Abstract
Rottlerin, a natural product isolated from Mallotus philippinensis, has been characterized as an effective chemoprevention agent in inhibiting tumor cell growth. Although multiple studies have revealed the role of rottlerin in tumorigenesis, the molecular mechanism of rottlerin-mediated anti-tumor activity has not been fully elucidated. It has been reported that Skp2 (S-phase kinase associated protein 2) plays an oncogenic role in human malignancies, indicating that inactivation of Skp2 could be a promising approach for the treatment of cancers. Therefore, in this study, we aim to investigate whether rottlerin exhibits its anti-tumor activities via targeting Skp2 pathway in pancreatic cancer. We found that rottlerin inhibited cell growth, induced apoptosis, arrested cell cycle, and retarded cell invasion and migration. Notably, we observed that the expression of Skp2 was significantly decreased in rottlerin-treated pancreatic cancer cells. Importantly, overexpression of Skp2 abrogated the anti-tumor function induced by rottlerin in pancreatic cancer cells. Consistently, depletion of Skp2 promoted rottlerin-mediated inhibition of cell growth and invasion. Collectively, our study demonstrated that rottlerin could suppress Skp2 expression and subsequently exert its tumor suppressive function in pancreatic cancer cells, suggesting that rottlerin might be a potential therapeutic compound for treating pancreatic cancer.
Keywords: Rottlerin, pancreatic cancer, Skp2, invasion, apoptosis, proliferation
Introduction
Pancreatic cancer (PC) is one of the common human malignancies. An estimated 53,070 people will be diagnosed with PC and 41,780 persons will die from this deadly disease in the United States in 2016 [1]. The 5-year relative survival of PC is currently 8% partly due to that some PC cases are diagnosed at a distant stage [1]. Although PC systemic therapies have been improved, the outcomes of patients with PC have not markedly changed [2]. For example, more than 80% of PC patients suffer disease relapse after surgery resection. Chemotherapeutic therapy has yielded only modest improvements in PC survival partly due to acquired drug resistance [3]. Chemoradiotherapy is used in locally advanced PC, but patients with PC failed to achieve big benefit from this treatment [2]. Therefore, it is urgent to discover new treatments to benefit patients with PC.
A large majority of available anti-cancer drugs are natural products or natural product-derived drugs or natural product mimics [4]. Rottlerin, also known as mallotoxin, is a natural compound isolated from the tree Mallotus phillippinensis [5]. Rottlerin was initially characterized as a PKCδ inhibitor and recently considered as an antioxidant and a potent inhibitor of NF-κB, a key regulator in controlling cell cycle and growth in human cancer cells [6]. Moreover, it has been demonstrated that rottlerin exhibited tumor suppressive function in cancer cells. For example, Lu et al. reported that rottlerin decreased LRP6 expression and its phosphorylation level, and suppressed Wnt/β-catenin and mTORC1 pathways, and subsequently down-regulated the expression of cyclin D1 and Survivin in prostate and breast cancer cells [7]. One study validated that rottlerin triggered autophagy through inhibition of mTORC1 activity in breast cancer cells [8]. Another group found that rottlerin triggered apoptosis and inhibited cell growth via targeting Akt, Notch and Shh signaling pathways in pancreatic cancer cells [9]. Although these studies have validated the role of rottlerin in tumorigenesis, further investigations are necessary to explore the molecular mechanism of rottlerin-mediated tumor suppressive function.
It has been documented that dysregulation of some genes such as K-ras, CDKN2A, TP53, Smad4, and ARID1A contributes to the development and progression of PC [10,11]. Ubiquitination by the ubiquitin proteasome system (UPS) is a post-translational modification, which controls protein degradation. Skp2, one of the well-characterized F-box proteins, functions as the substrate-recruiting component of the SCF (Skp1-Cullin1-F-box complex) type of E3 ubiquitin ligase complex [12]. Recently, S-phase kinase-associated protein 2 (Skp2) has been revealed to be critically involved in tumorigenesis including PC [13,14]. Skp2 has been reported to regulate cellular proliferation, cell cycle, apoptosis, and metastasis through targeting its substrates for ubiquitination and degradation [15]. The substrates of Skp2 include p21 [16], p27 [17], p57 [18], p53 [19], and Foxo1 [20]. Since these substrates are tumor suppressive proteins, Skp2 has been validated as an oncoprotein in tumorigenesis [21]. Indeed, overexpression of Skp2 was strongly associated with aggressive tumor behavior and poor clinical outcome in a wide range of human cancers [22]. Therefore, inactivation of Skp2 could be a promising approach to benefit the cancer patients.
In the present study, we explored whether Skp2 plays a pivotal role in regulation of cell growth, apoptosis, migration and invasion via overexpression or depletion of Skp2 in PC cells. We also investigated whether rottlerin exerts its anti-tumor activity in PC cells using multiple methods such as cell growth assay, FACS, wound healing assay, Transwell invasion analysis. Mechanistically, we determined whether rottlerin-mediated tumor suppressive activity is due to inactivation of Skp2 in PC cells by Western blotting and transfection. Our results demonstrated that the anti-tumor effects induced by rottlerin are partly through inhibition of Skp2 expression in PC cells, indicating that rottlerin could be a safe and effective agent for the treatment of PC.
Materials and methods
Cell culture and reagents
Human pancreatic cancer cell lines Patu8988 and Panc1 were cultured in DMEM supplemented with 10% fetal bovine serum and 1% penicillin and streptomycin in a 5% CO2 atmosphere at 37°C. Primary anti-Skp2 antibody was obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-p21 and anti-p27 antibodies were purchased from Cell Signaling Technology (Danvers, MA). All secondary antibodies were purchased from Thermo Scientific (Waltham, MA). Lipofectamine 2000 was obtained from Invitrogen. Monoclonal anti-β-actin and rottlerin (CAS number R5648, ≥ 85% rottlerin) were purchased from Sigma-Aldrich (St. Louis, MO). CTG (Cell Titer-Glo Luminescent) was obtained from Promega. Rottlerin was dissolved in DMSO to make a 10 mM stock solution and was added directly to the medium at different concentrations. Cells were treated with 0.1% DMSO as the control group.
Cell viability assay
Cells were seeded at 8 × 103 cells/well in 96-well plate for 24 h and treated with different concentrations of rottlerin. After 48 h and 72 h, 20 μl of the CellTiter-Glo® luminescence (CTG) solution was added to each well and incubated for 10 min at 37°C. Then, the reaction mixture was measured by the microplate.
Cell apoptosis analysis
Cells were cultured in six-well plate overnight and treated with various concentrations of rottlerin for 48 h. Then, cells were harvested and washed with PBS, resuspended in 500 μl binding buffer with 5 μl Propidium iodide (PI) and 5 μl FITC-conjugated anti-Annexin V antibody. Apoptosis was analyzed with a FACScalibur flow cytometer (BD, USA).
Clonogenic assay
Patu8988 and Panc1 cells were seeded in a 6-well plate and incubated overnight. After 72 h exposure to different concentrations of rottlerin, the viable cells were seeded into 100 mm dishes and incubated for 21 days at 37°C in a humidified 5% CO2 atmosphere. All the colonies were stained with 2% crystal violet.
