Abstract
Obesity is a major risk factor for cardiovascular disease in males and females. Whether obesity triggers cardiovascular disease via similar mechanisms in both the sexes is, however, unknown. In males, the adipokine leptin highly contributes to obesity-related cardiovascular disease by increasing sympathetic activity. Females secrete 3× to 4× more leptin than males, but do not exhibit high sympathetic tone with obesity. Nevertheless, females show inappropriately high aldosterone levels that positively correlate with adiposity and blood pressure (BP). We hypothesized that leptin induces hypertension and endothelial dysfunction via aldosterone-dependent mechanisms in females. Leptin control of the cardiovascular function was analyzed in female mice sensitized to leptin via the deletion of protein tyrosine phosphatase 1b (knockout) and in agouti yellow obese hyperleptinemic mice (Ay). Hypersensitivity to leptin (wild-type, 115±2; protein tyrosine phosphatase 1b knockout, 124±2 mm Hg; P<0.05) and obesity elevated BP (a/a, 113±1; Ay, 128±7 mm Hg; P<0.05) and impaired endothelial function. Chronic leptin receptor antagonism restored BP and endothelial function in protein tyrosine phosphatase 1b knockout and Ay mice. Hypersensitivity to leptin and obesity reduced BP response to ganglionic blockade in both strains and plasma catecholamine levels in protein tyrosine phosphatase 1b knockout mice. Hypersensitivity to leptin and obesity significantly increased plasma aldosterone levels and adrenal CYP11B2 expression. Chronic leptin receptor antagonism reduced aldosterone levels. Furthermore, chronic leptin and mineralocorticoid receptor blockade reduced BP and improved endothelial function in both leptin-sensitized and obese hyperleptinemic female mice. Together, these data demonstrate that leptin induces hypertension and endothelial dysfunction via aldosterone-dependent mechanisms in female mice and suggest that obesity leads to cardiovascular disease via sex-specific mechanisms.
Keywords: hypertension, leptin, mineralocorticoid receptor, obesity, sex
Obesity, which affects more women than men in the United States,1,2 is a major risk factor for cardiovascular disease (CVD), including hypertension and endothelial dysfunction. Notably, obesity has recently been identified as the cause of the 3-fold increase in the risk for hypertension in premenopausal women3,4 and of the rising number of school girls diagnosed with hypertension5,6 observed during the last 2 decades. Despite these alarming statistics, and the fact that similar increases in body weight induce larger elevations in blood pressure (BP) in premenopausal women compared with men,7 the mechanisms whereby body weight gain leads to hypertension and endothelial dysfunction remain unknown, in females.
A significant body of evidence demonstrates that the adipocyte-derived hormone leptin greatly contributes to the development of obesity-associated hypertension and endothelial dysfunction by increasing sympathetic activity, in males.8–13 Healthy premenopausal women secrete 3× to 4× more leptin than men,14–16 but have lower sympathetic tone than men of the same age.17–19 In response to obesity, women exhibit exaggerated increases in leptin levels compared with men, again with a mild16,20 to no increase in sympathetic tone.16,21–23 In addition, reduction in body weight decreases sympathetic activity in males only.16 These observations, which tend to minimize the contribution of the sympathetic tone as a source of hypertension in females, raise the question of mechanisms whereby obesity induces CVD in females.
Although our group recently discovered that leptin is a new direct regulator of adrenal aldosterone synthase (CYP11B2) expression and an inducer of aldosterone production,24 obesity has been identified as a source of inappropriately high aldosterone levels. Aldosterone levels have been reported to correlate with the degree of adiposity and hypertension in obese women but not in men.25–27 In addition, antagonism of aldosterone action via mineralocorticoid receptor (MR) blockade is more effective at preventing CVD in women compared with men.25–27 On the basis of these observations, we hypothesized that leptin induces hypertension and endothelial dysfunction via aldosterone-dependent mechanisms in lean and obese female mice. This hypothesis was first tested in protein tyrosine phosphatase 1b (Ptp1b) knockout (KO) mice. The Ptp1b KO mouse is a lean mouse well described for its increased leptin sensitivity,11,28,29 which allows the study of the physiological role of leptin independently of the confounding factors of obesity.11 The relevance of the data gathered with the Pt1b KO mice, to obesity, was investigated with the agouti yellow obese mice (Ay). The ectopic expression of the agouti peptide in Ay mice induces a hyperphagia promoting the development of obesity, raising leptin levels, and making these mice a good model to study the human pathology.30
Methods
Animal Models
Ptp1b null mice (KO) provided by Dr Michel Tremblay (Goodman Cancer Center of McGill University)11,28,31 were bred in our laboratory and compared with their wild-type control. Lean (a/a, stock no. 2168) and agouti yellow obese (Ay, stock no. 2468) mice on a KK background were purchased from Jackson laboratory (Bar Harbor, ME). Experiments were conducted in littermate male and female mice of 10 to 12 weeks of age. All animals were fed standard mouse chow (0.2% sodium), and tap water was provided ad libitum. Mice were housed in an American Association of Laboratory Animal Care—approved animal care facility at the Medical College of Georgia. Experiments were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and the Institutional Animal Care and Use Committee approved all protocols.
Leptin Sensitivity and Cardiovascular Phenotype
A detailed description of the methods used is available in the online-only Data Supplement.
Statistics
All data are presented as means±SEM. P values <0.05 were considered significant. Differences in means among groups for nonrepeated variables were compared by t test when normality was verified by a Kolmogorov–Smirnov test. The nonparametric Mann–Whitney test was used in the absence of normality. One-way ANOVA and Kruskal–Wallis test were used to assess significance of multiple data sets for parametric and nonparametric data, respectively. Differences in means among groups and treatments, with repeated variables, were compared by 2-way ANOVA with repeated measures, when appropriate. Tukey test was used as the post hoc test in ANOVA (GraphPad).
