Abstract
Paclitaxel plus cisplatin and 5-fluorouracil plus cisplatin treatments are effective strategies for patients with advanced esophageal squamous cell carcinoma. This study was to evaluate the safety and efficacy of paclitaxel plus cisplatin and 5-fluorouracil plus cisplatin as first-line chemotherapy for patients with advanced esophageal squamous cell carcinoma. A total of 398 patients with advanced esophageal squamous cell carcinoma who received chemotherapy were included and divided into 2 groups: paclitaxel plus cisplatin group and 5-fluorouracil plus cisplatin group. 195 patients received paclitaxel plus cisplatin and 203 patients received 5-fluorouracil plus cisplatin. The objective response rates were 42.5% and 38.4% for paclitaxel plus cisplatin group and 5-fluorouracil plus cisplatin group, respectively (P=0.948). The median progression-free survival was 7.85 months (95% CI, 6.77-8.94 months) for the paclitaxel plus cisplatin group and 6.53 months (95% CI, 5.63-7.43 months) for the 5-fluorouracil plus cisplatin group with significant difference (P=0.02). The median overall survival was 13.46 months (95% CI, 12.01-14.91 months) for the paclitaxel plus cisplatin group and 12.67 months (95% CI, 11.87-13.47 months) for the 5-fluorouracil plus cisplatin group (P=0.204). The first-line chemotherapy of paclitaxel plus cisplatin had better median progression-free survival than 5-fluorouracil plus cisplatin in patients with advanced esophageal squamous cell carcinoma with tolerable toxicities.
Keywords: Paclitaxel, cisplatin, 5-fluorouracil, squamous cell esophageal cancer, first-line treatment
Introduction
Esophageal carcinoma is the eighth most common cancer and the sixth most common cause of cancer related deaths worldwide with developing nations making up more than 80% of total cases and deaths [1]. In China, esophageal squamous cell carcinoma (ESCC) is the predominant histologic type (90%-95%), while the incidence of esophageal adenocarcinoma remains extremely low [2]. More than two-thirds of patients diagnosed with esophageal cancer had unresectable disease. Postoperative local recurrence or metastasis could be detected in 43.3-54.5% patients within 5 years [3,4]. Patients with unresectable or metastatic ESCC have a particularly dismal prognosis, with an overall survival (OS) of 3.0-8.0 months [5]. So far, there is no standard regimen for advanced or metastatic ESCC partially due to the limited number of patients included in the various treatment plans which have been shown to be effective to some extent. For example, Cisplatin-based regimens were used as effective treatment. The combination of 5-fluorouracil and cisplatin (CF) achieved a response rate of 13-35% with the median OS of 5.5-6.7 months [6-8]. Single agent paclitaxel has shown promising response for esophageal and gastric carcinoma [9-11]. Several phase II studies have demonstrated that paclitaxel plus cisplatin (TP) was an effective treatment strategy and resulted in significantly improved OS by 13.0 to 17.0 months in the first-line chemotherapy of advanced or metastatic ESCC [12-15]. It is worth reiterating that all the previous trials only include small number of patients (20-92 patients).
As mentioned above, TP and CF have been shown to be effective on separate trials. A side-by-side study of TP and CF is still much needed in that it would offer the advantage of direct comparison under standardized treatment schedule and response criteria. In this regard, our retrospective study was conducted to investigate the efficacy and toxicity of TP vs. CF treatment to afford enough clinical evidence for first-line chemotherapy in patients with unresectable or metastatic ESCC. Our study also represents so far the largest clinical trial with 398 enrolled patients in total compared to the previous trials (20-92 patients), providing more convincing conclusions and treatment schedules.
Patients and methods
Patients
This was a retrospective analysis of patients with advanced ESCC who received TP or CF as first line chemotherapy. Patients had to be at least 18 years of age at the time of registration and have histologically or cytologically confirmed ESCC, which was surgically unresectable or recurrent. They also had to have an Eastern Cooperative Oncology Group (ECOG) performance status of 0-2, a life expectancy of >12 weeks with sufficient bone marrow, liver, renal and cardiovascular function. Written informed consent for chemotherapy was obtained from all patients.
