Abstract
Dual specificity phosphatase 5 (DUSP5) is a negative regulator of Mitogen-activated protein kinase (MAPK) signaling pathway and has recently been identified as a tumor suppressor in several human malignancies. However, its clinical significance in colorectal cancer (CRC) remains unclear. In this study, we aimed to investigate the potential utility of DUSP5 as a novel biomarker for progression indication and chemotherapy benefit in CRC patients. Through quantitative real time-polymerase chain reaction and western blot, we determined that DUSP5 expression is dramatically lower in CRC tissues than that in matched normal tissues. The statistical analysis based on immunohistochemistry revealed that DUSP5 expression is significantly correlated with tumor differentiation, TNM stage, lymph node metastasis and distant metastasis. For the whole study cohort, patients with high DUSP5 expression had a better CRC-specific and disease-free survival than those with low DUSP5 expression and DUSP5 expression is an independent prognostic factor for patient survival. In subgroup analysis, DUSP5 has no prognostic significance in low-risk stage II patients, but could predict treatment response in high-risk stage II and stage III/IV patients who received standard FOLFOX chemotherapy scheme. Finally, the correlation analysis suggested that DUSP5 expression is associated with Epithelial-to-Mesenchymal Transition (EMT) phenotype in CRC tissues, suggesting that downregulated DUSP5 may contribute to poor prognosis partly by involving EMT. Taken together, our study proposes that DUSP5 is a promising biomarker for predicting CRC progression and advanced patients with high DUSP5 expression appear to benefit from standard FOLFOX chemotherapy scheme.
Keywords: DUSP5, colorectal cancer, prognosis, EMT
Introduction
Colorectal cancer (CRC) is the third most commonly diagnosed human malignancy in males and the second in females worldwide [1]. According to the latest report from American Cancer Society, it will account for approximately 134,490 new cases and 49,190 deaths in the United States in 2016 [2]. In China, with the changed lifestyle such as high fat diet, an increasing trend in its incidence as well as mortality rate has been observed during the past years [3]. Although the encouraging advances have been achieved in population screen and therapeutic techniques, approximately 56% of CRC patients are found in regional/distant metastasis stage at diagnosis, and the 5-Year relative survival rate of CRC patients has not been satisfactorily improved over the past decades, from 60% in 1989 to 66% in 2011 [2]. Precision Medicine, focusing on establishing accurate molecular profiling for individual patient with cancer, has been widely proposed as a promising concept for matching the right clinical management to the right patient [4]. However, so far, only few molecular biomarkers have been identified and applied in accurately distinguishing risk of patients within the same stage. Therefore, for further promoting the development of Precision Medicine in CRC, it is of great necessity to search and identify novel molecular indicators.
Mitogen-activated protein kinase (MAPK) signaling pathway is a well-known molecular event involved in malignant progression and therapeutic resistance of CRC [5]. Several regulatory molecules in this pathway have already been suggested to be implicated in prognostic evaluation and therapy decision, such as BRAF and KRAS. However, recent studies have found those biomarkers may not have clinical value for some CRC patients, especially for those in stage II/III [6,7]. Therefore, it needs to investigate that whether other molecules in MAPK pathway have any clinical significance and can be valuable candidates added into current molecular profiling for disease management. Dual specificity phosphatases (DUSPs), a group of proteins functioning in dephosphorylation of threonine/serine and tyrosine residues, have been reported to regulate MAP kinases in cancerous transformation, implying their potential to be developed into clinical targets or biomarkers [8]. For example, researchers have found that DUSP1 is closely correlated with chemotherapy resistance in lung and ovarian cancer [9,10]. Hypermethylated DUSP2 has been detected in most skin/lung cancer cell lines, suggesting epigenetic inactivation of DUSP2 may be involved in carcinogenesis of these tumor types [11]. Moreover, DUSP4 has been recently identified as a novel invasion suppressor inactivating ERK signaling in pancreatic cancer [12].
Previously, a comprehensive study by Slattery et al has assessed clinical value of DUSPs (including DUSP1, DUSP2, DUSP4, DUSP6 and DUSP7) in CRC and found DUSP2 may influence survival of colon cancer patients after diagnosis [13]. However, whether DUSP2 could indicate therapy response and other DUSPs such as DUSP3 or DUSP5 have any clinical significance for CRC patients, remains unclear. Recently, we identified DUSP5 as a dominant target of Zinc-finger protein X-linked (ZFX) using microarray analysis, which is responsible for ZFX-mediated malignant progression and 5-FU resistance in CRC [14]. Therefore, DUSP5 may have potential to be a useful biomarker and additional efforts are required to further confirm its clinical significance in CRC patients.
To achieve this aim, in this study, we firstly compared expression of DUSP5 between CRC and matched normal tissues using RT-PCR and western blot. Then, immunohistochemistry was conducted to further investigate the clinical correlation of DUSP5 with advanced CRC patients. Finally, since the role of DUSP family in breast cancer recently has been closely linked to Epithelial-to-Mesenchymal Transition (EMT), which is also a well-acknowledged molecular program driving CRC development, we thus analyzed the possible correlations between DUSP5 and EMT markers in CRC [15,16]. In sum, this study will not only identify DUSP5 as a novel prognostic biomarker for advanced CRC patients, but also suggest its potential to serve as an effective target for preventing disease progression and chemotherapy resistance.
