neurons in the rostral ventrolateral medulla, leading to sympathetic‐mediated increases in HR and SBP that are independent of baroreflex. Other studies have demonstrated increased excitability of rostral ventrolateral medulla neurons in HT compared to NT rats (Chan et al. 1991; Ito et al. 2000; Matsuura et al. 2002) and this increased activity is related to the increased sympathetic activity in HT (Fisher & Paton, 2012; Kumagai et al. 2012). These pathways may explain the responses of the present study; however, they were not assessed. Thus, the results of the present study indicate that the greater metaboreflex‐induced delay in HRR observed in HT subjects may occur via a baroreflex independent mechanism, and this should be addressed in future investigations.
The response during the recovery of the occlusion session was also increased compared to the control session, which supports the role of metaboreflex in eliciting ventilatory responses, as demonstrated previously (Piepoli et al. 1995). Although controversial, some studies argue that an overactivation of the metaboreflex–respiratory pathway may help to explain ventilatory imbalance observed during exercise in some cardiovascular diseases (Piepoli et al. 2008; Bruce et al. 2016). In the present study, however, responses to the occlusion session were similar between the NT and HT groups, denying any overactivation of the ventilatory arm of the metaboreflex in hypertension.
It is important to highlight that the results of the present study cannot be directly extrapolated to women. However, even though some studies have demonstrated differences in metaboreflex control of circulation between men and women (Ettinger et al. 1996), it appears that the mechanisms involved in these responses are similar between the sexes (Laprad et al. 1999). Future studies should address these aspects in women. Another important point is the high prevalence of overweight and obese subjects in our sample. Hypertension is commonly associated with weight excess (Bramlage et al. 2004), and the presence of obese and overweight subjects in the sample increases the applicability of the results. However, because obesity may affect cardiovascular and autonomic responses to exercise (Dimkpa & Oji, 2010), it may be argued that obesity partially explains the effects of metaboreflex on HRR in both groups. However, previous studies suggest that obesity reduces instead of increases metaboreflex sensitivity (Negrao et al. 2001; Trombetta et al. 2003). Thus, this factor probably does not explain the present results. In addition, because the presence of being overweight and obese was properly balanced between groups, this factor may not explain the differences between the groups.
An important limitation of the present study is that circulatory occlusion caused pain in the subjects. Thus, it could be speculated that the delayed HRR in the occlusion session was a result of cardiovascular responses to pain instead of to metaboreflex activation. However, increased BLC after the occlusion session, compared to the control session, indicates that circulatory arrest was effective in promoting metabolic stimulus. In addition, Iellamo et al. (1999) demonstrated that leg circulatory occlusion without previous exercise, despite promoting pain, was unable to increase HR. Finally, we performed an additional experiment with five HT and four NT men. They underwent a short exercise bout (∼5 min) at ∼70% of HRmax, and post‐exercise circulatory occlusion was applied for 5 min. Compared to the results of the present study, this protocol generated lower metabolic activation (BLC = 1.9 ± 0.6 mm l−1) and similar pain levels (6 ± 2) and resulted in faster HRR (HRR60s = 30 ± 6 beats min–1 and HRR300s = 40 ± 17 beats min–1). Taken together, such evidence supports the major role of metaboreflex, instead of pain, on the cardiovascular responses observed in the present study. In addition, despite greater pain in the occlusion than in the control session, pain perception was similar between the NT and the HT groups, showing that the greater metaboreflex‐induced delay in HRR for the HT group could not be attributed to pain mechanisms.
