Abstract
Key points
Inhibiting Nox2 reactive oxygen species (ROS) production reduced in vivo calcium influx in dystrophic muscle.
The lack of Nox2 ROS production protected against decreased in vivo muscle function in dystrophic mice.
Manganese‐enhanced magnetic resonance imaging (MEMRI) was able to detect alterations in basal calcium levels in skeletal muscle and differentiate disease status.
Administration of Mn2+ did not affect muscle function or the health of the animal, and Mn2+ was cleared from skeletal muscle rapidly.
We conclude that MEMRI may be a viable, non‐invasive technique to monitor molecular alterations in disease progression and evaluate the effectiveness of potential therapies for Duchenne muscular dystrophy.
Abstract
Duchenne muscular dystrophy (DMD) is an X‐linked progressive degenerative disease resulting from a mutation in the gene that encodes dystrophin, leading to decreased muscle mechanical stability and force production. Increased Nox2 reactive oxygen species (ROS) production and sarcolemmal Ca2+ influx are early indicators of disease pathology, and eliminating Nox2 ROS production reduces aberrant Ca2+ influx in young mdx mice, a model of DMD. Various imaging modalities have been used to study dystrophic muscle in vivo; however, they are based upon alterations in muscle morphology or inflammation. Manganese has been used for indirect monitoring of calcium influx across the sarcolemma and may allow detection of molecular alterations in disease progression in vivo using manganese‐enhanced magnetic resonance imaging (MEMRI). Therefore, we hypothesized that eliminating Nox2 ROS production would decrease calcium influx in adult mdx mice and that MEMRI would be able to monitor and differentiate disease status in dystrophic muscle. Both in vitro and in vivo data demonstrate that eliminating Nox2 ROS protected against aberrant Ca2+ influx and improved muscle function in dystrophic muscle. MEMRI was able to differentiate between different pathological states in vivo, with no long‐term effects on animal health or muscle function. We conclude that MEMRI is a viable, non‐invasive technique to differentiate disease status and might provide a means to monitor and evaluate the effectiveness of potential therapies in dystrophic muscle.
Keywords: Duchenne muscular dystrophy, MEMRI, sarcolemmal calcium influx
Key points
Inhibiting Nox2 reactive oxygen species (ROS) production reduced in vivo calcium influx in dystrophic muscle.
The lack of Nox2 ROS production protected against decreased in vivo muscle function in dystrophic mice.
Manganese‐enhanced magnetic resonance imaging (MEMRI) was able to detect alterations in basal calcium levels in skeletal muscle and differentiate disease status.
Administration of Mn2+ did not affect muscle function or the health of the animal, and Mn2+ was cleared from skeletal muscle rapidly.
We conclude that MEMRI may be a viable, non‐invasive technique to monitor molecular alterations in disease progression and evaluate the effectiveness of potential therapies for Duchenne muscular dystrophy.
Abbreviations
- BW
body weight
- Ca2+
calcium
- CT
computed tomography
- DMD
Duchene muscular dystrophy
- EDL
extensor digitorum longus
- ICP‐MS
inductively coupled plasma–mass spectrometry
- Mn2+
manganese
- MnCl2
manganese chloride
- MEMRI
manganese‐enhanced magnetic resonance imaging
- MnR
manganese Ringer
- MRI
magnetic resonance imaging
- mdx mouse
Duchenne muscular dystrophy mouse
- Nox2
NADPH oxidase 2
- NR
normal Ringer
- SNR
signal‐to‐noise ratio
- ROS
reactive oxygen species
- ROI
region of interest
- T1
longitudinal relaxation time constant
- T2
transverse relaxation time
- WT
wild‐type
Introduction
Duchene muscular dystrophy (DMD) is an X‐linked recessive disease, which affects one in every 3500 boys, resulting in progressive muscle atrophy, loss of ambulation and death from cardiac or respiratory failure (Levi et al. 2015). A variety of standard outcome measures, such as muscle strength, timed motor performance and pulmonary function tests, have been used to assess the functional ability and disease progression in DMD patients (Bushby & Connor, 2011). However, many of these tests are affected by the level of effort and mood of the patient, and most of the studies have evaluated ambulatory patients over the age of 6 years, leaving in question their application to patients of different ages with different levels of functional ability (Bushby & Connor, 2011; Rutkove et al. 2014).
Various imaging modalities, such as computed tomography (CT), magnetic resonance imaging (MRI) and ultrasound, have been used with the intent of removing patient subjectivity during testing in order to quantify and monitor disease progression non‐invasively over the entire range of ages and functional abilities (Rutkove et al. 2014; Willcocks et al. 2014, 2016; Shklyar et al. 2015; Zaidman et al. 2015; Hogrel et al. 2016). Computed tomography and ultrasound possess several limitations that hinder their use owing to concerns with radiation, depth of imaging and inter‐ and intra‐operator reliability (Ortolan et al. 2015). Magnetic resonance imaging (MRI) is a useful tool for imaging dystrophic muscle; however, early in disease progression the MRI scans may appear normal given that the technique relies upon the appearance of morphological alterations such as increased inflammation, fibrosis or fatty infiltrates (Ortolan et al. 2015). In addition, the morphological alterations can be influenced by ethnicity and adaptations due to exercise (Ortolan et al. 2015). Therefore, the ability to image molecular alterations in disease pathology, such as calcium influx, may provide a more sensitive measure for tracking disease progression throughout the life of the patient.
Muscular dystrophy is characterized by increased membrane permeability to calcium (Ca2+), resulting in greater Ca2+ influx across the sarcolemma (Tutdibi et al. 1999; De Backer et al. 2002; Pal et al. 2014), and increased NADPH oxidase 2 (Nox2)‐derived reactive oxygen species (ROS; Whitehead et al. 2010; Pal et al. 2014). Previous studies have shown that Ca2+ channel activity (Franco‐Obregon & Lansman, 1994; Vasquez et al. 2012) and Nox2 content and activity (Whitehead et al. 2010) are upregulated before the onset of other pathological symptoms (i.e. immune cell infiltration, oedema and fibrosis). Blocking Ca2+ channels or downregulating Nox2 ROS production in young (5‐week‐old) mdx mouse muscle reduces aberrant sarcolemmal Ca2+ influx and alleviates the pathophysiology associated with dystrophic muscle (Altamirano et al. 2013; Pal et al. 2014). These data support the idea that aberrant ROS production and sarcolemmal Ca2+ influx are crucial early events in the pathophysiology of DMD, prior to the onset of observable histological muscle damage and inflammation.
