Abstract
Craniosynostosis, the premature fusion of one or more of the cranial sutures, is estimated to occur in 1:1800-2500 births. Genetic murine models of craniosynostosis exist, but often imperfectly model human patients. Case, cohort, and surveillance studies have identified excess thyroid hormone as an agent that can either cause or exacerbate human cases of craniosynostosis. Here we investigate the influence of in utero and in vitro exogenous thyroid hormone exposure on a murine model of craniosynostosis, Twist 1 +/−. By 15 days post-natal there was evidence of coronal suture fusion in the Twist 1 +/− model, regardless of exposure. With the exception of craniofacial width, there were no significant effects of exposure; however, the Twist 1 +/− phenotype was significantly different from the wild type control. Twist 1 +/− cranial suture cells did not respond to thyroxine treatment as measured by proliferation, osteogenic differentiation, and gene expression of osteogenic markers. However, treatment of these cells did result in modulation of thyroid associated gene expression. Our findings suggest the phenotypic effects of the genetic mutation largely outweighed the effects of thyroxine exposure in the Twist 1 +/− model. These results highlight difficultly in experimentally modeling gene-environment interactions for craniosynostotic phenotypes.
Keywords: Thyroid Hormone, Cranial Suture, Craniosynostosis, Cephalometrics, Gene Expression
Introduction
Craniosynostosis is defined as the premature fusion of one or more of the cranial sutures prior to the completion of neural expansion. It is estimated that craniosynostosis occurs in 1:1800-2500 births (Delahyne et al., 2003; Robin, 1999). When it is part of a syndrome, craniosynostosis most often manifests with limb disorders and has strong genetic influence most commonly as part of a cascade of FGFR gain of function mutations. However, genomic cases are only a small percentage (10–15%) and most cases of craniosynostosis are classified as non-syndromic with unknown genetic etiology (Johnson and Wilkie, 2011; Morriss-Kay and Wilkie, 2005; Twigg and Wilkie, 2015; Wilkie, 1997; 2000; Wilkie et al., 2007; Wilkie and Wall, 1996). Surgical correction of the fused suture is often implemented to allow for proper neurocranial expansion and follow-up care and surgeries are necessary throughout craniofacial growth adding to both cost and risk associated with craniosynostosis (Parikh et al., 2015; Schaller et al., 2012; Simpson et al., 2016; Zakhary et al., 2014).
Specific genetic (Chen et al., 2003; Crane et al., 2005; Feng et al., 2013; Holmes and Basilico, 2012; Holmes et al., 2009; Morita et al., 2014; Perlyn et al., 2006; Roy et al., 1981; Shukla et al., 2007; Twigg et al., 2009; Wang et al., 2015) and likely non-syndromic animal models (Mooney et al., 1993; Mooney et al., 1998a; Mooney et al., 1998b) of craniosynostosis can be useful in the study of the pathogenesis of premature suture fusion. Current understanding of suture fusion progression has come from molecular differences in patent, fusing, and fused sutures during ontogeny (Coussens et al., 2007; Grova et al., 2012; Lenton et al., 2005; Levi et al., 2012; Rawlins and Opperman, 2008). Although specific to human gene mutations, most genetic murine models of craniosynostosis are not post-natal viable or present with a more aggressive phenotype than that found in human patients (Gong, 2012; Holmes et al., 2009; Motch Perrine et al., 2014; Nah et al., 2012; Twigg et al., 2009; Yeh et al., 2013). Several less severe models do exist and may lend themselves to the study of both growth and development after suture growth disruption, surgical correction, and molecular contribution and/or exacerbation of disorders (Huang et al., 2014; Liu et al., 2007; Mooney et al., 1993; Mooney et al., 1998b; Parsons et al., 2014; Veistinen et al., 2012).
Case, cohort, and surveillance studies have identified agents, environmental or pharmacological, that can either cause or exacerbate human cases of craniosynostosis (Alwan et al., 2007; Browne et al., 2011; Carmichael et al., 2008; Carmichael et al., 2010; Reefhuis et al., 2011). One such agent is aberrant exposure to thyroid hormone (hormone replacement therapy) or exposure to aberrant levels of thyroid hormone during development (maternal thyrotoxicosis) (Ardalan et al., 2012; Carmichael et al., 2015; Chawla et al., 2015; Cohen, 1988; Hashmi et al., 2012; Johnston and Bronsky, 1995; McNab and Ginsberg, 2005; Penfold and Simpson, 1975; Radetti et al., 2002; Rasmussen et al., 2007). Thyroid hormone is known to be important and necessary for limb growth and thyroid hormone receptor influence has been identified in the calvaria of preclinical models (Akita et al., 1996; Akita et al., 1994; O'Shea et al., 2005). However, the link to craniofacial disorders, specifically craniosynostosis, suggests a targeted effect either during a specific time in ontogeny, on a particular developing structure, the undifferentiated suture, or exacerbation of an underlying genetic defect. Here we investigate the influence of in utero and in vitro exogenous thyroid hormone exposure on the murine Twist 1 +/− genetic model of craniosynostosis. The Twist 1 +/− mutation in humans is linked to single suture synostoses as well as Saethre Chotzen syndrome (Hermann et al., 2012; Robin, 1999; Seto et al., 2007). The murine Twist 1 +/− model is considered a mild preclinical phenotype exhibiting targeted coronal suture fusion early in ontogeny and some syndactly. This suture fusion is easily identified, predictable, and has high penetrance allowing for aberration of growth due to environmental factors to be identified (Parsons et al., 2014). We are specifically testing the hypothesis that prenatal thyroxine exposure will exacerbate the craniofacial phenotype of Twist 1 +/− perinates and alter cellular activity and gene expression of cells garnered from the affected coronal suture.