Cell cycle analysis
Exponentially growing cells (2 × 105 cells/well) were seeded in a 6-well plate overnight and then treated with respective concentrations of rottlerin for 48 h. After 48 h, cells were collected and washed with cold PBS. Then, suspended cells with 70% cold alcohol were kept at 4°C overnight. Prior to analysis, the cells were washed with cold PBS, and re-suspended at 1 × 106 cells/ml in PBS. Cells were incubated with 0.1 mg/ml RNase I and 50 mg/ml Propidium iodide (PI) at 37°C for 30 min. Cell cycle was analyzed with a FACScalibur flow cytometer (BD, USA).
Cell scratch assay
Patu8988 cells and Panc1 cells were cultured in 6-well plate. After cells converged almost 100%, absorbed the supernatant and scratched the cells with a yellow pipette tip. Then washed the cells with PBS and added medium with rottlerin. The scratched area was photographed with a microscope at 0 h and 20 h, respectively, as described before [23].
Cell invasion assay
Cell invasion assay was conducted to test the invasive activity of Patu8988 and Panc1 cells treated with rottlerin or Skp2 transfection or combination [23]. Briefly, transfected cells were seeded in the upper chamber with 200 μl serum-free medium and there is 500 μl complete medium in the under chamber with the same concentration of rottlerin. After incubation for 24 h, the membrane of the chamber was strained with Giemsa and photographed with a microscope.
Transfection
Cells were seeded into 6-well plate and transfected with Skp2 cDNA or Skp2 siRNA or empty vector using lipofectamine 2000 following the instruction’s protocol [24]. Skp2 siRNA: sense 5’-GUG AUA GUG UCA UGC UAA ATT-3’; antisense 5’-UUU AGC AUG ACA CUA UCA CTT-3’. After the transfection, the cells were subjected to further analysis as described under the results sections.
Western blotting analysis
The harvested cells were washed by PBS and lysed with protein lysis buffer. The concentrations of the proteins were tested by BCA Protein Assay kit (Thermo Scientific, MA). Same amount of protein samples were separated by electrophoresis in Sodium Dodecyl Sulfonate (SDS)-polyacrylamide gel and then transferred onto a Polyvinylidene Fluoride (PVDF) membrane, and then incubated with primary antibody at 4°C overnight. After washed with TBST for three times and incubated with second antibody at room temperature for one hour. Then the expression of protein was detected by electrochemiluminescence (ECL) assay.
Statistical analysis
All statistical analyses were conducted using GraphPad Prism 5.0 (Graph Pad Software, La Jolla, CA). Student t-test was used to measure the difference between control group and single treatment group. ANOVA was performed to evaluate statistical significance between single treatment and combination treatments. All experiments have been done triple times. Results were presented as means ± SD. P < 0.05 was considered as statistically significant.
Results
Rottlerin inhibited cell proliferation
It has been reported that rottlerin suppressed cell growth in PC cells isolated from Kras (G12D) mice [9]. To determine whether rottlerin inhibited cell growth in PC cell lines, cell viability assay was performed to detect the effects of rottlerin on patu8988 and Panc1 cells. Our results showed that 2 μM rottlerin treatments led to 45% and 65% inhibition of cell growth at 48 hours and 72 hours, respectively, in Patu8988 cells (Figure 1A). Moreover, 4 μM rottlerin significantly inhibited 80% of cell growth in Patu8988 cells (Figure 1A). Our data further suggested that rottlerin markedly suppressed cell growth in time- and dose-dependent manners in Patu8988 cells. Similar results were observed in Panc1 cells (Figure 1A). Specifically, 1 μM and 3 μM rottlerin treatments resulted in 30% and 75% of cell growth inhibition, respectively, at 72 hours in Panc1 cells (Figure 1A). Collectively, rottlerin inhibited cell growth in both Patu8988 and Panc1 cells. To further validate the anti-proliferation function of rottlerin, colony formation assay was conducted to detect the colony numbers in rottlerin-treated PC cells. We observed that rottlerin significantly reduced colony numbers in both PC cell lines (Figure 1B), suggesting that rottlerin suppressed cell growth in PC cells.
Figure 1.
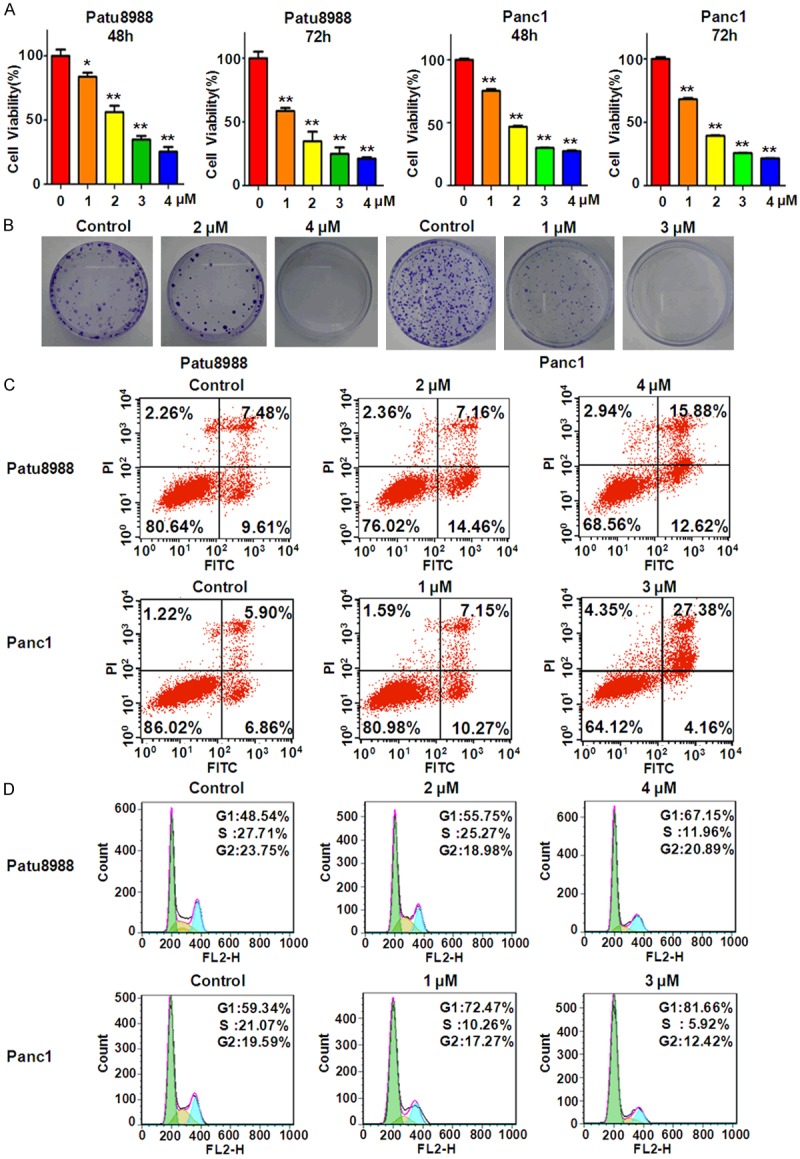
Rottlerin inhibited cell growth, induced apoptosis, and arrested cell cycle. A. Cell growth was measured by CellTiter-Glo® luminescence assay in PC cells after rottlerin treatment for 48 hours and 72 hours. *P < 0.05, **P < 0.01, compared to the control (DMSO treatment group). B. The colony activity was measured by Colony formation analysis in PC cells treated with different concentrations of rottlerin. C. Flow cytometry was conducted to detect the cell apoptosis in rottlerin-treated PC cells. D. Cell cycle was analyzed by Flow cytometry in PC cells treated with rottlerin.
