Abstract
Estrogen receptors (ER) play important roles in the development and progression of breast and ovarian cancers. ERs mediate transcriptional regulation through interaction with cofactors and binding to response elements within the regulatory elements of target genes. Here, we examined the expression and function of TBLR1/TBL1XR1, a core component of NCoR (nuclear receptor corepressor) and SMRT (silencing mediator of retinoic acid and thyroid receptor) corepressor complexes, in breast and ovarian cancers. We found that although TBLR1 is present in both the nucleus and cytoplasm of normal and neoplastic breast and ovarian cells, it is expressed at significantly higher levels in the nucleus of malignant breast and ovarian cells compared to benign cells. TBLR1 functions as an ER corepressor to inhibit ER-mediated transcriptional activation in both breast and ovarian cell lines, but it has no effect on androgen receptor (AR) mediated transcriptional activation in these cells. Furthermore, ectopic expression of nuclear TBLR1 in breast and ovarian cancer cells stimulates cell proliferation. The increased cell proliferation by nuclear TBLR1 is through both ER-independent and ER-dependent mechanisms as evidenced by increased growth in hormone-free medium and estrogen medium, as well as reduced growth with ER knockdown by siRNA. Nuclear TBLR1 overexpression also increased migration and invasion in both breast and ovarian cancer cells. Determining the functional relationship between TBLR1 and ER may provide insights to develop novel treatment strategies and improve response to hormonal therapy in breast and ovarian cancers.
Keywords: Breast cancer, ovarian cancer, estrogen receptor, nuclear TBLR1, TBL1XR1
Introduction
Transducin beta like related protein 1 (TBLR1/TBL1XR1) was initially identified as a core component of the SMRT/N-CoR corepressor complexes that are responsible for transcriptional repression mediated by numerous transcription factors and nuclear hormone receptors (NHRs) [1]. TBLR1 is a multifunctional protein [2] and has been shown to have both corepressor and coactivator activities [2-5] depending on nuclear receptor and cell types. TBLR1 is necessary for targeting of the N-CoR complex to chromatin through binding to hypoacetylated histones to facilitate repression but also has been shown when phosphorylated to target N-CoR for dismissal and initiate transcriptional activation [6]. Our recent study [7] has shown that TBLR1 is a transcriptional coactivator for androgen receptor (AR), a ligand-activated transcriptional factor, in prostate cancer cells. TBLR1 acts as a tumor suppressor in prostate cancer by selectively activating AR target genes important for growth suppression and differentiation but does not activate pro-proliferative AR target genes [7].
Estrogen is a steroid hormone that is responsible for sexual development, fertility and metabolic processes. Elevated estrogen level has been linked to breast and ovarian cancers. The effects of estrogen are mediated by ERs through both genomic and non-genomic mechanisms. ERs mediate transcriptional regulation through direct binding to estrogen responsive elements (ERE) within the regulatory elements of target genes and subsequent recruitment of cofactors. ER associated coactivators and corepressors have been implicated in breast [8,9] and ovarian cancer [10]. N-CoR and SMRT are two well-studied corepressors and play key roles in transcriptional repression by unliganded NHRs [1,11]. Unlike other NHRs, DNA binding sites are not occupied by ER in the absence of estrogen, and the corepressors are recruited to target genes in response to antagonists. The ratio of coactivators to corepressors facilitates the determination of the transcriptional responses to selective estrogen modulators. Our previous study showed that the subcellular localization of TBLR1 is important for its functions in prostate cancer [12]. A recent study showed that TBLR1 promotes proliferation in human breast cancer via activation of beta-catenin signaling [13]. However, the subcellular localization of TBLR1 in breast cancer and its expression in ovarian neoplasms have not been investigated. In addition to ER, AR is a prevalent sex steroid receptor expressed in female endocrine organ cancers including breast and ovarian cancers [14-18]. Thus, it is of interest to determine whether TBLR1 plays a role in breast and ovarian cancers through its interaction with ER and AR.
Here, we investigated the role of TBLR1 in breast and ovarian cancers with a focus on its interaction with ER. We examined TBLR1 expression and cellular localization in breast and ovarian cancers using a panel of cell lines and clinical samples. Our study showed that, consistent with increased nuclear TBLR1 expression in cancer cells, nuclear TBLR1 serves as corepressor of ER in breast and ovarian cancer cells and importantly, nuclear TBLR1 stimulated cell proliferation, migration and invasion in these cells.