Between January 2012 and June 2014, 398 patients with ESCC met these criteria and were included in this analysis in Henan Cancer Hospital and Nanyang City Center Hospital. Among them, 195 patients received TP and 203 patients received CF.
Treatment plan
Patients of TP group were treated with paclitaxel 175 mg/m2 over 3 hours on day 1 and cisplatin 75 mg/m2 day 1 every 3 weeks. Patients of CF group received FU 750 mg/m2/24 hours by continuous intravenous infusion day 1 through 5 and cisplatin 75 mg/m2 day 1 every 3 weeks. Patients receiving paclitaxel were premedicated with 10 mg of oral dexamethasone at 12 and 6 hours before the infusion and with intramuscular injection diphenhydramine and intravenous H2 receptor antagonist within 60 minutes before the infusion of paclitaxel to reduce the risk of hypersensitivity reaction. Cisplatin was administered with hydration and forced diuresis, and patients underwent routine monitoring of electrolytes, serum creatinine, and magnesium. The combination therapy was up to maximum 6 cycles. Treatment was discontinued until documented disease progression, unacceptable toxicity or patient’s refusal.
Statistical analysis
Response was assessed according to the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.0 as complete response (CR), partial response (PR), stable disease (SD) or progressive disease (PD) in patients with measurable lesions. Toxicities were graded according to the National Cancer Institute’s Common Terminology Criteria for Adverse Events (CTCAE) version 4.0. All comparisons of patients’ characteristics, response rates, and rates of toxic effects were performed with Fisher’s exact test. Progression-free survival (PFS) was measured from the initiation of TP or CF to the occurrence of progression, or death without evidence of progression. Overall survival (OS) was also measured from the first day of TP or CF administration to the day of death or to the final day of the follow up period. The Kaplan-Meier method was used to estimate overall survival curves and median survival and the two groups were compared using log-rank testing. All tests were two-sided, value of P<0.05 was accepted as statistically significant. The last follow-up was performed in June 2015.
Results
Patient characteristics
Between January 2012 and June 2014, a total of 398 patients were enrolled in this retrospective study. Patient characteristics for both groups are shown in Table 1. Median age was 61 years (35-75 years) in TP group and 63 years (32-75 years) in CF group. Total 183 patients underwent esophagectomy, including 84 patients of TP group and 99 patients of CF group. The two groups were similar in terms of other parameters including gender, ECOG performance status, histological type, prior treatment status, target site of metastasis. Both of the groups, the median total duration of treatment was 4 cycles (2-6 cycles). And the total treatment cycles were 794 and 896 cycles in TP group and CF group, respectively.
Table 1.
Baseline patient characteristics
| TP group N=195 | CF group N=203 | |||
|---|---|---|---|---|
|
|
|
|||
| Characteristic | No. | % | No. | % |
| Sex | ||||
| Male | 134 | 68.7 | 143 | 70.4 |
| Female | 61 | 31.3 | 60 | 29.6 |
| Age, years | ||||
| Range | 35-75 | 32-75 | ||
| Median | 61 | 63 | ||
| Performance status | ||||
| 0 | 125 | 64.1 | 137 | 67.5 |
| 1 | 58 | 29.7 | 51 | 25.1 |
| 2 | 12 | 6.2 | 15 | 7.4 |
| Metastases | ||||
| Lymph nodes | 98 | 50.3 | 128 | 63.1 |
| Mediastinum | 83 | 42.6 | 112 | 55.2 |
| Lung | 69 | 35.4 | 89 | 43.8 |
| Liver | 35 | 17.9 | 62 | 30.5 |
| Bone | 24 | 12.3 | 37 | 18.2 |
| Others | 7 | 3.6 | 10 | 4.9 |
| Esophagectomy | ||||
| Yes | 84 | 43.1 | 99 | 48.8 |
| No | 111 | 56.9 | 104 | 51.2 |
Abbreviations: TP, paclitaxel plus cisplatin; CF, fluorouracil plus cisplatin.