Materials and Methods
In Utero Exposure
Adult, wild type, C57BL6 control and Twist 1 +/− (002222) (Mus musculus, Jackson Laboratories, Bar Harbor, ME, USA) male and female mice were utilized to produce in utero thyroxine exposed and unexposed litters. C57BL6 males were bred with Twist 1 +/− females or Twist 1 +/− males were bred with C57BL6 females and separated at ~E13 of pregnancy. At this time, a dose of levothyroxine previously described to cause alteration to circulating thyroid hormone levels (Synthroid, Abbott Laboratories, Abbott, IL, USA) (control dose = no treatment; thyroxine exposed ~415 ng per day) was added to the drinking water provided to pregnant dams (Bowers et al., 1967; Capuco et al., 1999; Krause et al., 2015; Thordarson et al., 1992). Dose was carefully chosen to be less than replacement therapy shown to inhibit Thyroid Stimulating Hormone release, but likely to increase fetal exposure to the exogenous thyroid hormone. Treatment continued until birth of the litters at ~E20. Eleven pn 20 litters were sacrificed for cell isolation. The remaining mouse pups were grown to 15, 20, or 25 days post-natal (pn) when they were sacrificed and whole skulls were fixed with 4% paraformaldehyde for 48 hours and then switched to 70% Ethanol for micro computed tomography (μCT) analysis. Pn 20 pups were used for cell isolation as this time point allowed for collection of enough tissue to achieve cell isolation and expansion. Post-natal days 15, 20 and 25 were chosen for growth assessment as they offered the ability to employ 2D histomorphometric analysis and 3D μCT analysis over a range of time when fusion is predicted to occur in this model (Parsons et al., 2014). In addition, at sacrifice tail snips were procured and standard polymerase chain reaction (KAPA Biosystems, Wilmington MA, USA) with agarose gel electrophoresis was performed to confirm Twist 1 +/− genotypes (Forward: CTTGGGTGGAGAGGCTATTC; Reverse: AGGTGAGATGACAGGAGATC). Twist 1 −/− is embryonic lethal (Parsons et al., 2014). In order to avoid any uncharacterized craniofacial abnormality of the wild type littermates of Twist 1 +/− pups, separate wild type C57BL6 crosses were utilized to procure control wild type pups used in growth experiments. Animal Use Protocols were approved by Georgia Regents University Institutional Animal Care and Use Committee (2011-0365), and the Medical University of South Carolina Institutional Animal Care and Use Committee (AR#3341). All breeding procedures were carried out in an Association for Assessment and Accreditation of Laboratory Animal Care International accredited facility where all husbandry and related services are provided by the Division of Laboratory Animal Resources. All procedures and the reporting thereof are in compliance with the Animal Research: Reporting in Vivo Experiments (ARRIVE) guidelines (Kilkenny et al., 2010).
μCT
μCT images were obtained on 15, 20, and 25 day post-natal Twist 1 +/− mouse pup skulls with a SkyScan 1174 (Kontich, Belgium) at a 22.57 μm voxel resolution. Scans were obtained on 142 animals (Male=42.8%; Female=43.3%; Undetermined=13.9%) (Table 1). Mouse skulls were reconstructed with NRecon v1.6.4.8 (BrukermicroCT, Kontich, Belgium) as previously described and imported into Amira v5.0 where it was exposed to a Gaussian Smoothing image filter (r50.3 in X, Y, and Z dimensions; isometric kernel size53) to reduce extraneous noise in the images (Parsons et al., 2014). Threshold settings were then set to only visualize bone volume within the skull. Measurements of the length and width of the cranial vault were collected by a single experienced rater (TEP) from each reconstructed mouse skull. Landmarks can be visualized at the following website: http://getahead.psu.edu/viewer.html?id=Adult_Mouse_Skull. Measures were compared at each time point by split-plot ANOVA or Kruskal-Wallis (if data was not normally distributed) where appropriate for effects by dose, p<0.05 was considered significant for post-hoc Bonferonni analyses. Sex of the pups was recorded for future post-hoc investigation but was not considered a factor in the current analyses. All statistical analyses were completed using SPSS 23.0 (IBM, Armonk, NY).
Table 1.