Materials and methods
Cell culture, cell proliferation and in vitro matrigel invasion assays
The cell lines, MCF10A, MCF7, MDA-MB-231, SKBr3, T29, SKOV-3 and OVCAR-3 were obtained from the American Type Culture Collection (ATCC). MCF10A, an immortalized mammary epithelial cell line, was cultured in MEGM mammary epithelial cell growth medium. MCF7 and MDA-MB-231 cells were grown in DMEM medium containing 10% FBS and 1 U/ml of penicillin and 1 mg/ml streptomycin. The benign immortalized ovarian surface epithelial cell line T29 was maintained in medium 199 and MCDB105 (Sigma). The two ovarian cancer cell lines SKOV-3 and OVCAR-3 were cultured in McCoy’s 5a medium (Invitrogen) and RPMI 1640 (Gibco) respectively, supplemented with 10% FBS and 1 U/ml of penicillin and 1 mg/ml streptomycin. Cell proliferation was measured by the colorimetric WST-1 assay (Roche). Cells (1 × 104) were plated into 24-well plates and measured absorbance at 450 nm every other day on a plate reader. Invasion assays were performed using BD Matrigel invasion chamber and 5% FBS was used as the chemoattractant in the lower chamber. Cells (5 × 104) were incubated for 24 h prior to staining and counting. The percentage of invasion was expressed as the ratio of invading cells over cell number normalized on day 2 of the growth curve.
Luciferase assay
In vivo reporter transcription assay was performed using the Dual-reporter Luciferase Assay System (Promega) according to manufacturer’s instructions. Briefly, 5 × 104 cells per well were plated into 24-well plates 24 h before transfection. After being washed with phosphate-buffered saline, cells in each well were transfected with 5 ng of AR or 0.5 ng of ER expression vector, 100 ng of luciferase reporter plasmid, 1 ng of the pR-LUC internal control luciferase plasmid, and increasing amounts of pCDNA3.1-TBLR1 expression vector. The total amount of DNA was adjusted to 1 μg for each transfection with pcDNA3.1 empty vector as needed. Transfections were conducted in phenol red-free DMEM medium; 8 h later, the medium was changed to phenol red-free DMEM plus charcoal-treated fetal bovine serum (10%) containing 10 nM R1881 or 10 nM 17-estradiol. Cells were cultured for 48 h with ligand prior to luciferase reading. The light output was measured by Lumat LB9507 luminometer (Berthold).
Whole cell lysates, cell fractionation, and immunoblot analysis
Whole-cell extracts were prepared as described [7] from MCF10A, MCF7, MDA-MB-231, SKBr3, T29, OVCAR3 and SKOV3 cells in RIPA buffer (50 mM Tris-HCl pH 8.0, 150 mM NaCl, 1% NP-40, 1 mM EDTA, 0.1% SDS). For cell fractionation, cells were first lysed with cytoplasmic buffer (10 mM HEPES pH 7.6, 50 mM NaCl, 0.5 M sucrose, 1 mM DTT, 5 mM MgCl2, 0.1% Triton X-100). After centrifugation, the supernatant was collected as the cytoplasmic fraction and the nuclear pellet was washed 3 times with cytoplasmic buffer followed by lysis with RIPA buffer. Extracts were subjected to electrophoresis on SDS-PAGE and then transferred to nitrocellulose membranes for immunoblot analysis as described [19] with antibodies for TBLR1 (Santa Cruz antibodies), H2B and GAPDH (Cell Signaling).
Immunohistochemistry on breast and ovarian cancer tissue
Immunohistochemical study with antibody against TBLR1 was used to characterize the expression pattern of TBLR1 in invasive ductal carcinoma of the breast. Two sets of breast cancer cases are used in this study. The first study set included the whole tissue sections from 40 cases of breast adenocarcinoma. TBLR1 nuclear and cytoplasmic expression in the malignant glands was compared with the adjacent benign breast glands on the same slide. The second study set included 52 cases of breast adenocarcinoma on a tissue microarray (TMA). Each case was represented by three 0.6 mm cores on the TMA. Cases on the TMA were stratified by various clinicopathological factors. In both study sets, the levels of TBLR1 nuclear and cytoplasmic expression were scored semi-quantitatively: 0 as negative, 1 as weak, 2 as moderate and 3 as strong expression. 108 cases of ovarian epithelial carcinomas, including papillary serous carcinoma (PSC, n=50), serous borderline tumors (SBT, n=23), endometrioid carcinoma (EMC, n=10), ovarian clear cell carcinoma (CCC, n=13) and ovarian mucinous carcinoma (MUC, n=12), and 22 cases of normal fallopian tubes (FT) (as staining control), were retrieved, and a tissue microarray (TMA) was assembled. IHC with TBLR1 was performed (TBLR1 antibody clone L-08, Santa Cruz Biotechnology Sc-100908) and TBLR1 nuclear and cytoplasmic expression pattern was scored. The levels of TBLR1 expression were scored semi-quantitatively; 0 as negative, 1 as weak, 2 as moderate and 3 as strong expression.
Statistical analysis
Statistical analyses of the above results were performed by Student’s t-test using the SPSS program (version 11.0) (SPSS, USA). Differences are considered statistically significant if P < 0.05.