Regarding other limitations, it is not possible to rule out the hypothesis that deactivation of cardiopulmonary reflex as a result of reductions in venous return in the occlusion session might have played a role in the HRR delay observed in this session. However, this mechanism could not explain the increased in the occlusion session because deactivation of cardiopulmonary receptors presents a negligible influence on under normoxic conditions (Hildebrandt et al. 2000). Another aspect is that, as a result of technical difficulties, it was not possible to collect venous blood from the leg during the occlusion; however, the use of capillary blood samples is considered as a valid surrogate measure of venous lactate (Dassonville et al. 1998) and the 1 min interval between the cuff release and blood collection from the earlobe was sufficient for the spreading of lactate to the whole body, allowing an adequate evaluation of the impact of post‐exercise circulatory occlusion. Finally, we recognize a limitation when considering lactate as being responsible for metabolic acidosis (Robergs et al. 2004) and even as the main mediator of the metaboreflex responses (Vissing et al. 2001). However, studies have demonstrated that an increased lactate concentration is associated with other metabolites (Sahlin et al. 1976; Robergs et al. 2004) arguably promoting blood acidosis and triggering the metaboreflex response (Boushel, 2010). Thus, increases in BLC during the occlusion session might be considered as an indirect marker of metabolic stress and metaboreflex activation caused by the occlusion protocol.
In conclusion, in NT and HT men, metaboreflex activation after exercise delayed HRR, cardiac parasympathetic activation and sympathetic withdrawal, which was accompanied by a reduction in cBRS. The effect of metaboreflex on delaying HRR was greater in HT men, suggesting an increased metaboreflex sensitivity in this population. However, greater metaboreflex‐induced delay in HRR in HT men occurs independently of changes in cBRS responses.
Additional information
Competing interests
The authors declare that they have no competing interests.
Author contributions
TP, APA, GVS, DMJ and CLMF participated in the conception and design of the study. TP, LCB, RYF, PNS and NDSJ participated in the acquisition and analysis of the data. TP, LCB, RYF, PNS, NDSJ and CLMF were responsible for the interpretation of data. TP, LCB, RYF, PNS, NDSJ and CLMF contributed to drafting the paper. TP, APA, GVS, DMS and CLMF critically reviewed the manuscript. All authors approved the final version of the manuscript and agree to be accountable for all aspects of the work. All authors here listed qualify for authorship and all those who qualify for authorship are listed.
Funding
The present study was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP – 2013/04997‐0 and 2013/05519‐4), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq – 304003‐2014‐0) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES).
Acknowledgements
The authors thank Rhenan Bartels for his assistance with the mathematical procedures, Romulo Cássio de Moraes Bertuzzi for assistance with the lactate analysis, and all of the volunteers for their willingness to participate in the study.
References
- Aneni E, Roberson LL, Shaharyar S, Blaha MJ, Agatston AA, Blumenthal RS, Meneghelo RS, Conceicao RD, Nasir K & Santos RD (2014). Delayed heart rate recovery is strongly associated with early and late‐stage prehypertension during exercise stress testing. Am J Hypertens 27, 514–521. [DOI] [PubMed] [Google Scholar]