One in vitro method to assess Ca2+ influx across the sarcolemma is to monitor manganese (Mn2+) quench of the fluorescence of fura‐2 (Tutdibi et al. 1999; Pal et al. 2014), which is a sensitive indicator dye for measuring intracellular Ca2+. Mn2+ is a divalent cation similar in ionic radius and chemical properties to Ca2+ that can pass through various Ca2+ channels, and its paramagnetic properties make it a potent MRI spin lattice relaxation time constant (T1) contrast agent (Naruse & Sokabe, 1993; Dryselius et al. 1999; Takeda, 2003; Waghorn et al. 2009). Manganese‐enhanced MRI (MEMRI) has been used to assess various conditions associated with Ca2+ ions, such as brain activity (Cha et al. 2016; Schroeder et al. 2016), neuronal tract tracing and axonal transport (Inoue et al. 2011; Majid et al. 2014), injury (Rodriguez et al. 2016; Yang et al. 2016), ischaemia–reperfusion (Zhao et al. 2015) and cardiac function (Chen et al. 2012; Andrews et al. 2015). However, it has never been used to assess aberrant Ca2+ handling in dystrophic skeletal muscle. Therefore, we hypothesized that eliminating Nox2 ROS production would decrease aberrant Ca2+ influx and that MEMRI would be able to differentiate disease status in dystrophic muscle.
Methods
Ethical approval
All animal procedures were performed in accordance with National Institutes of Health guidelines and approved by the Institutional Animal Care and Use Committee of Baylor College of Medicine.
Animals
C57Bl/6J [wild‐type (WT)] and C57Bl/10ScSn‐Dmdmdx/J (mdx) mice were purchased from Jackson Laboratories (Bar Harbor, ME, USA) and bred following their breeding strategy. To generate mdx animals lacking Nox2 ROS production, mice lacking p47phox [B6(Cg)‐Ncf1m1J/J, JaxMice], a Nox2 subunit, were crossed with dystrophin‐deficient mice (C57BL/10ScSn‐Dmdmdx/J) to generate p47phox (−/−)/dystrophin‐deficient (−/y) mice (p47−/−/mdx; Pal et al. 2014). All mice had ad libitum access to food and water. At ∼5 months of age, mice were anaesthetized by isoflurane (2%) inhalation for either in vivo experiments or killed by rapid cervical dislocation for in vitro assays.
In vitro force measurements
The extensor digitorum longus (EDL) muscle was surgically dissected. One end of the EDL was attached to a fixed hook and the other to a force transducer (F30; Harvard Apparatus, Holliston, MA, USA) using silk suture (4–0). The muscle was placed in a physiological saline solution containing (mm): 2.0 CaCl2, 120.0 NaCl, 4.0 KCl, 1.0 MgSO4, 25.0 NaHCO3, 1.0 KH2PO4 and 10.0 glucose, pH 7.3, and continuously gassed with 95% O2–5% CO2 at 25°C. Each EDL was incubated at 30°C for 15 min, after which optimal muscle length (L o) and voltage (V max) were adjusted to elicit the maximal twitch force. The 95% O2–5% CO2 solution was changed to either a CaCl2 (NR) or a MnCl2‐modified Ringer (MnR) solution, which was at 30°C, containing (mm): 1.8 CaCl2 or MnCl2, 120.0 NaCl, 4.7 KCl, 0.6 MgSO4, 1.6 NaHCO3, 0.13 NaH2PO4, 7.8 glucose and 20.0 Hepes, pH 7.3. Each EDL was incubated in its respective solution for 2 min, followed by establishing a force–frequency relationship. Force–frequency characteristics were measured at stimulation frequencies of 1, 10, 20, 40, 80, 120 and 150 Hz every minute with pulse and train durations of 0.5 and 250 ms, respectively. At the end of the contractile protocol, muscle length was measured using hand‐held electronic callipers, and fibre bundles were trimmed of excess connective tissue, blotted dry and weighed. Muscle weight and L o were used to estimate cross‐sectional area, and absolute forces were expressed in newtons per centimetre squared (Close, 1972).
Manganese quench assay
Flexor digitorum brevis muscle was surgically isolated and incubated in minimal essential media containing 1% penicillin (10,000 U/ml) and streptomycin (10,000 micrograms/ml) (Pen Strep; Life Technologies, Grand Island, NY, USA) and 0.4% collagenase A (Roche Applied Science, Indianapolis, IN, USA) at 37°C for 2.0 h. To release single fibres, flexor digitorum brevis muscles were gently triturated in minimal essential media containing 10% fetal bovine serum and 1% Pen Strep and incubated in 5% CO2 at 37°C until used, typically 12–36 h later. The fibres were then plated on gel from Engelbreth–Holm–Swarm (ECM) murine sarcoma (Sigma‐Aldrich, St Louis, MO, USA)‐coated 96‐well culture plates (Greiner Bio‐one, Monroe, NC, USA), washed with a Ca2+‐based Hepes modified Ringer solution and incubated with fura‐2 AM (5 μm; TEFLabs, Austin, TX, USA) for 30 min at room temperature. Prior to microscopy, fibres were washed again with a Ca2+‐based Hepes modified Ringer solution, and the dye was allowed to de‐esterify for 20 min at room temperature. Fura‐2 excitation (360 nm/380 nm) and emission (510 nm) were monitored using the IonOptix Myocyte Calcium and Contractility Recording System (IonOptix, Westwood, MA, USA). Baseline fluorescence measurements were monitored for 1 min in a Ca2+‐based Hepes modified Ringer solution; the buffer was then replaced with a Mn2+‐based Hepes modified Ringer solution and monitored for an additional 3.5 min. Data were imported into OriginPro 2015 (OriginLab, Northampton, MA, USA) and, using the 360 nm signal, the background fluorescence rate was subtracted from the Mn2+ quench rate for each cell and normalized to the average rate calculated for WT cells.
Manganese‐enhanced MRI
Animal body weight (BW) was measured 2 days before, on the day of administration and 2 days after either Mn2+–bicine or bicine‐only control buffer. Mice were anaesthetized by inhalation of isoflurane (2%) and given a 40 mg kg−1 i.v. tail infusion (0.4 ml h−1) of manganese (II) chloride tetrahydrate (MnCl2; Sigma‐Aldrich, St Louis, MO, USA) dissolved in a bicine (Sigma‐Aldrich) buffer or a bicine‐only solution (50 mm). The hindlimb lower legs of each mouse were imaged 30 min and 2 days postinfusion. Mice were placed in the prone position on a custom‐built holder, and using a bite bar, the head was secured into a head/nose cone. The hindlimbs were extended, with the feet plantar flexed and individually secured to the holder using tape. The position of the mouse allowed for a head‐first entry as the custom‐built holder entered the magnet. While in the magnet, an air heating system (SA Instruments Inc., Stony Brook, NY, USA) was used to maintain body temperature at 37°C, and a rectal probe and a pressure pad, placed beneath the animal, were used to monitor temperature and respiratory rate, respectively, using the Model 1025 Small Animal Monitoring & Gating System software (SA Instruments Inc.).