Results
TBLR1 expression and subcellular localization in breast and ovarian cancer
We previously showed that TBLR1 localization is different in benign and malignant prostate tissue and cells [7]. In this study, we compared the expression levels and intracellular localization of TBLR1 in benign and malignant breast and ovarian cell lines. First, we performed cell lysate fractionation and analyzed TBLR1 expression by western blot across the three breast cell lines (Figure 1A) including the immortalized, non-transformed epithelial cell line MCF10A, and human breast adenocarcinoma cell lines, MCF7 and MDA-MB-231. The MCF7 cell line is positive both for ER and AR expression; in contrast, MCF10A and MDA-MB-231 cell lines are both AR and ER negative. We found that TBLR1 expression is present in both benign and malignant breast cells as full-length 55 kD protein. However, the pattern of TBLR1 localization is different between benign and malignant cell lines. MCF10A and MCF7 cells show higher nuclear TBLR1 expression compared to cytoplasmic expression (Figure 1A). We also examined the expression and localization of TBLR1 across three ovarian cell lines (Figure 1B). We compared benign immortalized ovarian surface epithelial cell line T29, and malignant ovarian cancer cell lines OVCAR-3 and SKOV3. Both the benign and malignant ovarian cell lines are positive for AR and ER expression. The TBLR1 expression level in both the nucleus and cytoplasm in OVCAR-3 cells was higher than T29 or SKOV3 cells. SKOV3 cells showed lowest level of nuclear TBLR1 of the three cell lines.
Figure 1.
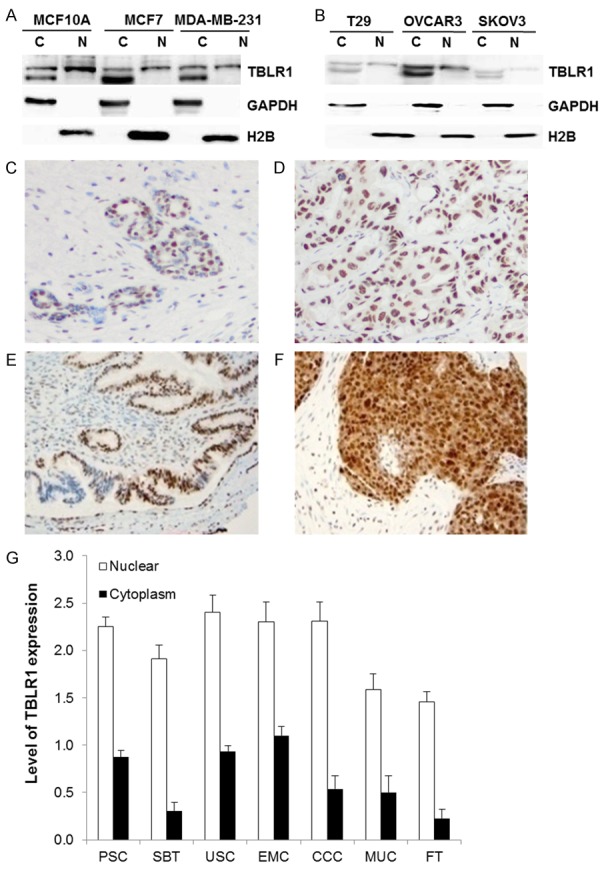
Expression of TBLR1 in breast and ovarian cancer. (A) Localization of TBLR1 expression in breast cells was examined by cell fractionation followed by western blot. GAPDH is used as a cytoplasm loading control. H2B is used as a nuclear loading control. (B) Localization of TBLR1 expression in ovarian cells was examined by cell fractionation followed by western blot. GAPDH is used as a cytoplasm loading control. H2B is used as a nuclear loading control. (C, D) Increased nuclear TBLR1 expression in breast cancer (D) compared to benign breast (C) cells. (E, F) Increased nuclear TBLR1 expression in ovarian cancer (F) compared to benign fallopian tubal (E) cells. (G) Average nuclear and cytoplasmic scoring of 108 cases of ovarian epithelial carcinomas, including papillary serous carcinoma (PSC, n=50), serous borderline tumors (SBT, n=23), endometrioid carcinoma (EMC, n=10), ovarian clear cell carcinoma (CCC, n=13) and ovarian mucinous carcinoma (MUC, n=12), with 15 cases of uterine serous carcinoma (USC) and 22 cases of normal fallopian tubes (FT).
We next examined the expression and localization of TBLR1 in 40 human breast cancer tissue samples. Expression was scored comparing malignant glands to the surrounding benign breast glands on the same tissue section. The expression of TBLR1 in the nucleus was significantly higher in the malignant glands compared to the adjacent benign breast glands (Figure 1C, 1D) with average score in tumor as 2.3 vs. benign as 1.8 (P < 0.01). Specifically, among the 40 cases of adenocarcinoma studied, the level of TBLR1 nuclear expression in malignant glands was higher in 20 cases, equal in 19 cases, and lower in 1 case. No significant difference between the level of TBLR1 cytoplasmic expression between malignant glands and benign glands was observed. When stratified with clinicopathological factors, the levels of TBLR1 nuclear expression were higher in androgen receptor (AR) positive cases (average score in AR (+) as 1.9 vs. AR (-) as 1.4). The level of TBLR1 nuclear expression is also higher in progesterone receptor (PR) positive cases with average score for PR (+) as 2.0 vs. PR (-) as 1.6. The level of TBLR1 nuclear expression is not significantly correlated with age at diagnosis, tumor size, ER or Her-2 status. No correlation between the level of TBLR1 cytoplasmic expression and the above mentioned clinicopathological factors were observed.