- Best SA, Bivens TB, Palmer MD, Boyd KN, Galbreath MM, Okada Y, Carrick‐Ranson G, Fujimoto N, Shibata S & Hastings JL (2014). Heart rate recovery after maximal exercise is blunted in hypertensive seniors. J Appl Physiol 117, 1302–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonde‐Petersen F, Rowell LB, Murray RG, Blomqvist GG, White R, Karlsson E, Campbell W & Mitchell JH (1978). Role of cardiac output in the pressor responses to graded muscle ischemia in man. J Appl Physiol 45, 574–580. [DOI] [PubMed] [Google Scholar]
- Boushel R (2010). Muscle metaboreflex control of the circulation during exercise. Acta Physiol (Oxf) 199, 367–383. [DOI] [PubMed] [Google Scholar]
- Bramlage P, Pittrow D, Wittchen HU, Kirch W, Boehler S, Lehnert H, Hoefler M, Unger T & Sharma AM (2004). Hypertension in overweight and obese primary care patients is highly prevalent and poorly controlled. Am J Hypertens 17, 904–910. [DOI] [PubMed] [Google Scholar]
- Bruce RM, Turner A & White MJ (2016). Ventilatory responses to muscle metaboreflex activation in chronic obstructive pulmonary disease. J Physiol 594, 6025–6035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan RK, Chan YS & Wong TM (1991). Responses of cardiovascular neurons in the rostral ventrolateral medulla of the normotensive Wistar Kyoto and spontaneously hypertensive rats to iontophoretic application of angiotensin II. Brain Res 556, 145–150. [DOI] [PubMed] [Google Scholar]
- Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr, Jones DW, Materson BJ, Oparil S, Wright JT Jr & Roccella EJ (2003). The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 289, 2560–2572. [DOI] [PubMed] [Google Scholar]
- Choi HM, Stebbins CL, Lee OT, Nho H, Lee JH, Chun JM, Kim KA & Kim JK (2013). Augmentation of the exercise pressor reflex in prehypertension: roles of the muscle metaboreflex and mechanoreflex. Appl Physiol Nutr Metab 38, 209–215. [DOI] [PubMed] [Google Scholar]
- Choi HM, Stebbins CL, Nho H, Kim KA, Kim C & Kim JK (2012). Skeletal muscle metaboreflex is enhanced in postmenopausal women. Eur J Appl Physiol 112, 2671–2678. [DOI] [PubMed] [Google Scholar]
- Cole CR, Blackstone EH, Pashkow FJ, Snader CE & Lauer MS (1999). Heart‐rate recovery immediately after exercise as a predictor of mortality. N Engl J Med 341, 1351–1357. [DOI] [PubMed] [Google Scholar]
- Coote JH (2010). Recovery of heart rate following intense dynamic exercise. Exp Physiol 95, 431–440. [DOI] [PubMed] [Google Scholar]
- Coote JH, Hilton SM & Perez‐Gonzalez JF (1971). The reflex nature of the pressor response to muscular exercise. J Physiol 215, 789–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dassonville J, Beillot J, Lessard Y, Jan J, Andre AM, Le Pourcelet C, Rochcongar P & Carre F (1998). Blood lactate concentrations during exercise: effect of sampling site and exercise mode. J Sports Med Phys Fitness 38, 39–46. [PubMed] [Google Scholar]
- Delaney EP, Greaney JL, Edwards DG, Rose WC, Fadel PJ & Farquhar WB (2010). Exaggerated sympathetic and pressor responses to handgrip exercise in older hypertensive humans: role of the muscle metaboreflex. Am J Physiol Heart Circ Physiol 299, H1318–H1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimkpa U & Oji JO (2010). Association of heart rate recovery after exercise with indices of obesity in healthy, non‐obese adults. Eur J Appl Physiol 108, 695–699. [DOI] [PubMed] [Google Scholar]
- Eldridge FL, Millhorn DE & Waldrop TG (1981). Exercise hyperpnea and locomotion: parallel activation from the hypothalamus. Science 211, 844–846. [DOI] [PubMed] [Google Scholar]
- Erdogan D, Gonul E, Icli A, Yucel H, Arslan A, Akcay S & Ozaydin M (2011). Effects of normal blood pressure, prehypertension, and hypertension on autonomic nervous system function. Int J Cardiol 151, 50–53. [DOI] [PubMed] [Google Scholar]
- Ettinger SM, Silber DH, Collins BG, Gray KS, Sutliff G, Whisler SK, McClain JM, Smith MB, Yang QX & Sinoway LI (1996). Influences of gender on sympathetic nerve responses to static exercise. J Appl Physiol (1985) 80, 245–251. [DOI] [PubMed] [Google Scholar]
