Abstract
Tumor endothelial cells have been found to be associated with metastasis and cancer progression. In this study, we reported that human esophageal cancer endothelial cells (HECEC), unlike corresponding human esophageal normal endothelial cells (HENEC) displayed several distinct feature couple with unique gene expression profile. Further studies showed that HECEC can enhance migration, invasion and self-renewal properties of esophageal carcinoma cell in vitro by a direct cell-cell interaction. In vivo assay demonstrated that HECEC could significantly enhance the invasion and lung metastasis of esophageal cancer cells. To elucidate the molecular mechanisms of HECEC in esophageal carcinoma progression, we employed the microarray to analyze the gene expression profiles before and after treating with HECEC, HENEC or conditioned meium from HECEC. Among the highly expressed HECEC-regulated genes, we focused on Epiregulin (EREG). Further studies demonstrated that overexpression of EREG in EC9706 or Kyse30 cells can induce actin reorganization, sphere formation ability and a significantly enrichment of CD44+ cancer stem-like cells. Moreover, up-regulation of EREG in esophageal cancer cells could enhance lung metastasis and decrease the survival time in vivo. Further study indicated that EREG could induce activation of the Src and FAK. In addition, all these effects could also be inhibited by the function-blocking anti-EREG antibody in a dose dependent manner. Immunohistochemical analysis revealed that high level of EREG was significantly correlated with lymph node metastases and poor prognosis. In summary, HECEC play key roles in enhancing the invasion, migration, cancer stem cell phenotype and metastatic potential of esophageal cancer cells through Epiregulin.
Keywords: Esophageal cancer, EREG, cancer stem cell, CD44, tumor endothelial cells, metastasis
Introduction
Human esophageal carcinoma is one of the most common causes of cancer death worldwide, and is particularly prevalent in China. The poor prognosis of esophageal cancer is largely due to early-stage invasion of adjacent tissues and late-satge distant metastasis [1].
It has been reported that many types of cancer, including esophageal carcinoma, are initiated from and maintained by cancer stem cells (CSCs), which possesses the self-renewal capacity and can give rise to the heterogeneous lineages of daughter cancer cells [2]. Accumulating evidence has shown that cancer stem cells (CSCs) played essential roles in promoting tumor invasion and metastasis [3,4]. Thus, targeting cancer stem cell might be a promising therapeutic option.
The tumor microenvironment contains endothelial cells, immune cells as well as the soluble factors could maintain the cancer stem cell phenotype to facilitate cancer initiation, progression and distant metastasis [5,6]. Among them, tumor endothelial cells played essential roles in the tumor growth and survival. Unlike the endothelium in the normal “quiescent” tissues, tumor endothelial cells owned unique structure and functions [7]. Previous studies in our lab successfully isolated and harvested the HECEC from fresh samples of esophageal squamous cell carcinoma [8]. We also found that HECECs, not HUVEC, can significantly enhance the esophageal tumor growth when were co-injected with human esophageal cancer cells into nude mice [9]. Although there is a great deal of evidence that immortalized normal EC can enhance tumor growth, cancer stem cell (CSC) phenotype and pro-metastatic properties, the roles of tumor endothelial cell in tumor invasion and metastasis need further validation [10,11]. In this study, we investigated the mechanism of the interaction between HECECs and esophageal cancer cells in promoting tumor invasion and metastasis. Here, we report that HECEC could promote esophageal cancer progression through elevated Epiregulin.
Materials and methods
Samples
All tissue specimens were collected from patients in the Department of Pathology in Cancer Hospital, Chinese Academy of Medical Sciences, Beijing, China. Patients did not receive any treatment before surgery, and signed informed consent forms for sample collection. For immunohistochemistry analysis, 120 paraffin-embedded esophageal carcinoma and paired adjacent normal esophageal tissues were randomly obtained from patients with clinical follow-up records during 1996-2005. 60 paraffin-embedded esophageal carcinoma and paired lymph node metastatic samples were randomly collected from patients during 1997-2002. For all the specimens, clinicopathological information (age, gender, pathology, differentiation, and TNM stage) was available. The study was approved by the medical ethics committee of Cancer Institute and Hospital, CAMS.
Cell culture
The HECECs and HENECs were isolated from human esophageal squamous carcinoma and paired adjacent normal tissues [9]. Briefly, The tissues were rinsed with 0.1 M Phosphate buffer saline for about 10 minutes and cut into slices. Then, they were digested with 0.1% collagenase at 37°C for 2 hr. Magnetic beads (Miltenyi Biotec, Germany) coupled with anti-CD31 (Endogen, Woburn, MA, USA) were added into the plates to bind to the endothelial cells. The cells were washed three times with D-MEM to discard excessive beads. After 10 mM EDTA/0.1% trypsin (Gibco) treatment, cells binding with magnetic beads were subfractionated by magnetic attraction. Finally, endothelial cells were incubated in plates coated by 2% Gelatin (Sigma) in D-MEM supplemented with 10% FBS and 100 μg/ml ECGS (endothelial cells growth supplement, Sigma) at 37°C, 5% CO2. The esophageal carcinoma cell (ESCC) line EC9706 (a gift from Dr. Minrong Wang, Chinese Academy of Medical Sciences Cancer Institute Hospital, Peking University Medical School, Beijing 100021, China) was cultured in RPMI 1640 supplemented with 10% fetal bovine serum. KYSE30 was generously provided by Dr. Shimada, Kyoto University, were maintained in the medium containing MEM (Gibco) with 10% FBS.