Results
General Physiological Parameters
As reported in Table, deletion of Ptp1b induced a slight increase in body weight, but no changes in plasma leptin levels, in female mice. Leptin sensitization with Ptp1b deletion was confirmed by demonstrating that female Ptp1b KO mice exhibit a larger decrease in body weight in response to leptin infusion compared with wild-type females (Figure S1). Female agouti yellow (Ay) mice are obese and exhibit a 4-fold increase in plasma leptin levels (Table).
Table.
General Physiological Parameters in Ptp1b and Agouti Yellow Obese Mice
| Mouse Strain | ||||
|---|---|---|---|---|
| WT | Ptp1b KO | a/a | Ay | |
| Weight, g | 25.1±0.7 | 27.2±0.4* | 29.7±0.7 | 39.4±0.5* |
| Leptin, pg/mL | 238±76 | 164±27 | 879±13 | 3717±26* |
Data are presented as mean±SEM. n=6 to 8 in WT and Ptp1b KO mice. n=3 in lean (a/a) and agouti yellow obese mice (Ay). KO indicates knockout; Ptp1b, protein tyrosine phosphatase 1b; and WT, wild-type.
P<0.05 vs respective control group.
Leptin Sensitization and Obesity Increase BP in Female Mice
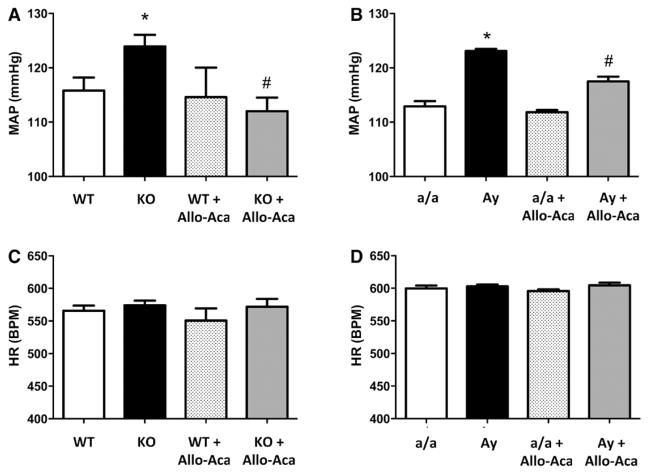
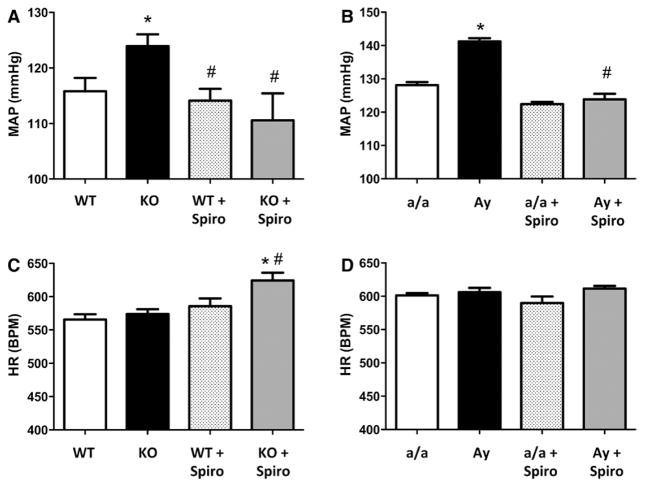
To determine whether leptin sensitization with Ptp1b deletion or high leptin levels in Ay mice affect BP control, BP was measured in conscious mice via telemetry. As reported in Figure 1, both Ptp1b deletion (Figure 1A) and Ay (Figure 1B) induced a significant increase in mean arterial pressure, but did not elevate heart rate (Figure 1C and 1D). Consistent with the literature,11,30,32 Ptp1b deletion and Ay increased MAP in male mice (Figure S2). Seven days of chronic treatment with the highly specific leptin receptor antagonist Allo-Aca restored BP to its baseline level, in female Ptp1b KO mice, and significantly reduced it in female Ay mice. This suggests that Ptp1b deletion and obesity increase BP via leptin-dependent mechanisms in female mice.
Figure 1.
Leptin sensitization and obesity increase blood pressure in female mice. Mean arterial pressure (MAP, A and B) and heat rate (HR, C and D) recorded in wild-type (WT) and protein tyrosine phosphatase 1b knockout (KO) mice as well as in lean (a/a) and agouti yellow obese (Ay) female mice treated or not with the leptin receptor antagonist Allo-Aca. Data are mean±SEM; n=6 to 8 for WT and KO mice and n=3 for a/a and Ay mice. *P<0.05 vs WT or a/a, #P<0.05 vs KO or Ay.