- Fadel PJ & Raven PB (2012). Human investigations into the arterial and cardiopulmonary baroreflexes during exercise. Exp Physiol 97, 39–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher JP, Adlan AM, Shantsila A, Secher JF, Sorensen H & Secher NH (2013). Muscle metaboreflex and autonomic regulation of heart rate in humans. J Physiol 591, 3777–3788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher JP & Paton JF (2012). The sympathetic nervous system and blood pressure in humans: implications for hypertension. J Hum Hypertens 26, 463–475. [DOI] [PubMed] [Google Scholar]
- Fisher JP, Young CN & Fadel PJ (2008). Effect of muscle metaboreflex activation on carotid‐cardiac baroreflex function in humans. Am J Physiol Heart Circ Physiol 294, H2296–H2304. [DOI] [PubMed] [Google Scholar]
- Goldberger JJ, Le FK, Lahiri M, Kannankeril PJ, Ng J & Kadish AH (2006). Assessment of parasympathetic reactivation after exercise. Am J Physiol Heart Circ Physiol 290, H2446–H2452. [DOI] [PubMed] [Google Scholar]
- Goodwin GM, McCloskey DI & Mitchell JH (1972). Cardiovascular and respiratory responses to changes in central command during isometric exercise at constant muscle tension. J Physiol 226, 173–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grassi G, Cattaneo BM, Seravalle G, Lanfranchi A & Mancia G (1998). Baroreflex control of sympathetic nerve activity in essential and secondary hypertension. Hypertension 31, 68–72. [DOI] [PubMed] [Google Scholar]
- Greaney JL, Matthews EL, Boggs ME, Edwards DG, Duncan RL & Farquhar WB (2014). Exaggerated exercise pressor reflex in adults with moderately elevated systolic blood pressure: role of purinergic receptors. Am J Physiol Heart Circ Physiol 306, H132–H141. [DOI] [PubMed] [Google Scholar]
- Gribbin B, Pickering TG, Sleight P & Peto R (1971). Effect of age and high blood pressure on baroreflex sensitivity in man. Circ Res 29, 424–431. [DOI] [PubMed] [Google Scholar]
- Hartwich D, Dear WE, Waterfall JL & Fisher JP (2011). Effect of muscle metaboreflex activation on spontaneous cardiac baroreflex sensitivity during exercise in humans. J Physiol 589, 6157–6171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrandt W, Ottenbacher A, Schuster M, Baisch F & Bartsch P (2000). Increased hypoxic ventilatory response during hypovolemic stress imposed through head‐up‐tilt and lower‐body negative pressure. Eur J Appl Physiol 81, 470–478. [DOI] [PubMed] [Google Scholar]
- Ichinose M, Saito M, Kondo N & Nishiyasu T (2008). Baroreflex and muscle metaboreflex: control of muscle sympathetic nerve activity. Med Sci Sports Exerc 40, 2037–2045. [DOI] [PubMed] [Google Scholar]
- Ichinose M, Saito M, Wada H, Kitano A, Kondo N & Nishiyasu T (2002). Modulation of arterial baroreflex dynamic response during muscle metaboreflex activation in humans. J Physiol 544, 939–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iellamo F, Pizzinelli P, Massaro M, Raimondi G, Peruzzi G & Legramante JM (1999). Muscle metaboreflex contribution to sinus node regulation during static exercise: insights from spectral analysis of heart rate variability. Circulation 100, 27–32. [DOI] [PubMed] [Google Scholar]
- Imai K, Sato H, Hori M, Kusuoka H, Ozaki H, Yokoyama H, Takeda H, Inoue M & Kamada T (1994). Vagally mediated heart rate recovery after exercise is accelerated in athletes but blunted in patients with chronic heart failure. J Am Coll Cardiol 24, 1529–1535. [DOI] [PubMed] [Google Scholar]
- Ito S, Komatsu K, Tsukamoto K & Sved AF (2000). Excitatory amino acids in the rostral ventrolateral medulla support blood pressure in spontaneously hypertensive rats. Hypertension 35, 413–417. [DOI] [PubMed] [Google Scholar]