Rottlerin induced apoptosis in PC cells
It has been reported that rottlerin stimulated apoptosis in PC cells by interacting with mitochondria and stimulating cytochrome c release [25]. Next, we investigated the effects of rottlerin on apoptosis in Patu8988 and Panc1 cells using PI-FITC-annexin assay. We found that 4 μM rottlerin treatments for 48 hours led to cell apoptosis from 17.1% to 28.5% in patu8988 cells (Figure 1C). Similarly, apoptotic cells were increased from 12.76% to 31.5% in Panc1 cells after 3 μM rottlerin treatment for 48 hours (Figure 1C). Our findings unraveled that rottlerin could trigger cell apoptosis in PC cell lines.
Rottlerin induced cell cycle arrest
To further explore the mechanism of rottlerin-mediated anti-proliferation in PC cells, cell cycle analysis was performed by PI staining and flow cytometry in both patu8988 and Panc1 cells after rottlerin treatment. We observed that rottlerin arrested cell cycle at G1 phase in both PC cells. For example, adding 4 μM rottlerin to Patu8988 cells caused G1 phase from 48% to 67% (Figure 1D). In line with this, 3 μM rottlerin led to increased G1 phase from 59% to 82% in Panc1 cells (Figure 1D). These results identified that rottlerin induced G1 phase arrest in PC cells, which could contribute to cell growth inhibition induced by rottlerin.
Rottlerin retarded cell migration and invasion
To investigate whether rottlerin has any effect on cell migration and invasion in PC cells, the wound healing assay and invasion analysis were performed using scratch approach and matrigel-coated membrane method, respectively. The results from wound healing assay clearly showed that rottlerin remarkably retarded cell migration in a dose-dependent manner in both patu8988 and Panc1 cells (Figure 2A). In support of this, our invasion assay demonstrated that rottlerin decreased the cell numbers of penetration of PC cells through the matrigel-coated membrane (Figure 2B). Altogether, rottlerin could inhibit cell migratory activity and invasion in PC cells.
Figure 2.
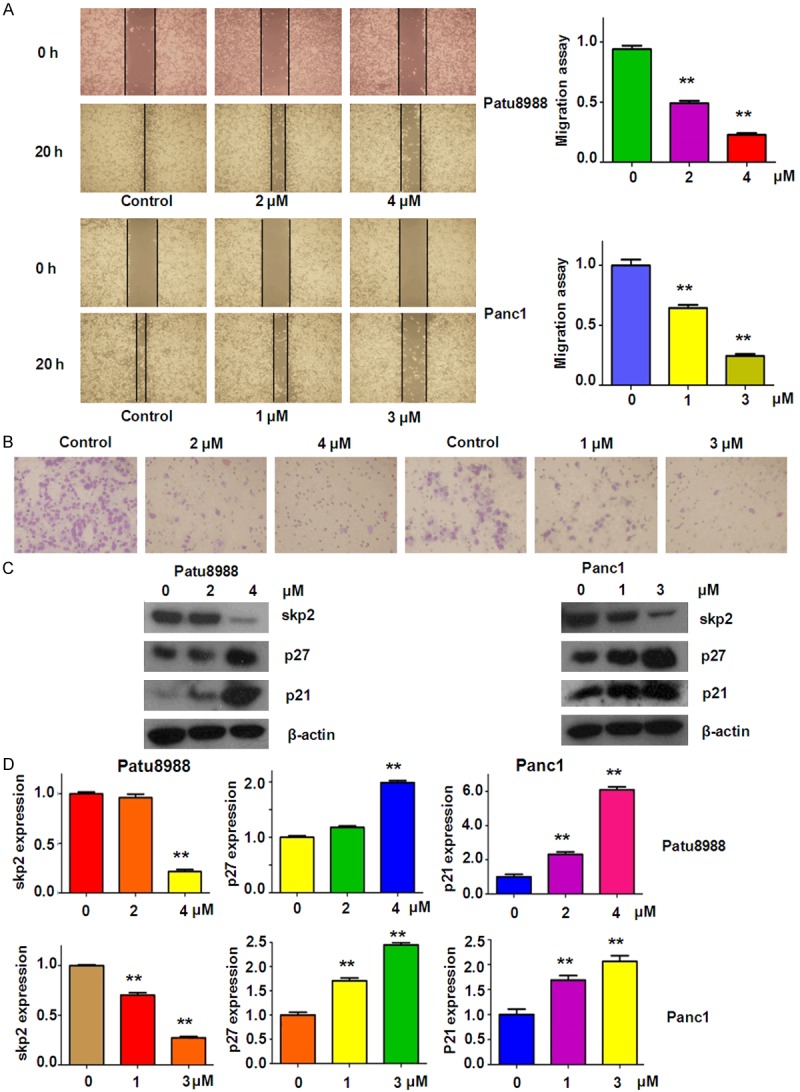
Rottlerin inhibited cell migratory activity and suppressed the expression of Skp2 on PC cells. (A) Left panel: cell migration was measured by wound healing assay in Patu8988 cells (top panel) and Panc1 cells (bottom panel) after rottlerin treatment. Right panel: Quantitative results are illustrated for the degree of wound healing. **P < 0.01, vs control (DMSO treatment group). (B) Transwell chambers assay was conducted to measure the inhibitory effect on Patu8988 cells (left panel) and Panc1 cells (right panel) after rottlerin treatment. (C) The expression of Skp2, p27 and p21 was determined by Western blotting analysis in Patu8988 and Panc1 cells after rottlerin treatment. (D) Quantitative results are illustrated for (C). **P < 0.01 vs control.
Rottlerin decreased Skp2 expression
It has been reported that overexpression of Skp2 was observed in PC patients [26], indicating that inactivation of Skp2 could be helpful for achieving better outcome of PC. Therefore, we detected whether rottlerin could inactivate the expression of Skp2 in PC cells by Western blotting analysis. We found that Skp2 expression was significantly down-regulated by rottlerin treatment in dose-dependent manner in both PC cell lines (Figure 2C and 2D). To further confirm the inhibition of Skp2 by rottlerin, we also measured the expression of p27 and p21, two key targets of Skp2, in PC cells treated with rottlerin. As expected, we observed the up-regulation of p27 and p21 in both PC cells after rottlerin treatment (Figure 2C and 2D). These results suggest that rottlerin could suppress Skp2 expression in PC cells.
Over-expression of Skp2 abrogated rottlerin-induced cell growth inhibition
To determine whether rottlerin exhibited its anti-tumor physiological function via inactivation of Skp2, PC cells were transfected with Skp2 cDNA and then treated with rottlerin for 48 hours. In the following experiments, 3 μM rottlerin and 4 μM rottlerin were used for Panc1 and Patu8988 cells, respectively. We found that overexpression of Skp2 enhanced cell growth in both patu8988 and Panc1 cells (Figure 3A). More importantly, Skp2 cDNA transfection rescued cell growth inhibition induced by rottlerin in both PC cells (Figure 3A), demonstrating that rottlerin inhibited cell growth partly through down-regulation of Skp2 in PC cells.