Leptin Sensitization and Obesity Induce Endothelial Dysfunction in Female Mice
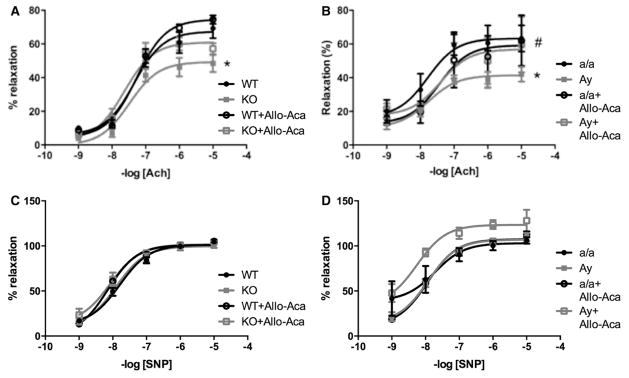
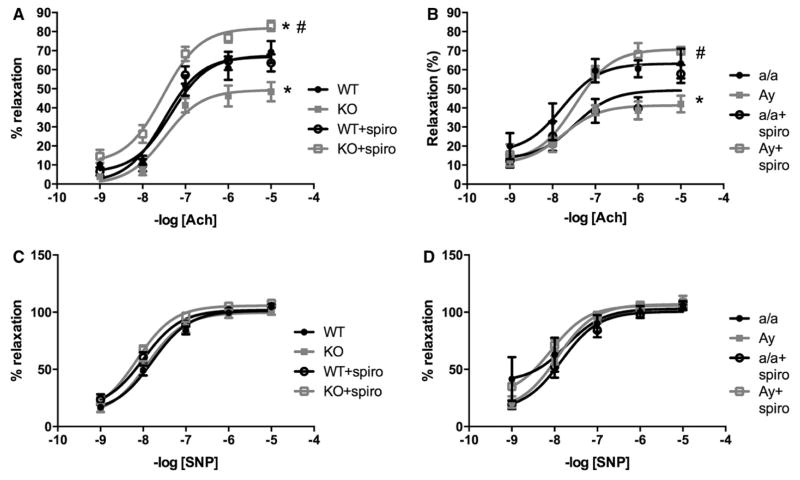
Endothelial dysfunction is a precursor and major consequence of CVD. As reported in Figure 2, leptin sensitization in Ptp1b KO mice reduced acetylcholine-induced relaxation (Figure 2A) but preserved sodium nitroprusside–mediated dilatation (Figure 2C), supporting a dysfunction at the level of the endothelium only, in female mice. Similarly female Ay mice exhibit a reduced acetylcholine-mediated dilation (Figure 2B) with no alteration of sodium nitroprusside–mediated relaxation (Figure 2D). Consistent with the literature,11,33 Ptp1b deletion did not impair endothelial function in male mice, despite hypertension. Similarly, obesity and hypertension did not reduce acetylcholine-mediated relaxation in male Ay mice (Figure S3). Seven days of treatment with the highly specific leptin receptor antagonist Allo-Aca fully restored endothelial function in both Ptp1b KO and Ay females (Figure 2A and 2B), whereas having no effects in wild-type and a/a females nor on the sodium nitroprusside–mediated relaxation (Figure 2C and 2D).
Figure 2.
Leptin sensitization and obesity induce endothelial dysfunction in female mice. Aortic ring relaxation to acetylcholine (ACh, A and B) and sodium nitroprusside (SNP, C and D) measured in wild-type (WT) and protein tyrosine phosphatase 1b knockout (KO) mice as well as in lean (a/a) and agouti yellow obese (Ay) female mice treated or not with the leptin receptor antagonist Allo-Aca. Data are mean±SEM; n=6 to 8 for WT and KO mice and n=3 for a/a and Ay mice. *P<0.05 vs WT or a/a, #P<0.05 vs KO or Ay.
Leptin Sensitization and Obesity Reduce Neurogenic Control of BP in Female Mice
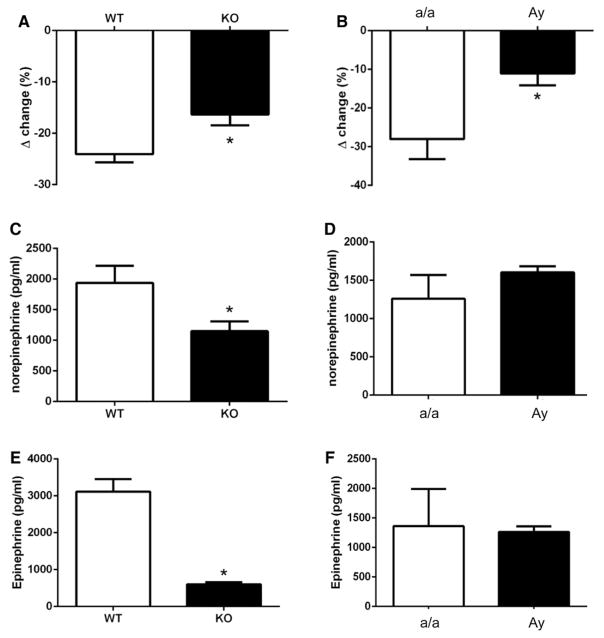
We analyzed sympathetic contribution to the control of BP by measuring BP response to ganglionic blockade in conscious mice and revealed that leptin sensitization (Figure 3A) and Ay (Figure 3B) significantly lowered BP response to mecamylamine, in females, whereas it significantly increased BP response to mecamylamine in male animals (Figure S4). Leptin sensitization with Ptp1b deletion also significantly reduced circulating norepinephrine (Figure 3C) and epinephrine (Figure 3E) levels in females, whereas no change in plasma catecholamine levels was reported in Ay females (Figure 3D and 3F).
Figure 3.
Leptin sensitization and obesity reduce neurogenic control of blood pressure. Blood pressure response to ganglionic blockade in (A) wild-type (WT) and leptin-sensitized (protein tyrosine phosphatase 1b knockout [KO]) mice as well as in (B) lean (a/a) and agouti yellow obese (Ay) female mice. Plasma (C and D) norepinephrine and (E and F) epinephrine levels. Data are mean±SEM; n=6 to 8 for WT and KO mice and n=3 for a/a and Ay mice. *P<0.05 vs WT or a/a.