Ex Vivo Cephalometric Specimens
| Description | 15 days PN | 20 Days PN | 25 Days PN |
|---|---|---|---|
| Wild Type (Twist +/+) | 26 | 19 | 23 |
| Twist +/− Unexposed | 18 | 13 | 8 |
| Twist +/− Exposed | 8 | 16 | 11 |
Whole Mount Alizarin Red
A random selection of 3 skulls per group (Wild type, Twist 1 +/−, Twist 1 +/− exposed) per time point were used to determine gross dysmorphology and fusion of cranial sutures. Briefly, calvaria were defleshed, dissected, debrained, and placed in 0.5% potassium hydroxide (KOH) for 24 hours. Calvaria were then washed with de-ionized water and placed in 1.6% KOH with 33mg/liter alizarin red (Amresco, Solon, OH, USA) for 24 hours. Skulls were then washed in de-ionized water and placed in a clearing solution (2 parts glycerin, 1 part benzyl alcohol, and 1 part 70% Ethanol) for 3 hours, heated to 45°C for one hour, moved to a final storing solution (1 part glycerin, 1 part 70% Ethanol) and captured using a stereoscope with attached camera (Motic, British Columbia, Canada).
Cell Isolation
After sacrifice at pn 20, 11 litters denoted for cell isolation were separated by sex and genotype and tissue harvested to isolate the native suture cells (Cooper et al., 2010). Soft tissue was removed from the skull and the coronal suture was excised utilizing surgical scissors. The bone pieces were incubated in 5 mL of a 4% collagenase I solution at 37°C on an orbital shaker for 30 minutes, then centrifuged at 5000*g for 5 minutes, and then the supernatant was aspirated. Bone fragments were then resuspended and transferred into 75 mm2 flasks with standard culture media, Dulbecco's Modified Eagle's Medium (DMEM; Lonza, Allendale, NJ, USA) containing 10% fetal bovine serum (FBS; Atlanta Biologics, Atlanta, GA, USA), 1% penicillin/streptomycin (penstrep; Lonza), and 0.2% amphotericin B (Lonza) (Cooper et al., 2010). Primary, wild type and Twist 1 +/− coronal suture cells were cultured in standard media with changes twice a week until 95% confluence was reached. At the time of confluence, cells were seeded at a density of 4,000 cells per well for cell proliferation and alkaline phosphatase (ALP) assays, and 65,000 cells/well for RNA collection. Cells were treated with standard media only or standard media supplemented with thyroxine. Thyroxine concentrations corresponded to clinical high, normal, and low circulating serum levels (780ng/ml, 78 ng/ml, or 7.8 ng/ml respectively) (Kronenberg and Williams, 2008).
Bioassays
After 3 or 7 days in culture cell viability (proliferation) was assessed with the colorimetric MTS assay (Promega, Madison, WI, USA) (Cory et al., 1991). Briefly, 20 μL of the MTS solution was added to each well and incubated at 37 °C for 1 hour. The 96 well plate was read at 490 nm on a Gen5 plate reader for absorbance (BioTek, Winooski, VT, USA). Quantitative ALP activity was assessed at 3 and 7 days. Cells were lysed in a 0.1% Triton X lysis buffer and whole cell lysate was measured using SigmaFast p-Nitrophenyl phosphate kit (Sigma-Aldrich, St. Louis, MO, USA) by adding para-nitrophenylphosphate (pNPP) as a substrate assay buffer containing MgCl2 for 30 minutes, and the kinetic of absorbance was read at 405 nm. Standard t-test and ANOVA with post-hoc Bonferonni analyses were conducted on quantitative data (MTS, ALP) where appropriate. Violations of homogeneity of variation resulted in the use of Welch's correction. A total of n=8 wild-type control cell populations were used in the bioassays. For Twist +/− we had n=8 for MTS but some cell population loss for our ALP assay resulting in an n=5 for 3day and n=7 for our 7day Twist +/− data.
qrt-PCR
For RNA expression studies cells were treated with standard proliferation media or a high concentration of thyroxine (780 ng/ml) for 3 or 7 days in culture. Cells were detached, lysates pelleted and RNA isolated using the RNeasy Plus Kit (Qiagen, Valencia, CA, USA). Quantity and quality of RNA was assessed using a Spectrophotometer (Nanodrop 1000, Wilmington, DE, USA). All ratios of absorbance at 260 and 280 nm were greater than 2.0. Experiments were run using a one-step kit for cDNA synthesis and gene expression (TaqMan® RNA-to-Ct™ 1-Step Kit, Life Technologies, Thermo-Fisher Scientific, Walltham, MA, USA) following manufacturer instructions. A master mix was made from nuclease free water, rt-mix, enzyme mix and commercially prepared probe/primer sets (Taqman Gene Expression Assays, Table 2). A total of 50ng of RNA from each sample was added to the master mix for each reaction (run in duplicate) for each gene product for each condition time (3 or 7 day) by treatment (media only control or media supplemented with thyroxine). Data were normalized to 18S ribosomal RNA expression by ΔCT. Quantitative data were compared for gene expression change due to treatment by ΔΔCT methodology. We used statistical analyses for qrt-PCR data as previously published to determine statistical differences for gene expression (ΔΔCT and ΔΔCT standard deviation) after thyroxine treatment for targets of interest at time of exposure (Yuan et al., 2006). Differences were considered statistically significant if p≤0.05. A resulting total of n=4 WT control cell populations and n=9 Twist +/− were used for gene expression studies.
