Abstract
Dome-shaped macula is described as an inward bulge of the macula within a posterior staphyloma in highly myopic eyes. Choroidal neovascularization is a known complication that can cause visual loss in dome-shaped macula. Herein, we describe a patient who presented with features of polypoidal choroidal neovascularization that developed on a background of high myopia with dome-shaped macula.
Keywords: Dome-shaped macula, Polypoidal, Polypoidal choroidal vasculopathy, Polypoidal choroidal neovascularization, High myopia, Neovascularization, Imaging
Background
Dome-shaped macula (DSM) is an inward bulge of the macula within a posterior staphyloma in highly myopic patients [1]. Recently, Imamura et al. [2] utilized enhanced depth optical coherence tomography (EDI-OCT) to demonstrate that the anterior bulge was consequent to relative increases in scleral thickness beneath the macula. Gaucher et al. [1] reported that the prevalence of dome-shaped macula was approximately 10.7% in highly myopic eyes. However, recent reports have utilized multimodal imaging to show that the rate of DSM is higher and in the range between 15 and 20% [3, 4].
Choroidal neovascularization (CNV) is a known complication of DSM and occurs at a rate of approximately 20% [4, 5]. Deobhakta et al. [6] recently demonstrated that some eyes with DSM will manifest a shallow pigment epithelial detachment (PED) overlying an area of abrupt change in choroidal thickness typically occurring at the edge of the dome. They hypothesized that some of these flat irregular PEDs might harbor type 1 neovascularization. Herein, we describe the multimodal imaging findings in a patient with DSM who presented with type 1 neovascularization with polypidal lesions. To our knowledge, polypoidal type 1 neovascularization has not been documented in the setting of DSM.
Case presentation
A 50-year-old white female with a history of high myopia presented with metamorphopsia and visual deterioration in her left eye that had developed gradually over many months. She had previously been diagnosed with central serous chorioretinopathy and had received photodynamic therapy to the left eye after which two intravitreal injections of bevacizumab were administered (last treatment was over 1 year previously). She was taking metformin for glucose intolerance and was otherwise healthy.
Visual acuities were 20/250 in the right eye, improving to 20/100 with pinhole and 20/800 in the left eye, improving to 20/100 with pinhole. Her refractive error was −7.5 and −9.0 diopters right and left, respectively.
The anterior segments were unremarkable and intraocular pressures were 15 mmHg in both eyes. Ophthalmoscopic examination showed peripapillary atrophy and retinal pigment epithelial changes that were consistent with myopic degeneration (Figure 1). There was no hemorrhage.
Figure 1.
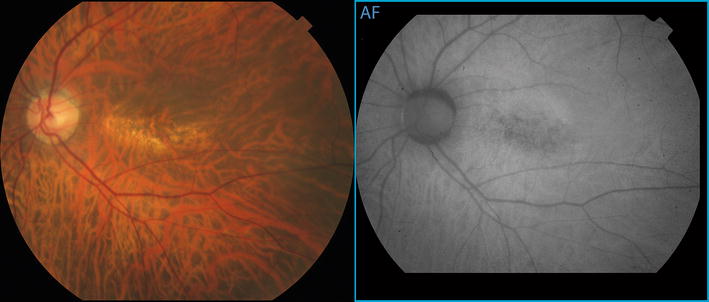
Color photograph and fundus autofluorescence images of the posterior pole of the left eye show a rectangular area of mottled retinal pigment epithelial atrophy arranged with its long axis aligned horizontally.
Horizontal OCT (Heidelberg Spectralis, Heidelberg Engineering, Germany) raster scans did not overtly illustrate any dome configuration of the patient’s macula. However, a combination of horizontal and vertical raster scans enabled a radially asymmetric three-dimensional dome-shaped configuration to be appreciated (Figure 2). Spectral domain OCT showed thin choroids in both eyes with subfoveal choroidal thickness of 98 and 95 um, respectively. The vertically oriented OCT line scans showed an abrupt change in choroidal thickness occurring at the inferior edge of the dome (Figure 3). EDI-OCT confirmed an increase in scleral thickness beneath the site of the macular dome.
Figure 2.
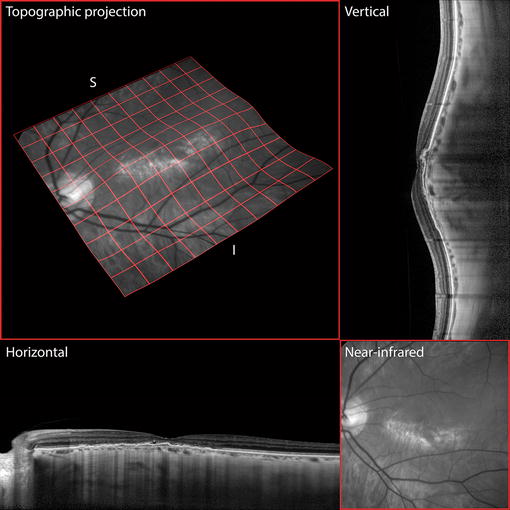
Topological representations of the posterior pole. Vertical and horizontal raster spectral domain optical coherence tomography line scans show that the long axis of the elliptical dome lies in the horizontal meridian, as demonstrated also by a quad-mesh projection onto an elevation-mapped near-infrared image plane. The region of atrophy lies at the inferior border of the crest of the elliptical dome, where the choroid is thinnest.