Magnetic resonance images were acquired on a 9.4 T Bruker AvanceBiospec Spectrometer, 21‐cm‐bore horizontal scanner with a 35 mm volume resonator (Bruker BioSpin, Billerica, MA, USA) with Paravision 5.1 software (Bruker Biospin). Once positioned in the magnet, a FLASH tripilot multisequence scan was performed to ensure that the legs were ideally positioned for the volume coil. Legs were imaged using a T1‐weighted 2D RARE sequence with fat suppression. Imaging parameters for MEMRI scans were as follows: repetition time (TR) = 614.3 ms, echo time (TE) = 7.6 ms, radio frequency (RF) = 2, field of view (FOV) = 4 cm × 3 cm, slice thickness = 1 mm with no interslice gap, matrix size = 256 × 256, 15 slice, 3 m, 55 s and 910 ms. The default Paravision software fat suppression method was employed using the a −3.5 ppm offset with a gauss512 pulse shape, a 1040.1 Hz bandwidth, a spoiler duration of 2 ms and a spoiler strength of 20%.
Magnetic resonance image processing
To measure the volume of muscle with enhanced Mn2+ contrast, images were imported into Amira 5.1, and regions of interest (ROI) for the soft tissue were masked for the right and left legs. A background four corners mask was also made for the signal‐to‐noise ratio (SNR) calculation. Scans were reconstructed with absolute mapping to raw 2dseq files, and the 2dseq files and mask files were imported into Matlab (Mathworks, Natick, MA, USA) for processing. Analysis for enhancement consisted of application of an SNR cut‐off and distribution analysis of that ROI on each slice. The SNR was calculated using the four corners background and ROI mask method using SNR = R × S ROI/SDAIR where R is the Rician distribution factor, S ROI is the mean signal in the ROI being analysed and SDAIR is the standard deviation of the air signal found using the background four corners mask (Dietrich et al. 2007). If the SNR was below R × 25, that ROI slice was not used for analysis. Evaluation of post‐SNR processed data revealed a >94% inclusion of voxels for analysis. Semi‐automatic selection of muscle enhancement was applied by using a threshold of two standard deviations above the ROI median signal intensity (Durmus et al. 2012; Vohra et al. 2016). Each ROI was processed individually for SNR and threshold, and enhanced voxels were calculated as the number of voxels above the previously described threshold divided by ROI size, without slices discounted by SNR. Owing to the focal, inhomogeneous and differential involvement of pathology within the same muscle in mdx mice, the lower leg muscle enhancement was calculated as the number of enhanced voxels divided by the total processed volume.
In vivo force measurements
Dorsiflexor (tibialis anterior and extensor digitorum longus) force was measured using a dual‐mode lever system (305C‐LR‐FP) with electrical stimulation (701C; Aurora Scientific Inc., Aurora, ON, Canada) 2 days prior to, immediately after and 2 days after buffer administration. On the day of buffer infusion, force was measured within 2 h of buffer administration and immediately after MRI. While anaesthetized, mice were placed on their back, the knee joint was immobilized, and the foot was secured into a footplate. Needle electrodes were inserted below the knee joint, just underneath the skin but above the tibialis anterior. A 1 Hz stimulus was administered to determine optimal electrode placement, and after 1 min the force was measured using a 150 Hz stimulus. Data were analysed using the dynamic muscle control and analysis software (Aurora Scientific Inc.).
Elemental analysis of Mn2+
Inductively coupled plasma–mass spectrometry (ICP‐MS) was used to obtain the Mn2+ concentration quantitatively in different tissues. Using the same MnCl2 administration protocol as MEMRI, Mn2+ levels in the muscles of the lower leg (gastrocnemius, soleus, tibialis anterior and EDL) and the kidneys were measured in non‐infused and MnCl2‐infused mice after 30 min and 2 days. After the mice were killed, all tissues were frozen in liquid nitrogen and stored at −80°C. Tissue samples were lyophilized for 48 h and subsequently placed in glass scintillation vials. Seventy per cent HNO3, trace‐metal grade (Sigma‐Aldrich) was regularly added to the samples while under heat to digest the organic matter. After 7–10 days of constant acid exposure, samples appear as a clear or light yellow liquid. Samples were diluted to 5 ml with an aqueous solution of 2% HNO3, trace‐metal grade and 2% ethanol HPLC grade (CHROMASOLV®; Sigma‐Aldrich), and filtered using a syringe filter (0.22 μm pore size). All samples were analysed with a NexION 300 ICP‐MS (PerkinElmer, Waltham, MA, USA), using lutetium as the internal standard.
Data analysis
A one‐way ANOVA was used to measure statistical differences between groups for the Mn2+ quench assay and frequency specific force production. Two‐way and two‐way repeated‐measures ANOVAs were used where appropriate to determine statistical differences between groups for all other data. Tukey's post hoc test was used when statistical differences were identified. A linear regression analysis was performed to assess the correlation between enhanced voxels assessed by MEMRI and in vivo muscle function. Statistical analysis was performed in Origin Pro (OriginLab Corporation, Northhampton, MA, USA), with significance set a priori at P ≤ 0.05. Data are reported as the means ± SEM, unless otherwise specified.
Results
Genetic deletion of Nox2 activity attenuates Mn2+ influx and recovers force deficits in adult mdx skeletal muscle
Sarcolemmal Ca2+ influx is measured indirectly by Mn2+ entry into the cell and quantified using the rate of dye quench (Tutdibi et al. 1999; De Backer et al. 2002; Pal et al. 2014). Skeletal muscles of mdx mice are known to have elevated Mn2+/Ca2+ influx (Pal et al. 2014) and decreased force production (Pal et al. 2014). Alterations in ROS production have been shown to affect both Ca2+ handling and force production (Pal et al. 2014). Here, we demonstrated that adult mdx mice had an increase in sarcolemmal Mn2+ influx and that eliminating Nox2 ROS production reduced that influx back to WT levels (P ≤ 0.05; Fig. 1 A). The lack of Nox2 ROS production improved EDL muscle function at or above 80 Hz compared with mdx animals (Fig. 1 B). In addition, Mn2+ had no effect on EDL muscle function for any of the genotypes at any tested frequency (Fig. 1 B). Taken together, these data indicate that reducing Nox2 ROS production improves mdx pathology (decreased sarcolemmal Ca2+ influx and increased muscle force production) and provide evidence that Mn2+ administration should not alter muscle function following MEMRI.
Figure 1. Eliminating Nox2 reactive oxygen species (ROS) production reduces Mn2+ influx and protects against force decrements in mdx muscle .
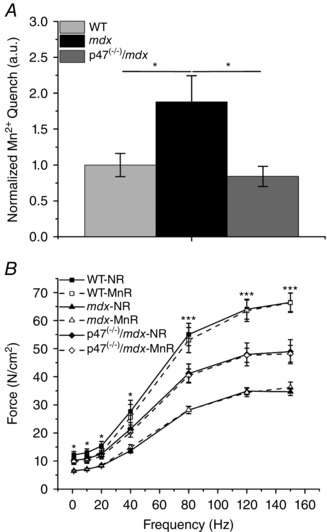
A, sarcolemmal Mn2+ influx is elevated in flexor digitorum brevis fibres of adult mdx skeletal muscle compared with both wild‐type (WT) and p47−/−/mdx muscle, * P ≤ 0.05. B, All three genotypes were significantly different from one another at stimulation frequencies ≥80 Hz, *** P ≤ 0.05. Extensor digitorum longus (EDL) force from WT mice only was greater than that of mdx mice at stimulation frequencies <40 Hz, * P ≤ 0.05. There were no significant differences in force production between EDL muscle in normal Ringer solution (NR; filled symbols and continuous lines) and manganese Ringer solution (MnR; open symbols and dashed lines). Data (means ± SEM) are representative of at least five fibres from at least three animals for Mn2+ quench and at least five animals for force frequency.
