Abstract
Autophagy has been proved to be involved in metastasis of cancers. However, the detailed mechanisms are still unclear. In this work, we aim to provide the first study of the role that autophagy plays in migration and invasion in ovarian cancer cells. Transwell chamber was used to examine migration and invasion capacities. Western blotting and immunofluorescence were performed to investigate the expressions of mesenchymal markers (Vimentin, N-cadherin), epithelial marker (Keratin), transcript factor (Zeb1) and HO-1. Small interfering RNA (siRNA) was used to generate autophagy defect cells (A2780 Atg7 siRNA and Skov-3 Atg7 siRNA cells). Reactive oxygen species (ROS) were examined by flow cytometry. We found Skov-3 cells exhibited a fibroblastoid like phenotype and more invasive ability with lower level of autophagy than A2780. Transwell chamber showed that autophagy inhibition promoted migration and invasion capacities of autophagy defect cells. Western blotting showed that the expressions of mesenchymal markers and transcript factor were up-regulated, while, the expression of epithelial marker was down-regulated in autophagy defect cells. Conversely, autophagy induction could impair the migration and invasion through reversing epithelial-mesenchymal transition (EMT) in A2780 and Skov-3 cells. Besides, autophagy defect could increase the level of intracellular ROS and the expression of HO-1. NAC (ROS scavenging agent) could inhibit the migration and invasion through reversing EMT and decrease the expression of HO-1. What’s more, Znpp (HO-1 inhibitor) impaired the migration and invasion through reversing EMT. In conclusion, our results suggest that autophagy inhibition may promote EMT through ROS/HO-1 pathway in ovarian cancer cells.
Keywords: Autophagy defect, EMT, HO-1, Zeb1, ovarian cancer
Introduction
Ovarian cancer is the leading cause of death among all gynecological malignancies, accounting for 5% of all the new deaths [1]. Though the overall five-year survival rate has been raised up from 36% to 46%, the high rate of distant metastasis is still considered to be the leading cause of recurrence and death in ovarian cancer patients [2]. Based on data from Surveillance, Epidemiology, and End Results Program (SEER) 18 2006-2012, the five-year survival rate was up to 92.1% of patients diagnosed with Stage I. However, over 75% of ovarian cancer patients were diagnosed with metastasis. As a result, the five-year survival rate declined sharply to 17% (stage IV). Hence, it’s of great importance for identifying the molecules or signaling pathways involved in metastasis to improve treatment efficiency and prognosis in ovarian cancer.
Epithelial-mesenchymal transition (EMT) process was firstly identified in the context of embryogenesis, where it made epithelial cells transform to mesenchymal cells [3]. Recently, it has been suggested that EMTs could be classified into three different sub types: a) Type 1 EMT, participating in embryonic development; b) Type 2 EMT, involved in tissue damage, regeneration and organ fibrosis; c) Type 3 EMT, associated with cancer progression and metastasis [4]. Particularly, EMT plays a critical role in tumor spreading and dissemination, which tumor cells lose their epithelial morphology and detach from the primary site and invade surrounding tissues and blood vessels [5]. Emerging evidence has been confirmed that EMT process participates in the onset of metastasis in epithelial ovarian cancer (EOC) [6,7]. Therefore, the targeted treatment that can reverse EMT may benefit EOC patients.
Autophagy has reported to be vital in the maintenance of cellular homeostasis by cellular cleaning through the removal of intracellular components in lysosomes such as long-lived proteins and old or damaged organelles [8-10]. It’s also been proved that autophagy defect is involved in many diseases including diabetes mellitus, neurodegenerative disease and cancer [9,11,12]. However, it’s still highly controversial about the roles of autophagy in cancer onset and metastasis. Up to now, only a few studies report the relationship between autophagy and metastasis. Gulhati et al reported that autophagy could impair the migration and invasion of colon cancer by inhibiting the expressions of RhoA and Rac1 pathways [13]. Later then, autophagy inhibiting migration and invasion of cancers were identified in breast cancer, melanoma, gastric cancer and glioblastoma [14,18]. Meanwhile, it has been clarified that autophagy could reverse EMT by degrading transcript factors of EMT such as Snail, Slug and Twist [14,16]. However, it’s still unknown whether autophagy can impair the migration and invasion in ovarian cancer through reversing EMT.
Reactive oxygen species (ROS) are products of intracellular metabolism in aerobic organisms. In physiological conditions, ROS play a vital role in intracellular signal transduction [19]. Abnormal excess ROS can cause genomic instability, resulting in malignancy and death of cells [20]. It has been demonstrated that autophagy defect increases the intracellular ROS level [21]. Moreover, ROS have been proposed as modulators of the EMT process [4]. Magdalena et al reported that ROS could promote breast cancer cells metastasis through inducing EMT by ROS/NF-κB/Snail pathway [22]. Another study showed EMT could be induced by ROS/NF-κB/HIF-1α pathway, and this could be reversed by ROS scavenging agent (NAC) [18]. Therefore, we speculate that autophagy defect promotes EMT may also by ROS in ovarian cancer.
Heme oxygenase-1 (HO-1) catalyzes the first rate-limiting step in the degradation of cellular heme to liberate free iron, carbon monoxide (CO) and biliverdin in mammalian cells [23]. Under physiological conditions, HO-1 can be induced by different signals and transcript factors such as ROS, NRF-2, NF-κB, AP2 and so on, participating in the maintenance of cellular homeostasis [24]. However, emerging evidence has proved that HO-1 overexpression is found in many cancers and associated with poor prognosis and tumor stage, including renal carcinoma, rectal cancer, thyroid carcinoma and glioblastoma [25,29]. Moreover, it has been reported HO-1 promotes the migration and invasion in pulmonary cancer [30]. However, the mechanisms about HO-1 involved in the metastasis of cancers are not clearly understood. Therefore, whether HO-1 is associated with the migration and invasion caused by autophagy defect needs to be studied in ovarian cancer.
Here we show the first study of the role that autophagy plays in migration and invasion in ovarian cancer cells. Overall, our study supposes that targeting autophagy may become an effective treatment for inhibiting the metastasis of ovarian cancer.
Methods and materials
Cell culture
Human ovarian cancer cell lines, A2780 and Skov-3 cells, were purchased from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China). The A2780 cells were cultured in RPMI 1640 medium (Hyclone, Utah, USA) with 10% fetal bovine serum (Gibco, Australia), 2.0 g/L NaHCO3. The Skov-3 cells were cultured in McCoy’s 5A medium (Hyclone, Utah, USA) with 10% fetal bovine serum (Gibco, Australia), 2.2 g/L NaHCO3. Both cells were cultured at 37°C in 5% CO2 and 95% air.
For autophagy induction, cells were treated with 20 nM Rapamycin (Sigma, Aldrich) or cultured in Earle’s Balanced Salt Solution (Sigma, Aldrich).
As indicated, 20 μM Chloroquine (Sigma, Aldrich), 10 mM N-acetylcysteine (Solarbio, China) and 10 μM Zinc-protoporphyrin (Santa Cruz Biotechnology, Texas, USA) were added to the media.