Leptin Sensitization and Obesity Increase Aldosterone Signaling in Female Mice
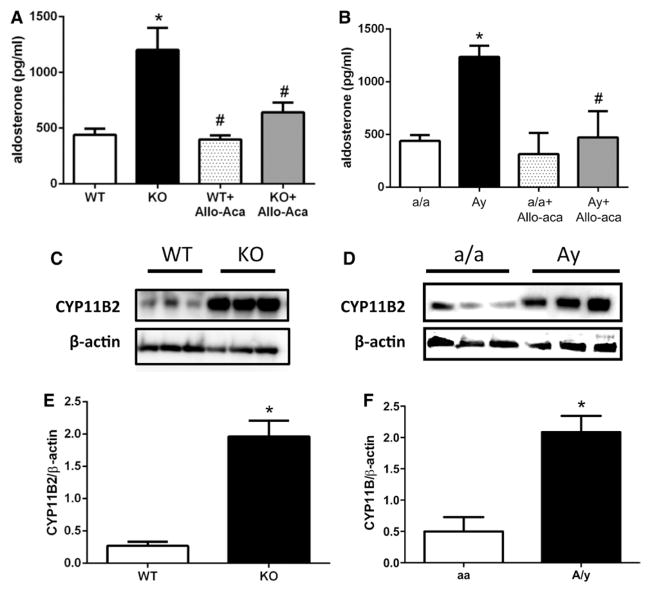
As reported in Figure 4, leptin sensitization with Ptp1b deletion (Figure 4A) and Ay (Figure 4B) induced a 2- to 3-fold increase in plasma aldosterone levels in female mice. No increase in plasma aldosterone levels was observed in male Ptp1b and Ay animals (Figure S5). Western blot quantification of adrenal aldosterone synthase (CYP11B2) expression revealed a significant increase with leptin sensitization (Figure 4C and 4E) and Ay (Figure 4D and 4F) in female mice. Leptin sensitization significantly increased adrenal CYP11B2 expression in male mice, whereas trends toward an increase were observed in male Ay mice (Figure S5). Chronic leptin receptor antagonism with Allo-Aca significantly reduced plasma aldosterone in both Ptp1b (Figure 4A) and Ay female mice (Figure 4B), supporting a direct link between leptin and aldosterone secretion.
Figure 4.
Leptin sensitization and obesity increase aldosterone signaling. Plasma aldosterone levels measured in (A) wild-type (WT) and protein tyrosine phosphatase 1b knockout (KO) mice as well as in (B) lean (a/a) and agouti yellow obese (Ay) female mice treated or not with the leptin receptor antagonist Allo-Aca. C and D, Representative Western blot image for CYP11B2 and β-actin. E and F, Quantification of the Western blot for CYP11B2. Data are mean±SEM; n=6 to 12 for WT and KO mice and n=3 for a/a and Ay mice. *P<0.05 vs WT or a/a, #P<0.05 vs KO or Ay.
Leptin Sensitization and Obesity Elevate BP Via MR-Dependent Mechanisms in Female Mice
The functional relevance of the increased aldosterone signaling reported in Figure 4 was tested by analyzing BP response to a chronic inhibition of aldosterone action via MR blockade. Figure 5 shows that MR blockade restored BP to control levels in leptin-sensitized (Figure 5A) and in Ay female mice (Figure 5B) and elevated HR in Ptp1b KO female mice (Figure 5C). Spironolactone treatment did not reduce BP in male Ptp1b KO mice (Figure S6).
Figure 5.
Leptin sensitization and obesity elevate blood pressure via mineralocorticoid receptor (MR)–dependent mechanisms. Mean arterial pressure (MAP, A and B) and heat rate (HR, C and D) recorded in wild-type (WT) and protein tyrosine phosphatase 1b knockout (KO) mice as well as in lean (a/a) and agouti yellow obese (Ay) female mice treated or not with the MR blocker spironolactone (spiro). Data are mean±SEM; n=6 to 8 for WT and KO mice and n=3 for a/a and Ay mice. *P<0.05 vs WT or a/a, #P<0.05 vs KO or Ay.
Leptin Sensitization and Obesity Impair Endothelial Function Via MR-Dependent Mechanisms in Female Mice
To determine whether leptin sensitization and Ay induce endothelial dysfunction via aldosterone-dependent mechanisms, endothelial function was analyzed in female mice chronically treated with the MR blocker spironolactone for 7 days. As reported in Figure 6, spironolactone restored endothelial function in leptin-sensitized (Figure 6A) and agouti yellow obese female mice (Figure 6B). Spironolactone treatment did not affect endothelium-independent (sodium nitroprusside) relaxation in any mouse strain (Figure 6C and 6D).
Figure 6.
Leptin sensitization and obesity impair endothelial function via mineralocorticoid receptor (MR)–dependent mechanisms. Aortic ring relaxation to acetylcholine (ACh, A and B) and sodium nitroprusside (SNP, C and D) measured in wild-type (WT) and protein tyrosine phosphatase 1b knockout (KO) mice as well as in lean (a/a) and agouti yellow obese (Ay) female mice treated or not with the MR blocker spironolactone (spiro). Data are mean±SEM; n=6 to 8 for WT and KO mice and n=3 for a/a and Ay mice. *P<0.05 vs WT or a/a, #P<0.05 vs KO or Ay.
Discussion
The goal of this study was to determine whether leptin, a major player in obesity-related CVD in males, contributes to hypertension and endothelial dysfunction in female mice. The main findings are that: (1) leptin sensitization with Ptp1b deletion increases BP, aldosterone signaling, and leads to endothelial dysfunction in female mice; (2) endogenous increases in plasma leptin levels with obesity elevate BP, enhance aldosterone signaling and trigger endothelial dysfunction, in female mice; and (3) leptin receptor antagonism and MR blockade restore BP and endothelial function, in obese and leptin-sensitized female mice. Together, these observations highlight the role of leptin and aldosterone in obesity-related CVD in females.