Eliminating Nox2 ROS production decreases aberrant sarcolemmal Ca2+ permeability in dystrophic muscle: differentiation of disease status by MEMRI
Mn2+ can enter skeletal muscle fibres, as indicated by our Mn2+ quench data, is paramagnetic, and has been used as an MRI contrast agent (Naruse & Sokabe, 1993; Dryselius et al. 1999; Takeda, 2003; Waghorn et al. 2009; Inoue et al. 2011; Majid et al. 2014). In this study, we used MEMRI to determine the volume of muscle with enhanced Mn2+ contrast. Representative MEMRI images illustrate enhanced contrast in the lower leg of both mdx and p47−/−/mdx mice compared with WT mice or bicine controls (Fig. 2 A). Quantification revealed in vivo enhanced contrast in the lower limb of mdx mice compared with both WT and p47−/−/mdx animals 30 min postinfusion (P ≤ 0.05; Fig. 2 B). Although the p47−/−/mdx animals showed greater enhanced contrast compared with WT mice, the contrast level was lower than that observed with the mdx animals (P ≤ 0.05; Fig. 2 B). Two days after MnCl2 injection, both mdx and p47−/−/mdx contrast was reduced to WT levels and the values were not different from bicine‐injected control mice, indicating that animals had returned to baseline levels (Fig. 2 B).
Figure 2. Nox2 ROS promote sarcolemmal Ca2+ permeability .
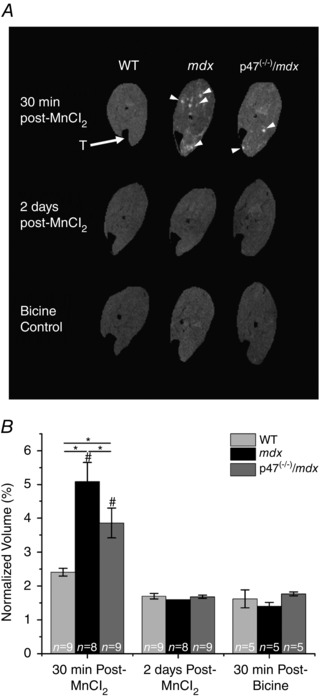
A, representative magnetic resonance images. Both mdx and p47−/−/mdx mice displayed enhanced contrast (arrowheads) 30 min after MnCl2 administration compared with WT mice. Enhanced contrast was almost completely dissipated 2 days postadministration in all three genotypes and was similar to the bicine control buffer images at 30 min after bicine administration. T (arrow) indicates the tibia. B, at 30 min postinfusion, the lower leg of mdx mice showed enhanced contrast compared with WT mice, while the p47−/−/mdx mice demonstrated reduced enhanced voxels compared with mdx mice, * P ≤ 0.05. Enhanced contrast was significantly different between 30 min post‐ and 2 days post‐MnCl2 administration for both mdx and p47−/−/mdx mice, # P ≤ 0.05. All mice returned to baseline values 2 days later, given that there were no differences between the 2 days post‐MnCl2 and 30 min post‐bicine control buffer. Data are means ± SEM unless otherwise noted.
Inductively coupled plasma–mass spectrometry confirmed an increase in lower leg muscle Mn2+ content 30 min postinjection. Muscle Mn2+ content in mdx mice was elevated above WT levels (P ≤ 0.05) and showed a trend to be elevated above p47−/−/mdx mice (P = 0.07), in agreement with the MEMRI data (Fig. 3). The Mn2+ levels were reduced to near baseline levels 2 days postinjection (Fig. 3).
Figure 3. Reduced muscle Mn2+ concentration upon inhibition of Nox2 ROS .
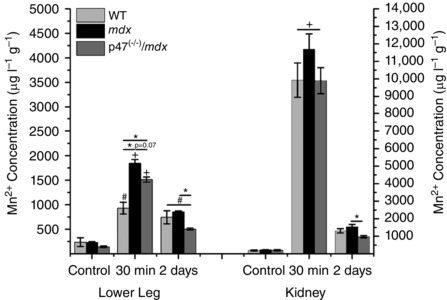
The concentration of Mn2+ increased 30 min postinfusion in both the lower leg and the kidney. The mdx mice showed greater Mn2+ accumulation than WT, whereas p47−/−/mdx demonstrated a trend to have less Mn2+ accumulation than mdx mice in the lower leg; P ≤ 0.05 # vs. control, + vs. control and 2 days, and * between groups. Data (means ± SEM) are representative of at least three animals.
The kidney participates in overall Mn2+ clearance from the body (Kato, 1963; Gerdin, 1985; Hu et al. 2011); therefore, using ICP‐MS we evaluated the Mn2+ concentration in the kidney as an indicator of Mn2+ clearance. Inductively coupled plasma–mass spectrometry data demonstrated elevated Mn2+ content in the kidney 30 min postinjection that was reduced to baseline levels by 2 days (Fig. 3), indicating that the kidney is not accumulating Mn2+ and is likely clearing it from the system.
Nox2 ROS promotes in vivo torque loss in dystrophic muscle
Change in BW is used to assess animal health/disease severity, with a 10–15% decrease in BW within a few days taken to be crucial to the animal's health and a criterion for euthanasia (Foltz & Ullman‐Cullere, 1999; Ray et al. 2010). Although there were initial differences in BW between the various genotypes (Fig. 4 A), the administration of either MnCl2 or bicine had no effect on BW over the course of the protocol (Fig. 4 B). These data indicate that MnCl2 injection had no negative effects on animal health over the course of the study.
Figure 4. Inhibition of Nox2 ROS protects against in vivo torque loss in dystrophic muscle .