Figure 3.
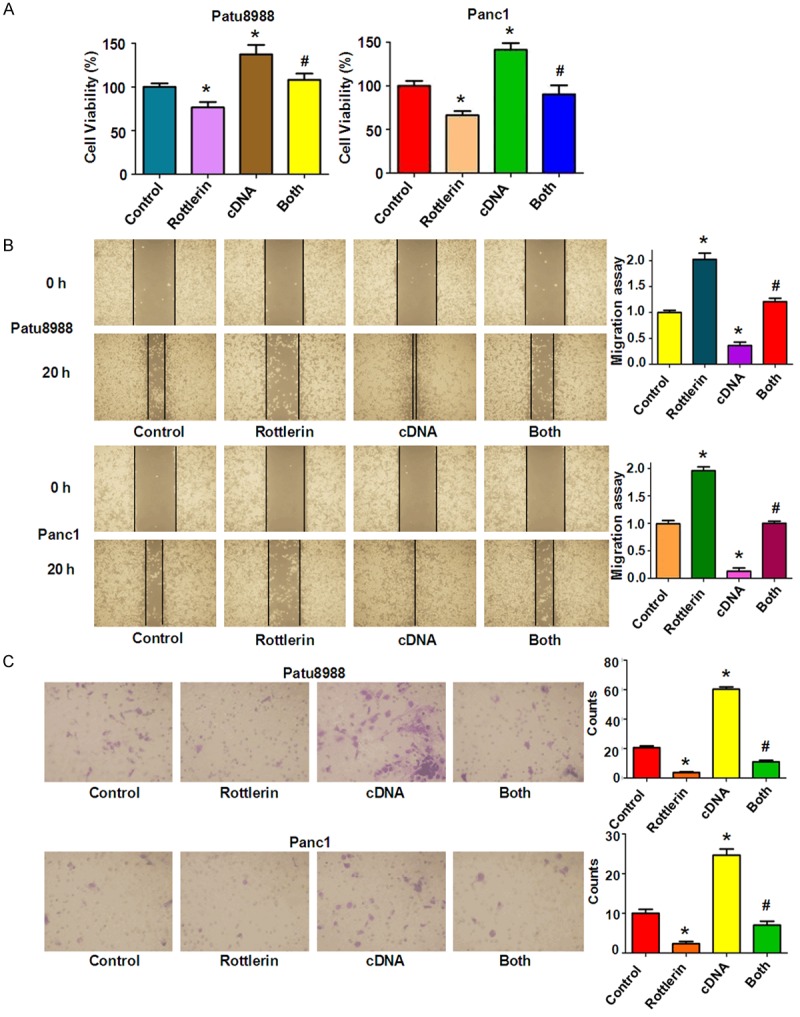
Over-expression of Skp2 abrogated rottlerin-induced inhibition of cell growth, migration and invasion. A. CellTiter-Glo® luminescence assay was used to investigate cell growth in PC cells after rottlerin treatment in combination with Skp2 cDNA transfection. *P < 0.05, compared with control; #P < 0.05, compared with rottlerin treatment alone or Skp2 cDNA transfection alone. Control: cDNA control (pcDNA 3.1); cDNA: Skp2 cDNA; Both: rottlerin + Skp2 cDNA. B. Left panel: The wound healing assay was conducted to test the cell migration in PC cells after Skp2 cDNA transfection plus rottlerin treatment. Right panel: Quantitative results are illustrated for left panel. *P < 0.05 vs control. C. Left panel: The invasive ability was detected by Transwell chambers assay in Patu8988 and Panc1 cells treated with rottlerin and Skp2 cDNA transfecton. Right panel: Quantitative results are illustrated for the numbers of invasion cells. *P < 0.05, compared with control; #P < 0.05 compared with rottlerin treatment alone or Skp2 cDNA transfection alone.
Over-expression of Skp2 enhanced cell motility and inhibited cell apoptosis
Next, we determine the cell motility of PC cells treated with rottlerin in combination with Skp2 cDNA transfection. Our wound healing assay results showed that overexpression of Skp2 promoted cell migration in both PC cell lines (Figure 3B). Moreover, over-expressing Skp2 cells abrogated the rottlerin-mediated cell migration inhibition to a great degree in PC cells (Figure 3B). Consistently, our invasion assay showed that Skp2 cDNA transfection markedly enhanced cell invasion compared with the control group (Figure 3C). Strikingly, Skp2 overexpression rescued the rottlerin-triggered inhibition of cell invasion in PC cells (Figure 3C). In addition, we observed that overexpression of Skp2 significantly inhibited cell apoptosis in both PC cell lines (Figure 4A). Importantly, Skp2 overexpression abrogated rottlerin-induced cell apoptosis in PC cells (Figure 4A). Notably, we found that Skp2 cDNA remarkably increased the expression of Skp2 in PC cells (Figure 4B). Furthermore, down-regulation of Skp2 by rottlerin was rescued by Skp2 cDNA transfection in PC cells (Figure 4B). We also observed that overexpression of Skp2 abrogated rottlerin-mediated induction of p21 level in PC cells (Figure 4B).
Figure 4.
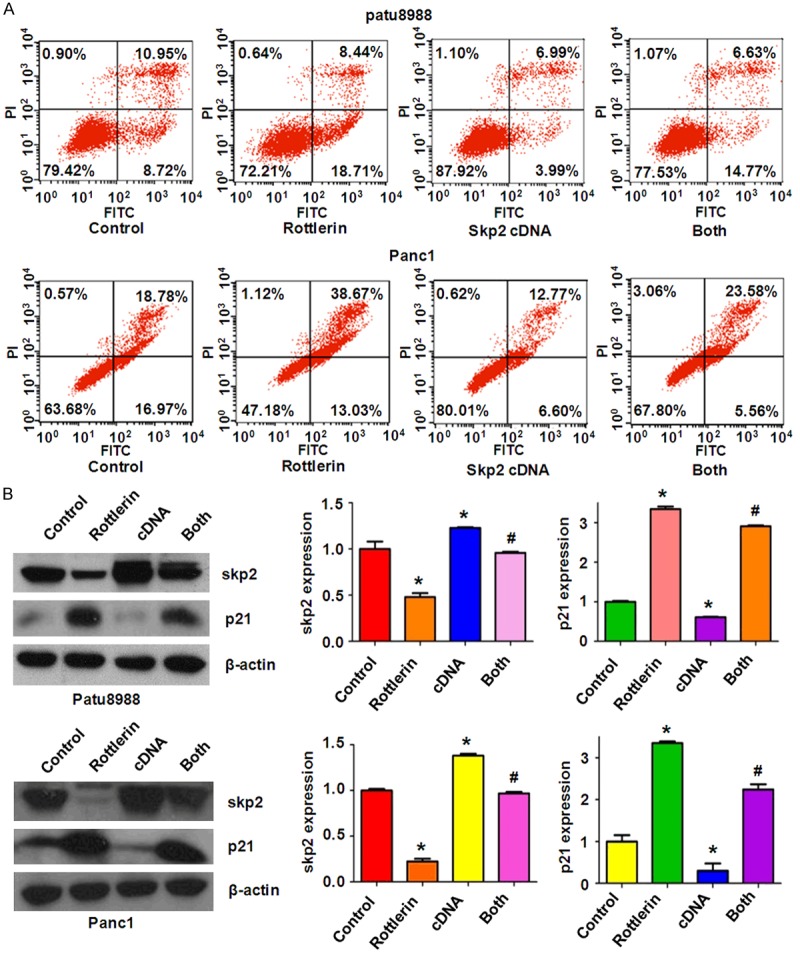
Skp2 overexpression inhibited rottlerin-induced apoptosis in PC cells. A. Cell apoptosis was measured by Flow cytometry in PC cells transfected with Skp2 cDNA and treated with rottlerin. B. Left panel, the expression of Skp2 and its target p21 was measured by Western blotting in PC cells with Skp2 cDNA transfection and rottlerin treatment. Right panel, Quantitative results are illustrated for left panel. Control: cDNA control (pcDNA 3.1); cDNA: Skp2 cDNA; Both: rottlerin + Skp2 cDNA. *P < 0.05, compared with control; #P < 0.05 compared with rottlerin treatment alone or Skp2 cDNA transfection alone.