In vitro angiogenesis assay
As described previously, 96-well plates were coated with 50 ul/well ice-cold Matrigel (BD Biosciences, San Jose, CA, USA), which was allowed to polymerize for 30 min at 37°C. 2 × 103 HECECs, HENEC and HUVEC were seeded into a 96-well culture plate pre-coated with Matrigel and then cultured in the culture condition. After 24 h incubation, tube formation was observed and quantified by using a phase contrast microscopy [8].
Invasion assay
Invasion assays were carried out in a 24-well transwell unit on polycarbonate filter (40 um) coated with Matrigel. Esophageal cancer cells were placed into the upper well, cultured for 24 h and allowed to invade into the Matrigel layer. After a 24 h incubation period, the cancer cells that had passed through the filter into the lower wells were stained by 4’,6-diamidino-2-phenylindole (DAPI), counted and photographed. All experiments were performed in triplicate.
Immunohistochemistry
The avidin-biotin-complex method was used for immunohistochemical analysis. Endogenous reactivity for peroxidase was inactivated before antigen retrieval with citrate buffer. After blocking with 10% goat serum at 37°C for 30 min, the slides were incubated with primary antibody at 37°C for 2 hours. Standard avidin-biotin complex peroxidase immunohistochemical staining was performed with anti-EREG Polyclonal Antibody (AF1195, R&D Systems) at 10 μg/mL. Biotinylated secondary antibodies were used to visualize the specific markers by avidin-HRP/DAB reaction. Tissues with no staining were rated as 0, with faint staining or moderate to strong staining in 10% of cells as 1, with moderate staining or strong staining in 10% to 50% of cells as 2, and with strong staining in > 50% of cells as 3. Esophageal carcinoma tissues that registered levels 0 and 1 were defined as negative for expression, whereas samples at levels 2 or 3 were defined as positive.
RT-PCR
Cells were harvested in Trizol® reagent (Invitrogen), and total RNA was isolated according to the manufacturer’s instructions. Single-stranded cDNA was synthesized from 4 µg total RNA using M-MLV reverse transcriptase (Invitrogen), with an oligo(dT) 18-mer as the primer, in a final reaction volume of 25 µl.
EREG: 5-AGCTTCCCTTCTAGGCTGACA-3, 5-CGAGTATTCAGACTTGCGGC-3; AREG: 5-AAGCTTACTCGCTCTTCCAAC-3, 5-CTATGCTATAGCATGTACATTTCC-3; MMP1: 5-AGCTTAGCCATCACTTACCTTG-3, 5-AGCTCGAGCCAATTTTTCCTGC-3; MMP3: 5-CGAGCTAAGTAAAGCCAG-3, 5-AGCCAACAATTAAGCCAGC-3; CYR61: 5-GCAAAGCTTCTTGTTGGCGTCT-3, 5-CAGGCTCGAGAAAGTCCCTAAATT-3; IL6: 5-ATGTCCTTCTCCACAAGCGC-3, 5-GAAGAGCCCTCAGGCTGGACTG-3; IL8: 5-GATGACGAGTTGTGGTCCCT-3, 5-GATTGGCCTTGGAAGATGAA-3; CD44: 5-ATCTACCCCAGCAACCCTAC-3, 5-GCCTGCTGAGATGGTATTTG-3; GAPDH: 5-TGGTATCGTGGAAGGACTCATGAC-3, 5-ATGCCAGTGAGCTTCCCGTTCAGC-3.
Western blotting
For western blot analysis, cellular proteins were extracted in 40 mM Tris-HCl (pH 7.4) containing 150 mM NaCl and 1% (v/v) Triton X-100, supplemented with a cocktail of protease inhibitors. Equal amounts of protein were resolved on 10% SDS-PAGE gels, then transferred to a PVDF membrane. After blocking with 5% non-fat milk, the membranes were incubated, first with primary antibody at 4°C overnight, then with HRP-conjugated sheep anti-rabbit or anti-mouse IgG secondary antibodies (Vector, Burlingame, CA). After washing, the blots were developed using the Super Enhanced chemiluminescence detection kit (Applygen Technologies Inc., Beijing, China). Protein bands were visualized after exposure of the membrane to Kodak X-ray film. Antibodies against EREG (AF1195, 0.1 μg/mL) was purchased from R&D Systems. EGFR (44796G, used at 1:1000), EGFR [pY1086] (44790G, used at 1:1000), FAK (34Q36, used at 1 μg/mL), FAK [pY576] (44-652G, used at 1 μg/mL), SRC (44-655G, used at 1:1000), SRC [pY418] (44660G, used at 1:1000) were purchased from Invitrogen.
Immunofluorescence
Cells were cultured for 24 hours. After rinsing with PBS, cells were fixed in PBS-4% paraformaldehyde for 30 minutes and were permeabilized with 0.1% Triton X-100 (Roche Diagnostics GmbH, Mannheim, Germany) for 10 minutes at 4°C. F-actin of the cells were then dyed with Phallotoxin (A12381).
Animal experiments
All the animal experiments were performed in full compliance with institutional guidelines and with the approval of the Animal Care and Use Committee, Cancer Institute/Hospital, CAMS and PUMC. Female nu/nu mice from the Jackson Laboratory (Vitalriver, China) were kept in a pathogen-free facility at Cancer Hospital, Chinese Academy of Medical Sciences. The facility is accredited for animal care by the Chinese Association for Accreditation of Laboratory Animal Care. For co-transplantation of Tumor Cells with Endothelial Cells model, 1 × 106 tumor cells (KYSE30s or EC9706) mixed with 4 × 106 endothelial cells (HECECs or HENECs) were transplanted into nude mice. Mice were divided into groups of six animals each, according to the number and kind of cells injected. Cell mixture was subcutaneously inoculated on the back by one injection per mouse. Mice were sacrificed 30 days after injected with the mixture of KYSE30 or EC9706 cells with HECECs, and KYSE30 or EC9706 cells with HENECs to measure the invasive pattern and distant metastatic foci. For experimental metastases model, EC9706, EC9706-EREG and vector cells were subcutaneously injected into nude mice with 1 × 106 cells per animal for each group. 43 days after injection, 24 mice were killed and invasive pattern were analyzed with hematoxylin and eosin (H&E) staining. The lung with metastasis foci was used for pathological confirmation.