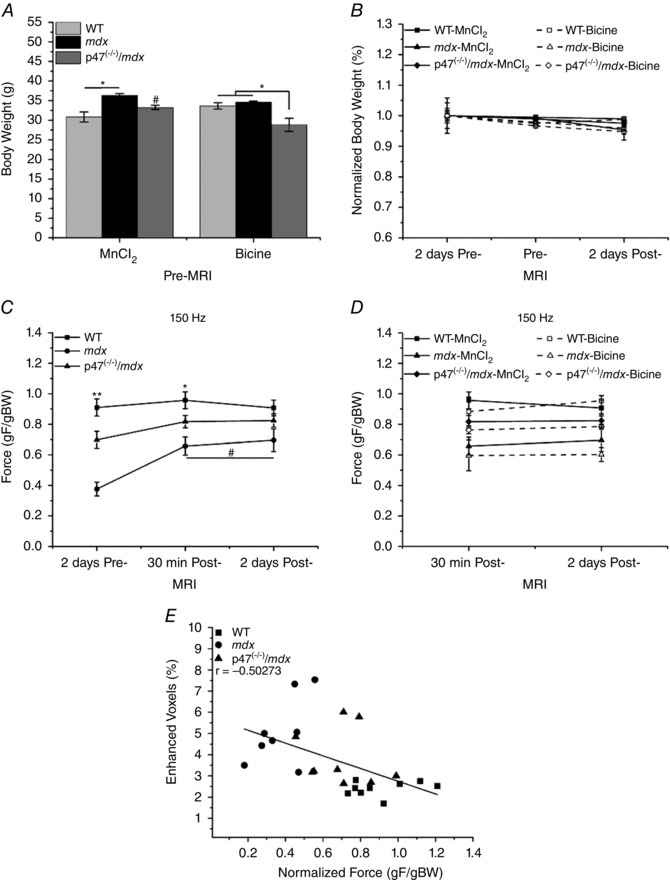
A, initial body weight (BW) was different between WT and mdx mice within the MnCl2 group and between p47−/−/mdx and both WT and mdx mice in the bicine control group, * P ≤ 0.05. Initial BW was different between p47−/−/mdx MnCl2 and p47−/−/mdx bicine‐only groups, # P ≤ 0.05. B, within each genotype, administration of either MnCl2 (filled symbols and continuous lines) or the bicine control buffer (open symbols and dashed lines) had no effect on BW over the course of the study. C, prior to buffer administration, WT, mdx and p47−/−/mdx muscle force production were statistically different from one another, ** P ≤ 0.05. After MnCl2 administration at the 30 min post‐MRI, only WT was different from mdx, * P ≤ 0.05. There were no significant differences in force production between groups after MnCl2 administration at the 2 days post‐MRI. Force production increased in mdx mice after MnCl2 injection at the 30 min post‐ and 2 days post‐MRI compared with pre‐MRI force production, # P ≤ 0.05. D, there were no significant differences in force production within any genotype after MnCl2 (filled symbols and continuous lines) or bicine administration (open symbols and dashed lines) at the 30 min post‐ or 2 days post‐MRI. E, there was a significant correlation between enhanced contrast and normalized force (P ≤ 0.05). Force was normalized to BW (gF/gBW) because of the initial BW differences observed in C. Data are from at least five animals and are represented as means ± SEM.
We have shown protections against decreased in vitro force production in both diaphragm (Pal et al. 2014) and EDL (Fig. 1 B) upon eliminating Nox2 ROS in mdx mice. Here, we demonstrated a 42% loss of in vivo dystrophic muscle function compared with a 20% decrement in the p47−/−/mdx mice, a 53% protection against dystrophy‐induced torque loss (Fig. 4 C). Administration of MnCl2 had no effect on in vivo muscle function compared with the bicine controls (Fig. 4 D). Torque production in WT and p47−/−/mdx mice did not change over the course of the study; however, mdx animals demonstrated an increase in torque following MnCl2 administration that was maintained 2 days postinjection (Fig. 4 C). In addition, we demonstrated a significant correlation (r = −0.50273) between enhanced contrast and muscle function (Fig. 4 E). Taken together, we demonstrated that eliminating Nox2 ROS production protected against in vivo torque loss in mdx skeletal muscle, MnCl2 had no negative effects on muscle function, and decreased muscle function was associated with enhanced sarcolemmal Mn2+ permeability.
Discussion
Dystrophic muscle is characterized by elevated Mn2+/Ca2+ influx across the sarcolemma. Previously, we have shown in young mdx mice that eliminating Nox2 ROS production reduced this influx to WT levels (Pal et al. 2014). Here, we confirmed the increased in vitro Mn2+/Ca2+ influx in adult mdx muscle and extended these findings to include increased in vivo Mn2+/Ca2+ accumulation using a novel technique in skeletal muscle, MEMRI. In addition, eliminating Nox2 ROS in dystrophic muscle protected against that aberrant Ca2+ influx both in vitro and in vivo and recovered significant muscle function compared with dystrophic muscle. Using MEMRI, we were able to monitor changes in contrast intensity over time and differentiate between dystrophic muscle and a treatment group which partly protected against the functional deficits observed in dystrophic muscle. Although it has been possible to detect differences in dystrophic pathology with other imaging modalities (Fan et al. 2014; Rutkove et al. 2014; Willcocks et al. 2014, 2016; Shklyar et al. 2015; Zaidman et al. 2015; Hogrel et al. 2016), they are dependent upon alterations in muscle volume, the development of fibrosis or an increase in fatty infiltrates to detect changes in muscle quality. Although it has been possible to detect molecular alterations in dystrophic muscle with positron emission tomography (Ahmad et al. 2011), to the best of our knowledge the present study is the first investigation to demonstrate the ability of an MRI modality to use molecular alterations in dystrophic muscle to detect differences between dystrophic and healthy muscle as well as to differentiate between dystrophic muscle and a treatment group genetically modified to reduce the pathology.
Although we were able to detect differences in the disease status of dystrophic muscle with MEMRI, it was important to determine whether MnCl2 accumulated or adversely affected muscle function, given that muscle function in dystrophic patients is progressively compromised (Beenakker et al. 2005). Previously, Dodd et al. (2005) have shown that acute MnCl2 administration had no effect on grip strength or endurance, in agreement with our in vitro and in vivo muscle function data. In addition, we found a slight increase in in vivo force production in mdx mice following MnCl2 administration that was sustained 2 days postinjection. Dystrophic skeletal muscle is characterized by increased ROS production (Whitehead et al. 2008, 2010; Pal et al. 2014; Kozakowska et al. 2015), and Mn2+ has been shown to possess an antioxidant capacity (Coassin et al. 1992; Barandier et al. 1998; Eybl & Kotyzová, 2010). In addition, Mn2+ has been used as the backbone in several synthetic antioxidants that demonstrated beneficial effects on muscle function (Doctrow et al. 2002; Kim & Lawler, 2012; Yamada et al. 2015). We are currently investigating whether the improved muscle function observed in mdx mice with Mn2+ administration is attributable to alterations in redox balance.