Transwell invasion assay
24-well transwell chambers (8 μm pore size; Corning Costar, Cambridge, MA) were used to perform cell invasion assay with Matrigel (100 μl, 1:8 dilution in serum free medium, BD Biosciences, San Jose, CA) and migration assay without Matrigel. 200 μl serum-free medium with 3×105/mL A2780 cells or 1×105/mL Skov-3 cells were plated into the upper chamber, while, medium with 20% FBS as a chemoattractant was added into the lower chamber. When compared the basic level of migration and invasion capacities, we plated 200 μl serum-free 1×105/mL of both cells into the upper chamber. After 24 h incubation with different treatments, non-invaded cells were removed. The cells on the underside of chambers were fixed in methanol and 3.7% formaldehyde solution, each for 5 min. Then the invaded cells were stained with Giemsa for 30 min. The invaded cells were pictured using Olympus IX51 (Olympus Optical, Melville, NY) inverted microscope and counted in five individual fields. Three independent experiments were done for statistical analysis.
Western blotting
The proteins after different treatment were extracted using RIPA lysis buffer (Beyotime, Jiangsu, China) with 1% PMSF (Thermo Fisher Scientific Inc., Waltham, MA) and 1% NAF (Beyotime, Jiangsu, China). The proteins were then separated on a 10% or 12% polyacrylamide gel and transferred to a pure nitrocellulose blotting membrane. Then, according to the manufacturer’s instruction, we incubated proteins with appropriate primary antibodies and secondary antibodies. Primary antibodies of EMT markers (Keratin, Vimentin, N-cadherin and Zeb1) and the loading control (GAPDH) were purchased from Cell Signaling Technology (USA). HO-1 antibody was obtained from Abcam. P62, Beclin-1 and LC3 were products of Cell Signaling Technology. The results were analyzed by ImageJ software. Three independent experiments were done for statistical analysis.
Immunofluorescence assay
Cold methyl alcohol was used to fix cells for 15 min. Then cells were permeabilized in 0.5% Triton X-100 (Solarbio, Beijing, China) for 10 min and blocked with 3% BSA for 1 h at room temperature. After washing with PBS three times, cells were incubated with primary antibodies against Vimentin and Keratin at 4°C overnight and FITC-conjugated secondary antibody (GeneCopoeia, USA) at 37°C for 1 h. DAPI (Solarbio, Beijing, China) was used to nuclear staining for 3 min at room temperature. Pictures were acquired using Olympus IX51 inverted microscope.
SiRNA transfection
The sequence targeting Atg7 and scrambled negative siRNA control were purchased from GenePharma (Shanghai, China). The Atg7 siRNA sequences were as follows: 5’-GGUCAAAGGACGAAGAUAATTUUAUCUUCGUCCUUUGACCTT-3’. After cells were seeded in 6-well plates overnight, reaching 50%-60% confluence, the transfection processes were performed according to the manufacturer’s instruction. After 6 h, the cells were incubated in complete medium with different treatments as indicated. Then, the transwell chamber assay and western blotting were performed after 48 h.
Measurement of intracellular reactive oxygen species (ROS)
The measurement of ROS was conducted according to the manufacturer’s instruction provided by Beyotime (Shanghai, China). Cells were seeded in 6-well plates and reached 70%-80% confluence. DCFH-DA was added and incubated with cells for 30 min at 37°C. The treated cells were prepared in ice-cold PBS for flow cytometry analysis, after being washed with serum-free medium for 3 times. The results were analyzed by Flow Jo software.
Statistical analysis
All experiments were performed at least three times for statistical analysis. The values of results were shown as mean ± SEM. The data were analyzed with GraphPad Prism Version 5.01 (GraphPad Software, Inc., La Jolla, CA, USA) by two-tailed Student’s t test. P < 0.05 was considered as significant.
Results
Skov-3 cells exhibit a fibroblastoid like phenotype and more invasive ability with lower level of autophagy than A2780
As shown in Figure 1A, Skov-3 cells lacked typical apico-basal polarity and had a typical spindle-shaped morphology, while, A2780 cells showed apical-basolateral polarization and a more regular polygon-shaped morphology. Then we used the transwell chamber to detect the migration and invasion capacities. The results were in accord with their morphology (Figure 1A and 1B). Furthermore, we examined the autophagy and EMT associated markers (Beclin-1, P62, LC3, Keratin, Vimentin, N-cadherin and Zeb1). Skov-3 cells had a lower level of Beclin-1 and LC3 with a higher level of P62 than A2780 cells (Figure 1C and 1D). The level of epithelial marker (Keratin) in Skov-3 cells was lower than A2780 cells; however, the level of mesenchymal markers (Vimentin, N-cadherin and Zeb1) in Skov-3 cells were higher than A2780 cells (Figure 1E and 1F).
Figure 1.
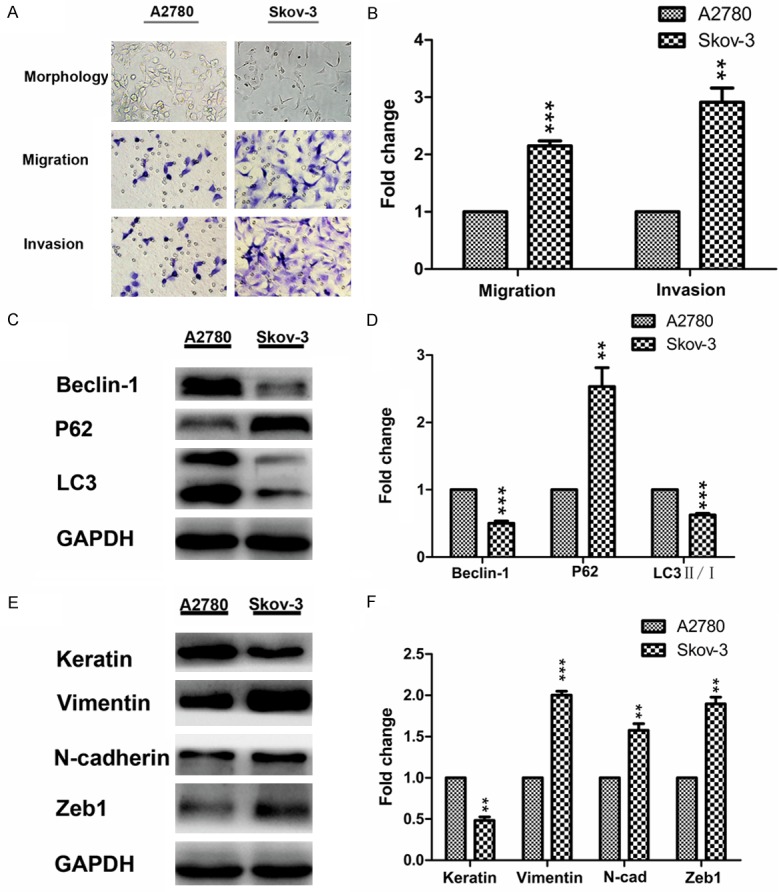
Skov-3 cells exhibit a fibroblastoid like phenotype and more invasive ability with lower level of autophagy than A2780 cells. A: Representative morphology of A2780 and Skov-3 cells (original magnification, ×200); representative transwell migration and invasion assay of A2780 and Skov-3 cells (original magnification, ×200). B: Quantification of migration and invasion abilities of A2780 and Skov-3 cells (**P < 0.01 and ***P < 0.001). C and D: Western blotting showing and quantitative analysis of the expressions of autophagy markers in A2780 and Skov-3 cells (**P < 0.01 and ***P < 0.001). E and F: Western blotting showing and quantitative analysis of the expressions of EMT markers in A2780 and Skov-3 cells (**P < 0.01 and ***P < 0.001). EMT, epithelial-mesenchymal transition.