Figure 3.

Multimodal imaging findings of choroidal neovascularization. Early and transit phase indocyanine green angiographic frames demonstrate perfusion of a choroidal vascular loop at the fovea (green arrowhead) followed by hyperfluorescence of at least three polypoidal lesions (red arrowheads) and their feeding network (yellow arrowhead). Magnified horizontal and vertical raster enhanced depth spectral domain optical coherence tomographic (OCT) line scans through these areas show the polypoidal lesions as peaked pigment epithelial detachments (PEDs) and the feeding network as an adjacent shallow irregular PED. The presence of pathologically dilated choroidal vessels is noted with overlying loss of choriocapillaris tissue, especially at the crest of the dome. En face OCT (3 × 3 mm) through the shallow PED reveals the spherical morphology of at least three polypoidal lesions. En face OCT angiography (3 × 3 mm) through the PED isolates the type 1 neovascular tissue from the rest of the choroid and shows significant flow through the feeder vessels and within the polypoidal lesions.
A complex irregular pigment epithelial detachment was noted at the left macula consistent with a polypoidal lesion and its associated type 1 branching vascular network (BVN) (Figures 2, 3). Shallow subretinal fluid was also seen on these scans. Indocyanine green angiography (TRC-50FX, Topcon Corporation, Tokyo, Japan) and split spectrum amplitude decorrelation angiography (Avanti, Optovue, Fremont, CA, USA) demonstrated the polypoidal lesion and its corresponding feeding vessels (Figure 3). The ICGA also showed relatively larger caliber choroidal vessels in the thicker choroid at the superior edge of the dome.
DSM is associated with high myopia [1] and previous reports have shown that this diagnosis can be missed if the macula is evaluated with a single OCT scan orientation. Liang et al. [4] reported that vertical and horizontal OCT scans are necessary to achieve diagnostic sensitivity for DSM. In their paper, they showed that of those eyes with DSM, the diagnosis could be made using only the vertical section in 77% of cases and using only the horizontal section in 2% of cases. These findings are reflected in our patient in whom the diagnosis of DSM was confirmed only after integration of vertical and horizontal OCT scans to construct a three-dimensional structure.
Vision loss from DSM can be consequent to serous neurosensory detachment masquerading as central serous chorioretinopathy [5]. Foveal retinoschisis [4, 7] and choroidal neovascularization [1, 7] are other known complications that can arise during the natural course of DSM. The rate of CNV in DSM is approximately 20%, however, little information is available concerning the morphology of these lesions. Utilizing OCT angiography and other imaging modalities, we show that CNV in DSM can manifest a polypoidal configuration. Polypoidal neovascular changes have also been described in central serous chorioretinopathy [8, 9], choroidal nevi [10, 11], peripheral exudative hemorrhagic chorioretinopathy [12] and optic nerve melanocytoma [13].
Conclusion
Scleral thickness at the site of DSM is increased in contradiction to what is usually found in high myopia [14]. Previous authors used this observation to postulate that control of ocular expansion in myopia may be more complex than initially proposed [2]. Polypoidal changes to type 1 neovascularization in the setting of DSM may reflect the unique stretch and mechanical forces being applied to the macula and choroid in this condition. It will be important to study other cases of CNV in DSM to validate this hypothesis.
Consent
Written informed consent was obtained from the patient for publication of this Case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Authors’ contributions
JN—acquisition of data, conceptualization of manuscript, review of literature, drafting/editing of the report. KD—acquisition of data, conceptualization of manuscript, editing the report, preparation of image panels. CB—conceptualization of manuscript, editing the report, review of literature. KBF—design of project, conceptualization of manuscript, editing the report, analysis and interpretation of data, final approval of manuscript. All authors have contributed significantly. All authors read and approved the final manuscript.
Compliance with ethical guidelines
Competing interests K. Bailey Freund: is a consultant to Genentech, ThromboGenics, Ohr Pharmaceutical, Optos, Optovue, and Heidelberg Engineering (honorarium for each). The other authors have no conflicting interests to disclose.
Financial support The LuEsther T. Mertz Retinal Research Center and The Macula Foundation, New York. The funding bodies had no role in the design or conduct of this research or the decision to publish.
Statement of originality The work submitted here is original and has not been presented or published elsewhere.