Although Mn2+ administration had no negative effect on muscle function, there was concern that Mn2+ might accumulate within cells and result in toxicity. The clearance of Mn2+ from most organ systems, except for the brain, appears to occur quickly (1–3 days) following an acute i.v. injection of MnCl2 (Kato, 1963; Ni et al. 1997; Takeda et al. 1998). Our MEMRI and ICP‐MS data are in agreement, because both skeletal muscle and the kidneys demonstrated a rapid clearance of Mn2+ by 2 days postinjection. In the brain, acute dosing results in a biphasic increase in brain Mn2+ levels (Sotogaku et al. 2000) that peaks around 24 h (Gallez et al. 1997; Ni et al. 1997; Sotogaku et al. 2000), followed by a slow gradual decline in Mn2+ levels (Gallez et al. 1997; Takeda et al. 1998). Using similar MnCl2 concentrations, Dodd et al. (2005) found minimal to no neurological deficits after tail vein injection. However, Ponzoni et al. (2002) found significant functional motor impairment after direct injection into the brain. Differences in the results may be explained by the different methods of administration, with tail vein injection (Dodd et al. 2005) resulting in a lower concentration delivered to the brain vs. direct injection into the brain (Ponzoni et al. 2002). Therefore, the amount of Mn2+ necessary in an acute dose to induce neurotoxic effects may need to exceed a specific threshold (Ponzoni et al. 2002). In support of this notion, Lee et al. (2005) demonstrated a dose‐dependent response after acute Mn2+ administration, with doses above 88 mg kg−1 resulting in systemic toxicity due to the inability of the animal to regulate body temperature. Therefore, given that the doses used in our study were significantly less (40 mg kg−1), it is highly unlikely that our mice were at risk for acute Mn2+ toxicity. In addition, multiple injections over 8 or 12 days using comparable doses had minimal to no effect on animal health, locomotion or endocrine response (Grunecker et al. 2010), indicating that repeat MEMRI scans may be possible without development of Mn2+ toxicity.
A variety of imaging modalities have been used to evaluate muscular dystrophy in humans and in animal models. Although many imaging techniques have proved clinically relevant, they rely upon the appearance of morphological alterations in muscle, such as increased fatty infiltrates or fibrosis (Rutkove et al. 2014; Willcocks et al. 2014, 2016; Shklyar et al. 2015; Zaidman et al. 2015; Hogrel et al. 2016), and are limited in their ability to detect differences in individuals where these alterations are minimal or have not yet begun to take place. Here, we demonstrated that MEMRI was able to detect differences in dystrophic muscle as well as differences between dystrophic muscle and a mouse model genetically modified to alleviate the dystrophic pathology. Although this demonstrates the feasibility of using MEMRI to monitor the volume of muscle with enhanced Mn2+ contrast, we do not know the concentration of Mn2+ within the muscle. We are currently using T1 mapping and T1 relaxivity to determine the concentration of Mn2+ within the muscle. We showed that Mn2+ clearance from muscle occurred relatively quickly, which could allow for repeated imaging without an impact on muscle function or accumulation of Mn2+ and toxicity. Mn2+ appears to clear from most tissues quickly; however, future work is needed to establish the lowest dose necessary to detect differences with MEMRI and evaluate neurological function. Given that MEMRI uses the movement of Mn2+ (surrogate for Ca2+) into a cell, an early event in the dystrophic process, it may allow for imaging of molecular alterations in dystrophic muscle across all age groups. We are currently investigating whether MEMRI can be used to differentiate disease status in young animals. Transverse relaxation time (T2)‐weighted MRI imaging is a standard in the field and has been used to assess replacement of muscle with adipose tissue and oedema (Mathur et al. 2011; Heier et al. 2014). Future studies combing T2 imaging and MEMRI would provide unprecedented details of the pathological progression of the dystrophic process.
Our data indicate that eliminating Nox2 ROS in adult mice continued to protect against in vitro and in vivo aberrant calcium influx and functional deficits observed in dystrophic muscle. In combination with our previous data (Pal et al. 2014), enhanced Nox2‐dependent ROS production in dystrophic muscle appears to play a major role in contributing to the observed alterations in calcium influx and subsequent muscle dysfunction. MEMRI is a viable, non‐invasive technique to monitor dystrophic muscle and is sensitive enough to differentiate between healthy muscle, dystrophic muscle and dystrophic muscle modified to alleviate the pathology. A single administration of MnCl2 was quickly cleared, did not appear to induce any toxic effects on the animal's health and had no negative effects on muscle function. Therefore, MEMRI might allow for patients to be imaged frequently, without negative consequences, and throughout their lives to evaluate and monitor disease progression. In addition, the ability of MEMRI to delineate differences in disease severity would provide a tool with which to evaluate and monitor the effectiveness of potential therapies for DMD.
Additional information
Competing interests
None declared.
Author contributions
G.G.R., J.A.L., R.G.P., G.R.S. and L.J.W. were involved with the conception and design of the experiments. J.A.L., G.R.S., W.T.R. and M.H.‐R. acquired the data. All authors were involved in the analysis and interpretation of the data and provided critical input to the writing and revisions of the manuscript. All authors approved the final version of the manuscript and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Funding
Research reported in this publication was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health (R01 AR061370, to G.G.R.), the National Institute of Neurological Disorders and Stroke of the National Institutes of Health (R21 NS085208, to R.G.P. and G.G.R.), the National Heart, Lung, and Blood Institute of the National Institutes of Health (T32 HL007676, to J.A.L.) and a Gillson Longenbaugh Foundation Award (G.G.R.). Additional support was provided by the Welch Foundation (C‐0627, to L.J.W.).
Acknowledgements
The authors would like to thank Drs Glen Walter and Joe Kornegay for their critical discussions and suggestions.