Down-regulation of Skp2 by its siRNA promoted rottlerin-induced anti-tumor activity
To dissect the role of Skp2 in rottlerin-mediate anti-cancer activity, PC cells were transfected with Skp2 siRNA and then treated with rottlerin for 48 hours. In line with the oncogenic role of Skp2, we found that the depletion of Skp2 inhibited cell growth in both PC cells (Figure 5A). Moreover, down-regulation of Skp2 significantly enhanced cell growth inhibition induced by rottlerin (Figure 5A). Our wound healing assay results revealed that down-regulation of Skp2 retarded cell migration (Figure 5B). We also found that depletion of Skp2 inhibited cell invasion in both PC cell lines (Figure 5C). Notably, Skp2 siRNA transfection in combination with rottlerin treatment retarded cell migration and invasion to a greater degree compared with rottlerin alone or siRNA treatment alone (Figure 5B and 5C). We also found that depletion of Skp2 induced apoptosis in PC cells (Figure 6A). Skp2 siRNA transfected cells were significantly more sensitive to rottlerin-induced apoptosis (Figure 6A). Our results also showed that Skp2 siRNA transfection increased p27 and p21 levels in PC cells (Figure 6B). Rottlerin treatment plus Skp2 siRNA upregulated p27 and p21 expression to more degree compared to rottlerin alone or siRNA transfection alone (Figure 6B). These findings indicate that rottelrin exerted its anti-tumor activity partly through down-regulation of Skp2 in PC cells.
Figure 5.
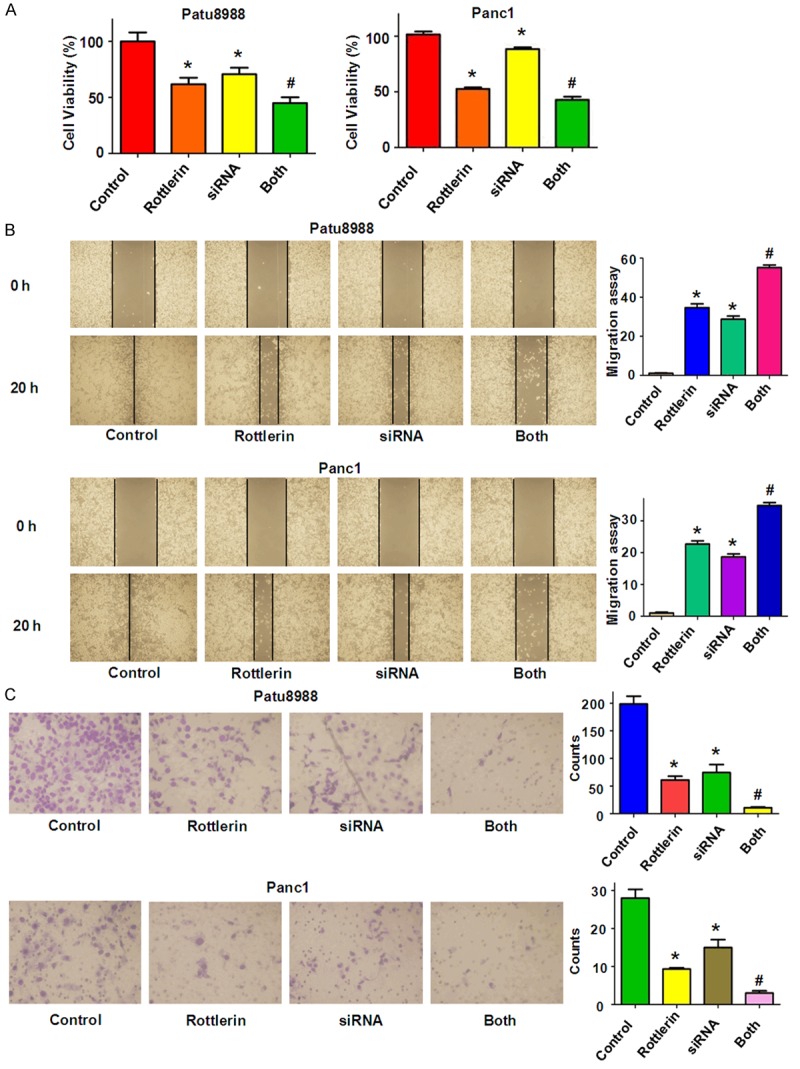
Skp2 siRNA promoted rottlerin-induced inhibition of cell growth, migration and invasion. A. Cell growth was analyzed by CellTiter-Glo® luminescence assay in PC cells after Skp2 siRNA transfection in combination with Rottlerin treatment. *P < 0.05, compared with control; #P < 0.05 compared with rottlerin treatment or Skp2 siRNA transfection. Control: siRNA control; siRNA: Skp2 siRNA; Both: rottlerin + Skp2 siRNA. B. Left panel: Cell migration was investigated by wound healing assay in PC cells after Skp2 siRNA transfection and rottlerin treatment. Right panel: Quantitative results are illustrated for left panel. *P < 0.05, compared with control; #P < 0.05 compared with rottlerin or siRNA treatment. C. Invasion assay was performed in PC cells after Skp2 siRNA transfection plus rottlerin treatment. Quantitative results are illustrated for the numbers of invasion cells. *P < 0.05, compared with control; #P < 0.05 compared with rottlerin treatment alone or siRNA transfection alone.
Figure 6.