Statistical analysis
The SPSS, version 15, software package (SPSS Inc., Chicago, IL) was used for statistical analysis. The two-sided t-test method was used for analysis of the number of lung metastatic foci among groups. The association between the immunoreactive markers and the clinicopathologic features was analyzed using the Chi-square test or the two-sided t-test, as appropriate. The survival rates were assessed by the Kaplan-Meier method and compared by the log-rank test. P-value < 0.05 was considered statistically significant.
Results
Tumor endothelial cells from human esophageal carcinoma tissues displayed the distinct gene pattern
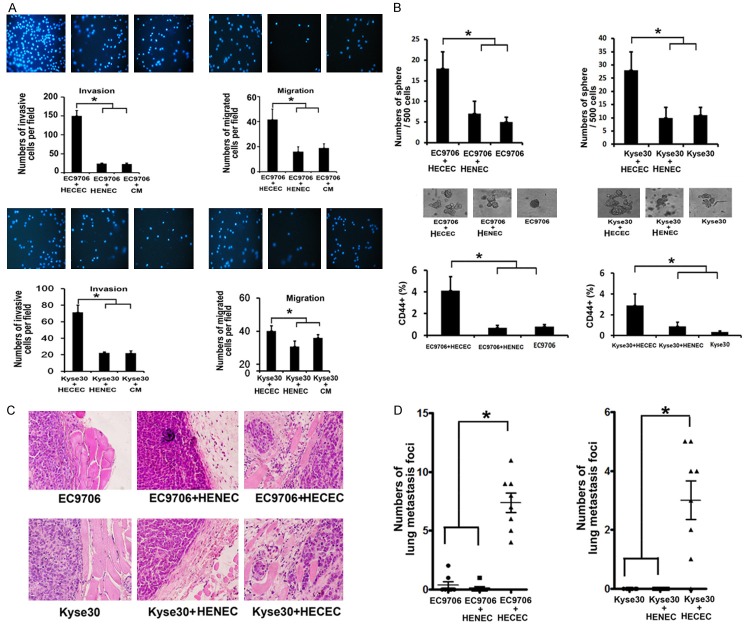
We previously reported that HECECs displayed spindle-shaped endothelial cell morphology with EC-specific markers and enhanced the tumor growth in vivo [9]. To explore the biological significance of angiogenesis, we used HECECs, HENECs and HUVEC for tube-formation assays, and found that they can form the tube in the Matrigel by the Tube formation assay for six hours at 37°C (Figure 1A). Cell proliferation assay using CCK-8 showed that HECECs proliferated more quickly than HENECs did. Also, HECECs can be continually passaged for at least 25 times or longer than HENECs (Figure 1B). To further analyze the different gene expression profiles between HECECs and HENECs, we identified 55 genes including 29 up-regulated genes and 26 down-regulated genes with respect to HENECs (cutoff of > 3 fold or < 0.2-fold) (Figure 1C). Functional clustering analysis by using DAVID database demonstrated that these differentially expressed genes were highly related to extracellular matrix factors (Figure 1D). We speculated that HECECs can facilitate tumor growth and metastatic dissemination by maintaining tumor microenvironment.
Figure 1.
The characterics of HECECs. A. The level of CD31 and the tube formation of HECECs, HENEC and HUVEC (× 20). B. The growth curve and passage time of HECECs and HENEC. C. Heat map of microarray data between HECECs and HENEC. D. Different Functional Gene Clusters.