Materials and methods
Patient data and specimens
A total of 369 paired tumor tissues and matched normal tissues were collected from CRC patients who received surgical treatment between January 2008 and October 2015 at Sixth People’s Hospital affiliated with Shanghai JiaoTong University and Tenth People’s Hospital affiliated with Tongji University. None of the patients received preoperative radiotherapy or chemotherapy. For high risk stage II, stage III and stage IV patients, the systemic chemotherapy was conducted based on FOLFOX scheme (5-Fu + Oxaliplatin + leucovorin). High risk stage II patients were defined as those complicated with T4 stage, obstruction/perforation, high histological grade, lymphovascular/perineural invasion, undefined circumferential resection margin and fewer harvested lymph nodes (<12). Postoperative pathological stage was classified according to Union for International Cancer Control Tumor-node-metastasis (TNM) staging system (7th edition). Routine follow-up was performed every 3 months for the first 2 years and then every 6 months for the next 3 years, with laboratory test, radiological examinations and telephone interview. CRC-specific survival is defined as the time from surgery to death caused by CRC. Disease-free survival (DFS) is defined as the time from surgery to CRC recurrence or regional/distant metastasis. The study was approved by the ethics committee of the both hospitals and written informed consents were obtained from patients for using their specimens in scientific studies.
Real-time reverse transcription-polymerase chain reaction (RT-PCR)
Total RNA was extracted from fresh CRC/matched normal tissues using Trizol according to the manufacturer’s instructions (Takara, Japan). The acquired RNA was then reverse-transcribed to complementary DNA (cDNA) using reverse transcriptase (RT) enzymes (Thermo Fisher Scientific, USA). Finally, the synthesized cDNA was applied to PCR reaction on Real-Time PCR System (Thermo Fisher Scientific) using SYBR Green Mix (Takara). The reaction condition was taken as follows: 40 cycles of 95°C for 15 sec, 60°C for 20 sec and 72°C for 20 sec. The melt condition was taken as follows: 71 cycles of 60.0°C-95.0°C for 30 sec. The analysis was performed on 2-ΔΔT method and β-actin was employed as the control gene. The primers for DUSP5 and β-actin were used as follows: DUSP5, forward 5’-GCAAGGTCCTGGTCCACTGT-3’ and reverse, 5’-AGGCGGAACTGCTTGGT CTT-3’; β-actin, forward 5’-AAGGTGACAGCAGTCGGTT-3’ and reverse, 5’-TGTGTGGACTTGGGAGAGG-3’. Each experiment was independently repeated three times.
Western blot
Total protein was extracted from fresh CRC/matched normal tissues using RIPA buffer according to the manufacturer’s instructions (Jrdun Biotechnology, China). The protein concentration was detected by BCA method routinely. Then, protein samples were separated on a 10% SDS-PAGE and electrophoretically transferred to polyvinylidene difloride membranes (Millipore, USA). After blocking in 5% skim milk for 1 h at room temperature, the membranes were incubated overnight at 4°C with the following primary antibodies: anti-DUSP5 (1:1000, Abcam, UK) and β-actin (1:2000, Abmart, USA). After three washes with TBST solution, horseradish peroxidase-conjugated secondary antibody (1:5000, Santa Cruz Biotechnology, USA) was applied at 37°C for 1 h. Finally, protein expression was detected by the Amersham ECL SelectTM detection system (GEHealthcare Life Sciences, USA) and β-actin was utilized as the internal control. Quantification of protein expression was performed using Quantity One software.
Immunohistochemistry and staining evaluation
Immunohistochemistry analysis on paraffin-embedded tissues was performed as described previously [17]. Briefly, the tissue sections were deparaffinized in xylene and rehydrated in graded alcohols (from 100% to 70%). After antigen retrieval by microwaving, the sections were incubated with 0.3% hydrogen peroxidase for 10 min to block endogenous peroxidase activity. Then, the sections were incubated with following primary antibodies at 4°C overnight: anti-DUSP5 (1:250, Abcam, UK), anti-E-cadherin (1:150, Epitomics, USA), anti-N-cadherin (1:150, Epitomics, USA) and anti-vimentin (1:200, Bioworld, USA). After three washes with PBS solution, the sections were incubated with secondary antibody (1:250, Abcam, UK) for 25 min at 37°C. Finally, protein detection was performed using diaminobenzidine reagent and the sections were counterstained with hematoxylin. The sections incubated with PBS solution instead of primary antibody were prepared as negative controls.
Staining evaluation was blindly conducted by two independent researchers and any discrepant cases were determined by an experienced pathologist. Five bright fields were randomly selected for scoring the stains. The scoring principle was based on a product of Staining Intensity (SI) and Percentage of Positive cells (PP). SI was categorized as follows: Negative (score 0), weak (score 1), moderate (score 2) and strong (score 3). PP was categorized as follows: <5% (score 0), 5-25% (score 1), 25%-50% (score 2), 50%-75% (score 3), >75% (score 4). For statistical analysis, the cut-off score of each protein was used for defining high/low expression, and determined by receiver operating characteristic (ROC) curve. The result demonstrated that the cut-off score is 2.5 for DUSP5, 1.5 for E-cadherin, 3.5 for N-cadherin and vimentin respectively, suggesting that staining score ≥3 for DUSP5, ≥2 for E-cadherin, ≥4 for N-cadherin and vimentin were indicating high expression.
