Abstract Abstract
Karyotypes of two species of the African annual killifish genus Nothobranchius Peters, 1868, Nothobranchius brieni Poll, 1938 and Nothobranchius sp. from Kasenga (D.R. Congo) are described. Both species displayed diploid chromosome number 2n = 49/50 for males and females respectively with multiple-sex chromosome system type X1X2Y/X1X1X2X2. The karyotypes of studied species are considerably different from those previously reported for the genus Nothobranchius and similar to the Actinopterygii conservative karyotype.
Keywords: Africa, chromosome number, karyotype, killifish, Nothobranchius
Introduction
Annual killifishes belonging to the genus Nothobranchius Peters, 1868 are mainly distributed in eastern Africa but several species are found in central Africa (Wildekamp 2004). They inhabit temporary pools that dry out during the dry season and have specific adaptations for extreme environments. Annual fishes are characterised by specific life history traits of extremely short lifespan and diapause in embryonic development (Furness 2015, Nagy 2015). Their unique biology makes them a model taxon with which to investigate aging, embryonic development, ecology, and natural selection (Cellerino et al. 2015).
Killifishes of the genus Nothobranchius comprise 71 valid species (FishBase 2015). In this genus karyologicaly were described only 23 species (Arai 2011). These species have variable karyotypes with diploid chromosome numbers (2n) ranging from 2n = 16 for Nothobranchius rachovii Ahl, 1926 to 2n = 43 for Nothobranchius thierryi (Ahl, 1924) (Scheel 1990). More than 60% of karyotypes in Nothobranchius are characterised by a modal diploid number of 2n = 36-38.
A multiple-sex chromosome system of X1X1X2X2/X1X2Y type has been reported for only one species of Nothobranchius, Nothobranchius guentheri (Pfeffer, 1893) with a female karyotype consisting of 36 chromosomes and the male karyotype consisting of 35 chromosomes (Ewulonu et al. 1985).
In this paper, the karyotypes of two species, Nothobranchius brieni Poll, 1938 and Nothobranchius sp. from Kasenga, were studied, bringing the number of species studied to 25.
Material and methods
Specimens of Nothobranchius brieni were collected from a large ephemeral swamp in the Lualaba drainage, near the village of Bukama in Katanga province (Democratic Republic of Congo, 09°11.374'S 25°51.334'E) on 2 April 2013 by E. Abwe, B. Katemo Manda, and B. Nagy, whereas specimens of Nothobranchius sp. from Kasenga (Nothobranchius sp. ‘Kasenga’) were collected in an ephemeral swamp in the Luapula drainage, near Kasenga, a village in Katanga province (D.R. Congo, 10°31.360'S, 28°27.368'E) on 17 April 2015, by E. Abwe, A. Chocha Manda, B. Katemo Manda, and T. Popp (Fig. 1).
Figure 1.
Localities of specimen collections in the Democratic Republic of Congo (1 Nothobranchius brieni 2 Nothobranchius sp. ‘Kasenga’).
Cytogenetic analysis
Chromosomes were prepared according to the Kligerman and Bloom method (1974). The chromosome preparations were obtained from head kidney tissue. Before preparation fish were treated intraperitoneally with 0.1% colchicine for 3–4 hours. The hypotonisation lasted 20–30 min at room temperature in 0.075 M KCl. Then tissue samples were fixed in 3:1 methanol : acetic acid for 24 hours. Six specimens of Nothobranchius brieni (three males and three females) and three specimens of Nothobranchius sp. ‘Kasenga’ (one male and three females) were karyotyped with this method. Meiotic chromosome preparations of Nothobranchius brieni were acquired from testes by the same technique.
Slides were dried by air and stained with 2% Giemsa solution in phosphate buffer at pH 6.8 for 10 min. Karyotypes were analysed under microscope “AxioImager” Karl Zeiss (Germany) equipped with CCD camera and “KaryoImage” Metasystems Software (Germany). In each specimen the chromosome number and type was determined on metaphase plate. Chromosome morphology was determined according to Levan et al. (1964). The chromosomes were classified as (M), (SM), and (A). To determine the (NF), chromosomes of the M and SM groups were considered bi-armed and those of group A as uni-armed.