Response and survival
The objective response rate (ORR) among patients with measurable disease are summarized in Table 2. In TP group, CR was observed in 3 patients (1.5%), and 80 patients (41.0%) achieved partial response. In contrast, 2 CR were obtained in CF group, and PR was observed in 76 patients (37.4%). The ORR were 42.5% for the TP group and 38.4% for the FP group (P=0.948). At the end of follow-up, 190 patients (97.4%) in TP group and 198 patients (97.50%) in CF group had died. The median PFS and OS were shown in Figures 1 and 2, respectively. The median PFS was 7.85 months (95% CI, 6.77-8.94 months) for TP group and 6.53 months (95% CI, 5.63-7.43 months) for CF group with significant difference (P=0.02) (Figure 1). The median OS was 13.46 months (95% CI, 12.01-14.91 months) for TP group and 12.67 months (95% CI, 11.87-13.47 months) for CF group (Figure 2). However, no significant difference in OS was identified between the two groups (P=0.204).
Table 2.
Response by treatment group
| Response | TP group (N=195) | CF group (N=203) | ||
|---|---|---|---|---|
|
|
|
|||
| No. | % | No. | % | |
| Complete response | 3 | 1.5 | 2 | 1.0 |
| Partial response | 80 | 41.0 | 76 | 37.4 |
| Stable disease | 59 | 30.3 | 52 | 25.6 |
| Progression disease | 53 | 27.2 | 73 | 36.0 |
| Response rate | 42.5 | 38.4 | ||
| Disease control rate | 72.8 | 64.0 | ||
Abbreviations: TP, paclitaxel plus cisplatin; CF, fluorouracil plus cisplatin.
Figure 1.
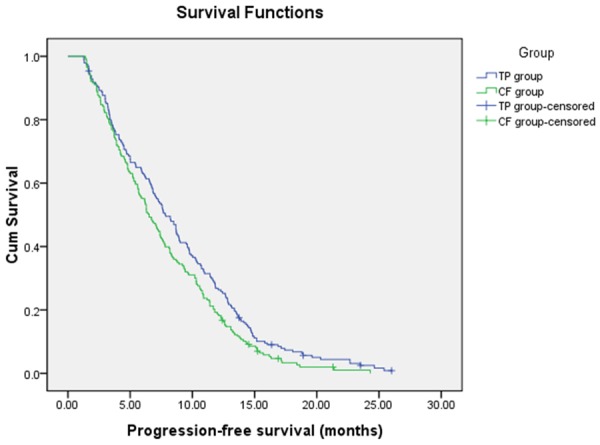
Progression-Free Survival.
Figure 2.
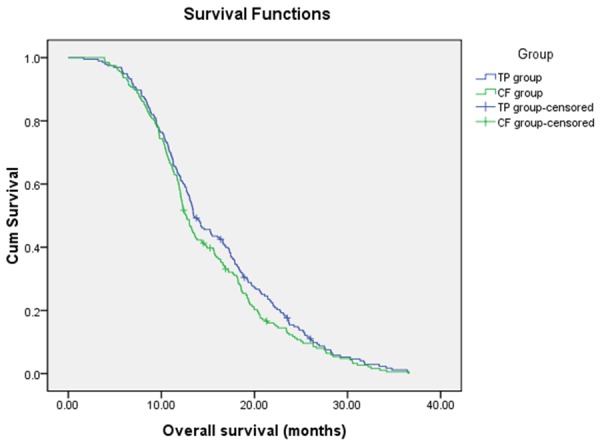
Overall Survival.
Toxicity
Toxicities graded according to CTCAE version 4.0 are listed in Table 3. Grade 3/4 hematologic toxicities were as follows: leucopenia (29.8% in TP group, 21.7% in CF group, P=0.566), neutropenia (27.7% in TP group, 19.7% in CF group, P=0.061), thrombocytopenia (6.1% in TP group, 3.5% in CF group, P=0.206), anemia (6.2% in TP group, 2.0% in CF group, P=0.034). The most common grade 3/4 non-hematologic toxicities were nausea, and vomiting. Twelve patients (5.9%) experienced grade 3/4 mucositis in CF group. While, grade 3/4 mucositis was not observed in TP group. One death was considered related to the treatment in TP group. The patient died of grade 4 neutropenia and pneumonia. There was no treatment-related death in CF group.
Table 3.