Abbreviations
- DSM
dome-shaped macula
- EDI-OCT
enhanced depth imaging optical coherence tomography
- CNV
choroidal neovascularization
- PED
pigment epithelial detachment
- OCT
optical coherence tomography
- BVN
branching vascular network
Contributor Information
Jonathan Naysan, Email: jnaysan@gmail.com.
Kunal K Dansingani, Email: kkd@doctor.com.
Chandrakumar Balaratnasingam, Email: balaratnasingam@gmail.com.
K Bailey Freund, Email: kbfnyf@aol.com.
References
- 1.Gaucher D, Erginay A, Lecleire-Collet A, Haouchine B, Puech M, Cohen SY, et al. Dome-shaped macula in eyes with myopic posterior staphyloma. Am J Ophthalmol. 2008;145(5):909–914. doi: 10.1016/j.ajo.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 2.Imamura Y, Iida T, Maruko I, Zweifel SA, Spaide RF. Enhanced depth imaging optical coherence tomography of the sclera in dome-shaped macula. Am J Ophthalmol. 2011;151(2):297–302. doi: 10.1016/j.ajo.2010.08.014. [DOI] [PubMed] [Google Scholar]
- 3.Chebil A, Ben Achour B, Chaker N, Jedidi L, Mghaieth F, El Matri L (2014) Choroidal thickness assessment with SD-OCT in high myopia with dome-shaped macula. J francais d’ophtalmologie. 37(3):237–241 [DOI] [PubMed]
- 4.Liang IC, Shimada N, Tanaka Y, Nagaoka N, Moriyama M, Yoshida T, et al. Comparison of clinical features in highly myopic eyes with and without a dome-shaped macula. Ophthalmology. 2015 doi: 10.1016/j.ophtha.2015.04.012. [DOI] [PubMed] [Google Scholar]
- 5.Viola F, Dell’Arti L, Benatti E, Invernizzi A, Mapelli C, Ferrari F, et al. Choroidal findings in dome-shaped macula in highly myopic eyes: a longitudinal study. Am J Ophthalmol. 2015;159(1):44–52. doi: 10.1016/j.ajo.2014.09.026. [DOI] [PubMed] [Google Scholar]
- 6.Deobhakta A, Ross AH, Helal J, Maia A, Freund KB. Localized choroidal thickness variation and pigment epithelial detachment in dome-shaped macula with subretinal fluid. Ophthalmic Surg Lasers Imag Retina. 2015;46(3):391–392. doi: 10.3928/23258160-20150323-18. [DOI] [PubMed] [Google Scholar]
- 7.Ellabban AA, Tsujikawa A, Matsumoto A, Yamashiro K, Oishi A, Ooto S, et al. Three-dimensional tomographic features of dome-shaped macula by swept-source optical coherence tomography. Am J Ophthalmol. 2013;155(2):320–328. doi: 10.1016/j.ajo.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 8.Toyama T, Ohtomo K, Noda Y, Ueta T. Polypoidal choroidal vasculopathy and history of central serous chorioretinopathy. Eye. 2014;28(8):992–997. doi: 10.1038/eye.2014.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang LH, Jonas JB, Wei WB (2015) Conversion of central serous chorioretinopathy to polypoidal choroidal vasculopathy. Acta Ophthalmol. doi:10.1111/aos.12606 [DOI] [PubMed]
- 10.Asao K, Hashida N, Nishida K. Choroidal nevus in an eye with polypoidal choroidal vasculopathy. Case Rep Ophthalmol. 2014;5(3):463–467. doi: 10.1159/000370044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peiretti E, Pozzoni MC, Fossarello M, Spaide RF. Polypoidal choroidal vasculopathy in association with choroidal nevus. Retinal Cases Brief Rep. 2009;3(1):12–14. doi: 10.1097/ICB.0b013e318166bd70. [DOI] [PubMed] [Google Scholar]
- 12.Goldman DR, Freund KB, McCannel CA, Sarraf D. Peripheral polypoidal choroidal vasculopathy as a cause of peripheral exudative hemorrhagic chorioretinopathy: a report of 10 eyes. Retina. 2013;33(1):48–55. doi: 10.1097/IAE.0b013e31825df12a. [DOI] [PubMed] [Google Scholar]
- 13.Bartlett HM, Willoughby B, Mandava N. Polypoidal choroidal vasculopathy in a patient with melanocytoma of the optic nerve. Retina. 2001;21(4):396–399. doi: 10.1097/00006982-200108000-00024. [DOI] [PubMed] [Google Scholar]
- 14.Hayashi M, Ito Y, Takahashi A, Kawano K, Terasaki H. Scleral thickness in highly myopic eyes measured by enhanced depth imaging optical coherence tomography. Eye. 2013;27(3):410–417. doi: 10.1038/eye.2012.289. [DOI] [PMC free article] [PubMed] [Google Scholar]