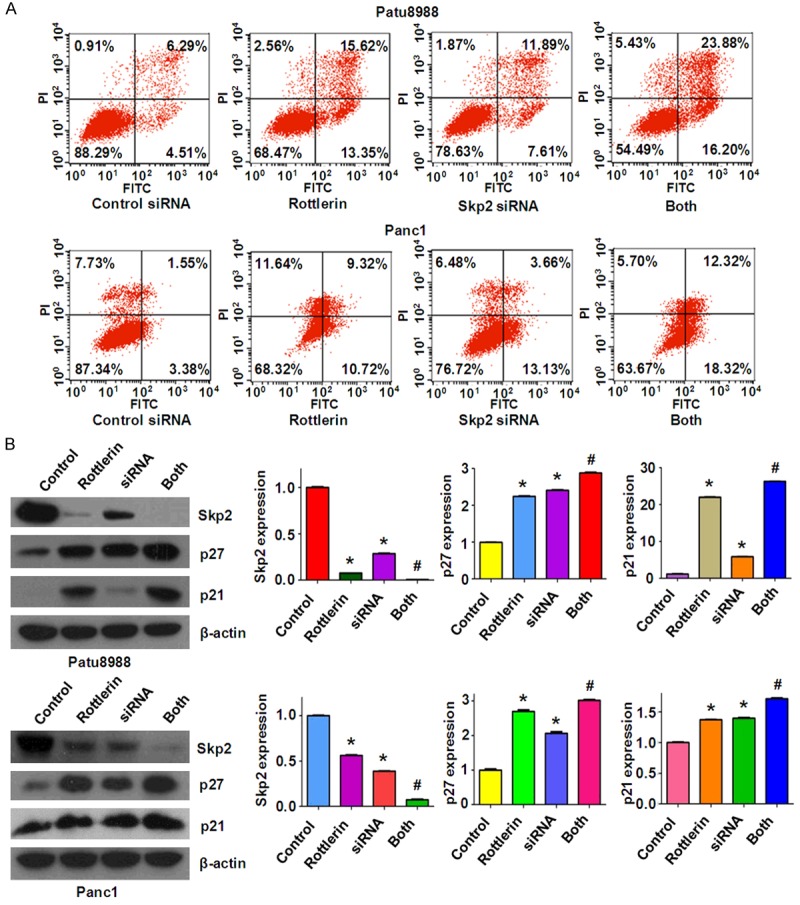
Down-regulation of Skp2 promoted rottlerin-induced apoptosis in PC cells. A. Apoptosis was determined by Flow cytometry in Patu8988 and Panc1 cells with Skp2 siRNA transfection and rottlerin treatment. Both: rottlerin + Skp2 siRNA. B. Left panel: Skp2 expression was tested by Western blotting analysis in PC cells with Skp2 siRNA transfection and rottlerin treatment. Right panel: Quantitative results are illustrated for left panel. *P < 0.05, vs control; #P < 0.05 vs rottlerin treatment or Skp2 siRNA transfection.
Discussion
Emerging evidence has validated that Skp2 was critically involved in tumorigenesis via promoting tumor growth and invasion mainly through targeting its substrates [13]. Clinically, high level of Skp2 was observed in a variety of human cancers including PC [26]. Overexpression of Skp2 was reported to be associated with the extent of lymph node metastasis, higher histological grade and poorer outcome in PC patients [26]. Several lines of evidence show that Skp2 promoted tumor progression and metastasis by down-regulation of p27 tumor suppressor protein and blocking p53-mediated apoptosis in PC cells [27,30]. Moreover, one study found that Skp2 conferred resistance of PC cells towards TRAIL (tumor necrosis factor-related apoptosis-inducing ligand) -induced apoptosis [31]. Recently, it has been reported that depletion of Y-box binding protein-1 inhibited tumor growth and arrested cell cycle via downregulation of Skp2 and consequent p27 accumulation in PC cells [32]. In current study, we also observed that overexpression of Skp2 enhanced cell growth, migration and invasion, whereas depletion of Skp2 inhibited cell growth and retarded migration and invasion. Altogether, Skp2 could play an oncogenic role in pancreatic tumorigenesis.
Since oncoprotein Skp2 is overexpressed in PC, inactivation of Skp2 could block development and progression of PC. Several groups have discovered multiple Skp2 inhibitors for inactivation of Skp2. For example, Singh R et al. have found that 5-bromo-8-toluylsulfonamidoquinoline-1 inhibited the Cks1-Skp2 protein-protein interaction, and subsequently increased p27 accumulation and inhibited cell growth of lung tumor cells [33]. Another group developed compound M1 as a Skp2 inhibitor, which could target the p300-binding site of Skp2 and thus blocks the Skp2/p300 interaction, leading to induction of p53-mediated apoptosis and cell death in cancer cells [34]. Additionally, arsenic trioxide has been found to exert anti-cancer activity in PC cells by down-regulating of Skp2 and upregulation of skp2 downstream targets such as FOXO1 and p53 [27]. Lin et al. reported that caffeic acid phenethyl ester inhibited cell growth and induced cell cycle arrest via regulation of Skp2 and its targets including p53, p21 and p27 in prostate cancer cells [35]. Recently, several natural compounds have been discovered as Skp2 inhibitors [35-38]. Flavokawain A (FKA), a chalcone from the kava plant, selectively down-regulated Skp2 in a proteasome dependent and ubiquitination mediated manner, and subsequently upregulated p27 level in prostate cancer [39]. Huang et al. reported that quercetin, curcumin and lycopene induced cell cycle arrest in breast cancer cells through downregulation of Skp2 protein [36]. Similarly, curcumin was found to exhibit cytotoxic effects on breast cancer cells via targeting PI3K/Akt-SKP2-Cip/Kips pathway [37,38]. Our previous study also showed that curcumin inhibited cell growth and induced apoptosis through inhbition of Skp2 in glioma cells [40]. In the present study, we observed that rottlerin downregulated Skp2 expression and subsequently accumulated its target genes levels including p21 and p27 in PC cells, suggesting that rottlerin could be a potential inhibitor of Skp2 in pancreatic cancer cells (Figure 7).
Figure 7.
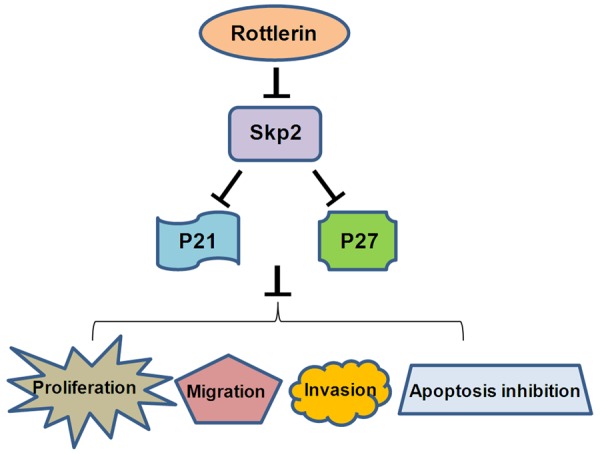
A schematic illustration of how rottlerin exerts its anti-tumor activity via targeting Skp2 in PC cells.
Several laboratories have demonstrated that rottlerin plays a key role in regulation of cell cycle and apoptosis through multiple signal pathways, including NF-κB-dependent and PKCδ/ERK-dependent pathway in human cancers [41,42]. Rottlerin has also been reported to inhibit autophagy in pancreatic cancer cells [43]. Recently, further studies have discovered that rottlerin could regulate multiple pathways, including Akt, Notch and Shh signaling pathways [9], Bcl-2 family [25], eEF-2K (elongation factor-2 kinase) [44] and PI3K/Akt/mTOR [45] pathway in PC cells. These reports argue that rottlerin could govern the expression of some genes. Therefore, further in-depth investigations are required to explore which pathway is pivotal for rottlerin-mediated anti-tumor activity in PC cells. More molecular studies are necessary to investigate how rottlerin inhibits Skp2 expression in PC cells. Furthermore, in vivo study is important to detect whether rottlerin retards tumor growth via inhibition of Skp2 in PC mouse models. In conclusion, our study identified that anti-tumor activities by rottlerin in PC cells could be partly through inactivation of Skp2, suggesting that inhibiting Skp2 by rottlerin could be a potential effective strategy to treat PC.