References
- Ahmad N, Welch I, Grange R, Hadway J, Dhanvantari S, Hill D, Lee TY & Hoffman LM (2011). Use of imaging biomarkers to assess perfusion and glucose metabolism in the skeletal muscle of dystrophic mice. BMC Musculoskelet Disord 12, 127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altamirano F, Valladares D, Henríquez‐Olguín C, Casas M, López JR, Allen PD & Jaimovich E (2013). Nifedipine treatment reduces resting calcium concentration, oxidative and apoptotic gene expression, and improves muscle function in dystrophic mdx mice. PLoS ONE 8, e81222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews M, Giger ML & Roman BB (2015). Manganese‐enhanced MRI detection of impaired calcium regulation in a mouse model of cardiac hypertrophy. NMR Biomed 28, 255–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barandier CE, Boucher FR & de Leiris JP (1998). Manganese reduces myocardial reperfusion injury on isolated rat heart. J Mol Cell Cardiol 30, 837–847. [DOI] [PubMed] [Google Scholar]
- Beenakker EA, Maurits NM, Fock JM, Brouwer OF & van der Hoeven JH (2005). Functional ability and muscle force in healthy children and ambulant Duchenne muscular dystrophy patients. Eur J Paediatr Neurol 9, 387–393. [DOI] [PubMed] [Google Scholar]
- Bushby K & Connor E (2011). Clinical outcome measures for trials in Duchenne muscular dystrophy: report from International Working Group meetings. Clin Investig (Lond) 1, 1217–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha M, Lee K, Lee C, Cho JH, Cheong C, Sohn JH & Lee BH (2016). Manganese‐enhanced MR imaging of brain activation evoked by noxious peripheral electrical stimulation. Neurosci Lett 613, 13–18. [DOI] [PubMed] [Google Scholar]
- Chen Y, Payne K, Perara VS, Huang S, Baba A, Matsuda T & Yu X (2012). Inhibition of the sodium–calcium exchanger via SEA0400 altered manganese‐induced T 1 changes in isolated perfused rat hearts. NMR Biomed 25, 1280–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Close RI (1972). Dynamic properties of mammalian skeletal muscles. Physiol Rev 52, 129–197. [DOI] [PubMed] [Google Scholar]
- Coassin M, Ursini F & Bindoli A (1992). Antioxidant effect of manganese. Arch Biochem Biophys 299, 330–333. [DOI] [PubMed] [Google Scholar]
- De Backer F, Vandebrouck C, Gailly P & Gillis JM (2002). Long‐term study of Ca2+ homeostasis and of survival in collagenase‐isolated muscle fibres from normal and mdx mice. J Physiol 542, 855–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich O, Raya JG, Reeder SB, Reiser MF & Schoenberg SO (2007). Measurement of signal‐to‐noise ratios in MR images: influence of multichannel coils, parallel imaging, and reconstruction filters. J Magn Reson Imaging 26, 375–385. [DOI] [PubMed] [Google Scholar]
- Doctrow SR, Huffman K, Marcus CB, Tocco G, Malfroy E, Adinolfi CA, Kruk H, Baker K, Lazarowych N, Mascarenhas J & Malfroy B (2002). Salen–manganese complexes as catalytic scavengers of hydrogen peroxide and cytoprotective agents: structure–activity relationship studies. J Med Chem 45, 4549–4558. [DOI] [PubMed] [Google Scholar]
- Dodd CA, Ward DL & Klein BG (2005). Basal ganglia accumulation and motor assessment following manganese chloride exposure in the C57BL/6 mouse. Int J Toxicol 24, 389–397. [DOI] [PubMed] [Google Scholar]
- Dryselius S, Grapengiesser E, Hellman B & Gylfe E (1999). Voltage‐dependent entry and generation of slow Ca2+ oscillations in glucose‐stimulated pancreatic β‐cells. Am J Physiol Endocrinol Metab 276, E512–E518. [DOI] [PubMed] [Google Scholar]
- Durmus T, Schilling R, Doeblin P, Huppertz A, Hamm B, Taupitz M & Wagner M (2012). Gadobutrol for magnetic resonance imaging of chronic myocardial infarction: intraindividual comparison with gadopentetate dimeglumine. Invest Radiol 47, 183–188. [DOI] [PubMed] [Google Scholar]
- Eybl V & Kotyzová D (2010). Protective effect of manganese in cadmium‐induced hepatic oxidative damage, changes in cadmium distribution and trace elements level in mice. Interdiscip Toxicol 3, 68–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Z, Wang J, Ahn M, Shiloh‐Malawsky Y, Chahin N, Elmore S, Bagnell CR Jr, Wilber K, An H, Lin W, Zhu H, Styner M & Kornegay JN (2014). Characteristics of magnetic resonance imaging biomarkers in a natural history study of golden retriever muscular dystrophy. Neuromuscul Disord 24, 178–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foltz CJ & Ullman‐Cullere MH (1999). Guidelines for assessing the health and condition of mice. Lab Animal 28, 28–32. [PubMed] [Google Scholar]
- Franco‐Obregon A Jr & Lansman JB (1994). Mechanosensitive ion channels in skeletal muscle from normal and dystrophic mice. J Physiol 481, 299–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallez B, Baudelet C, Adline J, Geurts M & Delzenne N (1997). Accumulation of manganese in the brain of mice after intravenous injection of manganese‐based contrast agents. Chem Res Toxicol 10, 360–363. [DOI] [PubMed] [Google Scholar]
- Gerdin BB (1985). Selective tissue accumulation of manganese and its effect on regional blood flow and haemodynamics after intravenous infusion of its chloride salt in the rat. Int J Tissue React 7, 373–380. [PubMed] [Google Scholar]
- Grunecker B, Kaltwasser SF, Peterse Y, Samann PG, Schmidt MV, Wotjak CT & Czisch M (2010). Fractionated manganese injections: effects on MRI contrast enhancement and physiological measures in C57BL/6 mice. NMR Biomed 23, 913–921. [DOI] [PubMed] [Google Scholar]
- Heier CR, Guerron AD, Korotcov A, Lin S, Gordish‐Dressman H, Fricke S, Sze RW, Hoffman EP, Wang P & Nagaraju K (2014). Non‐invasive MRI and spectroscopy of mdx mice reveal temporal changes in dystrophic muscle imaging and in energy deficits. PLoS One 9, e112477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogrel JY, Wary C, Moraux A, Azzabou N, Decostre V, Ollivier G, Canal A, Lilien C, Ledoux I, Annoussamy M, Reguiba N, Gidaro T, Le Moing AG, Cardas R, Voit T, Carlier PG & Servais L (2016). Longitudinal functional and NMR assessment of upper limbs in Duchenne muscular dystrophy. Neurology 86, 1022–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu TCC, Chuang K‐H, Yanasak N & Koretsky A (2011). Relation between blood and cardiac manganese during manganese‐enhanced magnetic resonance imaging (MEMRI) with T1 mapping in the rodent. NMR Biomed 24, 46–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue T, Majid T & Pautler RG (2011). Manganese enhanced MRI (MEMRI): neurophysiological applications. Rev Neurosci 22, 675–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato M (1963). Distribution and excretion of radiomanganese administered to the mouse. Q J Exp Physiol Cogn Med Sci 48, 355–369. [DOI] [PubMed] [Google Scholar]
- Kim JH & Lawler JM (2012). Amplification of proinflammatory phenotype, damage, and weakness by oxidative stress in the diaphragm muscle of mdx mice. Free Radic Biol Med 52, 1597–1606. [DOI] [PubMed] [Google Scholar]
- Kozakowska M, Pietraszek‐Gremplewicz K, Jozkowicz A & Dulak J (2015). The role of oxidative stress in skeletal muscle injury and regeneration: focus on antioxidant enzymes. J Muscle Res Cell Motil 36, 377–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Silva AC, Merkle H & Koretsky AP (2005). Manganese‐enhanced magnetic resonance imaging of mouse brain after systemic administration of MnCl2: dose‐dependent and temporal evolution of T 1 contrast. Magn Reson Med 53, 640–648. [DOI] [PubMed] [Google Scholar]