Results
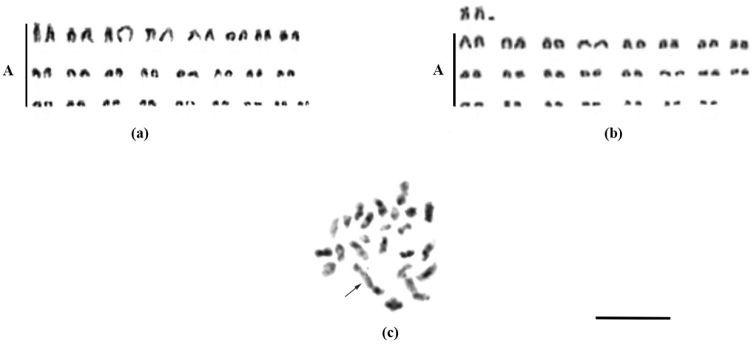
The diploid chromosome numbers of Nothobranchius brieni were 2n = 49 for males and 2n = 50 for females with NF = 50/50 respectively. The female karyotype consisted of 25 pairs of acrocentric chromosomes gradually decreasing in size (Fig. 2a). The male karyotype consisted of 23 pairs of acrocentric chromosome and one bi-armed pair and two unpaired acrocentric chromosomes (Fig. 2b). In the first meiotic chromosomes during spermatogenesis 23 bivalents and a trivalent were observed at diakinesis (Fig. 2c).
Figure 2.
Karyotypes of Nothobranchius brieni a somatic chromosomes of female b somatic chromosomes of male c meiotic metaphase I (testicular). (A). Note trivalent chromosome (arrowed). Scale bar: 10 µm.
The karyotype Nothobranchius sp. ‘Kasenga’ had diploid number 2n = 49 for males and 2n = 50 for females with NF = 68/68 respectively. The female karyotype had two pairs of metacentric, seven pairs of sub-metacentric, and 16 pair of acrocentric chromosomes varying in size from large to small (Fig. 3a). The male karyotype had 23 pair of chromosomes similar to the female with one bi-armed and two unpaired acrocentric chromosomes (Fig. 3b).
Figure 3.
Karyotypes of Nothobranchius sp. ‘Kasenga’ a somatic chromosomes of female b somatic chromosomes of male (M, SM, A). Scale bar: 10 µm.
Discussion
Cytogenetic characteristics
The described karyotypes stand apart from those already reported for species of genus Nothobranchius. The karyotype of Nothobranchius brieni has the chromosomal number 2n = 49/50 and 25 pairs of uni-armed chromosomes in female (50A) and 23 pairs of uni-armed homomorphic and three heteromorphic chromosomes in male (1M + 48A). The karyotype of Nothobranchius sp. ‘Kasenga’ has the same diploid number 2n = 49/50 but a different karyotype structure possessing metacentric, sub-metacentric, and uni-armed chromosomes with 4M + 14SM + 32A for females and 5M + 14SM + 30A for males, while other species of the genus have a considerably lower modal diploid number of only 36 chromosomes (Table 1).
Table 1.
The diploid number (2n) of Nothobranchius species (from Arai 2011 with modifications). *sex chromosome system of the type X1X2Y/X1X1X2X2.