Statistical analysis
The data were presented as the mean ± standard deviation and analyzed using 17.0 SPSS statistical software. Student’s t test was used for comparing mRNA/protein expression of DUSP5 between CRC tissues and matched normal tissues. The χ2 test was used for determining the correlation between DUSP5 expression and clinicopathological parameters of CRC patients. The Kaplan-Meier model was constructed for depicting survival curves and the log-rank test was used for comparing intergroup differences. Significant prognostic indicators for patient survival were identified by univariate and multivariate analyses based on the Cox proportional hazards regression model. A non-parametric Spearman’s rank correlation coefficient was used for determining the correlation between DUSP5 expression and EMT phenotype in CRC tissues. For all analysis, a p-value <0.05 was considered statistically significant.
Results
Expression of DUSP5 in CRC tissues and adjacent normal tissues
Quantitative Real-Time RT-PCR was employed to detect the mRNA expression of DUSP5 in 30 pairs of CRC tissues and adjacent normal tissues. As a result, we found the mRNA expression of DUSP5 was significantly higher in adjacent normal tissues than that in CRC tissues (0.82±0.28 vs 0.05±0.04, P<0.05, Figure 1A). This intergroup difference was then confirmed by western bolt (1.90±0.41 vs 0.54±0.20, P<0.05, Figure 1B) and the representative protein bands were demonstrated in Figure 1C. Furthermore, through immunohistochemistry analysis, we found the expression of DUSP5 was mainly located in nucleus of CRC cells and its high expression was prominently observed in 42.5% (157/369) of CRC tissues. The representative results of immunohistochemistry staining were demonstrated in Figure 1D.
Figure 1.
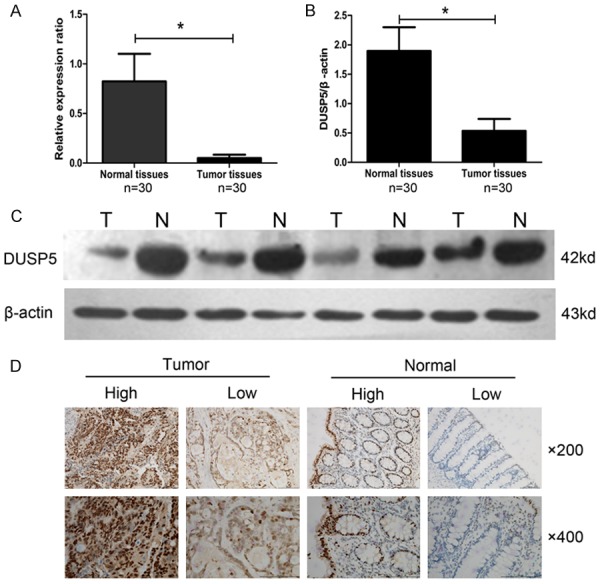
Expression of DUSP5 in CRC and matched normal tissues. A: The mRNA expression of DUSP5 in CRC tissues was significantly lower than that in matched normal tissues (0.82±0.28 vs 0.05±0.04, n=30, P<0.05). B and C: The protein expression of DUSP5 in CRC tissues was also significantly lower than that in matched normal tissues (1.90±0.41 vs 0.54±0.20, n=30, P<0.05). D: The representative nuclear staining of DUSP5 in tissues detected by immunohistochemistry.
Correlations between DUSP5 expression and clinicopathological characteristics
The correlations between DUSP5 expression and clinicopathological characteristics were summarized in Table 1. Generally, according to staining evaluation, there is a statistically significant correlation between DUSP5 expression and tumor differentiation (P=0.042), TNM stage (P<0.001), lymph metastasis (P<0.001) and distant metastasis (P=0.007). However, no statistically significant correlation was observed between DUSP5 expression and other clinical characteristics including gender (P=0.521), age (P=0.291), tumor location (P=0.114), tumor size (P=0.341) and invasion (P=0.738).
Table 1.
Correlations between DUSP5 expression and clinicopathological Characteristics
| Characteristics | Total | DUSP5 expression | P value | |
|---|---|---|---|---|
|
| ||||
| High | Low | |||
| Gender | ||||
| Male | 219 | 90 | 129 | 0.521 |
| Female | 150 | 67 | 83 | |
| Age | ||||
| ≤60 | 170 | 67 | 103 | 0.291 |
| >60 | 199 | 90 | 109 | |
| Tumor location | ||||
| Colon | 174 | 82 | 92 | 0.114 |
| Rectal | 195 | 75 | 120 | |
| Tumor differentiation | ||||
| Well/moderate | 217 | 102 | 115 | 0.042 |
| Poor | 152 | 55 | 97 | |
| Tumor size | ||||
| ≤5 cm | 205 | 92 | 113 | 0.341 |
| >5 cm | 164 | 65 | 99 | |
| TNM stage | ||||
| II | 184 | 98 | 86 | <0.001 |
| III and IV | 185 | 59 | 126 | |
| Tumor invasion | ||||
| T1-T2 | 41 | 16 | 25 | 0.738 |
| T3-T4 | 328 | 141 | 187 | |
| Lymph node metastasis | ||||
| Absent | 188 | 99 | 89 | <0.001 |
| Present | 181 | 58 | 123 | |
| Distant metastasis | ||||
| Absent | 323 | 146 | 177 | 0.007 |
| Present | 46 | 11 | 35 | |
Prognostic significance of DUSP5 in CRC patients
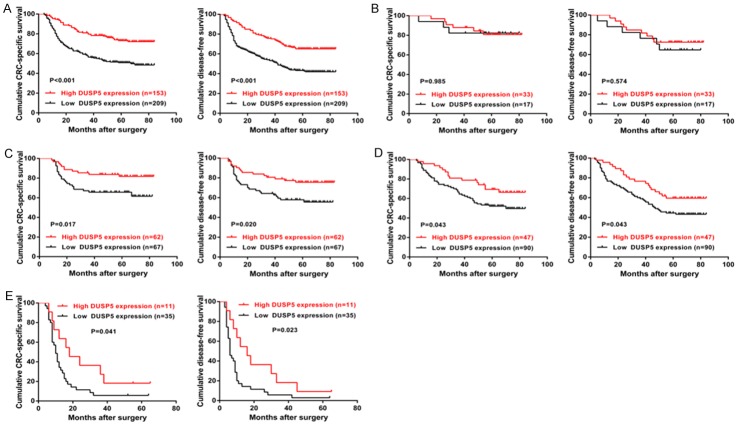