HECECs enhance the invasion, migration and cancer stem-like cell self-renewal of esophageal cancer cells in vitro
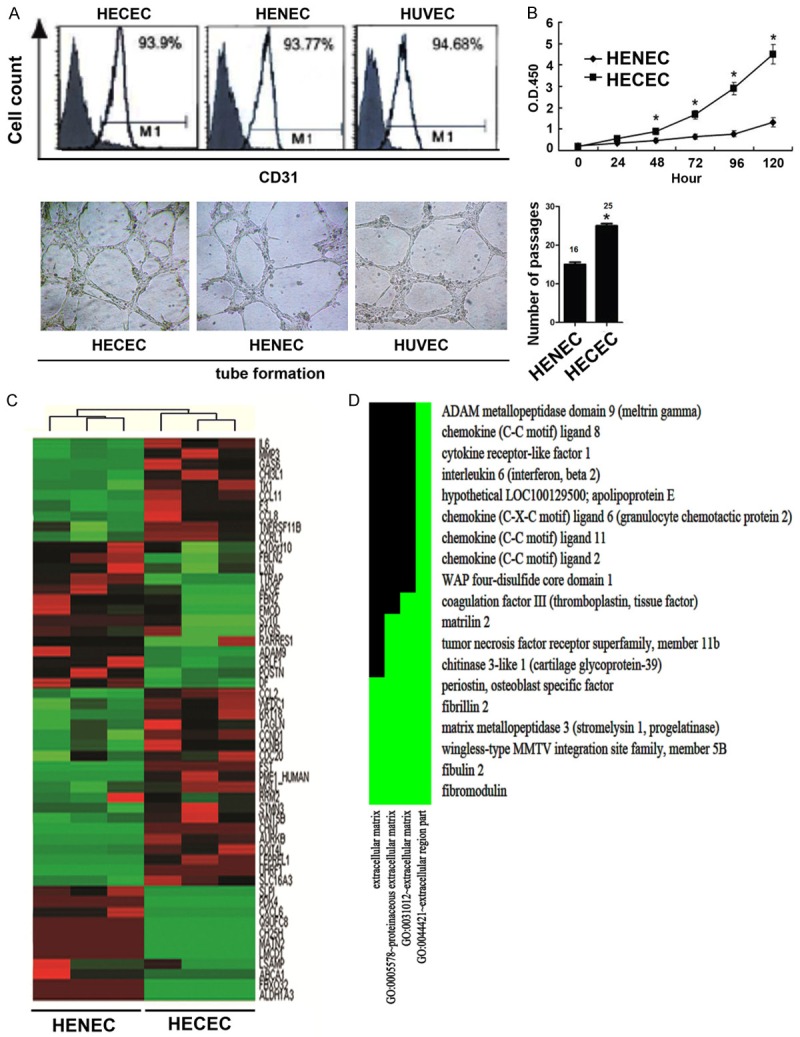
To investigate the effect of HECECs on esophageal cancer cells, we established a tumor-endothelial cells co-culture experiment [12]. Briefly, HECECs and HUVECs were grown as a monolayer to confluence on a galetin-coated flask. EC9706 and KYSE30 cells were plated on them. After 72 hours, the cancer cells began to penetrate the monolayer of endothelium, and ultimately grew up to cancer cell nests. We then removed endothelium by taking advantage of the fact that they were much more easily trypsinized than the esophageal cancer cells. The surviving tumor cells were subjected to analyze for several biological properties, and the cancer cells treated with HECEC cultural medium (CM) were as a control. As shown in Figure 2A, HECECs significantly accelerated the invasion and migration of EC9706 and Kyse30 cells, while CM from HECEC hardly affected the corresponding phenotype. To examine the effects of tumor endothelial cells on the self-renewal of CSCs, esophageal cancer cells interacting with tumor endothelial cells were cultured in serum-free medium and sphere formation was observed. We found that HECEC could increase sphere-forming capability of EC9706 or Kyse30 cells by nearly 3-fold as compared to parental cells or co-culture with HENEC (Figure 2B). It has been reported that CD44 was a CSC marker in esophageal cancer [13,14]. Further fluorescence-activated cell sorting (FACS) analysis indicated that percentage of CD44+ cells was significantly increased by co-culturing with HECEC (Figure 2B).
Figure 2.
The HECECs enhance ESCC cell malignant phenotype. A. The migration and invasion of EC9706 and Kyse30 after co-culturing with HECECs or HENEC or treated by CM from HECECs. B. The sphere number of EC9706 and Kyse30 in serum free medium after co-culturing with HECECs or HENEC. The percentage of CD44 in EC9706 and Kyse30 after co-culturing with HECECs or HENEC. C. H&E staining of EC9706 and Kyse30 xenograft tumor containing HECECs or HENEC. The showed a high invasive pattern in the EC9706 and Kyse30 co-injecting with HECECs. D. EC9706 and Kyse30 xenograft tumor containing HECECs significantly increased the lung metastatic foci number. (Statistical plots of lung metastasis foci).
HECECs promotes esophageal cancer cell invasion and lung metastasis in vivo
To investigate the role of HECEC in tumor progression, we inoculated the cell mixture containing KYSE30 cells or EC9706 with HECECs or HENECs into nude mice, and collected the lungs along with primary tumor tissues at the end of the experiment. The result demonstrated that Kyse30 or EC9706 cancer cells containing HECEC exhibited the highly invasive growth patterns into the surrounding muscles (Figure 2C). More importantly, we found that Kyse30 or EC9706 cells with HECEC displayed significantly more lung metastasis foci as compared to esophageal cancer cells with HENEC (Figure 2D).
HECEC-regulated genes gene expression analysis
To identify the HECEC-regulated genes, we employed microarrays to analyze the gene profile and identified 15 up-regulated genes in EC9706 cells directly contacting with HECEC (P < 0.01, cutoff of > 3-fold) (Figure 3A). Functional analysis revealed that most of them were involved in tumor invasion and metastasis. Furthermore, we selected 8 genes to validate microarray results by RT-PCR. The results showed that the expression changes of 8 selected genes were in consistence with the microarray data, and Epiregulin (EREG) was selected for further study (Figure 3B).
Figure 3.
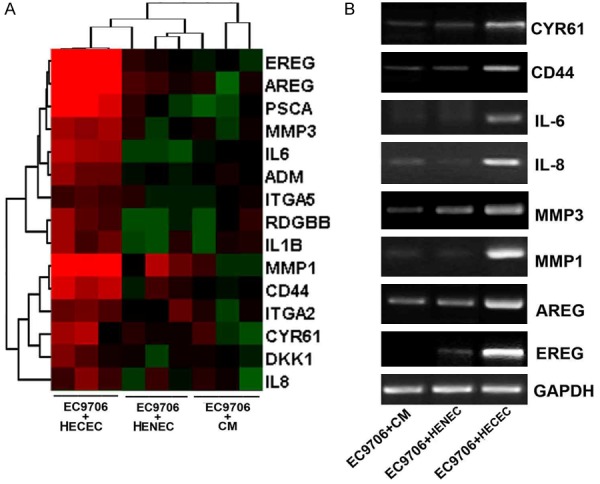
ECEC-regulating genes profile. A. The heat map of the dysregulated genes in the EC9706 before and after co-culturing with HECECs or HENEC or treated by CM from HECECs. B. Reverse transcription-PCR analysis of the selected genes.