Table 2.
Quantitative qRT-PCR TaqMan Assay (Applied Biosystems)
| Gene Symbol | Gene Name | Assay ID |
|---|---|---|
| Runx2 | Runt Related Transcription Factor 2 | Mm00501584_m1 |
| Alp | Alkaline Phosphatase | Mm00475834_m1 |
| Bglap | Bone Gamma-Carboxyglutamate Protein 3 | Mm01741771_g1 |
| Htra1 | HtrA Serine Peptidase 1 | Mm00479892_m1 |
| Igf1 | Insulin Like Growth Factor 1 | Mm00439560_m1 |
| Ehd1 | EH-Domain Containing 1 | Mm01236839_m1 |
| Dio3 | Deiodinase, Iodothyronine Type III | Mm00548953_s1 |
| Pappa | Pregnancy-Associated Plasma Protein A | Mm01259244_m1 |
| 18S | 18S ribosomal RNA | Mm03928990_g1 |
Results
Growth after In Utero Thyroxine Exposure
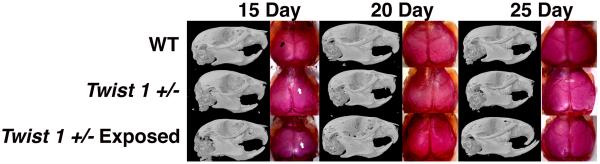
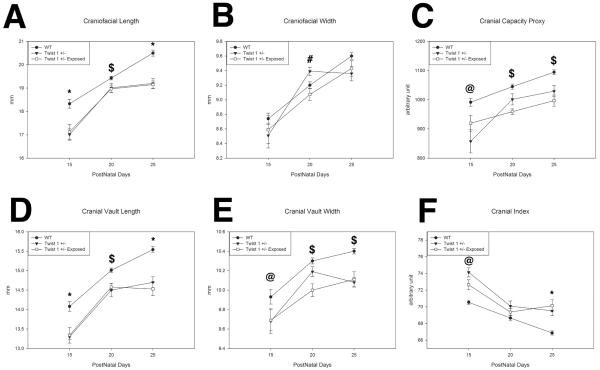
Figure 1 constitutes micro computed tomography (μCT) and alizarin red whole mount representatives for 15, 20, and 25 day post-natal control wild-type unexposed, control Twist 1 +/− unexposed, and Twist 1 +/− exposed in utero to ~415 ng per day of exogenous thyroid hormone. Note the gross dysmorphology including fused sutures of the Twist 1 +/− animals for both unexposed and thyroxine exposed animals. By 15 days there is clear evidence of coronal suture fusion in the Twist 1 +/− model, regardless of thyroxine exposure. We examined several parameters of calvaria growth to determine if thyroxine altered the growth trajectory of our Twist 1 +/− craniosynostotic model. For craniofacial length (Figure 2A), there were significant differences at 15, 20, and 25 days postnatal (p<0.001, p=0.020, p<0.001 respectively). Post-hoc analysis showed wild type control to have greater craniofacial length than both Twist 1 +/− unexposed (p<0.001) and exposed (p=0.027) at 15 days, Twist 1 +/− exposed at 20 days (p=0.032), and both Twist unexposed (p=0.002) and exposed (p<0.001) at 25 days postnatal. No significant differences in craniofacial length between Twist 1 +/− unexposed and exposed groups were found at any time point. There was a significant difference at 20 days post-natal (p=0.013) for craniofacial width (Figure 2B). Post hoc analysis showed the Twist 1 +/− unexposed group to have greater width than Twist 1 +/− exposed (p=0.012). Cranial capacity (Figure 2C) showed significant differences at 15, 20, and 25 days postnatal (p=0.05, p<0.001, p<0.001 respectively). Post-hoc analyses indicated that wild type controls have greater cranial capacity measures than Twist 1 +/− unexposed at 15 days postnatal (p=0.05) and Twist 1 +/− exposed at 20 and 25 days postnatal (p<0.001 for both). Significant differences were found for cranial vault length (Figure 2D) at 15, 20, and 25 days postnatal (p<0.001, p=0.004, p<0.001 respectively). Post-hoc analysis showed wild-type control to have greater cranial length than both Twist 1 +/− unexposed (p<0.001) and exposed (p=0.05) at 15 days, Twist 1 +/− exposed at 20 days (p=0.001), and both Twist 1 +/− unexposed (p=0.002) and exposed (p<0.001) at 25 days postnatal. For cranial vault width (Figure 2E), there were significant differences at 15, 20, and 25 days postnatal (p=0.05, p=0.002, p<0.001 respectively) and post-hoc analysis showed wild-type control to have greater cranial width than Twist 1 +/− unexposed at 15 days postnatal (p=0.028) and Twist 1 +/− exposed at 20 (p=0.001) and 25 days (p=0.001) postnatal. Cranial index measures (Figure 2F) showed significant differences at 15 (p=0.017) and 25 days postnatal (p<0.001). Post-hoc analysis showed the Twist 1 +/− unexposed group to have a larger cranial index than wild type control (p=0.023). Both Twist 1 +/− unexposed and Twist 1 +/− exposed had larger cranial indices than wild type control at 25 days postnatal (p=0.001 and p<0.001 respectively). No significant differences in craniofacial length, cranial capacity, cranial vault length, cranial vault width, or cranial index were found between Twist 1 +/− unexposed and Twist 1 +/− exposed groups at any time point.