Grade 3 to 4 Toxicities by group
| TP group (N=195) | CF group (N=203) | |||
|---|---|---|---|---|
|
|
|
|||
| Toxicities | Grade 3 (%) | Grade 4 (%) | Grade 3 (%) | Grade 4 (%) |
| Hematologic | ||||
| Leucopenia | 38 (19.5) | 20 (10.3) | 32 (15.8) | 12 (5.9) |
| Neutropenia | 30 (15.4) | 24 (12.3) | 26 (12.8) | 14 (6.9) |
| Anemia | 12 (6.2) | 0 | 4 (2.0) | 0 |
| Thrombocytopenia | 9 (4.6) | 3 (1.5) | 5 (2.5) | 2 (1.0) |
| Gastrointestinal | ||||
| Nausea | 11 (5.6) | 0 | 9 (4.4) | 0 |
| Vomiting | 8 (4.1) | 0 | 13 (6.4) | 0 |
| Diarrhea | 1 (0.5) | 0 | 6 (3.0) | 0 |
| Others | ||||
| Fatigue | 2 (1.0) | 0 | 3 (1.5) | 0 |
| Mucositis | 0 | 0 | 10 (4.9) | 2 (1.0) |
| Liver | 0 | 0 | 1 (0.5) | 0 |
Abbreviations: TP, paclitaxel plus cisplatin; CF, fluorouracil plus cisplatin.
Discussion
Palliative treatment is the only option for patients with advanced esophageal cancer with the goal of prolonging survival and improving quality of life. In this regard, double-agent chemotherapy of CF and TP have been the most widely used cytotoxic agents in patients with advanced ESCC. In the absence of adequately powered phase III trials for many years, previous data of CF or TP for advanced ESCC were from phase II studies with the sample size of 20 to 92 patients. This is the first study to directly compare efficacy and safety of CF and TP for the initial treatment of 398 patients with advanced ESCC.
In this retrospective study, the proportion of patients who received all four cycles of chemotherapy was similar in the two groups (approximately 70 percent), and thus the observed difference in survival is not thought to be attributable to a difference in the actual delivery of treatment. The 195 patients with advanced ESCC who were treated with TP had a median PFS of 7.85 months (95% confidence interval: 6.77-8.94 months), whereas the group that received CF had a median PFS of 6.53 months (95% confidence interval: 5.63-7.43 months) (p=0.02). The OS of the two groups was 13.46 months (95% confidence interval: 12.01-14.91 months) and 12.67 months (95% confidence interval: 11.87-13.47 months), respectively (p=0.204). There was no statistical difference in OS between the two groups. Importantly, a favorable PFS effect was observed in patients receiving TP.
In terms of efficacy, differences in ORR were similar between the two groups. The combination of paclitaxel and cisplatin protocol displayed an ORR of 42.5% with a disease control rate (DCR) of 72.8%. Recently, more studies have emerged utilizing nedaplatin in place of cisplatin plus paclitaxel as the initial treatment for metastatic ESCC. Patients received the treatment of 175 mg/m2 of paclitaxel, followed by nedaplatin 80 mg/m2 on day 1, every 3 weeks repeatedly had an ORR of 41.7-47.7% with PFS of 6.1-7.1 months and OS of 11.5-12.4 months [16-18]. The results of TP regimen for advanced ESCC were consistent with paclitaxel-nedaplatin regimen. In CF group, patients with advanced ESCC yielded an ORR of 38.4% and DCR of 64.0%. The first randomized phase II study of 5-fluorouracil plus cisplatin versus single cisplatin was cisplatin 100 mg/m2 on day 1 and 5-FU 1,000 mg/m2/d by continuous infusion or single cisplatin alone. Patients in CF group achieved an ORR of 35% and 7 treatment-related deaths (16%) were observed [6]. In another single arm study, a dose of 20 mg/m2 of cisplatin and 800 mg/m2 of 5-FU was given by continuous infusion for 24h on days 1-5 every 4 weeks. The ORR was 33.3% with good tolerance and a median OS of 201.5 days [7]. The combination of low dose-intensity CF seems to be an effective treatment with moderate toxicity in patients with advanced ESCC. As suggested by the data presented above, the combination of TP or CF is effective regimen as first-line therapy for patients with advanced ESCC.