- Kannankeril PJ, Le FK, Kadish AH & Goldberger JJ (2004). Parasympathetic effects on heart rate recovery after exercise. J Invest Med 52, 394–401. [DOI] [PubMed] [Google Scholar]
- Kaufman MP, Longhurst JC, Rybicki KJ, Wallach JH & Mitchell JH (1983). Effects of static muscular contraction on impulse activity of groups III and IV afferents in cats. J Appl Physiol Respir Environ Exerc Physiol 55, 105–112. [DOI] [PubMed] [Google Scholar]
- Kim KA, Stebbins CL, Choi HM, Nho H & Kim JK (2015). Mechanisms underlying exaggerated metaboreflex activation in prehypertensive men. Med Sci Sports Exerc 47, 1605–1612. [DOI] [PubMed] [Google Scholar]
- Kumagai H, Oshima N, Matsuura T, Iigaya K, Imai M, Onimaru H, Sakata K, Osaka M, Onami T, Takimoto C, Kamayachi T, Itoh H & Saruta T (2012). Importance of rostral ventrolateral medulla neurons in determining efferent sympathetic nerve activity and blood pressure. Hypertens Res 35, 132–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laprad SL, Augustyniak RA, Hammond RL & O'Leary DS (1999). Does gender influence the strength and mechanisms of the muscle metaboreflex during dynamic exercise in dogs? Am J Physiol Regul Integr Comp Physiol 276, R1203–R1208. [DOI] [PubMed] [Google Scholar]
- Malliani A, Pagani M, Lombardi F & Cerutti S (1991). Cardiovascular neural regulation explored in the frequency domain. Circulation 84, 482–492. [DOI] [PubMed] [Google Scholar]
- Mancia G & Grassi G (2014). The autonomic nervous system and hypertension. Circ Res 114, 1804–1814. [DOI] [PubMed] [Google Scholar]
- Matsuura T, Kumagai H, Kawai A, Onimaru H, Imai M, Oshima N, Sakata K & Saruta T (2002). Rostral ventrolateral medulla neurons of neonatal Wistar–Kyoto and spontaneously hypertensive rats. Hypertension 40, 560–565. [DOI] [PubMed] [Google Scholar]
- McCloskey DI & Mitchell JH (1972). Reflex cardiovascular and respiratory responses originating in exercising muscle. J Physiol 224, 173–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNulty CL, Moody WE, Wagenmakers AJ & Fisher JP (2014). Effect of muscle metaboreflex activation on central hemodynamics and cardiac function in humans. Appl Physiol Nutr Metab 39, 861–870. [DOI] [PubMed] [Google Scholar]
- Minson CT, Halliwill JR, Young TM & Joyner MJ (2000). Influence of the menstrual cycle on sympathetic activity, baroreflex sensitivity, and vascular transduction in young women. Circulation 101, 862–868. [DOI] [PubMed] [Google Scholar]
- Negrao CE, Trombetta IC, Batalha LT, Ribeiro MM, Rondon MU, Tinucci T, Forjaz CL, Barretto AC, Halpern A & Villares SM (2001). Muscle metaboreflex control is diminished in normotensive obese women. Am J Physiol Heart Circ Physiol 281, H469–H475. [DOI] [PubMed] [Google Scholar]
- Ng J, Sundaram S, Kadish AH & Goldberger JJ (2009). Autonomic effects on the spectral analysis of heart rate variability after exercise. Am J Physiol Heart Circ Physiol 297, H1421–H1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Leary DS (1993). Autonomic mechanisms of muscle metaboreflex control of heart rate. J Appl Physiol 74, 1748–1754. [DOI] [PubMed] [Google Scholar]
- Pagani M & Lucini D (2001). Autonomic dysregulation in essential hypertension: insight from heart rate and arterial pressure variability. Auton Neurosci 90, 76–82. [DOI] [PubMed] [Google Scholar]
- Parati G, Di Rienzo M, Bertinieri G, Pomidossi G, Casadei R, Groppelli A, Pedotti A, Zanchetti A & Mancia G (1988) Evaluation of the baroreceptor‐heart rate reflex by 24‐hour intra‐arterial blood pressure monitoring in humans. Hypertension 12, 214–222. [DOI] [PubMed] [Google Scholar]
- Peçanha T, Brito L, Fecchio R, Sousa P, Silva‐Júnior N & Forjaz C (2015). Metaboreflex activation delays heart rate recovery after aerobic exercise. FASEB J 29:1054.4 (abstract). [Google Scholar]