In this study, 2 mouse models were used to determine whether leptin contributes to the control of BP in female mice: the Ptp1b KO mouse and the agouti yellow obese mouse. Ptp1b KO mice were chosen for the role of Ptp1b in the control of leptin signaling. Ptp1b is a critical regulator of leptin signaling.28,29 Increases in Ptp1b expression decrease leptin signaling,34 whereas Ptp1b deletion protects both male and female mice from obesity via increased leptin sensitivity.35,36 Recent work from our group demonstrated that deletion of Ptp1b induces hypertension via leptin-dependent mechanisms in male mice.11 Insulin, the sensitivity of which is also increased by the deletion of Ptp1b,31 seems to intervene in the control of BP in the absence of leptin receptors only and most likely via indirect control of the metabolic function.37,38 In this present study, we confirmed that Ptp1b deletion increases leptin sensitivity in female mice by demonstrating that leptin infusion induces a larger drop in body weight in KO compared with wild-type female mice. Together, these data present the Ptp1b KO mouse as an ideal model to study the role of leptin in the control of the cardiovascular function in the absence of the confounding effects of obesity. The agouti yellow obese mice were used to analyze the relevance of the data generated in the Ptp1b KO mice in a mouse model of obesity that preserves a functional leptin signaling toward the cardiovascular system.30,32 Chronic ectopic expression of the agouti peptide in agouti yellow mice blocks hypothalamic melanocortin-4 receptors and leads to hyperphagia and obesity.39 This model, which presents the characteristic to mimic human obesity, exhibit high leptin levels, which increase sympathetic activity and BP, in male mice.32,40 Although the mechanisms leading to hypertension have been identified in male agouti yellow obese mice, it was unknown whether obesity-associated hyperleptinemia induces hypertension in agouti yellow obese female mice and whether similar mechanisms contribute to hypertension in male and female mice.
With these 2 models we demonstrate for the first time that increasing leptin sensitivity with Ptp1b deletion, or endogenous leptin levels with obesity, elevates BP via aldosterone-dependent mechanisms in female mice. Indeed, although chronic leptin receptor blockade reduced aldosterone signaling and BP in Ptp1b KO and agouti yellow obese females, MR antagonism exerted the same beneficial effects on BP. These data present leptin and aldosterone as major contributors to obesity-induced hypertension in females and introduce the new concept according to which leptin induces hypertension via sex-specific mechanisms. Although novel, this concept is supported by the human literature. Indeed, women who secrete 3× to 4× more leptin than men14–16 exhibit exaggerated increases in leptin levels, inappropriately high aldosterone levels and increased response to MR antagonists compared with men,25–27 with obesity. Furthermore, our results that minimize the role of sympathetic activity in leptin-mediated hypertension in females are in accordance with studies reporting mild16,20 to no increase in sympathetic tone16,21–23 in obese women, and no decrease in sympathetic activity with body weight reduction16 in women. Together these data support the concept that leptin induces hypertension via increased sympathetic activity in males and via aldosterone-dependent mechanisms in females. On the basis of our recent work identifying leptin as a direct regulator of adrenal aldosterone secretion,24 we limited our investigation to the analysis of the contribution of the adrenal glands. The adrenal glands is, however, not the only source of aldosterone. A recent study by Briones et al,41 indeed identified the adipose tissue as a new source of aldosterone. Although our previous study minimized the contribution of the adipose tissue to obesity-associated hyperaldosteronism,24 the current work does not rule out a potential contribution to the adipose tissue to the elevated aldosterone levels. The origin of the preferential stimulation of adrenal aldosterone production over sympathetic activation, in females, remains however completely unknown and requires further investigation.
The second key finding of this study is the demonstration that leptin triggers endothelial dysfunction via aldosterone-dependent mechanisms. This demonstration was made with aortic rings. Although this conduit artery poorly contributes to BP control, previous studies from our group demonstrated that the aorta is a good model to study the changes in vascular reactivity induced by leptin and obesity.11,42 Because of the increase in cardiac output associated with obesity and to the consequent increase in shear stress, aorta seem less prone to endothelial dysfunction than resistance arteries.42 Therefore, a dysfunction of the endothelium at the level of the aorta might be indicative of a dysfunction at the level of the resistance arteries. Here, we showed that both chronic leptin receptor antagonism and MR blockade restored endothelial function in leptin-sensitized and obese hyperleptinemic mice. These data are consistent with the recent demonstration that low dose of MR blockade prevents arterial stiffening43 in obese female mice and provides one more piece of evidence to support the sex specificity of the mechanisms, whereby leptin controls cardiovascular function. Indeed, although leptin triggers endothelial dysfunction via aldosterone-dependent mechanisms in females, recent findings demonstrate that leptin induces endothelial dysfunction via sympathoactivation and oxidative stress in males.12,13 In association with the exaggerated increases in leptin and aldosterone levels reported in obese women,25–27 the results of this study might provide an explanation for the higher susceptibility of premenopausal women to develop endothelial dysfunction with obesity compared with men.7,44
The lack of identification of the detailed mechanisms whereby leptin-mediated aldosterone production triggers hypertension in female mice might represent a limitation to this current study. Although the pleiotropic effects of aldosterone complicate tremendously the determination of these mechanisms, it seems reasonable to speculate that leptin triggers hypertension via peripheral mechanisms, notably via endothelial dysfunction. Indeed, although aldosterone acts centrally to increase sympathetic activity in males, neither Ptp1b KO females nor agouti yellow obese female mice exhibit increased sympathetic activity, despite high circulating aldosterone levels, hence a potential role for endothelial dysfunction. The hypothesis of the contribution of the endothelial dysfunction is further supported by the lack of impaired endothelial function in male animals that exhibit similar BP levels to females, but may contrast with studies demonstrating that aldosterone infusion induces endothelial dysfunction without raising BP in male rats.45
In conclusion, this study demonstrates for the first time that leptin raises BP and induces endothelial dysfunction via aldosterone-dependent mechanisms in female mice and introduce the new concept according to which leptin controls cardiovascular function via sex-specific mechanisms.