Myelosuppression was the most frequent toxic effect in both groups. Grade 3 or 4 hematologic toxicities included leucopenia (29.8% in TP group, 21.7% in CF group, P=0.566), neutropenia (27.7% in TP group, 19.7% in CF group, P=0.061), thrombocytopenia (6.1% in TP group, 3.5% in CF group, P=0.206) and anemia (6.2% in TP group, 2.0% in CF group, P=0.034). However, there was a significantly higher incidence of all grade mucositis among the patients who received CF than among those who received TP. One treatment-related death in the TP group occurred after the second cycle of treatment and was attributed to grade 4 neutropenia. Generally, these toxicities were clinically manageable.
In conclusion, our results indicate that both TP and CF treatments are effective strategies for patients with advanced ESCC. The combination of TP had a more favorable PFS than CF as first-line treatment and was a clinically encouraging combination therapy for patients with advanced ESCC. Further assessment in a randomized, prospective, phase III study is required to obtain definitive evidence.
Acknowledgements
This work was supported by National Natural Science Foundation of China, (grant number 81201954). We wish to thank Dr. Peng Liu for assistance in preparation of the manuscript.
Disclosure of conflict of interest
None.
References
- 1.Herszenyi L, Tulassay Z. Epidemiology of gastrointestinal and liver tumors. Eur Rev Med Pharmacol Sci. 2010;14:249–258. [PubMed] [Google Scholar]
- 2.Wang AH, Liu Y, Wang B, He YX, Fang YX, Yan YP. Epidemiological studies of esophageal cancer in the era of genome-wide association studies. World J Gastrointest Pathophysiol. 2014;5:335–343. doi: 10.4291/wjgp.v5.i3.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mariette C, Balon JM, Piessen G, Fabre S, Van Seuningen I, Triboulet JP. Pattern of recurrence following complete resection of esophageal carcinoma and factors predictive of recurrent disease. Cancer. 2003;97:1616–1623. doi: 10.1002/cncr.11228. [DOI] [PubMed] [Google Scholar]
- 4.Nakagawa S, Kanda T, Kosugi S, Ohashi M, Suzuki T, Hatakeyama K. Recurrence pattern of squamous cell carcinoma of the thoracic esophagus after extended radical esophagectomy with three-field lymphadenectomy. J Am Coll Surg. 2004;198:205–211. doi: 10.1016/j.jamcollsurg.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 5.Vestermark LW, Sorensen P, Pfeiffer P. [Chemotherapy to patients with metastatic carcinoma of the esophagus and gastro-esophageal junction. A survey of a Cochrane review] . Ugeskr Laeger. 2008;170:633–636. [PubMed] [Google Scholar]
- 6.Bleiberg H, Conroy T, Paillot B, Lacave AJ, Blijham G, Jacob JH, Bedenne L, Namer M, De Besi P, Gay F, Collette L, Sahmoud T. Randomised phase II study of cisplatin and 5-fluorouracil (5-FU) versus cisplatin alone in advanced squamous cell oesophageal cancer. Eur J Cancer. 1997;33:1216–1220. doi: 10.1016/s0959-8049(97)00088-9. [DOI] [PubMed] [Google Scholar]
- 7.Hayashi K, Ando N, Watanabe H, Ide H, Nagai K, Aoyama N, Takiyama W, Ishida K, Isono K, Makuuchi H, Imamura M, Shinoda M, Ikeuchi S, Kabuto T, Yamana H, Fukuda H. Phase II evaluation of protracted infusion of cisplatin and 5-fluorouracil in advanced squamous cell carcinoma of the esophagus: a Japan Esophageal Oncology Group (JEOG) Trial (JCOG9407) Jpn J Clin Oncol. 2001;31:419–423. doi: 10.1093/jjco/hye090. [DOI] [PubMed] [Google Scholar]