Additionally, we tested nuclear and cytoplasmic TBLR1 expression across different ovarian cancer tissues. We found TBLR1 expression was significantly higher in the nucleus compared to the cytoplasm (P < 0.005) in all of the specimens tested (Figure 1F). We found a relatively high expression of TBLR1 in both the nucleus and cytoplasm when comparing all subtypes of ovarian epithelial carcinomas to normal fallopian tubes (Figure 1E) (P < 0.01). A significant higher nuclear and cytoplasm expression of TBLR1 was also noted in most subtypes of ovarian epithelial carcinomas compared to USC (P < 0.005) except in nuclear staining of EMC (P=0.226) and CCC (P=0.217). More importantly, TBLR1 nuclear expression was significantly higher in PSC and CCC compared to MUC and SBT (P < 0.0001).
TBLR1 acts as an ER transcriptional corepressor in both breast and ovarian cancer cells
Previously, we showed that TBLR1 serves as an AR coactivator in prostate cancer cells [7]. To determine the function of TBLR1 in breast and ovarian cancer cells, we cotransfected a luciferase reporter gene with three copies of androgen responsive elements (ARE) or estrogen responsive elements (ERE) in the promoter region, along with full length AR or ER and increasing amounts of TBLR1 in pcDNA vectors in MDA-MB-231 or OVCAR3 cells. Cells were incubated either in the presence of androgen or estrogen for 48 hours after transfection. TBLR1 inhibited ER-mediated transcriptional activation in both MDA-MB-231 (Figure 2A) and OVCAR3 cells (Figure 2C) in a dose-dependent manner. Similarly, we also found TBLR1 inhibits ER-mediated transcriptional activation in breast cancer MCF7 cells (Supplementary Figure 1A). Interestingly, nuclear TBLR1 also functions as ER corerepressor in prostate cancer PC3 cells (Supplementary Figure 1C). We observed no change in AR transcriptional activation both in MCF7 cells (Supplementary Figure 1B) and MDA-MB-231 (Figure 2B) as well as OVCAR3 cells (Figure 2D). All luciferase experiments were repeated in triplicate. These findings suggest that TBLR1 functions as an ER corepressor in breast, ovarian, and prostate cancer, but did not alter AR mediated transcription in breast or ovarian cancer cells.
Figure 2.
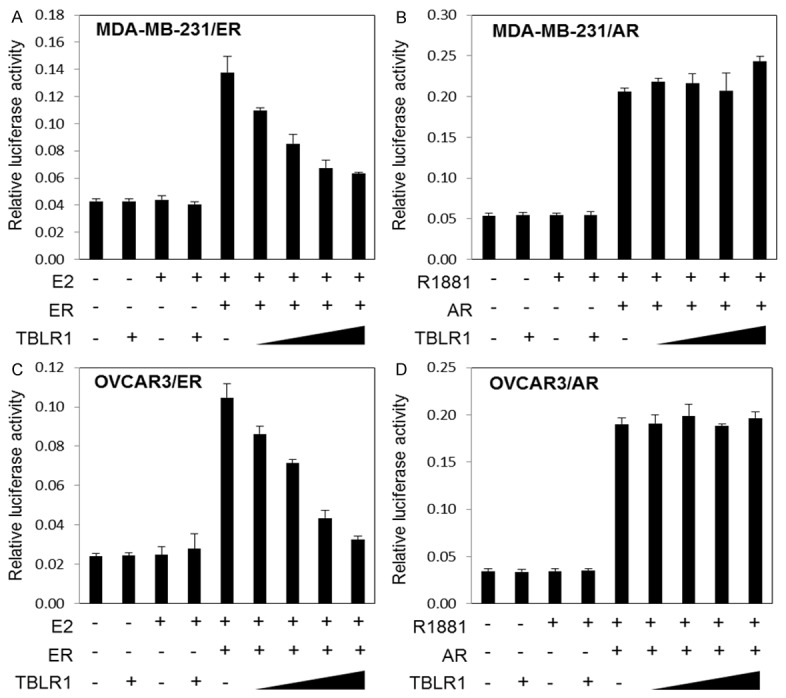
TBLR1 acts as an ER transcriptional corepressor in both breast and ovarian cancer cells. A. TBLR1 acts as a corepressor for ER in MDA-MB-231 breast cancer cells in a dose-dependent manner in the presence of 10 nM 17β-estradiol by dual luciferase assay with a 4X estrogen response element (ERE) luciferase reporter plasmid. B. Overexpression of TBLR1 showed no change in AR transcriptional activation in MDA-MB-231 breast cancer cells. C. TBLR1 acts as a corepressor for ER in OVCAR3 ovarian cancer cells in a dose-dependent manner in the presence of 10 nM 17β-estradiol by dual luciferase assay with a 4X estrogen response element (ERE) luciferase reporter plasmid. D. Overexpression of TBLR1 showed no change in AR transcriptional activation in OVCAR3 ovarian cancer cells. All experiments were performed in triplicate.