| Species | 2n | References |
|---|---|---|
| Nothobranchius brieni Poll, 1938* | 49♂/50♀ | Current study |
| Nothobranchius eggersi Seegers, 1982 | 36 | Scheel 1990 |
| Nothobranchius elongatus Wildekamp, 1982 | 38 | Scheel 1990 |
| Nothobranchius foerschi Wildekamp & Berkenkamp, 1979 | 34 | Ewulonu et al. 1985, Scheel 1990 |
| Nothobranchius furzeri Jubb, 1971 | 38 | Reichwald et al. 2009 |
| Nothobranchius guentheri (Pfeffer, 1893)* | 35♂/36♀ | Ewulonu et al. 1985, Scheel 1990 |
| Nothobranchius hengstleri Valdesalici, 2007 | 38 | Wildekamp et al. 2009 |
| Nothobranchius janpapi Wildekamp, 1977 | 38 | Scheel 1990 |
| Nothobranchius jubbi Wildekamp & Berkenkamp, 1979 | 34 | Scheel 1990 |
| Nothobranchius kirki Jubb, 1969 | 36 | Scheel 1990 |
| Nothobranchius korthausae Meinken, 1973 | 36 | Scheel 1990 |
| Nothobranchius krysanovi Shidlovskiy, Watters & Wildekamp, 2010 | 18 | Shidlovskiy et al. 2010 |
| Nothobranchius kuhntae (Ahl, 1926) | 38 | Scheel 1990 |
| Nothobranchius lucius Shidlovskiy, Watters & Wildekamp, 2010 | 36 | Wildekamp et al. 2009 |
| Nothobranchius makondorum Shidlovskiy, Watters & Wildekamp, 2010 | 38 | Wildekamp et al. 2009 |
| Nothobranchius melanospilus (Pfeffer, 1896) | 38 | Ewulonu et al. 1985 |
| Nothobranchius microlepis (Vinciguerra, 1897) | 24 | Scheel 1990 |
| Nothobranchius palmqvisti (Lönnberg, 1907) | 36 | Ewulonu et al. 1985, Scheel 1990 |
| Nothobranchius polli Wildekamp, 1978 | 36 | Ewulonu et al. 1985 |
| Nothobranchius patrizii (Vinciguerra, 1897) | 36 | Ewulonu et al. 1985 |
| Nothobranchius pienaari Shidlovskiy, Watters & Wildekamp, 2010 | 34 | Shidlovskiy et al. 2010 |
| Nothobranchius rachovii Ahl, 1926 | 16 | Ewulonu et al. 1985, Krysanov 1992 |
| Nothobranchius steinforti Wildekamp, 1977 | 36 | Scheel 1990 |
| Nothobranchius thierryi (Ahl, 1924) | 43 | Scheel 1990 |
| Nothobranchius sp. ‘Kasenga’* | 49♂/50♀ | Current study |
Sex chromosomes
The reduced diploid numbers and heteromorphic chromosomes in males suggest the occurrence of a multiple-sex chromosome system. A trivalent observation in the first meiotic chromosomes in Nothobranchius brieni and the presence of a bi-armed chromosome exclusively in the male karyotype indicate a multiple-sex chromosome system of the type X1X2Y/X1X1X2X2. One bi-armed neo-Y chromosome has most likely resulted from the Robertsonian fusion between the Y chromosome and an autosome, as has been described for other fish species (e.g., Kitano and Peichel 2012). In Nothobranchius brieni and Nothobranchius sp. ‘Kasenga’ the Y chromosomes is a large metacentric one, and X1 and X2 chromosomes are acrocentric of different sizes. The same-sex chromosome system has been reported only for Nothobranchius guentheri (Ewulonu et al. 1985) among the 23 previously karyotyped species.
Karyotype evolution
In the genus Nothobranchius and the related Aphyosemyon Mayers, 1924 the evolutionary trend to reduce the total number of chromosomes via acrocentric chromosome fusion was specified (Scheel 1990, Völker et al. 2005). This assumption has been confirmed by the data presented in Table 1. According to this hypothesis, basal taxa have higher chromosome numbers and more acrocentric chromosomes while derived taxa have lower numbers of chromosomes with metacentric chromosomes (Agnese et al. 2006). It is widely accepted that the hypothetical ancestral karyotype of teleostean fishes consisted of 2n = 48-50 acrocentric chromosomes (Ohno et al. 1969, Nakatani et al. 2007). The two species presented in this study have numbers and a structure of karyotype conservative for Actinopterygii fishes (Mank and Avise 2006, Molina et al. 2014). It is supposed that karyotype of Nothobranchius brieni is similar to that of the hypothetical ancestor of the genus Nothobranchius. There is a lack of molecular genetic data on this species, therefore we are not able to consider its phylogenetic position within the clade.
Acknowledgments
This research work was supported by RFBR (Russian Foundation for Basic Research) #16-04-01102.