Perspectives
Despite growing evidence demonstrating that CVD involve sex-specific mechanisms, guidelines for the treatment of hypertension and CVD do not account for sex, probably because of the gap in knowledge about the mechanisms involved. This study focused on obesity-associated CVD and identified leptin-mediated aldosterone secretion as a major contributor to hypertension and endothelial dysfunction in obese female mice. This work, which represents one of the first steps to understand the mechanisms underlying the rise of CVD in women, highlights the need for sex-specific therapies and provides potential new therapeutic avenues for the treatment of obesity-related CVD in women.
Novelty and Significance.
What Is New?
This study demonstrates for the first time that the adipocyte-derived hormone leptin is a major contributor to cardiovascular disease (CVD) in female mice and notably induces hypertension and endothelial dysfunction via aldosterone-dependent mechanisms.
This study shows for the first time that leptin and obesity induce hypertension and endothelial dysfunction via sex-specific mechanisms.
What Is Relevant?
Obesity is a worldwide epidemic that affects more women than men. Although growing evidence reports that obesity is a major source of CVD in premenopausal women, the mechanisms whereby an increase in body weight leads to CVD remain unknown in women. Using 2 different mouse models, we identified leptin-induced aldosterone secretion as a new mechanisms and potential leading factor to hypertension and endothelial dysfunction in obese women.
Summary
Results from this study show a novel mechanism by which the adipokine leptin triggers CVD and established that obesity leads to CVD via sex-specific mechanisms.
Acknowledgments
We thank Dr Micheal W. Brands and Mr Daniel Duggan for their technical assistance with the telemetry experiments. We also thank Dr Michel L. Tremblay (McGill University), for the Ptp1b KO mice, and Mrs Ruchi Anderson and Simone Kennard for their help with maintaining of the mouse colony.
Sources of Funding
This work was supported by a Scientist Development Grant from the American Heart Association (11SDG5060006 to E.J. Belin de Chantemèle) and Start-up funds from Georgia Regents University.
Footnotes
The online-only Data Supplement is available with this article at http://hyper.ahajournals.org/lookup/suppl/doi:10.1161/HYPERTENSIONAHA.115.06642/-/DC1.
Disclosures
None.
References
- 1.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity among adults: United states, 2011–2012. NCHS data brief. 2013:131. [PubMed] [Google Scholar]
- 2.Go AS, Mozaffarian D, Roger VL, et al. American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics–2013 update: a report from the American Heart Association. Circulation. 2013;127:e6–e245. doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999–2008. JAMA. 2010;303:235–241. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 4.Towfighi A, Zheng L, Ovbiagele B. Weight of the obesity epidemic: rising stroke rates among middle-aged women in the United States. Stroke. 2010;41:1371–1375. doi: 10.1161/STROKEAHA.109.577510. [DOI] [PubMed] [Google Scholar]
- 5.Lurbe E, Torro I, Aguilar F, Alvarez J, Alcon J, Pascual JM, Redon J. Added impact of obesity and insulin resistance in nocturnal blood pressure elevation in children and adolescents. Hypertension. 2008;51:635–641. doi: 10.1161/HYPERTENSIONAHA.107.099234. [DOI] [PubMed] [Google Scholar]
- 6.Sorof J, Daniels S. Obesity hypertension in children: a problem of epidemic proportions. Hypertension. 2002;40:441–447. doi: 10.1161/01.hyp.0000032940.33466.12. [DOI] [PubMed] [Google Scholar]
- 7.Wilsgaard T, Schirmer H, Arnesen E. Impact of body weight on blood pressure with a focus on sex differences: the Tromso Study, 1986–1995. Arch Intern Med. 2000;160:2847–2853. doi: 10.1001/archinte.160.18.2847. [DOI] [PubMed] [Google Scholar]
- 8.Hall JE, Hildebrandt DA, Kuo J. Obesity hypertension: role of leptin and sympathetic nervous system. Am J Hypertens. 2001;14(6 pt 2):103S–115S. doi: 10.1016/s0895-7061(01)02077-5. [DOI] [PubMed] [Google Scholar]
- 9.Mark AL. Selective leptin resistance revisited. Am J Physiol Regul Integr Comp Physiol. 2013;305:R566–R581. doi: 10.1152/ajpregu.00180.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Simonds SE, Pryor JT, Ravussin E. Leptin mediates the increase in blood pressure associated with obesity. Cell. 2014;159:1404–1416. doi: 10.1016/j.cell.2014.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Belin de Chantemele EJ, Muta K, Mintz J, Tremblay ML, Marrero MB, Fulton DJ, Stepp DW. Protein tyrosine phosphatase 1b, a major regulator of leptin-mediated control of cardiovascular function. Circulation. 2009;120:753–763. doi: 10.1161/CIRCULATIONAHA.109.853077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang J, Wang H, Luo W, Guo C, Wang J, Chen YE, Chang L, Eitzman DT. Leptin-induced endothelial dysfunction is mediated by sympathetic nervous system activity. J Am Heart Assoc. 2013;2:e000299. doi: 10.1161/JAHA.113.000299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bouloumie A, Marumo T, Lafontan M, Busse R. Leptin induces oxidative stress in human endothelial cells. FASEB J. 1999;13:1231–1238. [PubMed] [Google Scholar]