Acknowledgements
This work was supported by grant from National Natural Science Foundation of China (NSFC number 81572936) and the priority academic program development of Jiangsu higher education institutions.
Disclosure of conflict of interest
None.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Garrido-Laguna I, Hidalgo M. Pancreatic cancer: from state-of-the-art treatments to promising novel therapies. Nat Rev Clin Oncol. 2015;12:319–334. doi: 10.1038/nrclinonc.2015.53. [DOI] [PubMed] [Google Scholar]
- 3.Binenbaum Y, Na’ara S, Gil Z. Gemcitabine resistance in pancreatic ductal adenocarcinoma. Drug Resist Updat. 2015;23:55–68. doi: 10.1016/j.drup.2015.10.002. [DOI] [PubMed] [Google Scholar]
- 4.Newman DJ, Cragg GM, Snader KM. Natural products as sources of new drugs over the period 1981-2002. J Nat Prod. 2003;66:1022–1037. doi: 10.1021/np030096l. [DOI] [PubMed] [Google Scholar]
- 5.Soltoff SP. Rottlerin: an inappropriate and ineffective inhibitor of PKCdelta. Trends Pharmacol Sci. 2007;28:453–458. doi: 10.1016/j.tips.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 6.Maioli E, Torricelli C, Valacchi G. Rottlerin and cancer: novel evidence and mechanisms. ScientificWorldJournal. 2012;2012:350826. doi: 10.1100/2012/350826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lu W, Lin C, Li Y. Rottlerin induces Wnt co-receptor LRP6 degradation and suppresses both Wnt/beta-catenin and mTORC1 signaling in prostate and breast cancer cells. Cell Signal. 2014;26:1303–1309. doi: 10.1016/j.cellsig.2014.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Torricelli C, Daveri E, Salvadori S, Valacchi G, Ietta F, Muscettola M, Carlucci F, Maioli E. Phosphorylation-independent mTORC1 inhibition by the autophagy inducer Rottlerin. Cancer Lett. 2015;360:17–27. doi: 10.1016/j.canlet.2015.01.040. [DOI] [PubMed] [Google Scholar]
- 9.Huang M, Tang SN, Upadhyay G, Marsh JL, Jackman CP, Srivastava RK, Shankar S. Rottlerin suppresses growth of human pancreatic tumors in nude mice, and pancreatic cancer cells isolated from Kras(G12D) mice. Cancer Lett. 2014;353:32–40. doi: 10.1016/j.canlet.2014.06.021. [DOI] [PubMed] [Google Scholar]
- 10.Heestand GM, Kurzrock R. Molecular landscape of pancreatic cancer: implications for current clinical trials. Oncotarget. 2015;6:4553–4561. doi: 10.18632/oncotarget.2972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seicean A, Petrusel L, Seicean R. New targeted therapies in pancreatic cancer. World J Gastroenterol. 2015;21:6127–6145. doi: 10.3748/wjg.v21.i20.6127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frescas D, Pagano M. Deregulated proteolysis by the F-box proteins SKP2 and beta-TrCP: tipping the scales of cancer. Nat Rev Cancer. 2008;8:438–449. doi: 10.1038/nrc2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hao Z, Huang S. E3 ubiquitin ligase Skp2 as an attractive target in cancer therapy. Front Biosci. 2015;20:474–90. doi: 10.2741/4320. [DOI] [PubMed] [Google Scholar]
- 14.Wang Z, Liu P, Inuzuka H, Wei W. Roles of F-box proteins in cancer. Nature Rev Cancer. 2014;14:233–47. doi: 10.1038/nrc3700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bochis OV, Irimie A, Pichler M, Berindan-Neagoe I. The role of Skp2 and its substrate CDKN1B (p27) in colorectal cancer. J Gastrointestin Liver Dis. 2015;24:225–234. doi: 10.15403/jgld.2014.1121.242.skp2. [DOI] [PubMed] [Google Scholar]
- 16.Wei Z, Jiang X, Qiao H, Zhai B, Zhang L, Zhang Q, Wu Y, Jiang H, Sun X. STAT3 interacts with Skp2/p27/p21 pathway to regulate the motility and invasion of gastric cancer cells. Cell Signal. 2013;25:931–938. doi: 10.1016/j.cellsig.2013.01.011. [DOI] [PubMed] [Google Scholar]
- 17.Luo J, Zhou Y, Wang B, Li Q, Chen Y, Lan H. Immunohistochemically detected expression of Skp2, p27 kip1, and p-p27 (Thr187) in patients with cholangiocarcinoma. Tumour Biol. 2015;36:5119–5125. doi: 10.1007/s13277-015-3164-1. [DOI] [PubMed] [Google Scholar]
- 18.Yang C, Nan H, Ma J, Jiang L, Guo Q, Han L, Zhang Y, Nan K, Guo H. High Skp2/Low p57(Kip2) Expression is Associated with Poor Prognosis in Human Breast Carcinoma. Breast Cancer (Auckl) 2015;9:13–21. doi: 10.4137/BCBCR.S30101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yan J, Yao Z, Hu K, Zhong Y, Li M, Xiong Z, Deng M. Hepatitis B Virus Core Promoter A1762T/G1764A (TA)/T1753A/T1768A Mutations Contribute to Hepatocarcinogenesis by Deregulating Skp2 and P53. Dig Dis Sci. 2015;60:1315–1324. doi: 10.1007/s10620-014-3492-9. [DOI] [PubMed] [Google Scholar]
- 20.Wang H, Cui J, Bauzon F, Zhu L. A comparison between Skp2 and FOXO1 for their cytoplasmic localization by Akt1. Cell Cycle. 2010;9:1021–1022. doi: 10.4161/cc.9.5.10916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang G, Chan CH, Gao Y, Lin HK. Novel roles of Skp2 E3 ligase in cellular senescence, cancer progression, and metastasis. Chin J Cancer. 2012;31:169–177. doi: 10.5732/cjc.011.10319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zheng N, Zhou Q, Wang Z, Wei W. Recent advances in SCF ubiquitin ligase complex: Clinical implications. Biochim Biophys Acta. 2016;1866:12–22. doi: 10.1016/j.bbcan.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang Q, Wang Y, Lu X, Zhao Z, Zhu L, Chen S, Wu Q, Chen C, Wang Z. MiR-125b regulates epithelial-mesenchymal transition via targeting Sema4C in paclitaxel-resistant breast cancer cells. Oncotarget. 2015;6:3268–3279. doi: 10.18632/oncotarget.3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ma J, Fang B, Zeng F, Ma C, Pang H, Cheng L, Shi Y, Wang H, Yin B, Xia J, Wang Z. Down-regulation of miR-223 reverses epithelial-mesenchymal transition in gemcitabine-resistant pancreatic cancer cells. Oncotarget. 2015;6:1740–1749. doi: 10.18632/oncotarget.2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ohno I, Eibl G, Odinokova I, Edderkaoui M, Damoiseaux RD, Yazbec M, Abrol R, Goddard WA 3rd, Yokosuka O, Pandol SJ, Gukovskaya AS. Rottlerin stimulates apoptosis in pancreatic cancer cells through interactions with proteins of the Bcl-2 family. Am J Physiol Gastrointest Liver Physiol. 2010;298:G63–73. doi: 10.1152/ajpgi.00257.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Einama T, Kagata Y, Tsuda H, Morita D, Ogata S, Ueda S, Takigawa T, Kawarabayashi N, Fukatsu K, Sugiura Y, Matsubara O, Hatsuse K. High-level Skp2 expression in pancreatic ductal adenocarcinoma: correlation with the extent of lymph node metastasis, higher histological grade, and poorer patient outcome. Pancreas. 2006;32:376–381. doi: 10.1097/01.mpa.0000220862.78248.c4. [DOI] [PubMed] [Google Scholar]