EREG promotes invasion, sphere formation, actin reorganization and lung metastasis by activation of FAK and Src
To study the function of EREG, we established the EREG-overexpressed cells (EC9706-EREG and Kyse30-EREG) by using plasmid transfection. As shown in Figure 4A, EREG overexpression can induce a marked 3.9 fold increase in EC9706 cell invasion. The similar results also were found in KYSE30 cell line. There is evidence that reorganization of actin cytoskeleton contributed to the cancer cell migration and invasion. Immunofluorescence analysis revealed that over-expression of EREG can induce the formation of F-actin polymers and the filopodia at leading edges of cells (Figure 4B). Sphere formation assays in serum free culture medium demonstrated that the numbers of spheres were increased in both EC9706-EREG and Kyse30-EREG cells compared with vector or parental cancer cells, respectively (Figure 4C). CD44 was a candidate cancer stem cell marker in ESCC [13]. Then, we tested whether up-regulation of EREG could enhance the level of CD44+ cells by flow cytometric analysis. The result showed that high level of EREG enriched the CD44-positive cell population by 8 fold comparing to EC9706-vector cells. Consistently, overexpression of EREG in Kyse30 cells also enhance the percentage of CD44 positive cell by 7.3 fold (Figure 4D). To investigate the role of EREG in lung metastasis, EC9706-EREG cells were injected subcutaneously into the flanks of nude mice, and the number of lung metastatic foci was measured at the end of experiment. We found that EREG-EC9706 cells formed significantly more lung metastatic foci as compared to control (vector) cells (Figure 4D). In addition, the mice injected with EC9706-EREG cells showed a shorter survival time than mice with EC9706 cells (Figure 4E). EREG, a member of the epidermal growth factor family, can function as a ligand of EGFR. We found that the phospho-signals of EGFR (Y1068), Src (pY418) and FAK (pY576) were increased in Western blot. Meanwhile, we employed the specific EREG polyclonal inhibitory antibody and measured its effects. It could significantly suppress the phosphorylation of EGFR, Src and FAK in a dosage-dependent manner (Figure 4F).
Figure 4.
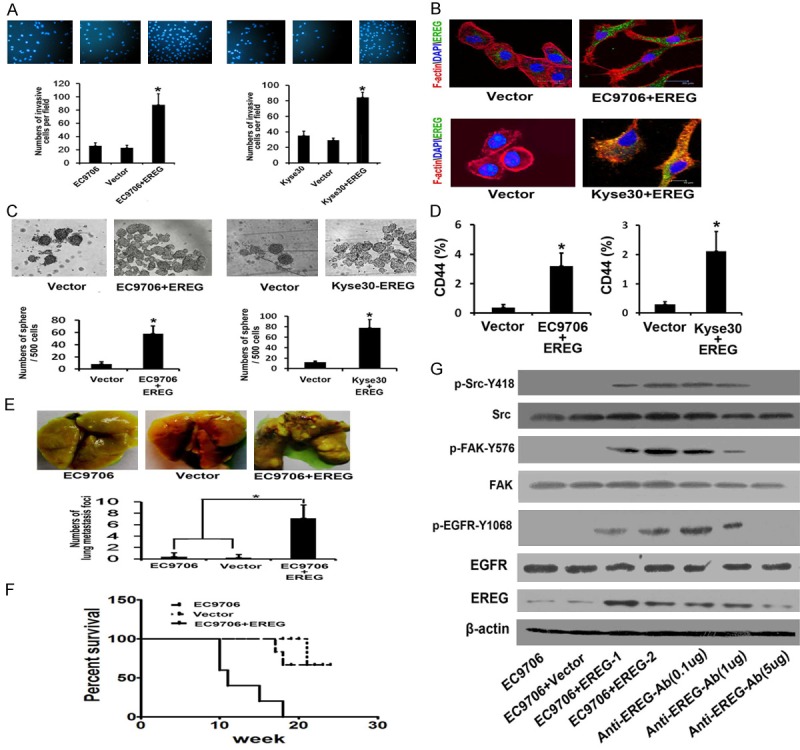
EREG overexpression enhanced cell migration, invasion, structure of actin cytoskeleton in vitro and lung metastasis in vivo. A. F-actin cytoskeleton structure of EC9706 and Kyse30 was visualized by immunofluorescence analysis. B. Representative images of spheroid colonies were shown. C. Self-renewal ability was investigated by mammosphere assays in serum free medium. D. Flow cytometry analysis of CD44+ population. E. Number of lung metastasis foci formed by EC9706-EREG, Vector and EC9706 parental cells in the nude mice. F. Kaplan-Meier survival curve of mice injected with EC9706-EREG, Vector and EC9706 parental cells. G. The effects of EREG on EGFR activation, phosphorylated EGFR, FAK or Src in EC9706 cells with or without overexpression of EREG were analyzed by Western Blot.