Figure 1. Effects of in utero thyroxine exposure on Twist 1 +/− post-natal craniofacial morphology.
Representative μCT and whole mount staining for pn 15 day (right) 20day (middle) and 25 day (left) for wild type (WT) (top), Twist 1 +/− unexposed (middle), and Twist 1 +/− exposed to ~415ng per day thyroxine in utero (bottom). Note the dysmorphology including fused coronal sutures in Twist 1 +/− animals (white arrows) regardless of exposure at all time points. Black arrow indicates patent coronal suture.
Figure 2. Effects of in utero thyroxine exposure Twist 1 +/− calvarial growth at 15, 20, and 25 days postnatal.
A. Wild types have more craniofacial length than Twist 1 +/− unexposed (p<0.001) and exposed (p=0.027) at 15 days, exposed at 20 days (p=0.032), and both unexposed (p=0.002) and exposed (p<0.001) at 25 days. B. Twist 1 +/− unexposed have greater craniofacial width than exposed (p=0.012). C. Wild types have greater cranial capacity than unexposed at 15 days (p=0.05), and exposed at 20 and 25 days (p<0.001). D. Wild types have greater cranial length than unexposed (p<0.001) and exposed (p=0.05) at 15 days, exposed at 20 days (p=0.001), and unexposed (p=0.002) and exposed (p<0.001) at 25 days. E. Wild types have greater cranial width than unexposed at 15 days (p=0.028), and exposed at 20 (p=0.001) and 25 days (p=0.001). F. Twist 1 +/− unexposed have a larger cranial index than wild type (p=0.023), and both unexposed and exposed have larger cranial indices than wild type at 25 days (p=0.001 and p<0.001). (*) Wild type different from unexposed and exposed. ($) wild type different from exposed. (#) Unexposed is different from exposed. (@) wild type different from unexposed.
Effects of Exogenous Thyroid Hormone on Twist 1 +/− Cranial Suture Cells
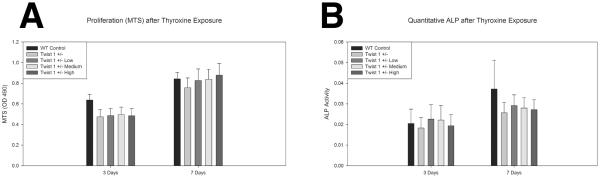
Figure 3 contains bioassay data for Twist 1 +/− derived suture cells (and wild type controls for comparison) treated with thyroxine. Data for MTS (proxy for cell proliferation) suggests no statistically significant differences in cell metabolic activity after 3 or 7 days in culture between wild type control, Twist 1 +/− untreated, or Twist 1 +/− treated with several concentrations of thyroxine (p=0.668, p=0.944). ALP data suggests Twist 1 +/− cells to have slightly decreased in vitro ALP activity under these culture conditions. There was greater overall ALP activity after 7 days compared to 3 days in culture. However, there were no significant differences after 3 or 7 days in culture between wild type control, Twist 1 +/− untreated, or Twist 1 +/− treated with several concentrations of thyroxine (p=0.987, p=0.828).
Figure 3. In vitro effects of exogenous thyroid hormone on Twist 1 +/− cranial suture cell metabolism and differentiation.
A. Assessment of cell proliferation (MTS) for wild type control, Twist 1 +/− untreated, and Twist 1 +/− cranial suture cells treated with low (7.8ng/ml), medium (78ng/ml), and high (780ng/ml) doses of thyroxine for 3 and 7 days demonstrates no significant differences in metabolic activity between groups. B. Assessment of alkaline phosphatase activity (ALP) shows Twist 1 +/− cells to have slightly decreased ALP activity at 7 days, however, there were no significant differences between groups at either time point.