Autophagy induction impairs cell migration and invasion by inhibiting EMT
Next, to further confirm that autophagy may participate in the migration and invasion in A2780 and Skov-3 cells, we incubated A2780 and Skov-3 cells with Earle’s Balanced Salt Solution (EBSS), the autophagic stimulus. By monitoring the increasing ratio of LC3II to LC3I and up-regulation of Beclin-1 with the down-regulation of P62, we found both cells showed a time-dependent induction of autophagy (Figure 2A and 2C). Consistent with these results, the mesenchymal markers (Vimentin, N-cadherin and Zeb1) were down-regulated and the epithelial marker (Keratin) was up-regulated in a time-dependent manner (Figure 2B and 2D). After incubating with EBSS for 24 h, immunostaining of Vimentin decreased with an increased expression of Keratin in both cells (Figure 3A and 3B). Then, we used Rapamycin (another autophagic stimulus) and CQ (autophagic inhibitor) to incubate A2780 and Skov-3 cells for 24 h. The results showed the migration and invasion properties were inhibited by incubating with EBSS or 20 nM Rap for 24 h; however, they were significantly reversed by incubating with 20 μM CQ together (Figure 4A-D). Meanwhile, we examined the EMT associated proteins. The western blotting results showed the mesenchymal markers such as Vimentin, N-cadherin and Zeb1 were down-regulated and Keratin, the epithelial marker was up-regulated after incubating with EBSS or 20 nM Rap for 24 h. However, they were also significantly reversed by incubating with 20 μM CQ together (Figure 5A-D). Overall, the findings suggested that autophagy induction impaired migration and invasion by inhibiting EMT and this could be reversed by autophagy inhibition.
Figure 2.
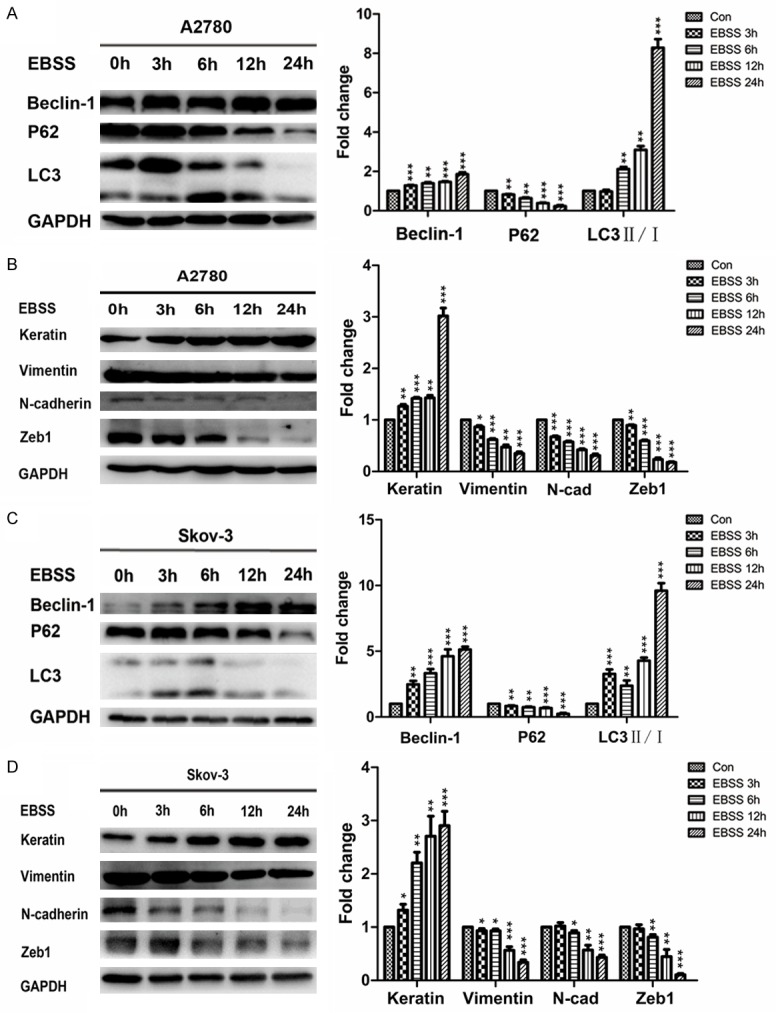
EBSS induces autophagy and inhibits the expressions of EMT markers in A2780 and Skov-3 cells. A and C: Western blotting showing and quantitative analysis of the expressions of autophagy markers in A2780 and Skov-3 cells after incubating with EBSS for 0 h, 3 h, 6 h, 12 h, 24 h (**P < 0.01 and ***P < 0.001). B and D: Western blotting showing and quantitative analysis of the expressions of EMT markers in A2780 and Skov-3 cells after incubating with EBSS for 0 h, 3 h, 6 h, 12 h, 24 h (*P < 0.05, **P < 0.01 and ***P < 0.001). EMT, epithelial-mesenchymal transition; EBSS, Earle’s Balanced Salt Solution.
Figure 3.

Autophagy induction increases the expression of epithelial marker (Keratin) and decreases the expression of mesenchymal marker (Vimentin). A and B: Immunofluorescence assay showing the expressions of EMT markers after incubating with EBSS for 24 h in A2780 and Skov-3 cells. Scale bar, 50 μm. EMT, epithelial-mesenchymal transition.
Figure 4.

Autophagy inhibition by CQ promotes the migration and invasion of the autophagy-induced A2780 and Skov-3 cells. A and B: Representative transwell migration and invasion assay of A2780 and Skov-3 cells (original magnification, ×200). C and D: Quantification of migration and invasion abilities of A2780 and Skov-3 cells (versus con: **P < 0.01 and ***P < 0.001; versus EBSS: §§P < 0.01 and §§§P < 0.001; versus Rap: ※※P < 0.01 and ※※※ P < 0.001). CQ, Chloroquine; EBSS, Earle’s Balanced Salt Solution; Rap, Rapamycin.
Figure 5.