During our follow-up, 7 patients were dead from cardiovascular disease instead of CRC and therefore we excluded them in the following prognostic analysis. To evaluate effects of DUSP5 in patient survival, The Kaplan-Meier model was employed to study the associations between DUSP5 expression, assessed at protein level, and CRC-specific survival/DFS of CRC patients. For the whole cohort, high expression of DUSP5 was associated with higher CRC-specific survival and DFS rate compared with low expression of DUSP5 (CRC-specific survival: P<0.001; DFS: P<0.001, Figure 2A). As shown in Tables 2 and 3, univariate analysis suggested that tumor differentiation, lymph node metastasis, distant metastasis and DUSP5 expression were significant factors affecting the CRC-specific survival and DFS of CRC patients (all P<0.01). However, in multivariate analysis, only lymph node metastasis, distant metastasis and DUSP5 expression were identified as independent prognostic factors affecting the CRC-specific survival and DFS of CRC patients (all P<0.05).
Figure 2.
Prognostic significance of DUSP5 in CRC patients. A: CRC-specific survival and Disease-free survival (DFS) curves of the whole study cohort. B: CRC-specific survival and DFS curves of low-risk stage II patients receiving no chemotherapy. C: CRC-specific survival and DFS curves of high-risk stage II patients receiving FOLFOX scheme. D: CRC-specific survival and DFS curves of stage III patients receiving FOLFOX scheme. E: CRC-specific survival and DFS curves of stage IV patients receiving FOLFOX scheme.
Table 2.
Univariate analysis and multivariate analysis for prognostic factors in CRC-sepcific survival
| Variables | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| HR | 95% CI | P value | HR | 95% CI | P value | |
| Age | 1.084 | 0.781-1.505 | 0.628 | |||
| Gender | 0.948 | 0.679-1.323 | 0.753 | |||
| Tumor location | 1.081 | 0.780-1.498 | 0.641 | |||
| Tumor size | 1.151 | 0.831-1.595 | 0.398 | |||
| Tumor invasion | 0.740 | 0.457-1.198 | 0.221 | |||
| Tumor differentiation | 1.901 | 1.371-2.634 | <0.001 | 1.381 | 0.984-1.937 | 0.062 |
| Lymph node metastasis | 2.676 | 1.886-3.797 | <0.001 | 1.671 | 1.147-2.435 | 0.008 |
| Distant metastasis | 7.693 | 5.286-11.194 | <0.001 | 5.757 | 3.857-8.592 | <0.001 |
| DUSP5 expression | 2.310 | 1.609-3.318 | <0.001 | 1.948 | 1.348-2.814 | <0.001 |
Table 3.
Univariate analysis and multivariate analysis for prognostic factors in disease-free survival
| Variables | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| HR | 95% CI | P value | HR | 95% CI | P value | |
| Age | 1.176 | 0.870-1.590 | 0.292 | |||
| Gender | 1.012 | 0.747-1.370 | 0.939 | |||
| Tumor location | 1.144 | 0.848-1.543 | 0.380 | |||
| Tumor size | 0.949 | 0.702-1.283 | 0.733 | |||
| Tumor invasion | 0.733 | 0.473-1.137 | 0.166 | |||
| Tumor differentiation | 1.639 | 1.216-2.209 | 0.001 | 1.214 | 0.889-1.656 | 0.222 |
| Lymph node metastasis | 2.241 | 1.644-3.056 | <0.001 | 1.483 | 1.062-2.071 | 0.021 |
| Distant metastasis | 7.190 | 5.021-10.297 | <0.001 | 5.784 | 3.942-8.485 | <0.001 |
| DUSP5 expression | 2.124 | 1.537-2.937 | <0.001 | 1.888 | 1.359-2.624 | <0.001 |
To further evaluate whether DUSP5 could serve as an effective biomarker for distinguishing disease progression and chemotherapy benefit within single stage, subgroup analysis based on Kaplan-Meier model was conducted. For the CRC-specific survival and DFS of low risk stage II patients (Figure 2B), no significant difference was found between DUSP5-high expression group and DUSP5-low expression group (CRC-specific survival: P=0.985 and DFS: P=0.574). However, for high risk stage II patients receiving FOLFOX chemotherapy scheme (Figure 2C), patients with high DUSP5 expression appeared to have a significantly better CRC-specific survival and DFS than those with low DUSP5 expression (CRC-specific survival: P=0.017 and DFS: P=0.020). This association remained statistically significant in stage III patients receiving the same chemotherapy (CRC-specific survival: P=0.043 and DFS: P=0.043, Figure 2D). With respect to stage IV patients receiving radical surgery combined with standard chemotherapy (Figure 2E), we also found high DUSP5 status was significantly associated with higher CRC-specific survival and DFS rate than low DUSP5 status (CRC-specific survival: P=0.041 and DFS: P=0.023).