Nuclear TBLR1 promotes growth in breast and ovarian cancer cells
To examine the effect of nuclear TBLR1 expression in breast and ovarian cells, we transfected MCF7 or OVCAR3 cells with pcDNA3.1 NLS-TBLR1 plasmid, a TBLR1 plasmid fused with the strong nuclear localization sequence PKKKRKV. The nuclear localization of NLS-TBLR1 was shown in Supplementary Figure 3. When compared with control cells, MCF7 cells transfected with pcDNA NLS-TBLR1 exhibited increased rate of proliferation in both estrogen medium (P=0.0029) and hormone-free medium (P=0.0031) (Figure 3A and 3C). When ER level was decreased by ER specific siRNA in MCF7 cells, proliferation was decreased as compared with control cells (P=0.0015). However, transfection with NLSTBLR1 plasmid partially rescued the negative proliferative effect of ER-knockdown in MCF7 cells (P=0.0211) (Figure 3B). These results indicated that nuclear TBLR1 may affect proliferation of MCF7 cells in both ER-dependent and ER-independent manners. We also overexpressed nuclear TBLR1 in OVCAR3 cells. In OVCAR3 cells, nuclear TBLR1 also increased proliferation in both estrogen medium (P=0.0018) and hormone-free medium (P=0.0357) (Figure 3D and 3F). In addition, ER knockdown in OVCAR3 cells also reduced proliferation and overexpression of nuclear TBLR1 partially rescued the negative proliferative effects (P=0.0291) (Figure 3E). These results confirmed TBLR1 proliferative effects in breast and ovarian cancer cells to be both ER- dependent and ER-independent. Additionally, we tested proliferative effects of nuclear TBLR1 in benign MCF10A and T29 cells in free and estrogen medium and found nuclear TBLR1 increased proliferation in both cell lines and in both conditions as well (Supplementary Figure 2A, 2B, 2D, 2E).
Figure 3.
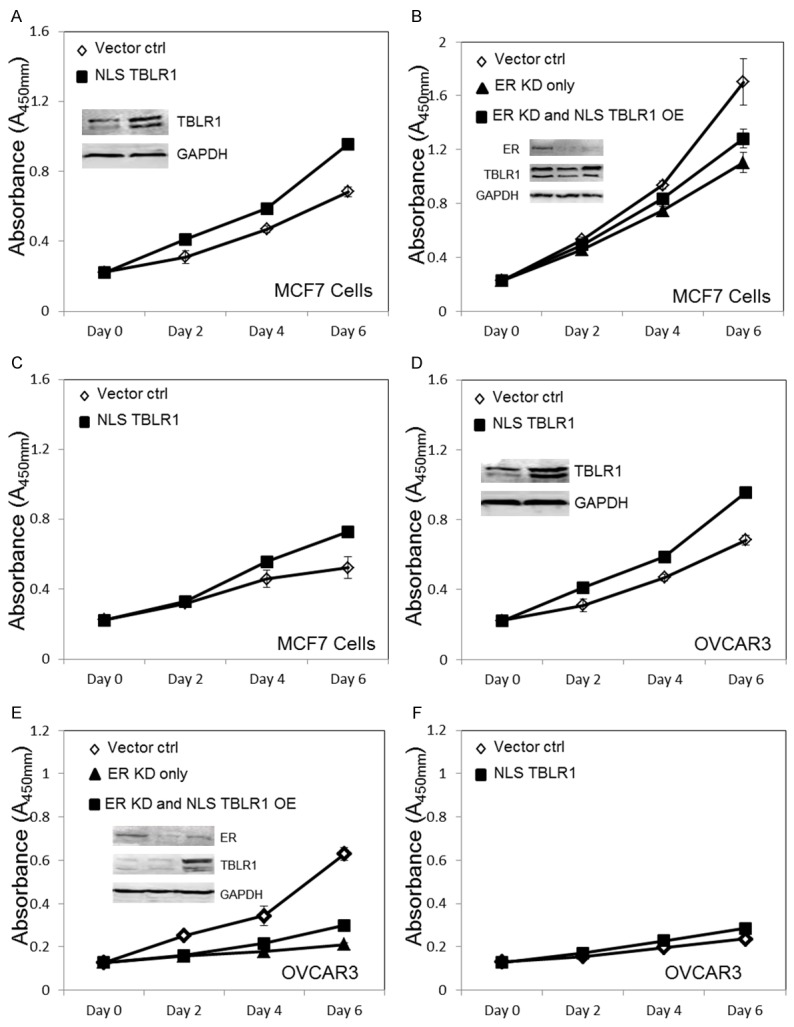
Nuclear TBLR1 effect on in vitro cell growth of breast and ovarian cancer cells. A. WST-1 cell proliferation assay comparing MCF7 cells with transiently overexpressed NLS-TBLR1 to MCF7 cells transfected with pcDNA control in 10 nM 17β estradiol media. The insert confirms TBLR1 overexpression by western blot, GAPDH is used as the loading control. B. WST-1 cell proliferation assay showing MCF7 cells transfected with control siRNA, ER specific siRNA, or ER siRNA + NLS-TBLR1 plasmid. The insert confirms ER knockdown and TBLR1 overexpression by western blot, GAPDH is used as the loading control. C. WST-1 cell proliferation assay comparing MCF7 cells with transiently overexpressed NLS-TBLR1 to MCF7 cells transfected with pcDNA control in hormone free media. D. WST-1 cell proliferation assay comparing OVCAR3 cells with transiently overexpressed NLS-TBLR1 to OVCAR3 cells transfected with pcDNA control in 10 nM 17β estradiol media. The insert confirms TBLR1 overexpression by western blot, GAPDH is used as the loading control. E. WST-1 cell proliferation assay showing OVCAR3 cells transfected with control siRNA, ER specific siRNA, or ER siRNA + NLS-TBLR1 plasmid. The insert confirms ER knockdown and TBLR1 overexpression by western blot, GAPDH is used as the loading control. F. WST-1 cell proliferation assay comparing OVCAR3 cells with transiently overexpressed NLS-TBLR1 to OVCAR3 cells transfected with pcDNA control in hormone free media.