- 14.Considine RV, Sinha MK, Heiman ML, Kriauciunas A, Stephens TW, Nyce MR, Ohannesian JP, Marco CC, McKee LJ, Bauer TL. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med. 1996;334:292–295. doi: 10.1056/NEJM199602013340503. [DOI] [PubMed] [Google Scholar]
- 15.Kennedy A, Gettys TW, Watson P, Wallace P, Ganaway E, Pan Q, Garvey WT. The metabolic significance of leptin in humans: gender-based differences in relationship to adiposity, insulin sensitivity, and energy expenditure. J Clin Endocrinol Metab. 1997;82:1293–1300. doi: 10.1210/jcem.82.4.3859. [DOI] [PubMed] [Google Scholar]
- 16.Lambert E, Straznicky N, Eikelis N, Esler M, Dawood T, Masuo K, Schlaich M, Lambert G. Gender differences in sympathetic nervous activity: influence of body mass and blood pressure. J Hypertens. 2007;25:1411–1419. doi: 10.1097/HJH.0b013e3281053af4. [DOI] [PubMed] [Google Scholar]
- 17.Matsukawa T, Sugiyama Y, Watanabe T, Kobayashi F, Mano T. Gender difference in age-related changes in muscle sympathetic nerve activity in healthy subjects. Am J Physiol. 1998;275(5 pt 2):R1600–R1604. doi: 10.1152/ajpregu.1998.275.5.R1600. [DOI] [PubMed] [Google Scholar]
- 18.Christou DD, Jones PP, Jordan J, Diedrich A, Robertson D, Seals DR. Women have lower tonic autonomic support of arterial blood pressure and less effective baroreflex buffering than men. Circulation. 2005;111:494–498. doi: 10.1161/01.CIR.0000153864.24034.A6. [DOI] [PubMed] [Google Scholar]
- 19.Burt VL, Whelton P, Roccella EJ, Brown C, Cutler JA, Higgins M, Horan MJ, Labarthe D. Prevalence of hypertension in the US adult population. Results from the Third National Health and Nutrition Examination Survey, 1988–1991. Hypertension. 1995;25:305–313. doi: 10.1161/01.hyp.25.3.305. [DOI] [PubMed] [Google Scholar]
- 20.Jones PP, Snitker S, Skinner JS, Ravussin E. Gender differences in muscle sympathetic nerve activity: effect of body fat distribution. Am J Physiol. 1996;270(2 pt 1):E363–E366. doi: 10.1152/ajpendo.1996.270.2.E363. [DOI] [PubMed] [Google Scholar]
- 21.Tank J, Heusser K, Diedrich A, Hering D, Luft FC, Busjahn A, Narkiewicz K, Jordan J. Influences of gender on the interaction between sympathetic nerve traffic and central adiposity. J Clin Endocrinol Metab. 2008;93:4974–4978. doi: 10.1210/jc.2007-2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Messina G, De Luca V, Viggiano A, Ascione A, Iannaccone T, Chieffi S, Monda M. Autonomic nervous system in the control of energy balance and body weight: personal contributions. Neurol Res Int. 2013;2013:639280. doi: 10.1155/2013/639280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bell C, Seals DR, Monroe MB, Day DS, Shapiro LF, Johnson DG, Jones PP. Tonic sympathetic support of metabolic rate is attenuated with age, sedentary lifestyle, and female sex in healthy adults. J Clin Endocrinol Metab. 2001;86:4440–4444. doi: 10.1210/jcem.86.9.7855. [DOI] [PubMed] [Google Scholar]
- 24.Huby AC, Antonova G, Groenendyk J, Gomez-Sanchez CE, Bollag WB, Filosa JA, Belin de Chantemèle EJ. Adipocyte-derived hormone leptin is a direct regulator of aldosterone secretion, which promotes endothelial dysfunction and cardiac fibrosis. Circulation. 2015;132:2134–2145. doi: 10.1161/CIRCULATIONAHA.115.018226. [DOI] [PubMed] [Google Scholar]
- 25.Calhoun DA, Sharma K. The role of aldosteronism in causing obesity-related cardiovascular risk. Cardiol Clin. 2010;28:517–527. doi: 10.1016/j.ccl.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kanashiro-Takeuchi RM, Heidecker B, Lamirault G, Dharamsi JW, Hare JM. Sex-specific impact of aldosterone receptor antagonism on ventricular remodeling and gene expression after myocardial infarction. Clin Transl Sci. 2009;2:134–142. doi: 10.1111/j.1752-8062.2009.00094.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vasan RS, Evans JC, Benjamin EJ, Levy D, Larson MG, Sundstrom J, Murabito JM, Sam F, Colucci WS, Wilson PW. Relations of serum aldosterone to cardiac structure: gender-related differences in the Framingham Heart Study. Hypertension. 2004;43:957–962. doi: 10.1161/01.HYP.0000124251.06056.8e. [DOI] [PubMed] [Google Scholar]
- 28.Cheng A, Uetani N, Simoncic PD, Chaubey VP, Lee-Loy A, McGlade CJ, Kennedy BP, Tremblay ML. Attenuation of leptin action and regulation of obesity by protein tyrosine phosphatase 1B. Dev Cell. 2002;2:497–503. doi: 10.1016/s1534-5807(02)00149-1. [DOI] [PubMed] [Google Scholar]
- 29.Zabolotny JM, Bence-Hanulec KK, Stricker-Krongrad A, Haj F, Wang Y, Minokoshi Y, Kim YB, Elmquist JK, Tartaglia LA, Kahn BB, Neel BG. PTP1B regulates leptin signal transduction in vivo. Dev Cell. 2002;2:489–495. doi: 10.1016/s1534-5807(02)00148-x. [DOI] [PubMed] [Google Scholar]
- 30.Mark AL, Shaffer RA, Correia ML, Morgan DA, Sigmund CD, Haynes WG. Contrasting blood pressure effects of obesity in leptin-deficient ob/ob mice and agouti yellow obese mice. J Hypertens. 1999;17(12 pt 2):1949–1953. doi: 10.1097/00004872-199917121-00026. [DOI] [PubMed] [Google Scholar]