- Pecanha T, Silva‐Junior ND & Forjaz CL (2014). Heart rate recovery: autonomic determinants, methods of assessment and association with mortality and cardiovascular diseases. Clin Physiol Funct Imaging 34, 327–339. [DOI] [PubMed] [Google Scholar]
- Perko MJ, Nielsen HB, Skak C, Clemmesen JO, Schroeder TV & Secher NH (1998). Mesenteric, coeliac and splanchnic blood flow in humans during exercise. J Physiol 513, 907–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piepoli M, Clark AL & Coats AJ (1995). Muscle metaboreceptors in hemodynamic, autonomic, and ventilatory responses to exercise in men. Am J Physiol Heart Circ Physiol 269, H1428–H1436. [DOI] [PubMed] [Google Scholar]
- Piepoli MF, Dimopoulos K, Concu A & Crisafulli A (2008). Cardiovascular and ventilatory control during exercise in chronic heart failure: role of muscle reflexes. Int J Cardiol 130, 3–10. [DOI] [PubMed] [Google Scholar]
- Polonia J, Amaral C, Bertoquini S & Martins L (2006). Attenuation of heart rate recovery after exercise in hypertensive patients with blunting of the nighttime blood pressure fall. Int J Cardiol 106, 238–243. [DOI] [PubMed] [Google Scholar]
- Potts JT (2006). Inhibitory neurotransmission in the nucleus tractus solitarii: implications for baroreflex resetting during exercise. Exp Physiol 91, 59–72. [DOI] [PubMed] [Google Scholar]
- Robergs RA, Ghiasvand F & Parker D (2004). Biochemistry of exercise‐induced metabolic acidosis. Am J Physiol Regul Integr Comp Physiol 287, R502–R516. [DOI] [PubMed] [Google Scholar]
- Rondon MU, Laterza MC, de Matos LD, Trombetta IC, Braga AM, Roveda F, Alves MJ, Krieger EM & Negrao CE (2006). Abnormal muscle metaboreflex control of sympathetic activity in never‐treated hypertensive subjects. Am J Hypertens 19, 951–957. [DOI] [PubMed] [Google Scholar]
- Rybicki KJ, Kaufman MP, Kenyon JL & Mitchell JH (1984). Arterial pressure responses to increasing interstitial potassium in hindlimb muscle of dogs. Am J Physiol Regul Integr Comp Physiol 247, R717–R721. [DOI] [PubMed] [Google Scholar]
- Sahlin K, Harris RC, Nylind B & Hultman E (1976). Lactate content and pH in muscle obtained after dynamic exercise. Pflügers Arch 367, 143–149. [DOI] [PubMed] [Google Scholar]
- Sausen MT, Delaney EP, Stillabower ME & Farquhar WB (2009). Enhanced metaboreflex sensitivity in hypertensive humans. Eur J Appl Physiol 105, 351–356. [DOI] [PubMed] [Google Scholar]
- Task‐Force (1996). Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Eur Heart J 17, 354–381. [PubMed] [Google Scholar]
- Trombetta IC, Batalha LT, Rondon MU, Laterza MC, Kuniyoshi FH, Gowdak MM, Barretto AC, Halpern A, Villares SM & Negrao CE (2003). Weight loss improves neurovascular and muscle metaboreflex control in obesity. Am J Physiol Heart Circ Physiol 285, H974–H982. [DOI] [PubMed] [Google Scholar]
- Vissing J, MacLean DA, Vissing SF, Sander M, Saltin B & Haller RG (2001). The exercise metaboreflex is maintained in the absence of muscle acidosis: insights from muscle microdialysis in humans with McArdle's disease. J Physiol 537, 641–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallin BG, Victor RG & Mark AL (1989). Sympathetic outflow to resting muscles during static handgrip and postcontraction muscle ischemia. Am J Physiol Heart Circ Physiol 256, H105–H110. [DOI] [PubMed] [Google Scholar]
- Watkins LL, Grossman P & Sherwood A (1996). Noninvasive assessment of baroreflex control in borderline hypertension. Comparison with the phenylephrine method. Hypertension 28, 238–243. [DOI] [PubMed] [Google Scholar]
- White DW & Raven PB (2014). Autonomic neural control of heart rate during dynamic exercise: revisited. J Physiol 592, 2491–2500. [DOI] [PMC free article] [PubMed] [Google Scholar]