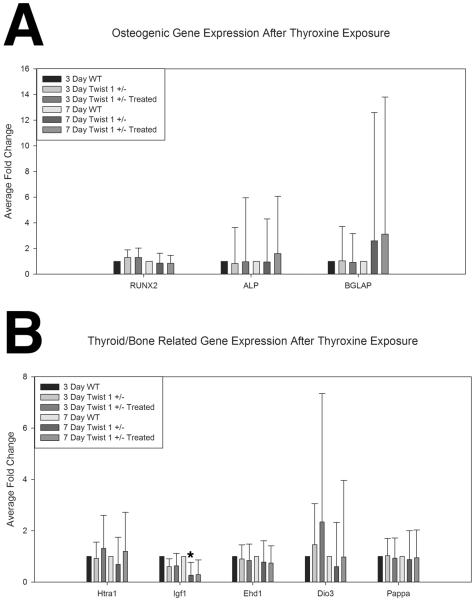
Investigation of osteogenic gene expression (Figure 4A) (Runx2, Alp, and Bglap) was conducted between wild type control and Twist 1 +/− untreated and treated cells. Supplemental Table 1 includes data reflecting the endogenous variability of the biological replicate samples. These data suggest the wild type control samples to have less variability than the Twist control or treated samples for the osteogenic gene expression. Fold change data for Runx2 showed slight modulation of this marker between wild type controls and Twist 1 +/− untreated and treated cells with a slight increase after 3 days in culture (1.3 fold each respectively), and a slight decrease after 7 days in culture (~.85 fold each respectively). There were not however any statistically significant changes for Runx2, an early marker of osteogenic commitment, between groups. Alp gene expression, was decreased after 3 days in culture for Twist 1 +/− untreated (.83 fold) compared to wild type control. By 7 days however, an increase in Alp gene expression for Twist 1 +/− cells treated with thyroxine compared to wild type (1.6 fold) was found. Twist 1 +/− treated cells had greater Alp expression at both 3 and 7 days in culture compared to Twist 1 +/− untreated cells (1.15 and 1.7 fold respectively). Interestingly, Bglap (osteocalcin) gene expression showed greater expression for both Twist 1 +/− untreated (2.6 fold) and treated cells (3.1 fold) compared to wild type controls after 7 days in culture. However, these results were variable, exhibiting great intragroup standard deviations; therefore, no statistically significant differences were observed.
Figure 4. In vitro effects of exogenous thyroid hormone on Twist 1 +/− cranial suture cell gene expression.
A. Osteogenic gene expression from wild type control (n=4), Twist 1 +/− untreated (n=9), and Twist 1 +/− (n=9) cranial suture cells treated with low (7.8ng/ml), medium (78ng/ml), and high (780ng/ml) doses of thyroxine for 3 and 7 days demonstrated a slight modulation of Runx2 activity between control and Twist 1 +/−, a slight increase in Alp associated with treatment, and an increase in Bglap (osteocalcin) expression associated with the Twist 1 +/− genotype. There were no statistically significant differences in the assessed osteogenic gene markers by treatment, group, or time. B. Thyroid related gene expression for all three groups of cranial suture cells demonstrated a slight effect of treatment for Htra1 as compared to wild type control and Twist 1 +/− untreated, and significantly more Igf1 expression in wild type control cells (*). Some modulation of Ehd1, and Dio3, were noted by treatment and genotype, but none was statistically significant. There was no significant change in Pappa gene expression noted between groups, or across times. Error bars reflect standard error of the fold change. Wild Type Control Samples measure of dispersion are included in Supplemental Table 1.
Investigation of thyroid related bone gene expression (Figure 4B, Supplemental Table 1) was conducted between wild type control and Twist 1 +/− untreated and treated cells Supplemental Table 1 includes data reflecting the endogenous variability of the biological replicate samples. These data suggest the wild type control samples to have similar variability as the Twist control or treated samples for thyroid related gene expression, with the exception of Igf1 and Ehd1. Fold change data for Htra1 shows decreased expression for Twist 1 +/− untreated compared to wild type control after 3 (.88 fold) and 7 days (.64 fold) in culture. However, it is observed that Twist 1 +/− treated cells have greater expression compared to wild type control (3 day 1.24 fold, 7 day 1.16 fold) and Twist 1 +/− untreated cells (3 day 1.41 fold, 7 day 1.82 fold) at both time points in culture. These results were not statistically significant. There was an interesting relationship for Igf1 gene expression in that wild type control cells had greater gene expression than both the Twist 1 +/− untreated and treated cells at both time points. After 7 days in culture the difference was statistically significant for Twist 1 +/− treated cells, p=0.05 (0.26 fold or greater than 3 fold downregulation) and trending for Twist 1 +/− untreated cells, p=0.06 (0.29 fold). There were no other statistically significant gene expression alterations for Igf1. Ehd1 expression was observed to be less for Twist 1 +/− untreated and treated samples compared to wild type controls especially after 7 days in culture (.78 and .74 fold respectively). Data for Dio3 shows increases in gene expression after 3 days in culture for Twist 1 +/− untreated (1.47 fold) and Twist 1 +/− treated (2.34 fold) compared to wild type controls. Expression was observed to be decreased for Twist 1 +/− unexposed (.60 fold) and normalized to similar levels for Twist 1 +/− treated cells compare to wild type controls after 7 days in culture. As expected Twist 1 +/− treated cells had greater gene expression than Twist 1 +/− untreated cells for both 3 (1.60 fold) and 7 day (1.63 fold) time points, but was not found to be statistically significant. For Pappa gene expression, great differences were not observed for wild type, Twist 1 +/− untreated, or Twist 1 +/− treated comparisons.