- Levi O, Genin O, Angelini C, Halevy O & Pines M (2015). Inhibition of muscle fibrosis results in increases in both utrophin levels and the number of revertant myofibers in Duchenne muscular dystrophy. Oncotarget 6, 23249–23260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majid T, Ali YO, Venkitaramani DV, Jang MK, Lu HC & Pautler RG (2014). In vivo axonal transport deficits in a mouse model of fronto‐temporal dementia. Neuroimage Clin 4, 711–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathur S, Vohra RS, Germain SA, Forbes S, Bryant ND, Vandenborne K & Walter GA (2011). Changes in muscle T2 and tissue damage after downhill running in mdx mice. Muscle Nerve 43, 878–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naruse K & Sokabe M (1993). Involvement of stretch‐activated ion channels in Ca2+ mobilization to mechanical stretch in endothelial cells. Am J Physiol Cell Physiol 264, C1037–C1044. [DOI] [PubMed] [Google Scholar]
- Ni Y, Petre C, Bosmans H, Miao Y, Grant D, Baert AL & Marchal G (1997). Comparison of manganese biodistribution and MR contrast enhancement in rats after intravenous injection of MnDPDP and MnCl2 . Acta Radiol 38, 700–707. [DOI] [PubMed] [Google Scholar]
- Ortolan P, Zanato R, Coran A, Beltrame V & Stramare R (2015). Role of radiologic imaging in genetic and acquired neuromuscular disorders. Eur J Transl Myol 25, 5014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal R, Palmieri M, Loehr JA, Li S, Abo‐Zahrah R, Monroe TO, Thakur PB, Sardiello M & Rodney GG (2014). Src‐dependent impairment of autophagy by oxidative stress in a mouse model of Duchenne muscular dystrophy. Nat Commun 5, 4425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponzoni S, Gaziri LCJ, Britto LRG, Barreto WJ & Blum D (2002). Clearance of manganese from the rat substantia nigra following intra‐nigral microinjections. Neurosci Lett 328, 170–174. [DOI] [PubMed] [Google Scholar]
- Ray MA, Johnston NA, Verhulst S, Trammell RA & Toth LA (2010). Identification of markers for imminent death in mice used in longevity and aging research. J Am Assoc Lab Anim Sci 49, 282–288. [PMC free article] [PubMed] [Google Scholar]
- Rodriguez O, Schaefer ML, Wester B, Lee YC, Boggs N, Conner HA, Merkle AC, Fricke ST, Albanese C & Koliatsos VE (2016). Manganese‐enhanced magnetic resonance imaging as a diagnostic and dispositional tool after mild‐moderate blast traumatic brain injury. J Neurotrauma 33, 662–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutkove SB, Geisbush TR, Mijailovic A, Shklyar I, Pasternak A, Visyak N, Wu JS, Zaidman C & Darras BT (2014). Cross‐sectional evaluation of electrical impedance myography and quantitative ultrasound for the assessment of Duchenne muscular dystrophy in a clinical trial setting. Pediatr Neurol 51, 88–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder MP, Weiss C, Procissi D, Wang L & Disterhoft JF (2016). Activity‐induced manganese‐dependent MRI (AIM‐MRI) and functional MRI in awake rabbits during somatosensory stimulation. NeuroImage 126, 72–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shklyar I, Geisbush TR, Mijialovic AS, Pasternak A, Darras BT, Wu JS, Rutkove SB & Zaidman CM (2015). Quantitative muscle ultrasound in Duchenne muscular dystrophy: a comparison of techniques. Muscle Nerve 51, 207–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotogaku N, Oku N & Takeda A (2000). Manganese concentration in mouse brain after intravenous injection. J Neurosci Res 61, 350–356. [DOI] [PubMed] [Google Scholar]
- Takeda A (2003). Manganese action in brain function. Brain Res Brain Res Rev 41, 79–87. [DOI] [PubMed] [Google Scholar]
- Takeda A, Sawashita J & Okada S (1998). Manganese concentration in rat brain: manganese transport from the peripheral tissues. Neurosci Lett 242, 45–48. [DOI] [PubMed] [Google Scholar]
- Tutdibi O, Brinkmeier H, Rüdel R & Föhr KJ (1999). Increased calcium entry into dystrophin‐deficient muscle fibres of MDX and ADR‐MDX mice is reduced by ion channel blockers. J Physiol 515, 859–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasquez I, Tan N, Boonyasampant M, Koppitch KA & Lansman JB (2012). Partial opening and subconductance gating of mechanosensitive ion channels in dystrophic skeletal muscle. J Physiol 590, 6167–6185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vohra RS, Mathur S, Bryant ND, Forbes SC, Vandenborne K & Walter GA (2016). Age‐related T2 changes in hindlimb muscles of mdx mice. Muscle Nerve 53, 84–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waghorn B, Yang Y, Baba A, Matsuda T, Schumacher A, Yanasak N & Hu TC (2009). Assessing manganese efflux using SEA0400 and cardiac T1‐mapping manganese‐enhanced MRI in a murine model. NMR Biomed 22, 874–881. [DOI] [PubMed] [Google Scholar]
- Whitehead NP, Pham C, Gervasio OL & Allen DG (2008). N‐Acetylcysteine ameliorates skeletal muscle pathophysiology in mdx mice. J Physiol 586, 2003–2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehead NP, Yeung EW, Froehner SC & Allen DG (2010). Skeletal muscle NADPH oxidase is increased and triggers stretch‐induced damage in the mdx mouse. PloS One 5, e15354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willcocks RJ, Arpan IA, Forbes SC, Lott DJ, Senesac CS, Senesac E, Deol J, Triplett W, Baligand C, Daniels MJ, Sweeney HL, Walter GA & Vandenborne K (2014). Longitudinal measurements of MRI‐T2 in boys with Duchenne muscular dystrophy: effects of age and disease progression. Neuromusc Disord 24, 393–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willcocks RJ, Rooney WD, Triplett WT, Forbes SC, Lott DJ, Senesac CR, Daniels MJ, Wang DJ, Harrington AT, Tennekoon GI, Russman BS, Finanger EL, Byrne BJ, Finkel RS, Walter GA, Sweeney HL & Vandenborne K (2016). Multicenter prospective longitudinal study of magnetic resonance biomarkers in a large Duchenne muscular dystrophy cohort. Ann Neurol 79, 535–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada T, Abe M, Lee J, Tatebayashi D, Himori K, Kanzaki K, Wada M, Bruton JD, Westerblad H & Lanner JT (2015). Muscle dysfunction associated with adjuvant‐induced arthritis is prevented by antioxidant treatment. Skelet Muscle 5, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Li Q, Wang M, Cao X, Ding Y, Wang G & Liao C (2016). Semiquantitative assessment of optic nerve injury using manganese‐enhanced MRI. Jpn J Radiol 34, 356–365. [DOI] [PubMed] [Google Scholar]
- Zaidman CM, Malkus EC & Connolly AM (2015). Muscle ultrasound quantifies disease progression over time in infants and young boys with Duchenne muscular dystrophy. Muscle Nerve 52, 334–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao DW, Zhang LT, Cheng HY, Zhang YL, Min JY, Xiao HL & Wang Y (2015). Monitoring dynamic alterations in calcium homeostasis by T1‐mapping manganese‐enhanced MRI (MEMRI) in the early stage of small intestinal ischemia‐reperfusion injury. NMR Biomed 28, 958–966. [DOI] [PubMed] [Google Scholar]