- 27.Gao JK, Wang LX, Long B, Ye XT, Su JN, Yin XY, Zhou XX, Wang ZW. Arsenic trioxide inhibits cell growth and invasion via down-regulation of Skp2 in pancreatic cancer cells. Asian Pac J Cancer Prev. 2015;16:3805–3810. doi: 10.7314/apjcp.2015.16.9.3805. [DOI] [PubMed] [Google Scholar]
- 28.Zhong L, Georgia S, Tschen SI, Nakayama K, Bhushan A. Essential role of Skp2-mediated p27 degradation in growth and adaptive expansion of pancreatic beta cells. J Clin Invest. 2007;117:2869–2876. doi: 10.1172/JCI32198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kouvaraki MA, Korapati AL, Rassidakis GZ, Tian L, Zhang Q, Chiao P, Ho L, Evans DB, Claret FX. Potential role of Jun activation domain-binding protein 1 as a negative regulator of p27kip1 in pancreatic adenocarcinoma. Cancer Res. 2006;66:8581–8589. doi: 10.1158/0008-5472.CAN-06-0975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schneider G, Saur D, Siveke JT, Fritsch R, Greten FR, Schmid RM. IKKalpha controls p52/RelB at the skp2 gene promoter to regulate G1- to S-phase progression. EMBO J. 2006;25:3801–3812. doi: 10.1038/sj.emboj.7601259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schuler S, Diersch S, Hamacher R, Schmid RM, Saur D, Schneider G. SKP2 confers resistance of pancreatic cancer cells towards TRAIL-induced apoptosis. Int J Oncol. 2011;38:219–225. [PubMed] [Google Scholar]
- 32.Shinkai K, Nakano K, Cui L, Mizuuchi Y, Onishi H, Oda Y, Obika S, Tanaka M, Katano M. Nuclear expression of Y-box binding protein-1 is associated with poor prognosis in patients with pancreatic cancer and its knockdown inhibits tumor growth and metastasis in mice tumor models. Int J Cancer. 2016;139:433–445. doi: 10.1002/ijc.30075. [DOI] [PubMed] [Google Scholar]
- 33.Singh R, Sran A, Carroll DC, Huang J, Tsvetkov L, Zhou X, Sheung J, McLaughlin J, Issakani SD, Payan DG, Shaw SJ. Developing structure-activity relationships from an HTS hit for inhibition of the Cks1-Skp2 protein-protein interaction. Bioorg Med Chem Lett. 2015;25:5199–5202. doi: 10.1016/j.bmcl.2015.09.067. [DOI] [PubMed] [Google Scholar]
- 34.Oh M, Lee JH, Moon H, Hyun YJ, Lim HS. A Chemical Inhibitor of the Skp2/p300 Interaction that Promotes p53-Mediated Apoptosis. Angew Chem Int Ed Engl. 2016;55:602–6. doi: 10.1002/anie.201508716. [DOI] [PubMed] [Google Scholar]
- 35.Lin HP, Lin CY, Huo C, Hsiao PH, Su LC, Jiang SS, Chan TM, Chang CH, Chen LT, Kung HJ, Wang HD, Chuu CP. Caffeic acid phenethyl ester induced cell cycle arrest and growth inhibition in androgen-independent prostate cancer cells via regulation of Skp2, p53, p21Cip1 and p27Kip1. Oncotarget. 2015;6:6684–6707. doi: 10.18632/oncotarget.3246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang HC, Lin CL, Lin JK. 1,2,3,4,6-penta-O-galloyl-beta-D-glucose, quercetin, curcumin and lycopene induce cell-cycle arrest in MDA-MB-231 and BT474 cells through downregulation of Skp2 protein. J Agric Food Chem. 2011;59:6765–6775. doi: 10.1021/jf201096v. [DOI] [PubMed] [Google Scholar]
- 37.Jia T, Zhang L, Duan Y, Zhang M, Wang G, Zhang J, Zhao Z. The differential susceptibilities of MCF-7 and MDA-MB-231 cells to the cytotoxic effects of curcumin are associated with the PI3K/Akt-SKP2-Cip/Kips pathway. Cancer Cell Int. 2014;14:126. doi: 10.1186/s12935-014-0126-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sun SH, Huang HC, Huang C, Lin JK. Cycle arrest and apoptosis in MDA-MB-231/Her2 cells induced by curcumin. Eur J Pharmacol. 2012;690:22–30. doi: 10.1016/j.ejphar.2012.05.036. [DOI] [PubMed] [Google Scholar]
- 39.Li X, Yokoyama NN, Zhang S, Ding L, Liu HM, Lilly MB, Mercola D, Zi X. Flavokawain A induces deNEDDylation and Skp2 degradation leading to inhibition of tumorigenesis and cancer progression in the TRAMP transgenic mouse model. Oncotarget. 2015;6:41809–24. doi: 10.18632/oncotarget.6166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang L, Ye X, Cai X, Su J, Ma R, Yin X, Zhou X, Li H, Wang Z. Curcumin suppresses cell growth and invasion and induces apoptosis by down-regulation of Skp2 pathway in glioma cells. Oncotarget. 2015;6:18027–18037. doi: 10.18632/oncotarget.4090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Valacchi G, Pecorelli A, Mencarelli M, Carbotti P, Fortino V, Muscettola M, Maioli E. Rottlerin: a multifaced regulator of keratinocyte cell cycle. Exp Dermatol. 2009;18:516–521. doi: 10.1111/j.1600-0625.2008.00816.x. [DOI] [PubMed] [Google Scholar]
- 42.Maioli E, Valacchi G. Rottlerin: bases for a possible usage in psoriasis. Curr Drug Metab. 2010;11:425–430. doi: 10.2174/138920010791526097. [DOI] [PubMed] [Google Scholar]
- 43.Akar U, Ozpolat B, Mehta K, Fok J, Kondo Y, Lopez-Berestein G. Tissue transglutaminase inhibits autophagy in pancreatic cancer cells. Mol Cancer Res. 2007;5:241–249. doi: 10.1158/1541-7786.MCR-06-0229. [DOI] [PubMed] [Google Scholar]
- 44.Ashour AA, Abdel-Aziz AA, Mansour AM, Alpay SN, Huo L, Ozpolat B. Targeting elongation factor-2 kinase (eEF-2K) induces apoptosis in human pancreatic cancer cells. Apoptosis. 2014;19:241–258. doi: 10.1007/s10495-013-0927-2. [DOI] [PubMed] [Google Scholar]
- 45.Singh BN, Kumar D, Shankar S, Srivastava RK. Rottlerin induces autophagy which leads to apoptotic cell death through inhibition of PI3K/Akt/mTOR pathway in human pancreatic cancer stem cells. Biochem Pharmacol. 2012;84:1154–1163. doi: 10.1016/j.bcp.2012.08.007. [DOI] [PubMed] [Google Scholar]