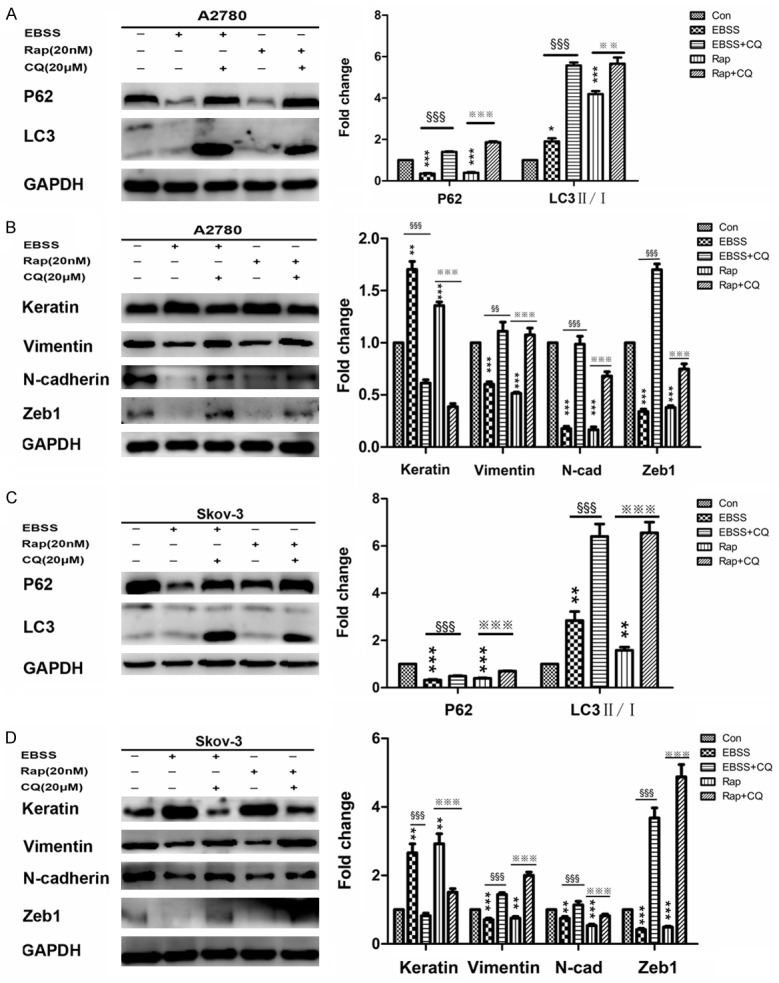
Autophagy inhibition by CQ impaired MET induced by autophagy in A2780 and Skov-3 cells. A and C: Western blotting showing and quantitative analysis of the expressions of autophagy markers in A2780 and Skov-3 cells (versus con: *P < 0.05, **P < 0.01 and ***P < 0.001; versus EBSS: § §§P < 0.001; versus Rap: ※※P < 0.01 and ※※※ P < 0.001). B and D: Western blotting showing and quantitative analysis of the expressions of EMT markers in A2780 and Skov-3 cells (versus con: **P < 0.01 and ***P < 0.001; versus EBSS: §§P < 0.01 and §§§P < 0.001; versus Rap: ※ ※※P < 0.001). MET, mesenchymal-epithelial transition; CQ, Chloroquine; EBSS, Earle’s Balanced Salt Solution; Rap, Rapamycin; EMT, epithelial-mesenchymal transition.
Autophagy defect increases the migration and invasion abilities of A2780 and Skov-3 cells by promoting EMT
Therefore, to further invest the mechanisms of autophagy involved in the migration and invasion abilities of ovarian cancer cells, we generated A2780 and Skov-3 cells with autophagy inhibition through knockdown of Atg7 (A2780 Atg7 siRNA and Skov-3 Atg7 siRNA cells) and the effect on autophagy was evaluated by western blotting for P62 and LC3II/I (Figure 7A and 7C). We used transwell chamber to investigate the migration and invasion capacities. The results showed autophagy defect increased the migration and invasion capacities of both cells (Figure 6A and 6B). Consistent with these results, the expressions of mesenchymal markers such as Vimentin, N-cadherin and Zeb1 were increased, while, the expression of Keratin, an epithelial marker, was suppressed in A2780 Atg7 siRNA and Skov-3 Atg7 siRNA cells than control cells (Figure 7B and 7D). Furthermore, we found autophagy defect also resulted in an increase in the expression of HO-1 (Figure 7B and 7D) and intracellular ROS level (Figure 6C and 6D). In summary, these results suggested that autophagy defect cause A2780 and Skov-3 cells more migratory and invasive by promoting EMT.
Figure 7.

Autophagy inhibition by knockdown of Atg7 promotes EMT in A2780 and Skov-3 cells. A and C: Western blotting showing and quantitative analysis of the expressions of autophagy markers in A2780 and Skov-3 cells (versus NC: *P < 0.05, **P < 0.01 and ***P < 0.001). B and D: Western blotting showing the expressions of HO-1 and EMT markers; Quantitative analysis of the expressions of HO-1 and EMT markers in A2780 and Skov-3 cells (versus NC: **P < 0.01 and ***P < 0.001). EMT, epithelial-mesenchymal transition.
Figure 6.
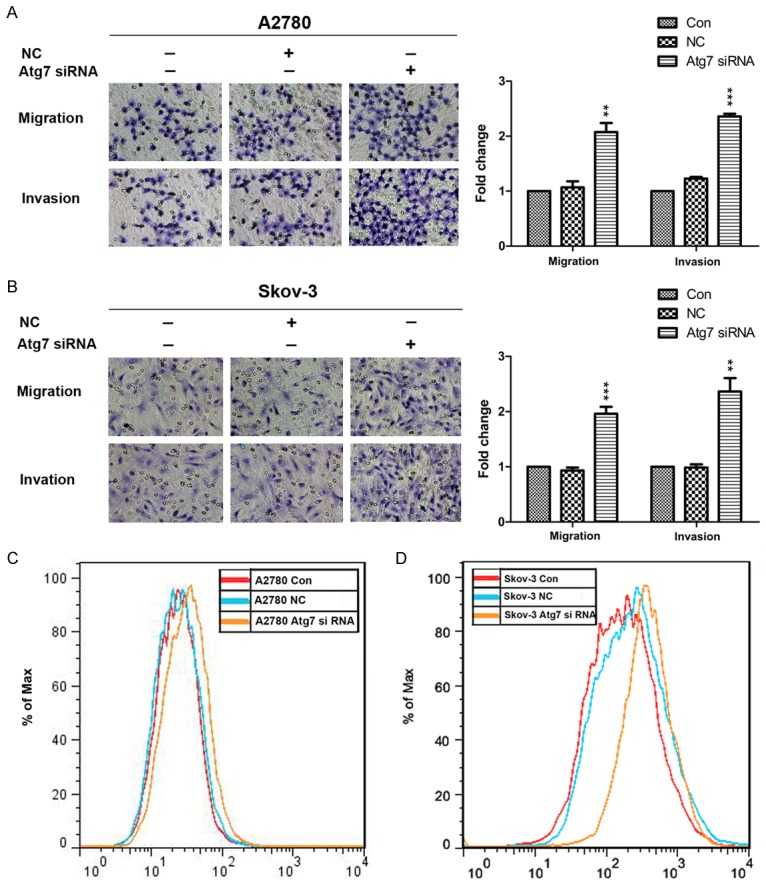
Autophagy inhibition by knockdown of Atg7 promotes migration and invasion and increases the intracellular ROS of A2780 and Skov-3 cells. A and B: Representative transwell migration and invasion assay of A2780 and Skov-3 cells (original magnification, ×200); Quantification of migration and invasion abilities of A2780 and Skov-3 cells (versus NC: **P < 0.01 and ***P < 0.001). C and D: Flow cytometry analysis of intracellular ROS level after pretreatment with DCFH-DA in A2780 and Skov-3 cells. ROS, reactive oxygen species.