Correlation between DUSP5 expression and EMT phenotype
The representative results of immunohistochemistry staining for EMT markers were demonstrated in Figure 3. Generally, high expression of E-cadherin and N-cadherin was dominantly found in the membrane of CRC cells, while high expression of vimentin was in CRC stroma. The correlation between DUSP5 expression and EMT phenotype was summarized in Table 4. According to immunohistochemistry evaluation, high membranous expression of E-cadherin and N-cadherin were detected in 34.1% (126/369) and 63.4% (234/369) of CRC patients respectively, while high stromal expression of vimentin was detected in 56.4% (208/369) of CRC patients. The correlation analysis indicated that DUSP5 expression was negatively correlated N-cadherin and vimentin expression (N-cadherin: r=-0.166, P=0.001; vimentin: r=-0.149, P=0.004), but positively correlated with E-cadherin expression in CRC tissues (r=0.432, P<0.001).
Figure 3.
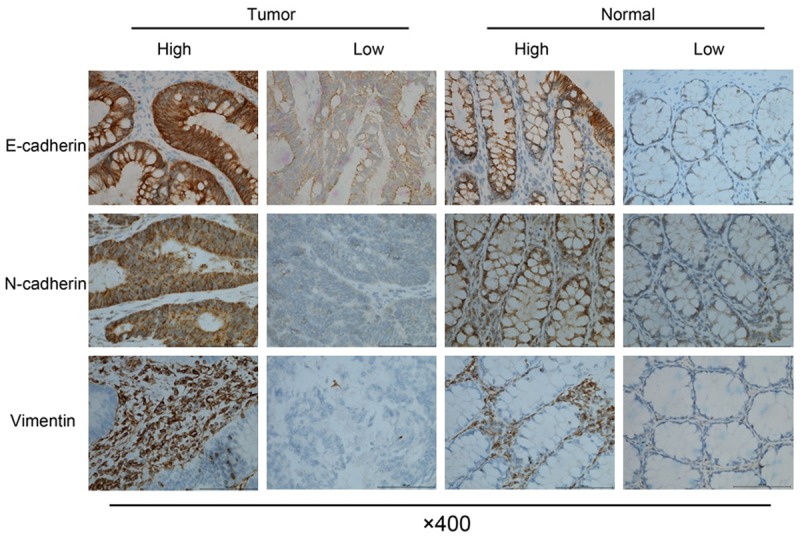
Representative images of immunohistochemical staining for Epithelial- Mesenchymal Transition (EMT) markers. Upper panel: membranous expression of E-cadherin in CRC and matched normal tissues. Middle panel: membranous expression of N-cadherin in CRC and matched normal tissues. Bottom panel: stromal expression of vimentin in CRC and matched normal tissues.
Table 4.
Correlations between DUSP5 expression and EMT phenotype
| EMT markers | Total | DUSP5 expression | r | P-value | |
|---|---|---|---|---|---|
|
| |||||
| Low (n) | High (n) | ||||
| E-cadherin | |||||
| Low | 243 | 177 | 66 | 0.432 | <0.001 |
| High | 126 | 35 | 91 | ||
| N-cadherin | |||||
| Low | 135 | 63 | 72 | -0.166 | 0.001 |
| High | 234 | 149 | 85 | ||
| Vimentin | |||||
| Low | 161 | 79 | 82 | -0.149 | 0.004 |
| High | 208 | 133 | 75 | ||
Discussion
Reliable molecular biomarkers are crucial to the clinical management of CRC, because they can offer guidance for risk stratification, progression indication and treatment decision [18]. Although traditional TNM stage, mutation profiling and differentiation grade are considered as key factors influencing clinical management, they have been suggested technically difficult in standardized detection and subjectively biased in feature indication [7,19,20]. Furthermore, novel clinical indicators such as micrometastasis volume and nutritional index have been proved as promising prognostic markers for CRC patients, but most of them remained to be further validated due to their inherent limitations [21,22]. Overall, it is widely suggested that clinicopathological features alone may be unable to accurately predict outcome of CRC patients and novel molecular biomarkers should be added into current evaluation system. Recent studies have identified numerous potential molecular biomarkers for CRC [18]. However, only few have been translated into clinical benefits because the majority of them are found difficult to predict different outcomes within a single stage category or identify patients who are sensitive to chemotherapy scheme [23]. Therefore, it is urgent and crucial for oncologists not only to discover useful biomarkers, but also to validate those which can actually help improve upon existing prognostic methods in CRC.
DUSP5 is originally proved as a specific negative regulator of MAPK signaling pathway, suggesting its potential role as a tumor suppressor [24]. This deduction was then confirmed by a recent work that DUSP5 could suppress the malignant progression of skin cancer by inactivating ERK1/2 [25]. However, with regard to its role in solid tumor, to our knowledge, few relevant studies are available and less is about gastrointestinal malignancy. Since our previous study suggested DUSP5 might be involved in CRC growth and 5-FU resistance, we thus made an effort to further investigate its clinical significance in this study [14]. As a result, we firstly found DUSP5 expression is significantly lower in tumor tissues than that in matched normal tissues, implying it may act as a negative regulator for CRC development. This finding is not only necessary but also of some significance to current studies because DUSPs expression has been recently suggested controversial in cancer and DUSP5 expression is poorly defined in particular [8,26]. Following Immunohistochemistry analysis, we also confirmed the above finding and demonstrated DUSP5 expression is significantly associated with tumor differentiation, TNM stage, lymph node metastasis and distant metastasis, strongly supporting its involvement in CRC development. This is somewhat in accordance with a recent work by Cai et al, who found DUSP5 expression was negatively associated with advanced pathological stage and high Gleason score in prostate cancer [27].