Nuclear TBLR1 promotes migration and invasion in breast and ovarian cancer cells
We next examined the effect of nuclear TBLR1 on migration and invasion ability of breast cancer cells, utilizing a dual chamber invasion assay with FBS as a chemoattractant. The number of cells that traversed the lower membrane were counted after 16 hour incubation. After transfection of NLSTBLR1 in MCF7 cells, we observed increased migration and invasion compared to transfection control cells (Figure 4A-C). The calculated invasion index was 1.5. We also observed increased migration and invasion in the benign breast cell line MCF10A (Supplementary Figure 2C).
Figure 4.
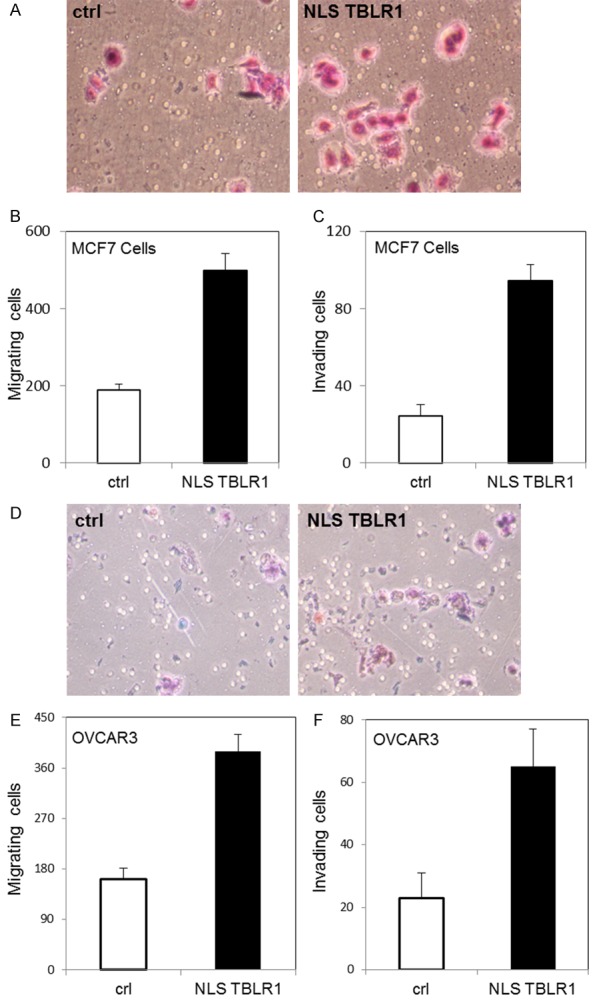
Nuclear TBLR1 effect on in vitro invasion of breast and ovarian cancer cells. A. Representative pictures of invasive MCF7 cells transfected with control plasmid (left) or NLSTBLR1 plasmid (right) by BD Matrigel invasion assay. B. Quantification of MCF7 cells with and without NLSTBLR1 transfection migrating across a membrane without Matrigel insert to measure migration ability. C. Quantification of MCF7 cells with and without NLSTBLR1 transfection migrating across a membrane containing Matrigel insert to measure invasive ability. D. Representative pictures of invasive OVCAR3 cells transfected with control plasmid (left) or NLSTBLR1 plasmid (right) by BD Matrigel invasion assay. E. Quantification of OVCAR3 cells with and without NLSTBLR1 transfection migrating across a membrane without Matrigel insert to measure migration ability. F. Quantification of OVCAR3 cells with and without NLSTBLR1 transfection migrating across a membrane containing Matrigel insert to measure invasive ability.
Additionally, we examined the effect of nuclear TBLR1 on migration and invasion ability on ovarian cancer cells. We overexpressed nuclear TBLR1 in OVCAR3 cells and assayed for changes in migration and invasion ability. We also observed a significant increase in migration and invasion in OVCAR3 cells with transfection of NLSTBLR1 (Figure 4D-F), with an invasion index of 1.2. We also observed the same effects on migration and invasion in the benign ovarian cell line T29 (Supplementary Figure 2F).