High level of EREG is associated with clinicopathological parameters
To investigate the expression and clinical significance of EREG, we evaluated the its expression in 120 esophageal carcinoma tissues samples paired adjacent normal tissues. The EREG immunopositivity was not observed in the normal tissues, but 85 of 120 primary lesions exhibited positive staining. Interestingly, we observed that cancer cells adjacent to ECs displayed higher level of EREG than in other location (Figure 5A). Associations of EREG expression with other variables were shown in Table 1. High level of EREG was significantly associated with depth of invasion (P = 0.000), lymph node metastasis (P = 0.022) and differentiation (P = 0.029). While there was no significant correlation between EREG expression and other parameter including Distant metastasis, Age and Gender. To further investigate the relationship between EREG expression and lymph node metastasis, we also collected the 60 esophageal carcinoma tissues samples and paired lymph node metastatic samples. We found that among these 60 paired samples, EREG was positive in 42 primary tumors (70.0%) and in 42 corresponding lymph node metastases (70%). Further analysis revealed that the levels of EREG were significantly higher in the lymph node metastases tissues than in the primary tissues (Figure 5B). The prognostic significance of EREG expression was determined by EREG staining and the corresponding clinical follow-up records. Kaplan-Meier survival analysis revealed a correlation between higher EREG expression levels and shorter overall survival times (P < 0.01) (Figure 5C). Taken together, these observations indicated that overexpression of EREG was significantly associated with poor prognosis in esophageal cancer.
Figure 5.
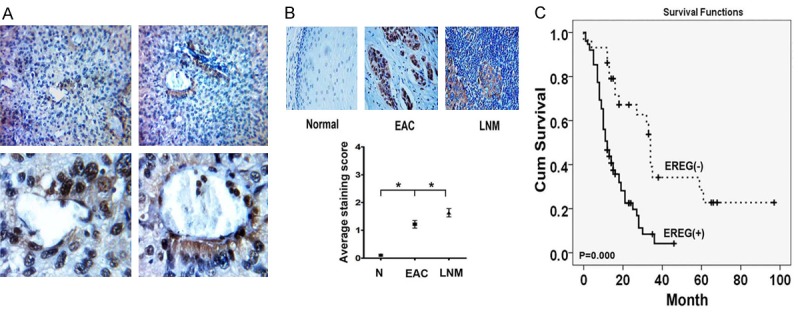
Expression of EREG in human esophageal carcinoma and Lymph node metastatic tissues. A. Representative images of EREG staining in perivascular regions of esophageal carcinoma tissues. B. IHC analysis of EREG expression in primary and Lymph node metastatic esophageal carcinoma. C. Kaplan Meier survival curve showed correlation between EREG level and overall survival.
Table 1.
Association between EREG expression and Clinicopathologic Characteristics of the 120 esophageal cancer patients
| Variables | EREG expression | P value | |
|---|---|---|---|
|
| |||
| Negative | Positive | ||
| Age | 59.2±10.7 | 61.7±10.1 | 0.231 |
| Gender | 0.502 | ||
| Male | 17 | 47 | |
| Female | 18 | 38 | |
| T | 0.000 | ||
| T1 | 8 | 9 | |
| T2 | 11 | 5 | |
| T3 | 13 | 57 | |
| T4 | 2 | 14 | |
| N | 0.022 | ||
| N0 | 18 | 26 | |
| N1 | 16 | 59 | |
| M | 0.36 | ||
| M0 | 35 | 83 | |
| M1 | 0 | 2 | |
| Grade | 0.029 | ||
| High | 5 | 17 | |
| Moderate | 23 | 42 | |
| Low | 4 | 25 | |
Discussion
Human esophageal cancer is one of the most common causes of cancer death worldwide, and is particularly prevalent in China [15]. The poor prognosis for esophageal cancer was largely due to early-stage invasion of adjacent tissue and distant metastasis [16]. Consequently, inhibition of tumor invasion and metastasis can significantly improve the survival of patients with esophageal cancer.
Cancer stem cell (CSC) is believed to possess the self-renewal capacity and can give rise to the heterogeneous lineages of daughter cancer cells [2]. CSC is not only responsible for radiation and chemotherapy resistance, but also are closely related to the migration, invasiveness and metastasis [17]. It has been reported that esophageal cancer tissues contained the cancer stem cell, and cancer stem cell marker CD44 can be utilized to efficiently enrich the CSC in esophageal cancer [13].
The tumor microenvironment including endothelial cells plays key a role in cancer progression. Recent evidence also proved that tumor endothelial cells could provide a niche to enrich cancer stem cell phenotype [6]. Tumor endothelial cells owned different structure and function, which is very different from endothelium in normal tissues. Previous studies demonstrated that immortalized normal EC can enhance tumor growth, cancer stem cell (CSC) phenotype and pro-metastatic properties [10]. However, the roles of tumor endothelial in invasion and metastasis are known very little nowadays.
In this study, we explored the characteristics of HECECs. Firstly, HECECs can form the tube in the Matrigel by the Tube formation assay, and can be continually passaged for at least 25 times or longer than HENECs. Secondly, we established a tumor-endothelial cells co-culture experiment, and used HENECs and cultural medium from HECEC as control. We found HECECs could enhance the invasion, migration and cancer stem-like cell self-renewal of esophageal cancer cells in vitro, and promoted esophageal cancer cell invasion and lung metastasis in vivo. Microarray analysis identified 15 HECEC up-regulated genes, and most of them were involved in tumor invasion and metastasis by functional cluster analysis. Among them, Epiregulin (EREG) was selected the candidate genes in this model. Previous studies suggested that Epiregulin plays an important role in cancer progression including bladder cancer [18], gastric cancer [19], colorectal cancer [19], breast cancer [20] and lung cancer [21]. But there is a limited number of reports suggested that EREG was involved in esophageal cancer progression. To investigate the function of EREG, we established the EREG-overexpressed cells. Functional assays revealed that overexpression of EREG in EC9706 or Kyse30 cells could significantly increase invasion, sphere-forming capability, the percentage of CD44-positive cell, and induce cancer cell actin reorganization as well in vitro. Furthermore, EC9706-EREG cells significantly developed more lung metastatic foci than parental cells in vivo.