Discussion
Overall our data suggest that the Twist 1 +/− mutation is a driving factor for aberration in craniofacial growth trajectory compared to controls. We were not able to establish segregating differences in growth parameters and observed few cell based gene activity alterations with physiological challenges of exogenous thyroid hormone. As the activity of cells within the cranial sutures is highly controlled to maintain fibrous tissue between osteogenic fronts allowing for proper craniofacial growth and development, even slight modulations of activity may be biologically important without being statistically significant. In our model of gene- environment interaction some predicted genes were modulated. Alp and Bglap were slightly modulated by thyroxine treatment and greater in Twist 1 +/− cells which we assume has a greater contribution of pre-osteoblasts and osteoblasts compared to stem cells or fibroblasts in the cell milieu isolated from the suture compared to wild type controls (Berendsen and Olsen, 2015; Long, 2012; Marini and Blissett, 2013). Runx2 expression was lower in our Twist 1 +/− suggesting overall greater contribution of osteoblasts in culture (Berendsen and Olsen, 2015; Long, 2012; Marini and Blissett, 2013). This would make sense as Twist is necessary for stemness of cells, particularly within the sutures of the skull (Miraoui and Marie, 2010; Zhao et al., 2015). Heterozygous loss of Twist signal, loss of stem cells, and increased osteogenesis are likely responsible for the resulting craniofacial phenotype.
Concerning thyroid related genes contributing to bone phenotype, Htra1 encodes for a protein that cleaves Igf from its receptor allowing for greater local and systemic circulating levels (Spyropoulou et al., 2015; Tiaden and Richards, 2013). This axis is viewed as pro-osteogenic and as thyroid hormone drives increases in these products, it is also viewed to be pro-osteogenic (Motomura and Brent, 1998). Here we see little modulation of Htra1 in vitro and an overall decrease in Igf signal in our Twist 1 +/− cells compared to wild type controls. It is known that osteoblast precursors are particularly susceptible to this axis, showing great increases in both products after in vitro administration of thyroxine (Bassett and Williams, 2003; Cray et al., 2013). It is likely that the presence of more mature cells, specifically osteoblasts in these cultures might lead to a lack of responsiveness of Twist 1 +/− cells in this culture system. Of the three other genes thought to directly modulate thyroid effects on bone or bony development, Dio3 was observed to have some modulation in this in vitro system. This gene is a marker of thyrotoxicosis (Hernandez et al., 2006), which we attempted to model here, and acts to inactivate thyroid hormone in several stages of development. We observe Dio3 expression highest early in our culture experiments (3 days), but it normalizes later in the time course (7 days). Overall we see no in vitro evidence of modulation or exacerbation of the Twist 1 +/− osteogenic or thyroid related profile.
With no observed in vivo or in vitro modulation of the Twist 1 +/− profile after exogenous thyroid challenge there are several limitations but also lessons to be learned from these experiments. There are multiple possible reasons why alterations in phenotype did not occur. In vivo dosing or timing of administration may have contributed to the lack of a phenotype. However, the dose is well described in the literature and is in a midrange of physiologically relevant doses that reflect the fluctuation observed during periods of thyrotoxicosis and unlike a more severe thyroid dysregulation such as Maternal Graves Disease (de Lima et al., 1999; McNab and Ginsberg, 2005; Radetti et al., 2002; Rasmussen et al., 2007). The timing of in utero exposure was targeted at calvarial development and has been used to elucidate other relevant pharmacological effects on craniofacial development and craniosynostosis (Cray et al., 2014; Durham et al., 2015). In addition, ages chosen for mouse craniofacial comparisons may have led us to miss relevant aberration in growth trajectory. Importantly, growth and morphology of the cranial vault is largely determined by the growth of the brain (Moss and Young, 1960; Riesenfeld, 1967; Sardi et al., 2007; Sun et al., 2004). In this case it is possible that the similarity between wild type and Twist 1 +/− cells in vitro and the dissimilarity between wild-type and Twist 1 +/− mice in vivo is reflective of the cranial sutures following rather than leading craniofacial growth trajectories in this model. Additionally, final form was similar between the Twist 1 +/− unexposed and exposed animals suggesting overall, the gene was, in this case, too predictive of morphological phenotype and not amenable to challenge with this agent.
In vitro arguments are also founded here. We chose in vitro doses relevant to circulating human levels to capture any alteration in cellular activity. None were observed, at least for proliferation and differentiation of paramount importance in craniosynostosis. For gene expression we do observe some indication of increased osteogenic markers in our Twist 1 +/− samples and even some modulation by thyroid hormone. However, these data were plagued by a great amount of variability between biological samples. This was not an unforeseen result considering the Twist 1 +/− phenotype presents with patent, fused, and fusing sutures (Miraoui and Marie, 2010; Parsons et al., 2014), compared to our wild type controls which all exhibit coronal suture patency. Thus we might expect to observe more variability within our Twist 1 +/− samples. In addition, although there were slight aberrations in the thyroid related genes studied here for the Twist 1 +/− cells, there were no great changes that might be suggestive of a positive or negative effect of the thyroid drug. Again, this may indicate that the suture area including the suture cells do not drive the aberrant craniofacial growth associated with the Twist 1 +/− model.