Autophagy defect promotes EMT and increases the expression of HO-1 through ROS
It’s known that autophagy defect results in an increase in intracellular ROS. Conforming to these studies, we found the level of intracellular ROS in A2780 Atg7 siRNA and Skov-3 Atg7 siRNA cells were higher than control cells (Figure 6C and 6D). To further clarify weather the increased intracellular ROS participate in EMT, we used the ROS scavenging agent (NAC). A2780 Atg7 siRNA and Skov-3 Atg7 siRNA cells were incubated with 10 mM NAC for 24 h. Then, we tested the migration and invasion properties by transwell chamber. The results showed that the migration and invasion properties were significantly inhibited in both cells after incubating with NAC for 24 h (Figure 8A and 8B). Meanwhile, the mesenchymal markers (Vimentin, N-cadherin and Zeb1) were down-regulated and the epithelial marker (Keratin) was up-regulated as shown by western blotting (Figure 8C and 8D). Furthermore, we found the expression of HO-1 was significantly suppressed after incubating with NAC. Altogether, these experiments demonstrated that autophagy defect could promote EMT and increase the expression of HO-1 through ROS.
Figure 8.
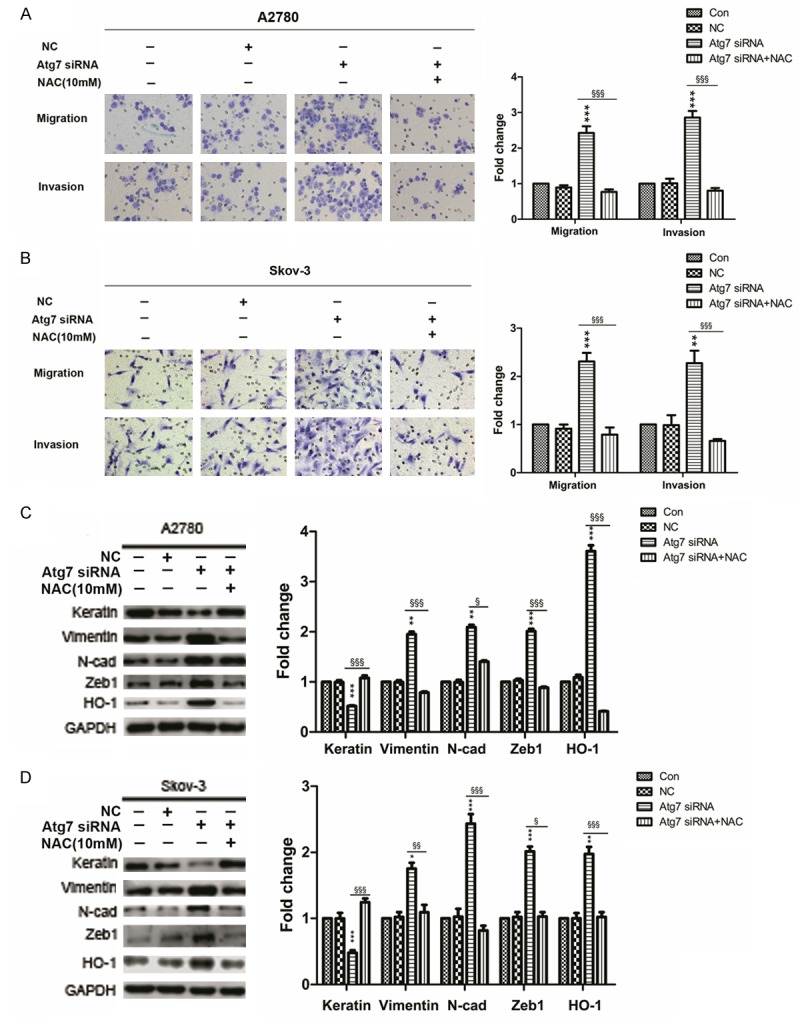
Autophagy defect promotes EMT and increases the expression of HO-1 through ROS. A and B: Representative transwell migration and invasion assay of A2780 and Skov-3 cells (original magnification, ×200); Quantification of migration and invasion abilities of A2780 and Skov-3 cells (versus NC: **P < 0.01 and ***P < 0.001; versus Atg7 siRNA: §§§P < 0.001). C and D: Western blotting showing the expressions of EMT markers and HO-1; Quantitative analysis of the expressions of EMT markers and HO-1 in A2780 and Skov-3 cells (versus NC: *P < 0.05, **P < 0.01 and ***P < 0.001; versus Atg7 siRNA: §P < 0.05, §§P < 0.01 and §§§P < 0.001). NAC, N-acetylcysteine; EMT, epithelial-mesenchymal transition; ROS, reactive oxygen species.
Autophagy defect promotes EMT by HO-1
It has been reported that HO-1 plays a vital role in metastasis in many other cancers. To clarify the mechanisms of HO-1 in promoting EMT of A2780 Atg7 siRNA and Skov-3 Atg7 siRNA cells, we used the HO-1 inhibitor (Znpp). After incubating A2780 Atg7 siRNA and Skov-3 Atg7 siRNA cells with 10 μM Znpp for 24 h, we examined the migration and invasion properties by transwell chamber. The results showed that the migration and invasion properties were significantly inhibited in both cells after incubating with Znpp for 24 h (Figure 9A and 9B). Next, we tested the EMT associated markers. The western blotting results showed the mesenchymal markers such as Vimentin, N-cadherin and Zeb1 were down-regulated and Keratin, the epithelial marker was up-regulated after incubating with Znpp for 24 h (Figure 9C and 9D). Taken together, our findings indicated that autophagy defect might promote EMT through ROS/HO-1 pathway.
Figure 9.
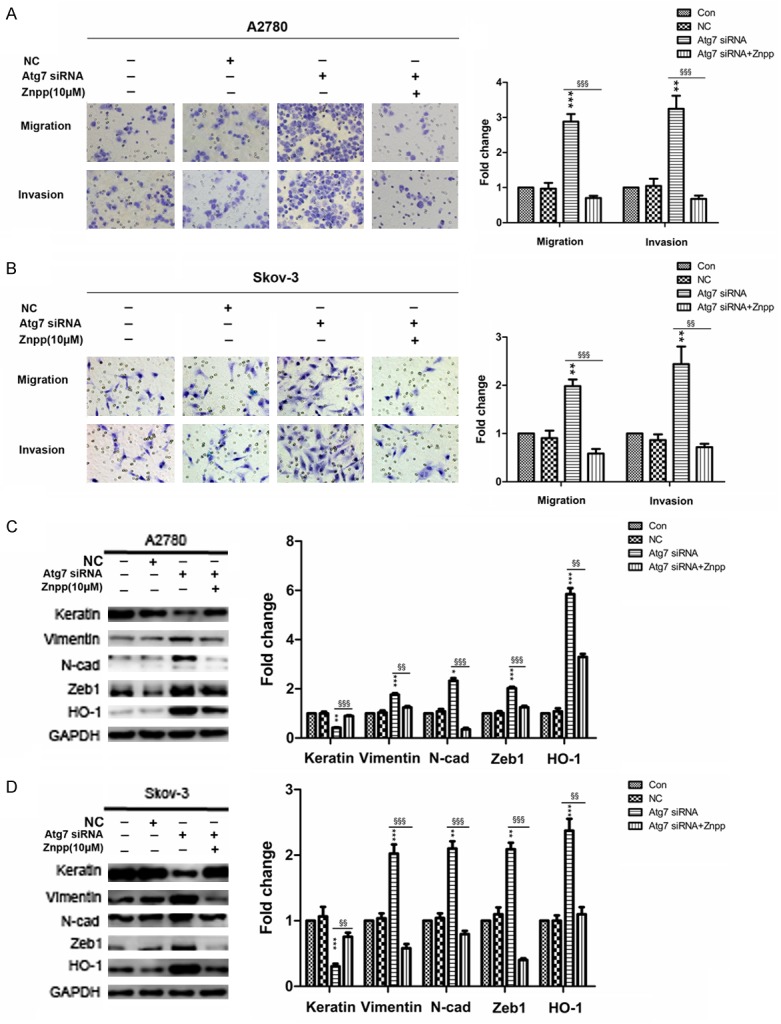
Autophagy defect promotes EMT by HO-1. A and B: Representative transwell migration and invasion assay of A2780 and Skov-3 cells (original magnification, ×200); Quantification of migration and invasion abilities of A2780 and Skov-3 cells (versus NC: **P < 0.01 and ***P < 0.001; versus Atg7 siRNA: §§P < 0.01 and §§§P < 0.001). C and D: Western blotting showing the expressions of EMT markers and HO-1; Quantitative analysis of the expressions of EMT markers and HO-1 in A2780 and Skov-3 cells (versus NC: *P < 0.05, **P < 0.01 and ***P < 0.001; versus Atg7 siRNA: §§P < 0.01 and §§§P < 0.001). Znpp, Zinc-protoporphyrin; EMT, epithelial-mesenchymal transition.