The prognostic significance of DUSP5 was subsequently assessed by Kaplan-Meier model and Cox proportional hazards regression model. The result revealed that high DUSP5 expression was associated with better CRC-specific survival and DFS than low DUSP5 expression. Moreover, DUSP5 was identified as an independent prognostic factor for the CRC-specific survival and DFS of CRC patients. These findings collectively suggested that DUSP5 may have the capacity to be a useful biomarker for prognostic indication. Shin et al found DUSP5 expression was dramatically downregulated by DNA methylation and DUSP5 methylation might serve as an independent prognostic parameter affecting the overall survival of gastric cancer patients, indirectly supporting our conclusion [28].
To further assess whether DUSP5 could actually help improve current prognostic methods, subgroup analysis based on TNM stage and chemotherapy receiving was performed. For stage II patients without receiving chemotherapy, we found DUSP5 expression was unable to distinguish patient outcome, largely due to the fact that these patients are clinically defined as low-risk and hardly likely to develop recurrence or metastasis. Therefore, we suggested that additional detection of DUSP5 expression for these patients might be of little practical significance for clinical management. Although adjuvant chemotherapy has been recommended to high-risk stage II patients in European Society for Medical Oncology (EMSO) since 2013, researchers found it could only improve clinical outcome of patients with T4 stage and those with other high-risk factors may be unable to benefit from it [29]. In this study, we also found not all of high-risk stage II patients could benefit from standard chemotherapy (FOLFOX scheme) and patients with high DUSP5 expression had a better CRC-specific survival/DFS than those with low DUSP5 expression. This finding indicated that DUSP5 could be a helpful biomarker on current high-risk stage II patient stratification for chemotherapy decision. With respect to stage III patients, traditional lymph node system has been proved to be low specific and sensitive in predicting survival [30]. In this study, we found high DUSP5 expression could predict better survival for stage III patients receiving chemotherapy, suggesting that additional detection of DUSP5 might help cover the shortage for traditional prognostic system in stage III patients. Finally, we found similar results in metastatic patients receiving surgery combined with standard chemotherapy, indicating that detecting DUSP5 expression in primary tumor tissues may also be useful for outcome prediction in patients with resectable metastatic CRC. However, since most metastatic CRC patients receive conservative chemotherapy instead of radical surgery and had relatively shorter survival, it is technically difficult to assess the expression of DUSP5 in primary CRC tissues for these patients and therefore further efforts may be made to investigate whether circulating DUSP5 level has any clinical significance for them.
To preliminarily explain the prognostic effect of DUSP5 on CRC patients, we performed correlation analysis between DUSP5 expression and EMT phenotype, based on the consideration that EMT is a well-established event driving CRC development and chemotherapy resistance [31]. This molecular event is also characterized as downregulation of epithelial markers (such as E-cadherin and cytoskeletal proteins) and upregulation of mesenchymal markers (such as N-cadherin and vimentin). In this study, we found DUSP5 expression is positively correlated with E-cadherin expression, but negatively correlated with N-cadherin and vimentin expression, suggesting it may be involved in regulation of EMT program. This finding can be indirectly supported by a recent comprehensive work proving that DUSP1, DUSP4, and DUSP6 are involved in EMT program in breast cancer cells [15]. Moreover, DUSP5 appears to inactive nuclear ERK signaling pathway, which is proved to induce chemotherapeutic resistance and liver metastases of CRC through EMT program [32,33]. Therefore, although no evidence directly linked DUSP5 with EMT phenotype, based on our clinical findings and previous relevant work, it is reasonable to speculate that downregulated DUSP5 may contribute to poor outcome of advanced CRC patients partly by participating in EMT program, which warrants further work for functional validation and mechanism clarification.
In summary, we firstly provide evidence that DUSP5 expression is profoundly downregulated in CRC tissues compared with that in matched normal tissues, and may be associated with malignant development of CRC. Then, we identify DUSP5 expression can serve as a useful prognostic biomarker for progression indication as well as chemotherapy benefit in advanced CRC patients. Finally, we suggest DUSP5 expression is correlated with EMT phenotype in CRC, which may partly explain its prognostic effect on patients but needs further molecular validation in vitro and in vivo. Overall, these findings collectively demonstrate that DUSP5 has great potential to be clinically translated into a reliable helper for accurate patient stratification and treatment decision making in CRC Precision Medicine.
Acknowledgements
This study is supported by the funding of Science and Technology Commission of Shanghai Municipality (NO. 124119a720), and CSCO-Merck Serono Oncology Research (NO. Y-MX2015-046). We thank Professor Yiming Chen (Department of Pathology, Renji Hospital affiliated to Shanghai Jiao Tong University) and Professor Yuping Gao (Center for Reproductive Medicine, Xinhua Hospital, School of Medicine, Shanghai Jiao Tong University) for their crucial guidance in Immunohistochemistry analysis.