- 31.Elchebly M, Payette P, Michaliszyn E, Cromlish W, Collins S, Loy AL, Normandin D, Cheng A, Himms-Hagen J, Chan CC, Ramachandran C, Gresser MJ, Tremblay ML, Kennedy BP. Increased insulin sensitivity and obesity resistance in mice lacking the protein tyrosine phosphatase-1B gene. Science. 1999;283:1544–1548. doi: 10.1126/science.283.5407.1544. [DOI] [PubMed] [Google Scholar]
- 32.Correia ML, Haynes WG, Rahmouni K, Morgan DA, Sivitz WI, Mark AL. The concept of selective leptin resistance: evidence from agouti yellow obese mice. Diabetes. 2002;51:439–442. doi: 10.2337/diabetes.51.2.439. [DOI] [PubMed] [Google Scholar]
- 33.Herren DJ, Herre DJ, Norman JB, Anderson R, Tremblay ML, Huby AC, Belin de Chantemèle EJ. Deletion of Protein Tyrosine Phosphatase 1B (PTP1B) Enhances Endothelial Cyclooxygenase 2 Expression and Protects Mice from Type 1 Diabetes-Induced Endothelial Dysfunction. PLoS One. 2015;10:e0126866. doi: 10.1371/journal.pone.0126866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lam NT, Covey SD, Lewis JT, Oosman S, Webber T, Hsu EC, Cheung AT, Kieffer TJ. Leptin resistance following over-expression of protein tyrosine phosphatase 1B in liver. J Mol Endocrinol. 2006;36:163–174. doi: 10.1677/jme.1.01937. [DOI] [PubMed] [Google Scholar]
- 35.Bence KK, Delibegovic M, Xue B, Gorgun CZ, Hotamisligil GS, Neel BG, Kahn BB. Neuronal PTP1B regulates body weight, adiposity and leptin action. Nat Med. 2006;12:917–924. doi: 10.1038/nm1435. [DOI] [PubMed] [Google Scholar]
- 36.Klaman LD, Boss O, Peroni OD, Kim JK, Martino JL, Zabolotny JM, Moghal N, Lubkin M, Kim YB, Sharpe AH, Stricker-Krongrad A, Shulman GI, Neel BG, Kahn BB. Increased energy expenditure, decreased adiposity, and tissue-specific insulin sensitivity in protein-tyrosine phosphatase 1B-deficient mice. Mol Cell Biol. 2000;20:5479–5489. doi: 10.1128/mcb.20.15.5479-5489.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Belin de Chantemele EJ, Ali MI, Mintz JD, Rainey WE, Tremblay ML, Fulton DJ, Stepp DW. Increasing peripheral insulin sensitivity by protein tyrosine phosphatase 1b deletion improves control of blood pressure in obesity. Hypertension. 2012;60:1273–1279. doi: 10.1161/HYPERTENSIONAHA.112.196295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ali MI, Ketsawatsomkron P, Belin de Chantemele EJ, Mintz JD, Muta K, Salet C, Black SM, Tremblay ML, Fulton DJ, Marrero MB, Stepp DW. Deletion of protein tyrosine phosphatase 1b improves peripheral insulin resistance and vascular function in obese, leptin-resistant mice via reduced oxidant tone. Circ Res. 2009;105:1013–1022. doi: 10.1161/CIRCRESAHA.109.206318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huszar D, Lynch CA, Fairchild-Huntress V, Dunmore JH, Fang Q, Berkemeier LR, Gu W, Kesterson RA, Boston BA, Cone RD, Smith FJ, Campfield LA, Burn P, Lee F. Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell. 1997;88:131–141. doi: 10.1016/s0092-8674(00)81865-6. [DOI] [PubMed] [Google Scholar]
- 40.Aizawa-Abe M, Ogawa Y, Masuzaki H, Ebihara K, Satoh N, Iwai H, Matsuoka N, Hayashi T, Hosoda K, Inoue G, Yoshimasa Y, Nakao K. Pathophysiological role of leptin in obesity-related hypertension. J Clin Invest. 2000;105:1243–1252. doi: 10.1172/JCI8341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Briones AM, Nguyen Dinh Cat A, Callera GE, Yogi A, Burger D, He Y, Corrêa JW, Gagnon AM, Gomez-Sanchez CE, Gomez-Sanchez EP, Sorisky A, Ooi TC, Ruzicka M, Burns KD, Touyz RM. Adipocytes produce aldosterone through calcineurin-dependent signaling pathways: implications in diabetes mellitus-associated obesity and vascular dysfunction. Hypertension. 2012;59:1069–1078. doi: 10.1161/HYPERTENSIONAHA.111.190223. [DOI] [PubMed] [Google Scholar]
- 42.Belin de Chantemele EJ, Mintz JD, Rainey WE, Stepp DW. Impact of leptin-mediated sympatho-activation on cardiovascular function in obese mice. Hypertension. 2011;58:271–279. doi: 10.1161/HYPERTENSIONAHA.110.168427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.DeMarco VG, Habibi J, Jia G, Aroor AR, Ramirez-Perez FI, Martinez-Lemus LA, Bender SB, Garro M, Hayden MR, Sun Z, Meininger GA, Manrique C, Whaley-Connell A, Sowers JR. Low-dose mineralocorticoid receptor blockade prevents Western Diet-induced arterial stiffening in female mice. Hypertension. 2015;66:99–107. doi: 10.1161/HYPERTENSIONAHA.115.05674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Safar ME, Balkau B, Lange C, Protogerou AD, Czernichow S, Blacher J, Levy BI, Smulyan H. Hypertension and vascular dynamics in men and women with metabolic syndrome. J Am Coll Cardiol. 2013;61:12–19. doi: 10.1016/j.jacc.2012.01.088. [DOI] [PubMed] [Google Scholar]
- 45.Blanco-Rivero J, Cachofeiro V, Lahera V, Aras-Lopez R, Márquez-Rodas I, Salaices M, Xavier FE, Ferrer M, Balfagón G. Participation of prostacyclin in endothelial dysfunction induced by aldosterone in normotensive and hypertensive rats. Hypertension. 2005;46:107–112. doi: 10.1161/01.HYP.0000171479.36880.17. [DOI] [PubMed] [Google Scholar]