Discussion
Under physiological conditions, both autophagy and EMT play vital roles in maintenance of basic physiological functions. In recent years, more and more studies have proposed that autophagy may inhibit EMT in fibrosis and cancer metastasis. However, the mechanisms are still unclear. Our work provides the first study that autophagy inhibition promotes epithelial-mesenchymal transition through ROS/HO-1 pathway in ovarian cancer cells.
In the preparatory work, we used Beclin-1, P62 and LC3 as autophagy markers and Keratin, Vimentin, N-cadherin, Zeb1 as EMT markers. Then we found that Skov-3 cells exhibit a fibroblastoid like phenotype and more invasive ability with lower level of autophagy than A2780, which was consistent with previous works. It has been reported that the sub clones of A2780 and Skov-3 cells with the lower level of Beclin-1 showed higher invasive/migratory capacities [31]. Moreover, it has also been demonstrated that decreased expression of Beclin-1 involved in poor prognosis of ovarian cancer [32]. Considering the fact that Beclin-1 is identified as being associated with autophagy and the marker of autophagy, we supposed that the level of autophagy was related to the migratory and invasive capacities. Then we verified that autophagy induction impaired cell migration and invasion by inhibiting EMT.
According with previous studies, autophagy induction inhibited migration and invasion through degrading EMT transcript factors such as Snail, Slug and Twist with increasing epithelial markers (Keratin and E-cadherin) and decreasing mesenchymal markers (Vimentin, N-cadherin) [14,16,33,34]. There are no reports about whether transcript factors Zeb1 and Zeb2 can be regulated by autophagy till now. It has been clarified that Zeb1 was involved in metastasis of various cancers such as breast cancer, colon cancer and thyroid cancer [35-37]. Moreover, Zeb1 has been proved being related to the distant metastasis, low chemotherapy response and poor prognosis in ovarian cancer [38,39]. Our work demonstrated that autophagy induction decreased the expression of Zeb1 and reversed EMT with inhibited migration and invasion capacities. However, the mechanism about autophagy decreasing the expression of Zeb1 is still unclear and needs more research.
We found autophagy defect caused an increase in intracellular ROS, which was consistent with previous reports [18,21]. Previous reviews summarized that ROS promoted EMT through ROS/HIF-1α, ROS/NF-κB, ROS/RAS, ROS/MAPK, ROS/PI3K pathway and so on [4]. However, there were only a few reports about the ROS and EMT in ovarian cancer. Alice et al reported that ROS participated in promoting EMT through stanniocalcin-2/ROS/ERK pathway in ovarian cancer cells [40]. Another study demonstrated that ROS promoted ovarian cancer progression by the HIF-1α/LOX/E-cadherin pathway. Our results showed that EMT promoted by autophagy defect was significantly reversed by NAC. It suggested that the autophagy defect promoted EMT, at least in part, due to the increased ROS.
Besides the increased ROS, we also found that autophagy defect caused the up-regulation of HO-1 expression, which was in agreement with previous study that increased expression of HO-1 induced by ROS promoted tumor invasion and metastasis in human lung epithelial cells [41]. HO-1 has been proposed as an emerging target of cancer therapy [24]. More and more studies have demonstrated that HO-1 participated in the metastasis of cancers. However, the mechanisms are still unclear. Seo et al showed that HO-1 promoted colorectal cancer metastasis by inhibiting antitumor immunity [42]. Dey et al confirmed that HO-1 expression was high in metastatic tumors with reduced overall survival of patients with lung adenocarcinoma and glioblastoma [43]. However, the role of HO-1 is still a controversial subject. Park et al reported inducing HO-1 expression inhibited invasion in breast cancer cells [44]. Another study proposed that HO-1 induction increased E-cadherin, favoring a less aggressive phenotype and supporting its anti-tumoral function in prostate cancer [45]. It has also been reported that HO-1 was an anti-inflammatory and anti-oxidant protein in protecting organs from fibrosis and impairing the onset of tumorigenesis [46]. We speculate that the role of HO-1 in metastasis may vary with different cancers and stages. Up to now, there are few studies demonstrating the role of HO-1 in ovarian cancer. For the first time, our work proposed that HO-1 may participated in the EMT induced by autophagy defect. We used HO-1 inhibitor (Znpp) incubating with A2780 Atg7 siRNA and Skov-3 Atg7 siRNA cells. The results showed the mesenchymal markers (Vimentin, N-cadherin) and transcript factor (Zeb1) was down-regulated, while, the epithelial marker (Keratin) was up regulated. What’s more, NAC could reverse the up-regulated HO-1 which was induced by autophagy defect associated ROS. Therefore, we speculated that autophagy defect promoted EMT may through ROS/HO-1 pathway. However, the mechanism about HO-1 regulating transcript factors such as Zeb1 needs to be further studied.
Conclusions
In conclusion, our work found that the lower level of autophagy was related to the higher migratory and invasive capacities of ovarian cancer cells. The mechanisms might be that autophagy defect increased ROS and then up-regulated HO-1, leading to an increased expression of transcript factor (Zeb1), resulting in the increased mesenchymal markers (Vimentin, N-cadherin) and decreased epithelial marker (Keratin). Our findings propose that targeting autophagy may become an effective treatment for inhibiting the metastasis of ovarian cancer.
Acknowledgements
This work was supported by grants from Development and Reform commission Project of Shandong Province (26010104081103).