Disclosure of conflict of interest
None.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 3.Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 4.Friedman AA, Letai A, Fisher DE, Flaherty KT. Precision medicine for cancer with next-generation functional diagnostics. Nat Rev Cancer. 2015;15:747–756. doi: 10.1038/nrc4015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burotto M, Chiou VL, Lee JM, Kohn EC. The MAPK pathway across different malignancies: a new perspective. Cancer. 2014;120:3446–3456. doi: 10.1002/cncr.28864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murcia O, Juarez M, Hernandez-Illan E, Egoavil C, Giner-Calabuig M, Rodriguez-Soler M, Jover R. Serrated colorectal cancer: Molecular classification, prognosis, and response to chemotherapy. World J Gastroenterol. 2016;22:3516–3530. doi: 10.3748/wjg.v22.i13.3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shen Y, Han X, Wang J, Wang S, Yang H, Lu SH, Shi Y. Prognostic impact of mutation profiling in patients with stage II and III colon cancer. Sci Rep. 2016;6:24310. doi: 10.1038/srep24310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Low HB, Zhang Y. Regulatory Roles of MAPK Phosphatases in Cancer. Immune Netw. 2016;16:85–98. doi: 10.4110/in.2016.16.2.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kang Y, Nagaraja AS, Armaiz-Pena GN, Dorniak PL, Hu W, Rupaimoole R, Liu T, Gharpure KM, Previs RA, Hansen JM, Rodriguez-Aguayo C, Ivan C, Ram P, Sehgal V, Lopez-Berestein G, Lutgendorf SK, Cole SW, Sood AK. Adrenergic Stimulation of DUSP1 Impairs Chemotherapy Response in Ovarian Cancer. Clin Cancer Res. 2016;22:1713–1724. doi: 10.1158/1078-0432.CCR-15-1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin YC, Lin YC, Shih JY, Huang WJ, Chao SW, Chang YL, Chen CC. DUSP1 expression induced by HDAC1 inhibition mediates gefitinib sensitivity in non-small cell lung cancers. Clin Cancer Res. 2015;21:428–438. doi: 10.1158/1078-0432.CCR-14-1150. [DOI] [PubMed] [Google Scholar]
- 11.Haag T, Richter AM, Schneider MB, Jimenez AP, Dammann RH. The dual specificity phosphatase 2 gene is hypermethylated in human cancer and regulated by epigenetic mechanisms. BMC Cancer. 2016;16:49. doi: 10.1186/s12885-016-2087-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hijiya N, Tsukamoto Y, Nakada C, Tung Nguyen L, Kai T, Matsuura K, Shibata K, Inomata M, Uchida T, Tokunaga A, Amada K, Shirao K, Yamada Y, Mori H, Takeuchi I, Seto M, Aoki M, Takekawa M, Moriyama M. Genomic Loss of DUSP4 Contributes to the Progression of Intraepithelial Neoplasm of Pancreas to Invasive Carcinoma. Cancer Res. 2016;76:2612–2625. doi: 10.1158/0008-5472.CAN-15-1846. [DOI] [PubMed] [Google Scholar]
- 13.Slattery ML, Lundgreen A, Wolff RK. MAP kinase genes and colon and rectal cancer. Carcinogenesis. 2012;33:2398–2408. doi: 10.1093/carcin/bgs305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yan X, Shan Z, Yan L, Zhu Q, Liu L, Xu B, Liu S, Jin Z, Gao Y. High expression of Zinc-finger protein X-linked promotes tumor growth and predicts a poor outcome for stage II/III colorectal cancer patients. Oncotarget. 2016;7:19680–19692. doi: 10.18632/oncotarget.7547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boulding T, Wu F, McCuaig R, Dunn J, Sutton CR, Hardy K, Tu W, Bullman A, Yip D, Dahlstrom JE, Rao S. Differential Roles for DUSP Family Members in Epithelial-to-Mesenchymal Transition and Cancer Stem Cell Regulation in Breast Cancer. PLoS One. 2016;11:e0148065. doi: 10.1371/journal.pone.0148065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yan X, Yan L, Liu S, Shan Z, Tian Y, Jin Z. N-cadherin, a novel prognostic biomarker, drives malignant progression of colorectal cancer. Mol Med Rep. 2015;12:2999–3006. doi: 10.3892/mmr.2015.3687. [DOI] [PubMed] [Google Scholar]
- 17.Shan ZZ, Yan XB, Yan LL, Tian Y, Meng QC, Qiu WW, Zhang Z, Jin ZM. Overexpression of Tbx3 is correlated with Epithelial-Mesenchymal Transition phenotype and predicts poor prognosis of colorectal cancer. Am J Cancer Res. 2015;5:344–353. [PMC free article] [PubMed] [Google Scholar]
- 18.Aghagolzadeh P, Radpour R. New trends in molecular and cellular biomarker discovery for colorectal cancer. World J Gastroenterol. 2016;22:5678–5693. doi: 10.3748/wjg.v22.i25.5678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chu QD, Zhou M, Medeiros KL, Peddi P, Kavanaugh M, Wu XC. Poor survival in stage IIB/C (T4N0) compared to stage IIIA (T1-2 N1, T1N2a) colon cancer persists even after adjusting for adequate lymph nodes retrieved and receipt of adjuvant chemotherapy. BMC Cancer. 2016;16:460. doi: 10.1186/s12885-016-2446-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuijpers CC, Sluijter CE, von der Thusen JH, Grunberg K, van Oijen MG, van Diest PJ, Jiwa M, Nagtegaal ID, Overbeek LI, Willems SM. Interlaboratory Variability in the Histologic Grading of Colorectal Adenocarcinomas in a Nationwide Cohort. Am J Surg Pathol. 2016;40:1100–1108. doi: 10.1097/PAS.0000000000000636. [DOI] [PubMed] [Google Scholar]