Epiregulin (EREG), a member of the epidermal growth factor family, can function as a ligand of EGFR to induce invasion, migration and cell survival through a complex signaling pathways such as Src and FAK activation [22-24]. On the basis of recent studies, we evaluated the related signaling targets including phospho-signals of EGFR (Y1068), Src (pY418) and FAK (pY576) by using western blot. We found that all three molecular targets were activated by EREG and subsequently inactivated by EREG polyclonal inhibitory antibody.
To determine the clinical significance of EREG expression in esophageal carcinoma tissues, we assessed the level of EREG in 120 esophageal carcinoma tissues samples that had clinical follow-up records by immunohistochemistry analysis. We found that there was no detectable staining of EREG in the normal epithelium, but 78% primary lesions exhibited positive staining. The result also demonstrated that the expression of EREG was correlated with Lymph node metastasis and was coupled with shorter overall survival. Furthermore, we also harvested the 60 esophageal carcinoma tissues and paired lymph node metastatic samples to evaluate the EREG level. We found that expression of EREG in the lymph node metastatic was noticeably higher than that in the matched primary colon cancer tissues. Interestingly, we also found that Esophageal carcinoma cells Adjacent to ECs in esophageal carcinoma tissues demonstrate over-expression of EREG. Taken together, these observations indicated that overexpression of EREG was significantly associated with esophageal carcinoma poor prognosis.
Taken together, the present study may have a variety of implications for understanding the role of HECEC in esophageal cancer progression. First, this is the first study defining a mechanism how HECECs regulated the invasion, migration, cancer stem cell phenotype and metastatic potential of esophageal cancer cells in a direct interaction. This study reports that HECEC can enhance the level of EREG in esophageal cancer and lead to activation of phosphorylation of EGFR/Src/FAK. At last, our findings suggest that overexpression of EREG was significantly associated with lymph node metastasis and was coupled with shorter overall survival. It is possible that targeting EREG may inhibit CSC and improve the outcomes of esophageal cancer.
Acknowledgements
Supported by grant from National High-tech R&D Program of China for Young Scholars (No: 2014AA020537). Beijing Gao Chuang Ji Hua (No: G02060050). Beijing Nova Program (No: Z1511000003150121).
Disclosure of conflict of interest
None.
References
- 1.Zhou Z, Ran YL, Hu H, Pan J, Li ZF, Chen LZ, Sun LC, Peng L, Zhao XL, Yu L, Sun LX, Yang ZH. TM4SF3 promotes esophageal carcinoma metastasis via upregulating ADAM12m expression. Clin Exp Metastasis. 2008;25:537–548. doi: 10.1007/s10585-008-9168-0. [DOI] [PubMed] [Google Scholar]
- 2.O’Brien CA, Kreso A, Jamieson CH. Cancer stem cells and self-renewal. Clin Cancer Res. 2010;16:3113–3120. doi: 10.1158/1078-0432.CCR-09-2824. [DOI] [PubMed] [Google Scholar]
- 3.Dalerba P, Clarke MF. Cancer Stem Cells and Tumor Metastasis: First Steps into Uncharted Territory. Cell Stem Cell. 2007;1:241–242. doi: 10.1016/j.stem.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 4.Wang MC, Jiao M, Wu T, Jing L, Cui J, Guo H, Tian T, Ruan ZP, Wei YC, Jiang LL, Sun HF, Huang LX, Nan KJ, Li CL. Polycomb complex protein BMI-1 promotes invasion and metastasis of pancreatic cancer stem cells by activating PI3K/AKT signaling, an ex vivo, in vitro, and in vivo study. Oncotarget. 2016;7:9586–9599. doi: 10.18632/oncotarget.7078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hu H, Sun L, Guo C, Liu Q, Zhou Z, Peng L, Pan J, Yu L, Lou J, Yang Z, Zhao P, Ran Y. Tumor Cell-Microenvironment Interaction Models Coupled with Clinical Validation Reveal CCL2 and SNCG as Two Predictors of Colorectal Cancer Hepatic Metastasis. Clin Cancer Res. 2009;15:5485–5493. doi: 10.1158/1078-0432.CCR-08-2491. [DOI] [PubMed] [Google Scholar]
- 6.Lu J, Ye X, Fan F, Xia L, Bhattacharya R, Bellister S, Tozzi F, Sceusi E, Zhou Y, Tachibana I, Maru DM, Hawke DH, Rak J, Mani SA, Zweidler-McKay P, Ellis LM. Endothelial cells promote the colorectal cancer stem cell phenotype through a soluble form of Jagged-1. Cancer Cell. 2013;23:171–185. doi: 10.1016/j.ccr.2012.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilson NS, Yang A, Yang B, Couto S, Stern H, Gogineni A, Pitti R, Marsters S, Weimer RM, Singh M, Ashkenazi A. Proapoptotic Activation of Death Receptor 5 on Tumor Endothelial Cells Disrupts the Vasculature and Reduces Tumor Growth. Cancer Cell. 2012;22:80–90. doi: 10.1016/j.ccr.2012.05.014. [DOI] [PubMed] [Google Scholar]