Disclosure of conflict of interest
None.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Vaughan S, Coward JI, Bast RC Jr, Berchuck A, Berek JS, Brenton JD, Coukos G, Crum CC, Drapkin R, Etemadmoghadam D, Friedlander M, Gabra H, Kaye SB, Lord CJ, Lengyel E, Levine DA, McNeish IA, Menon U, Mills GB, Nephew KP, Oza AM, Sood AK, Stronach EA, Walczak H, Bowtell DD, Balkwill FR. Rethinking ovarian cancer: recommendations for improving outcomes. Nat Rev Cancer. 2011;11:719–725. doi: 10.1038/nrc3144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Elloul S, Vaksman O, Stavnes HT, Trope CG, Davidson B, Reich R. Mesenchymal-to-epithelial transition determinants as characteristics of ovarian carcinoma effusions. Clin Exp Metastasis. 2010;27:161–172. doi: 10.1007/s10585-010-9315-2. [DOI] [PubMed] [Google Scholar]
- 4.Cannito S, Novo E, di Bonzo LV, Busletta C, Colombatto S, Parola M. Epithelial-mesenchymal transition: from molecular mechanisms, redox regulation to implications in human health and disease. Antioxid Redox Signal. 2010;12:1383–1430. doi: 10.1089/ars.2009.2737. [DOI] [PubMed] [Google Scholar]
- 5.Thompson EW, Williams ED. EMT and MET in carcinoma--clinical observations, regulatory pathways and new models. Clin Exp Metastasis. 2008;25:591–592. doi: 10.1007/s10585-008-9189-8. [DOI] [PubMed] [Google Scholar]
- 6.Liu R, Zheng J, Li C, Pang Y, Zheng Q, Xu X, Liu P. Celecoxib induces epithelial-mesenchymal transition in epithelial ovarian cancer cells via regulating ZEB1 expression. Arch Gynecol Obstet. 2015;291:1361–1369. doi: 10.1007/s00404-014-3555-3. [DOI] [PubMed] [Google Scholar]
- 7.Miow QH, Tan TZ, Ye J, Lau JA, Yokomizo T, Thiery JP, Mori S. Epithelial-mesenchymal status renders differential responses to cisplatin in ovarian cancer. Oncogene. 2015;34:1899–1907. doi: 10.1038/onc.2014.136. [DOI] [PubMed] [Google Scholar]
- 8.Mizushima N, Komatsu M. Autophagy: renovation of cells and tissues. Cell. 2011;147:728–741. doi: 10.1016/j.cell.2011.10.026. [DOI] [PubMed] [Google Scholar]
- 9.Sridhar S, Botbol Y, Macian F, Cuervo AM. Autophagy and disease: always two sides to a problem. J Pathol. 2012;226:255–273. doi: 10.1002/path.3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Choi AM, Ryter SW, Levine B. Autophagy in human health and disease. N Engl J Med. 2013;368:1845–1846. doi: 10.1056/NEJMc1303158. [DOI] [PubMed] [Google Scholar]
- 11.Kongara S, Karantza V. The interplay between autophagy and ROS in tumorigenesis. Front Oncol. 2012;2:171. doi: 10.3389/fonc.2012.00171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu H, Cao MM, Wang Y, Li LC, Zhu LB, Xie GY, Li YB. Endoplasmic reticulum stress is involved in the connection between inflammation and autophagy in type 2 diabetes. Gen Comp Endocrinol. 2015;210:124–129. doi: 10.1016/j.ygcen.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 13.Gulhati P, Bowen KA, Liu J, Stevens PD, Rychahou PG, Chen M, Lee EY, Weiss HL, O’Connor KL, Gao T, Evers BM. mTORC1 and mTORC2 regulate EMT, motility, and metastasis of colorectal cancer via RhoA and Rac1 signaling pathways. Cancer Res. 2011;71:3246–3256. doi: 10.1158/0008-5472.CAN-10-4058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lv Q, Wang W, Xue J, Hua F, Mu R, Lin H, Yan J, Lv X, Chen X, Hu ZW. DEDD interacts with PI3KC3 to activate autophagy and attenuate epithelial-mesenchymal transition in human breast cancer. Cancer Res. 2012;72:3238–3250. doi: 10.1158/0008-5472.CAN-11-3832. [DOI] [PubMed] [Google Scholar]
- 15.Qiang L, Zhao B, Ming M, Wang N, He TC, Hwang S, Thorburn A, He YY. Regulation of cell proliferation and migration by p62 through stabilization of Twist1. Proc Natl Acad Sci U S A. 2014;111:9241–9246. doi: 10.1073/pnas.1322913111. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 16.Catalano M, D’Alessandro G, Lepore F, Corazzari M, Caldarola S, Valacca C, Faienza F, Esposito V, Limatola C, Cecconi F, Di Bartolomeo S. Autophagy induction impairs migration and invasion by reversing EMT in glioblastoma cells. Mol Oncol. 2015;9:1612–1625. doi: 10.1016/j.molonc.2015.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liao H, Huang Y, Guo B, Liang B, Liu X, Ou H, Jiang C, Li X, Yang D. Dramatic antitumor effects of the dual mTORC1 and mTORC2 inhibitor AZD2014 in hepatocellular carcinoma. Am J Cancer Res. 2015;5:125–139. [PMC free article] [PubMed] [Google Scholar]
- 18.Qin W, Li C, Zheng W, Guo Q, Zhang Y, Kang M, Zhang B, Yang B, Li B, Yang H, Wu Y. Inhibition of autophagy promotes metastasis and glycolysis by inducing ROS in gastric cancer cells. Oncotarget. 2015;6:39839–39854. doi: 10.18632/oncotarget.5674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gough DR, Cotter TG. Hydrogen peroxide: a Jekyll and Hyde signalling molecule. Cell Death Dis. 2011;2:e213. doi: 10.1038/cddis.2011.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Costa A, Scholer-Dahirel A, Mechta-Grigoriou F. The role of reactive oxygen species and metabolism on cancer cells and their microenvironment. Semin Cancer Biol. 2014;25:23–32. doi: 10.1016/j.semcancer.2013.12.007. [DOI] [PubMed] [Google Scholar]
- 21.Mathew R, Karp CM, Beaudoin B, Vuong N, Chen G, Chen HY, Bray K, Reddy A, Bhanot G, Gelinas C, Dipaola RS, Karantza-Wadsworth V, White E. Autophagy suppresses tumorigenesis through elimination of p62. Cell. 2009;137:1062–1075. doi: 10.1016/j.cell.2009.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cichon MA, Radisky DC. ROS-induced epithelial-mesenchymal transition in mammary epithelial cells is mediated by NF-κB-dependent activation of Snail. Oncotarget. 2014;5:2827–2838. doi: 10.18632/oncotarget.1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maines MD. Heme oxygenase: function, multiplicity, regulatory mechanisms, and clinical applications. FASEB J. 1988;2:2557–2568. [PubMed] [Google Scholar]
- 24.Chau LY. Heme oxygenase-1: emerging target of cancer therapy. J Biomed Sci. 2015;22:22. doi: 10.1186/s12929-015-0128-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maines MD, Abrahamsson PA. Expression of heme oxygenase-1 (HSP32) in human prostate: normal, hyperplastic, and tumor tissue distribution. Urology. 1996;47:727–733. doi: 10.1016/s0090-4295(96)00010-6. [DOI] [PubMed] [Google Scholar]