- 21.Yamamoto H, Murata K, Fukunaga M, Ohnishi T, Noura S, Miyake Y, Kato T, Ohtsuka M, Nakamura Y, Takemasa I, Mizushima T, Ikeda M, Ohue M, Sekimoto M, Nezu R, Matsuura N, Monden M, Doki Y, Mori M. Micrometastasis Volume in Lymph Nodes Determines Disease Recurrence Rate of Stage II Colorectal Cancer: A Prospective Multicenter Trial. Clin Cancer Res. 2016;22:3201–3208. doi: 10.1158/1078-0432.CCR-15-2199. [DOI] [PubMed] [Google Scholar]
- 22.Yang Y, Gao P, Chen X, Song Y, Shi J, Zhao J, Sun J, Xu Y, Wang Z. Prognostic significance of preoperative prognostic nutritional index in colorectal cancer: results from a retrospective cohort study and a meta-analysis. Oncotarget. 2016 doi: 10.18632/oncotarget.10148. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dalerba P, Sahoo D, Paik S, Guo X, Yothers G, Song N, Wilcox-Fogel N, Forgo E, Rajendran PS, Miranda SP, Hisamori S, Hutchison J, Kalisky T, Qian D, Wolmark N, Fisher GA, van de Rijn M, Clarke MF. CDX2 as a Prognostic Biomarker in Stage II and Stage III Colon Cancer. N Engl J Med. 2016;374:211–222. doi: 10.1056/NEJMoa1506597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Buffet C, Catelli MG, Hecale-Perlemoine K, Bricaire L, Garcia C, Gallet-Dierick A, Rodriguez S, Cormier F, Groussin L. Dual Specificity Phosphatase 5, a Specific Negative Regulator of ERK Signaling, Is Induced by Serum Response Factor and Elk-1 Transcription Factor. PLoS One. 2015;10:e0145484. doi: 10.1371/journal.pone.0145484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rushworth LK, Kidger AM, Delavaine L, Stewart G, van Schelven S, Davidson J, Bryant CJ, Caddye E, East P, Caunt CJ, Keyse SM. Dual-specificity phosphatase 5 regulates nuclear ERK activity and suppresses skin cancer by inhibiting mutant Harvey-Ras (HRasQ61L)-driven SerpinB2 expression. Proc Natl Acad Sci U S A. 2014;111:18267–18272. doi: 10.1073/pnas.1420159112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shen J, Zhang Y, Yu H, Shen B, Liang Y, Jin R, Liu X, Shi L, Cai X. Role of DUSP1/MKP1 in tumorigenesis, tumor progression and therapy. Cancer Med. 2016;5:2061–2068. doi: 10.1002/cam4.772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cai C, Chen JY, Han ZD, He HC, Chen JH, Chen YR, Yang SB, Wu YD, Zeng YR, Zou J, Liang YX, Dai QS, Jiang FN, Zhong WD. Down-regulation of dual-specificity phosphatase 5 predicts poor prognosis of patients with prostate cancer. Int J Clin Exp Med. 2015;8:4186–4194. [PMC free article] [PubMed] [Google Scholar]
- 28.Shin SH, Park SY, Kang GH. Down-regulation of dual-specificity phosphatase 5 in gastric cancer by promoter CpG island hypermethylation and its potential role in carcinogenesis. Am J Pathol. 2013;182:1275–1285. doi: 10.1016/j.ajpath.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 29.Verhoeff SR, van Erning FN, Lemmens VE, de Wilt JH, Pruijt JF. Adjuvant chemotherapy is not associated with improved survival for all high-risk factors in stage II colon cancer. Int J Cancer. 2016;139:187–193. doi: 10.1002/ijc.30053. [DOI] [PubMed] [Google Scholar]
- 30.Mohan HM, Walsh C, Kennelly R, Ng CH, O'Connell PR, Hyland JM, Hanly A, Martin S, Gibbons D, Sheahan K, Winter DC. Lymph node ratio does not provide additional prognostic information compared with the N1/N2 classification in stage III colon cancer. Colorectal Dis. 2016 doi: 10.1111/codi.13410. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 31.Bhangu A, Wood G, Mirnezami A, Darzi A, Tekkis P, Goldin R. Epithelial mesenchymal transition in colorectal cancer: Seminal role in promoting disease progression and resistance to neoadjuvant therapy. Surg Oncol. 2012;21:316–323. doi: 10.1016/j.suronc.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 32.Kidger AM, Keyse SM. The regulation of oncogenic Ras/ERK signalling by dual-specificity mitogen activated protein kinase phosphatases (MKPs) Semin Cell Dev Biol. 2016;50:125–132. doi: 10.1016/j.semcdb.2016.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tanahashi T, Osada S, Yamada A, Kato J, Yawata K, Mori R, Imai H, Sasaki Y, Saito S, Tanaka Y, Nonaka K, Yoshida K. Extracellular signal-regulated kinase and Akt activation play a critical role in the process of hepatocyte growth factor-induced epithelial-mesenchymal transition. Int J Oncol. 2013;42:556–564. doi: 10.3892/ijo.2012.1726. [DOI] [PubMed] [Google Scholar]