Citation
Krysanov E, Demidova T, Nagy B (2016) Divergent karyotypes of the annual killifish genus Nothobranchius (Cyprinodontiformes; Nothobranchiidae). Comparative Cytogenetics 10(3): 439–445. doi: 10.3897/CompCytogen.v10i3.9863
References
- Agnèse JF, Zentz F, Legros O, Sellos D. (2006) Phylogenetic relationships and phylogeography of the Killifish species of the subgenus Chromaphyosemion (Radda, 1971) in West Africa, inferred from mitochondrial DNA sequences. Molecular Phylogenetics and Evolution 40(2): 332–346. doi: 10.1016/j.ympev.2006.03.018 [DOI] [PubMed] [Google Scholar]
- Arai R. (2011) Fish Karyotypes – A Check List. Springer, 340 pp. doi: 10.1007/978-4-431-53877-6 [Google Scholar]
- Cellerino A, Valenzano DR, Reichard M. (2015) From the bush to the bench: the annual Nothobranchius fishes as a new model system in biology. Biological Reviews 34(2): 511–533. doi: 10.1111/brv.12183 [DOI] [PubMed] [Google Scholar]
- Dorn A, Musilova Z, Platzer M, Reichwald K, Cellerino A. (2014) The strange case of East African annual fish: aridification correlates with diversification for a savannah aquatic group? BMC Evolutionary Biology 14: . doi: 10.1186/s12862-014-0210-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewulonu UV, Haas R, Turner BJ. (1985) A multiple sex chromosome system in the annual killifish, Nothobranchius guentheri. Copeia 2: 503–508. doi: 10.2307/1444868 [Google Scholar]
- FishBase (2015) FishBase. Avalible at http://www.fishbase.org
- Furness AI. (2015) The evolution of an annual life cycle in killifish: adaptation to ephemeral aquatic environments through embryonic diapause. Biological reviews of the Cambridge Philosophical Society 91(3): 796–812. doi: 10.1111/brv.12194 [DOI] [PubMed] [Google Scholar]
- Kitano J, Peichel C. (2012) Turnover of sex chromosomes and speciation in fishes. Environmental Biology of Fishes 94: 549–558. doi: 10.1007/s10641-011-9853-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kligerman AD, Bloom SE. (1977) Rapid chromosome preparations from solid tissues of fishes. Journal of the Fisheries Research Board of Canada 34(2): 266–269. doi: 10.1139/f77-039 [Google Scholar]
- Krysanov EYu. (1992) Aneuploidy in postnatal ontogenesis of fishes. Acta Zoologica Fennica 191: 177–182. [Google Scholar]
- Levan A, Fredga K, Sandberg A. (1964) Nomenclature for centromeric position on chromosomes. Hereditas 52: 201–220. doi: 10.1111/j.1601-5223.1964.tb01953.x [Google Scholar]
- Nagy B. (2015) Life history and reproduction of Nothobranchius fishes. Journal of the American Killifish Association 47(4/6): 182–192. [Google Scholar]
- Nakatani Y, Takeda H, Kohara Y, Morishita S. (2007) Reconstruction of the vertebrate ancestral genome reveals dynamic genome reorganization in early vertebrates. Genome Research 17(9): 1254–1265. doi: 10.1101/gr.6316407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno S, Muramoto J, Klein J, Atkin NB. (1969) Diploid-tetraploid relationship in clupeoid and salmon fish. Chromozómes Today 2: 139–147. [Google Scholar]
- Scheel JJ. (1990) Atlas of killifishes of the Old World. TFH. Publications, Neptune, 448 pp. [Google Scholar]
- Shidlowsky KM, Watters BR, Wildekamp RH. (2010) Notes on the annual killifish species Nothobranchius rachovii (Cyprinodontiformes; Nothobranchiidae) with the description of two new species. Zootaxa 2724: 37–57. [Google Scholar]
- Völker M, Ráb P, Kullmann H. (2005) Karyotype differentiation in Chromaphyosemion killifishes (Cyprinodontiformes, Nothobranchiidae). I: Chromosome banding patterns of C. alpha, C. kouamense and C. lugens. Genetica 125(1): 33–41. doi: 10.1111/j.1095-8312.2008.00967.x [DOI] [PubMed] [Google Scholar]
- Wildekamp RH, Shidlovskiy KM, Watters BR. (2009) Systematics of the Nothobranchius melanospilus species group (Cyprinodontiformes: Nothobranchiidae) with description of two new species from Tanzania and Mozambique. Ichthyological Exploration of Freshwaters 20: 237–254. [Google Scholar]
- Wildekamp RH. (2004) A world of killies – Atlas of the oviparous cyprinodontiform fishes of the world (Vol. 4). The American Killifish Association, Elyria, Ohio, 398 pp. [Google Scholar]