- 26.Goodman AI, Choudhury M, da Silva JL, Schwartzman ML, Abraham NG. Overexpression of the heme oxygenase gene in renal cell carcinoma. Proc Soc Exp Biol Med. 1997;214:54–61. doi: 10.3181/00379727-214-44069. [DOI] [PubMed] [Google Scholar]
- 27.Gandini NA, Fermento ME, Salomon DG, Obiol DJ, Andres NC, Zenklusen JC, Arevalo J, Blasco J, Lopez Romero A, Facchinetti MM, Curino AC. Heme oxygenase-1 expression in human gliomas and its correlation with poor prognosis in patients with astrocytoma. Tumour Biol. 2014;35:2803–2815. doi: 10.1007/s13277-013-1373-z. [DOI] [PubMed] [Google Scholar]
- 28.Yin H, Fang J, Liao L, Maeda H, Su Q. Upregulation of heme oxygenase-1 in colorectal cancer patients with increased circulation carbon monoxide levels, potentially affects chemotherapeutic sensitivity. BMC Cancer. 2014;14:436. doi: 10.1186/1471-2407-14-436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang TY, Liu CL, Chen MJ, Lee JJ, Pun PC, Cheng SP. Expression of haem oxygenase-1 correlates with tumour aggressiveness and BRAF V600E expression in thyroid cancer. Histopathology. 2015;66:447–456. doi: 10.1111/his.12562. [DOI] [PubMed] [Google Scholar]
- 30.Hsu FF, Yeh CT, Sun YJ, Chiang MT, Lan WM, Li FA, Lee WH, Chau LY. Signal peptide peptidase-mediated nuclear localization of heme oxygenase-1 promotes cancer cell proliferation and invasion independent of its enzymatic activity. Oncogene. 2015;34:2410–2411. doi: 10.1038/onc.2014.464. [DOI] [PubMed] [Google Scholar]
- 31.Bai H, Li H, Li W, Gui T, Yang J, Cao D, Shen K. The PI3K/AKT/mTOR pathway is a potential predictor of distinct invasive and migratory capacities in human ovarian cancer cell lines. Oncotarget. 2015;6:25520–25532. doi: 10.18632/oncotarget.4550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lin HX, Qiu HJ, Zeng F, Rao HL, Yang GF, Kung HF, Zhu XF, Zeng YX, Cai MY, Xie D. Decreased expression of Beclin 1 correlates closely with Bcl-xL expression and poor prognosis of ovarian carcinoma. PLoS One. 2013;8:e60516. doi: 10.1371/journal.pone.0060516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grassi G, Di Caprio G, Santangelo L, Fimia GM, Cozzolino AM, Komatsu M, Ippolito G, Tripodi M, Alonzi T. Autophagy regulates hepatocyte identity and epithelial-to-mesenchymal and mesenchymal-to-epithelial transitions promoting Snail degradation. Cell Death Dis. 2015;6:e1880. doi: 10.1038/cddis.2015.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Qiang L, He YY. Autophagy deficiency stabilizes TWIST1 to promote epithelial-mesenchymal transition. Autophagy. 2014;10:1864–1865. doi: 10.4161/auto.32171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kan JY, Yen MC, Wang JY, Wu DC, Chiu YJ, Ho YW, Kuo PL. Nesfatin-1/Nucleobindin-2 enhances cell migration, invasion, and epithelial-mesenchymal transition via LKB1/AMPK/TORC1/ZEB1 pathways in colon cancer. Oncotarget. 2016;7:31336–49. doi: 10.18632/oncotarget.9140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ma Z, Li Y, Xu J, Ren Q, Yao J, Tian X. MicroRNA-409-3p regulates cell invasion and metastasis by targeting ZEB1 in breast cancer. IUBMB Life. 2016;68:394–402. doi: 10.1002/iub.1494. [DOI] [PubMed] [Google Scholar]
- 37.Zhang Y, Liu G, Wu S, Jiang F, Xie J, Wang Y. Zinc finger E-box-binding homeobox 1: its clinical significance and functional role in human thyroid cancer. Onco Targets Ther. 2016;9:1303–1310. doi: 10.2147/OTT.S96723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Davidson B, Holth A, Hellesylt E, Tan TZ, Huang RY, Trope C, Nesland JM, Thiery JP. The clinical role of epithelial-mesenchymal transition and stem cell markers in advanced-stage ovarian serous carcinoma effusions. Hum Pathol. 2015;46:1–8. doi: 10.1016/j.humpath.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 39.Li X, Huang R, Li RH, Trope CG, Nesland JM, Suo Z. Expression of zinc finger E-box-binding homeobox factor 1 in epithelial ovarian cancer: A clinicopathological analysis of 238 patients. Mol Clin Oncol. 2016;4:18–22. doi: 10.3892/mco.2015.662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Law AY, Wong CK. Stanniocalcin-2 promotes epithelial-mesenchymal transition and invasiveness in hypoxic human ovarian cancer cells. Exp Cell Res. 2010;316:3425–3434. doi: 10.1016/j.yexcr.2010.06.026. [DOI] [PubMed] [Google Scholar]
- 41.Kang X, Kong F, Wu X, Ren Y, Wu S, Wu K, Jiang Z, Zhang W. High glucose promotes tumor invasion and increases metastasis-associated protein expression in human lung epithelial cells by upregulating heme oxygenase-1 via reactive oxygen species or the TGF-beta1/PI3K/Akt signaling pathway. Cell Physiol Biochem. 2015;35:1008–1022. doi: 10.1159/000373928. [DOI] [PubMed] [Google Scholar]
- 42.Seo GS, Jiang WY, Chi JH, Jin H, Park WC, Sohn DH, Park PH, Lee SH. Heme oxygenase-1 promotes tumor progression and metastasis of colorectal carcinoma cells by inhibiting antitumor immunity. Oncotarget. 2015;6:19792–19806. doi: 10.18632/oncotarget.4075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dey S, Sayers CM, Verginadis II, Lehman SL, Cheng Y, Cerniglia GJ, Tuttle SW, Feldman MD, Zhang PJ, Fuchs SY, Diehl JA, Koumenis C. ATF4-dependent induction of heme oxygenase 1 prevents anoikis and promotes metastasis. J Clin Invest. 2015;125:2592–2608. doi: 10.1172/JCI78031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Park SY, Jin ML, Kim YH, Lee SJ, Park G. Sanguinarine inhibits invasiveness and the MMP-9 and COX-2 expression in TPA-induced breast cancer cells by inducing HO-1 expression. Oncol Rep. 2014;31:497–504. doi: 10.3892/or.2013.2843. [DOI] [PubMed] [Google Scholar]
- 45.Gueron G, Giudice J, Valacco P, Paez A, Elguero B, Toscani M, Jaworski F, Leskow FC, Cotignola J, Marti M, Binaghi M, Navone N, Vazquez E. Heme-oxygenase-1 implications in cell morphology and the adhesive behavior of prostate cancer cells. Oncotarget. 2014;5:4087–4102. doi: 10.18632/oncotarget.1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen X, Wei SY, Li JS, Zhang QF, Wang YX, Zhao SL, Yu J, Wang C, Qin Y, Wei QJ, Lv GX, Li B. Overexpression of Heme Oxygenase-1 Prevents Renal Interstitial Inflammation and Fibrosis Induced by Unilateral Ureter Obstruction. PLoS One. 2016;11:e0147084. doi: 10.1371/journal.pone.0147084. [DOI] [PMC free article] [PubMed] [Google Scholar]