Discussion
TBLR1 has been shown to be increased in breast cancers compared to benign breast tissue [13]. However, the study did not define whether the increased TBLR1 is nuclear or cytoplasmic protein and TBLR1 may express different function based on cellular localizations [13]. In this study, we first examined the expression of TBLR1 protein and its subcellular localization in both breast and ovarian cell lines using western blot on cell fractionation and tissue by immunohistochemistry. All the cell lines examined express both nuclear and cytoplasmic TBLR1. TBLR1 was predominantly located in the nucleus in breast cancer and malignant glands expressed higher level of nuclear TBLR1 than the adjacent benign glands in 50% of cases although there was no correlation between nuclear TBLR1 and other clinicopathological parameters such as age at diagnosis, tumor size, and ER or Her2 status. Interestingly, the level of TBLR1 was higher in high-grade serous ovarian carcinoma (HGSOC) and clear cell carcinoma than in serous borderline tumors and mucinous carcinoma (Figure 1F). These findings suggest that nuclear TBLR1 may play a role in the development and progression of ovarian carcinomas and that TBLR1 may have prognostic values in ovarian cancers. The high levels of nuclear TBLR1 expression correlates well with our functional study results in breast and ovarian cancer.
Additionally, we studied the effects of TBLR1 on both ER and AR mediated transcriptional activation in both breast and ovarian cell lines using a dual reporter luciferase assay driven by ERE transcriptional elements. Our results showed that TBLR1 inhibited the transcription activation mediated by ER in a dose dependent manner in both breast and ovarian cell lines. Although in prostate cancer, TBLR1 acts as a coactivator for AR, TBLR1 had no effects on AR mediated transcriptional activation in breast and ovarian cancer cells by in vitro luciferase assays.
Ectopic expression of nuclear TBLR1 in malignant cell lines reveals that nuclear TBLR1 promotes the cell proliferation, migration, and invasion of breast and ovarian cells. Similar results were also observed in benign immortalized cell lines. The growth promotion process has both estrogen independent and dependent components. Nuclear TBLR1 facilitates increased cell growth in hormone free media. In the presence of estrogen, it also accelerates cell growth and this process is reversible upon ER knock down using siRNAs in ER positive cell lines, indicating at least partial ER-dependence of TBLR1 action. Interestingly, nuclear TBLR1 functions as an AR coactivator in prostate and suppresses prostate cancer cell growth, while it serves as an ER corepressor in breast and ovarian cells to promote cancer cell proliferation. Thus the oncogenic or tumor suppressive function of nuclear TBLR1 is dependent on both cell types and hormone receptor affected. Of note, there is a high level of nuclear TBLR1 expression in MCF10A, however ER expression is negative in this cell line, indicating the distinct function of nuclear TBLR1 in conjunction with ER despite its ER independent function. The mechanism of action of TBLR1 as an ER corepressor to promote cancer cell growth is of great interest and importance and will need to be examined in future studies. It is possible that TBLR1 inhibits ER target genes that regulate cell differentiation and tumor suppression.
In conclusion, our study shows that nuclear TBLR1 is significantly upregulated in breast and ovarian cancers. It serves as an ER corepressor in both breast and ovarian cells and functions as an oncogene by promoting cell proliferation, migration and invasion and the oncogenic roles of nuclear TBLR1 are partially dependent through ER. Further studies on the mechanism of TBLR1 function in breast and ovarian cancer will advance our understanding of mechanisms of tumor growth regulation and assist in the development of effective targeted therapies.
Acknowledgements
This material is supported in part by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development (Biomedical Laboratory Research and Development). The NYU Experimental Pathology Immunohistochemistry Core Laboratory is supported in part by the Laura and Isaac Perlmutter Cancer Center Support Grant (NIH/NCI P30CA016087) and the National Institutes of Health S10 Grants (NIH/ORIP S10OD01058 and S10OD018338).