- 8.Lorenzen S, Schuster T, Porschen R, Al-Batran SE, Hofheinz R, Thuss-Patience P, Moehler M, Grabowski P, Arnold D, Greten T, Muller L, Rothling N, Peschel C, Langer R, Lordick F. Cetuximab plus cisplatin-5-fluorouracil versus cisplatin-5-fluorouracil alone in first-line metastatic squamous cell carcinoma of the esophagus: a randomized phase II study of the Arbeitsgemeinschaft Internistische Onkologie. Ann Oncol. 2009;20:1667–1673. doi: 10.1093/annonc/mdp069. [DOI] [PubMed] [Google Scholar]
- 9.Ajani JA, Ilson DH, Daugherty K, Pazdur R, Lynch PM, Kelsen DP. Activity of taxol in patients with squamous cell carcinoma and adenocarcinoma of the esophagus. J Natl Cancer Inst. 1994;86:1086–1091. doi: 10.1093/jnci/86.14.1086. [DOI] [PubMed] [Google Scholar]
- 10.Ilson DH, Wadleigh RG, Leichman LP, Kelsen DP. Paclitaxel given by a weekly 1-h infusion in advanced esophageal cancer. Ann Oncol. 2007;18:898–902. doi: 10.1093/annonc/mdm004. [DOI] [PubMed] [Google Scholar]
- 11.Wilke H, Muro K, Van Cutsem E, Oh SC, Bodoky G, Shimada Y, Hironaka S, Sugimoto N, Lipatov O, Kim TY, Cunningham D, Rougier P, Komatsu Y, Ajani J, Emig M, Carlesi R, Ferry D, Chandrawansa K, Schwartz JD, Ohtsu A. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol. 2014;15:1224–1235. doi: 10.1016/S1470-2045(14)70420-6. [DOI] [PubMed] [Google Scholar]
- 12.Petrasch S, Welt A, Reinacher A, Graeven U, Konig M, Schmiegel W. Chemotherapy with cisplatin and paclitaxel in patients with locally advanced, recurrent or metastatic oesophageal cancer. Br J Cancer. 1998;78:511–514. doi: 10.1038/bjc.1998.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ilson DH, Forastiere A, Arquette M, Costa F, Heelan R, Huang Y, Kelsen DP. A phase II trial of paclitaxel and cisplatin in patients with advanced carcinoma of the esophagus. Cancer J. 2000;6:316–323. [PubMed] [Google Scholar]
- 14.Zhang X, Shen L, Li J, Li Y, Li J, Jin M. A phase II trial of paclitaxel and cisplatin in patients with advanced squamous-cell carcinoma of the esophagus. Am J Clin Oncol. 2008;31:29–33. doi: 10.1097/COC.0b013e3181131ca9. [DOI] [PubMed] [Google Scholar]
- 15.Huang J, Zhou Y, Zhang H, Qu T, Mao Y, Zhu H, Quan L, Xing P, Wang J, He J, Xu N, Sun Y. A phase II study of biweekly paclitaxel and cisplatin chemotherapy for recurrent or metastatic esophageal squamous cell carcinoma: ERCC1 expression predicts response to chemotherapy. Med Oncol. 2013;30:343. doi: 10.1007/s12032-012-0343-4. [DOI] [PubMed] [Google Scholar]
- 16.Cao W, Xu C, Lou G, Jiang J, Zhao S, Geng M, Xi W, Li H, Jin Y. A phase II study of paclitaxel and nedaplatin as first-line chemotherapy in patients with advanced esophageal cancer. Jpn J Clin Oncol. 2009;39:582–587. doi: 10.1093/jjco/hyp058. [DOI] [PubMed] [Google Scholar]
- 17.He YF, Ji CS, Hu B, Fan PS, Hu CL, Jiang FS, Chen J, Zhu L, Yao YW, Wang W. A phase II study of paclitaxel and nedaplatin as front-line chemotherapy in Chinese patients with metastatic esophageal squamous cell carcinoma. World J Gastroenterol. 2013;19:5910–5916. doi: 10.3748/wjg.v19.i35.5910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Du J, Hu C, Zhang Y, Hu B, Wang F, Zhang Y. A retrospective study of paclitaxel combining nedaplatin chemotherapy for esophageal cancer. Anticancer Drugs. 2015;26:101–105. doi: 10.1097/CAD.0000000000000170. [DOI] [PubMed] [Google Scholar]