- 8.Zhang Y, Ran Y, Yu L, Hu H, Zhou Z, Sun L, Lou J, Yang Z. Monoclonal Antibody to Human Esophageal Cancer Endothelium Inhibits Angiogenesis and Tumor Growth. Anticancer Res. 2006;26:2963–2970. [PubMed] [Google Scholar]
- 9.Hu H, Zhang Y, Ran Y, Zhou Z, Yu L, Lou J, Yang Z. Human esophageal cancer endothelial cells increase tumor growth by incorporating with mouse endothelium. Cancer Lett. 2007;252:123–130. doi: 10.1016/j.canlet.2006.12.015. [DOI] [PubMed] [Google Scholar]
- 10.Ghiabi P, Jiang J, Pasquier J, Maleki M, Abu-Kaoud N, Rafii S, Rafii A. Endothelial cells provide a notch-dependent pro-tumoral niche for enhancing breast cancer survival, stemness and pro-metastatic properties. PLoS One. 2014;9:e112424. doi: 10.1371/journal.pone.0112424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kumar P, Ning Y, Polverini PJ. Endothelial cells expressing Bcl-2 promotes tumor metastasis by enhancing tumor angiogenesis, blood vessel leakiness and tumor invasion. Lab Invest. 2008;88:740–749. doi: 10.1038/labinvest.2008.46. [DOI] [PubMed] [Google Scholar]
- 12.Hu H, Sun L, Guo C, Liu Q, Zhou Z, Peng L, Pan J, Yu L, Lou J, Yang Z, Zhao P, Ran Y. Tumor cell-microenvironment interaction models coupled with clinical validation reveal CCL2 and SNCG as two predictors of colorectal cancer hepatic metastasis. Clin Cancer Res. 2009;15:5485–5493. doi: 10.1158/1078-0432.CCR-08-2491. [DOI] [PubMed] [Google Scholar]
- 13.Zhao JS, Li WJ, Ge D, Zhang PJ, Li JJ, Lu CL, Ji XD, Guan DX, Gao H, Xu LY, Li EM, Soukiasian H, Koeffler HP, Wang XF, Xie D. Tumor Initiating Cells in Esophageal Squamous Cell Carcinomas Express High Levels of CD44. PLoS One. 2011;6:e21419. doi: 10.1371/journal.pone.0021419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rassouli FB, Matin MM, Bahrami AR, Ghaffarzadegan K, Cheshomi H, Lari S, Memar B, Kan MS. Evaluating stem and cancerous biomarkers in CD15+CD44+ KYSE30 cells. Tumour Biol. 2013;34:2909–2920. doi: 10.1007/s13277-013-0853-5. [DOI] [PubMed] [Google Scholar]
- 15.Zhao P, Dai M, Chen W, Li N. Cancer trends in China. Jpn J Clin Oncol. 2010;40:281–285. doi: 10.1093/jjco/hyp187. [DOI] [PubMed] [Google Scholar]
- 16.Hong L, Han Y, Zhang H, Fan D. Prognostic markers in esophageal cancer: from basic research to clinical use. Expert Rev Gastroenterol Hepatol. 2015;9:887–889. doi: 10.1586/17474124.2015.1041507. [DOI] [PubMed] [Google Scholar]
- 17.Han L, Shi S, Gong T, Zhang Z, Sun X. Cancer stem cells: therapeutic implications and perspectives in cancer therapy. Acta Pharm Sin B. 2013;3:65–75. [Google Scholar]
- 18.Nicholson BE, Frierson HF, Conaway MR, Seraj JM, Harding MA, Hampton GM, Theodorescu D. Profiling the evolution of human metastatic bladder cancer. Cancer Res. 2004;64:7813–7821. doi: 10.1158/0008-5472.CAN-04-0826. [DOI] [PubMed] [Google Scholar]
- 19.Wu WK, Tse TT, Sung JJ, Li ZJ, Yu L, Cho CH. Expression of ErbB Receptors and their Cognate Ligands in Gastric and Colon Cancer Cell Lines. Anticancer Res. 2009;29:229–234. [PubMed] [Google Scholar]
- 20.Vlaicu P, Mertins P, Mayr T, Widschwendter P, Ataseven B, Högel B, Eiermann W, Knyazev P, Ullrich A. Monocytes/macrophages support mammary tumor invasivity by co-secreting lineage-specific EGFR ligands and a STAT3 activator. BMC Cancer. 2013;13:197–197. doi: 10.1186/1471-2407-13-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang J, Iwanaga K, Choi KC, Wislez M, Raso MG, Wei W, Wistuba II, Kurie JM. Intratumoral Epiregulin Is a Marker of Advanced Disease in Non-Small Cell Lung Cancer Patients and Confers Invasive Properties on EGFR-Mutant Cells. Cancer Prev Res (Phila) 2008;1:201–207. doi: 10.1158/1940-6207.CAPR-08-0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tai YL, Chu PY, Lai IR, Wang MY, Tseng HY, Guan JL, Liou JY, Shen TL. An EGFR/Src-dependent β4 integrin/FAK complex contributes to malignancy of breast cancer. Sci Rep. 2015;5:16408. doi: 10.1038/srep16408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Long W, Yi P, Amazit L, LaMarca HL, Ashcroft F, Kumar R, Mancini MA, Tsai SY, Tsai MJ, O’Malley BW. SRC-3Δ4 mediates the interaction of EGFR with FAK to promote cell migration. Molecular cell. 2010;37:321–332. doi: 10.1016/j.molcel.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Demers MJ, Thibodeau S, Noel D, Fujita N, Tsuruo T, Gauthier R, Arguin M, Vachon PH. Intestinal epithelial cancer cell anoikis resistance: EGFR-mediated sustained activation of Src overrides Fak-dependent signaling to MEK/Erk and/or PI3-K/Akt-1. J Cell Biochem. 2009;107:639–654. doi: 10.1002/jcb.22131. [DOI] [PubMed] [Google Scholar]