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Burke LJ, Baniahmad A. Co-repressors 2000. FASEB J. 2000;14:1876–1888. doi: 10.1096/fj.99-0943rev. [DOI] [PubMed] [Google Scholar]
- 2.Tomita A, Buchholz DR, Shi YB. Recruitment of N-CoR/SMRT-TBLR1 corepressor complex by unliganded thyroid hormone receptor for gene repression during frog development. Mol Cell Biol. 2004;24:3337–3346. doi: 10.1128/MCB.24.8.3337-3346.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang XM, Chang Q, Zeng L, Gu J, Brown S, Basch RS. TBLR1 regulates the expression of nuclear hormone receptor co-repressors. BMC Cell Biol. 2006;7:31. doi: 10.1186/1471-2121-7-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Perissi V, Aggarwal A, Glass CK, Rose DW, Rosenfeld MG. A corepressor/coactivator exchange complex required for transcriptional activation by nuclear receptors and other regulated transcription factors. Cell. 2004;116:511–526. doi: 10.1016/s0092-8674(04)00133-3. [DOI] [PubMed] [Google Scholar]
- 5.Choi HK, Choi KC, Yoo JY, Song M, Ko SJ, Kim CH, Ahn JH, Chun KH, Yook JI, Yoon HG. Reversible SUMOylation of TBL1-TBLR1 regulates beta-catenin-mediated Wnt signaling. Mol Cell. 2011;43:203–216. doi: 10.1016/j.molcel.2011.05.027. [DOI] [PubMed] [Google Scholar]
- 6.Yoon HG, Choi Y, Cole PA, Wong J. Reading and function of a histone code involved in targeting corepressor complexes for repression. Mol Cell Biol. 2005;25:324–335. doi: 10.1128/MCB.25.1.324-335.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Daniels G, Li Y, Gellert LL, Zhou A, Melamed J, Wu X, Zhang X, Zhang D, Meruelo D, Logan SK, Basch R, Lee P. TBLR1 as an androgen receptor (AR) coactivator selectively activates AR target genes to inhibit prostate cancer growth. Endocr Relat Cancer. 2014;21:127–142. doi: 10.1530/ERC-13-0293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Qin L, Liu Z, Chen H, Xu J. The steroid receptor coactivator-1 regulates twist expression and promotes breast cancer metastasis. Cancer Res. 2009;69:3819–3827. doi: 10.1158/0008-5472.CAN-08-4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O'Malley BW, Kumar R. Nuclear receptor coregulators in cancer biology. Cancer Res. 2009;69:8217–8222. doi: 10.1158/0008-5472.CAN-09-2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chakravarty D, Roy SS, Babu CR, Dandamudi R, Curiel TJ, Vivas-Mejia P, Lopez-Berestein G, Sood AK, Vadlamudi RK. Therapeutic targeting of PELP1 prevents ovarian cancer growth and metastasis. Clin Cancer Res. 2011;17:2250–2259. doi: 10.1158/1078-0432.CCR-10-2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yoon HG, Chan DW, Huang ZQ, Li J, Fondell JD, Qin J, Wong J. Purification and functional characterization of the human N-CoR complex: the roles of HDAC3, TBL1 and TBLR1. EMBO J. 2003;22:1336–1346. doi: 10.1093/emboj/cdg120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Daniels G, Zhang X, Zhong X, Santiago L, Wang LH, Wu X, Zhang JY, Liang F, Li X, Neubert TA, Steinke L, Shen Y, Basch R, Schneider R, Levy DE, Lee P. Cytoplasmic, full length and novel cleaved variant, TBLR1 reduces apoptosis in prostate cancer under androgen deprivation. Oncotarget. 2016;7:39556–39571. doi: 10.18632/oncotarget.9005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li X, Liang W, Liu J, Lin C, Wu S, Song L, Yuan Z. Transducin (beta)-like 1 X-linked receptor 1 promotes proliferation and tumorigenicity in human breast cancer via activation of beta-catenin signaling. Breast Cancer Res. 2014;16:465. doi: 10.1186/s13058-014-0465-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cardillo MR, Petrangeli E, Aliotta N, Salvatori L, Ravenna L, Chang C, Castagna G. Androgen receptors in ovarian tumors: correlation with oestrogen and progesterone receptors in an immunohistochemical and semiquantitative image analysis study. J Exp Clin Cancer Res. 1998;17:231–237. [PubMed] [Google Scholar]
- 15.Edmondson RJ, Monaghan JM, Davies BR. The human ovarian surface epithelium is an androgen responsive tissue. Br J Cancer. 2002;86:879–885. doi: 10.1038/sj.bjc.6600154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lehmann BD, Bauer JA, Chen X, Sanders ME, Chakravarthy AB, Shyr Y, Pietenpol JA. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest. 2011;121:2750–2767. doi: 10.1172/JCI45014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Collins LC, Cole KS, Marotti JD, Hu R, Schnitt SJ, Tamimi RM. Androgen receptor expression in breast cancer in relation to molecular phenotype: results from the Nurses’ Health Study. Mod Pathol. 2011;24:924–931. doi: 10.1038/modpathol.2011.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guedj M, Marisa L, de Reynies A, Orsetti B, Schiappa R, Bibeau F, MacGrogan G, Lerebours F, Finetti P, Longy M, Bertheau P, Bertrand F, Bonnet F, Martin AL, Feugeas JP, Bieche I, Lehmann-Che J, Lidereau R, Birnbaum D, Bertucci F, de The H, Theillet C. A refined molecular taxonomy of breast cancer. Oncogene. 2012;31:1196–1206. doi: 10.1038/onc.2011.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li Y, Wang L, Zhang M, Melamed J, Liu X, Reiter R, Wei J, Peng Y, Zou X, Pellicer A, Garabedian MJ, Ferrari A, Lee P. LEF1 in androgen-independent prostate cancer: regulation of androgen receptor expression, prostate cancer growth, and invasion. Cancer Res. 2009;69:3332–3338. doi: 10.1158/0008-5472.CAN-08-3380. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