In conclusion, we cannot claim a successful modeling of a gene-environment interaction between Twist 1 +/− craniosynostosis and exogenous thyroid hormone exposure. If anything, in this milder case of a craniosynostotic phenotype, the gene won. There was no additive effect of this potential teratogenic challenge. This is an important point as there is no evidence in the literature that is suggestive of specific interactions between lack of thyroid hormone homeostasis and Twist 1 +/− driven craniosynostosis. However, from an additive modeling perspective the Twist 1+/− murine model provides an available alternative to a non-syndromic craniosynostosis model and has the necessary features for such study (mild phenotype, postnatal viability). Although, this is one sole test of the gene-environment interaction or additivity (Tabery, 2007), other models with more severe phenotypes might be expected to show even less effect of an “environmental” challenge. It is of potentially more translational impact to study pure environmental challenges for craniosynostosis (i.e. thyroid hormone in the wild type murine system) similar to that which was clinically impactful for other craniofacial disorders (Lammer et al., 1985; Lau and Li, 1995; Yasuda et al., 1986).
Supplementary Material
Acknowledgments
This study utilized the facilities and resources of the MUSC Center for Oral Health Research (COHR). ELD and RNH are funded through a training grant from the National Institutes of Health National Institute of Dental and Craniofacial Research [5T32DE017551]. The MUSC Center for Oral Health Research (COHR), is partially supported by the National Institutes of Health National Institute of General Medicine [P30GM103331].
Grant Sponsor: National Institute of Dental and Craniofacial Research [R03DE023350A to JJC], Cleft Palate Foundation Cleft/Craniofacial Anomalies Grant Award [to JJC], National Institute of Aging (NIA) [1P01AG036675 to ME].
Footnotes
Disclosure Statement: The authors have declared that no conflict of interest exists and there is nothing to disclose.
Literature Cited
- Akita S, Hirano A, Fujii T. Identification of IGF-I in the calvarial suture of young rats: histochemical analysis of the cranial sagittal sutures in a hyperthyroid rat model. Plast Reconstr Surg. 1996;97(1):1–12. doi: 10.1097/00006534-199601000-00001. [DOI] [PubMed] [Google Scholar]
- Akita S, Nakamura T, Hirano A, Fujii T, Yamashita S. Thyroid hormone action on rat calvarial sutures. Thyroid. 1994;4(1):99–106. doi: 10.1089/thy.1994.4.99. [DOI] [PubMed] [Google Scholar]
- Alwan S, Reefhuis J, Rasmussen SA, Olney RS, Friedman JM, National Birth Defects Prevention S Use of selective serotonin-reuptake inhibitors in pregnancy and the risk of birth defects. N Engl J Med. 2007;356(26):2684–2692. doi: 10.1056/NEJMoa066584. [DOI] [PubMed] [Google Scholar]
- Ardalan M, Rafati A, Nejat F, Farazmand B, Majed M, El Khashab M. Risk factors associated with craniosynostosis: a case control study. Pediatr Neurosurg. 2012;48(3):152–156. doi: 10.1159/000346261. [DOI] [PubMed] [Google Scholar]
- Bassett JH, Williams GR. The molecular actions of thyroid hormone in bone. Trends Endocrinol Metab. 2003;14(8):356–364. doi: 10.1016/s1043-2760(03)00144-9. [DOI] [PubMed] [Google Scholar]
- Berendsen AD, Olsen BR. Bone development. Bone. 2015;80:14–18. doi: 10.1016/j.bone.2015.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers CY, Schally AV, Reynolds GA, Hawley WD. Interactions of L-thyroxine or L-triiodothyronine and thyrotropin-releasing factor on the release and synthesis of thyrotropin from the anterior pituitary gland of mice. Endocrinology. 1967;81(4):741–747. doi: 10.1210/endo-81-4-741. [DOI] [PubMed] [Google Scholar]
- Browne ML, Hoyt AT, Feldkamp ML, Rasmussen SA, Marshall EG, Druschel CM, Romitti PA. Maternal caffeine intake and risk of selected birth defects in the National Birth Defects Prevention Study. Birth Defects Res A Clin Mol Teratol. 2011;91(2):93–101. doi: 10.1002/bdra.20752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capuco AV, Kahl S, Jack LJ, Bishop JO, Wallace H. Prolactin and growth hormone stimulation of lactation in mice requires thyroid hormones. Proc Soc Exp Biol Med. 1999;221(4):345–351. doi: 10.1046/j.1525-1373.1999.d01-91.x. [DOI] [PubMed] [Google Scholar]
- Carmichael SL, Ma C, Rasmussen SA, Cunningham ML, Browne ML, Dosiou C, Lammer EJ, Shaw GM. Craniosynostosis and risk factors related to thyroid dysfunction. Am J Med Genet A. 2015;167A(4):701–707. doi: 10.1002/ajmg.a.36953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmichael SL, Ma C, Rasmussen SA, Honein MA, Lammer EJ, Shaw GM, National Birth Defects Prevention S Craniosynostosis and maternal smoking. Birth Defects Res A Clin Mol Teratol. 2008;82(2):78–85. doi: 10.1002/bdra.20426. [DOI] [PubMed] [Google Scholar]
- Carmichael SL, Rasmussen SA, Lammer EJ, Ma C, Shaw GM, National Birth Defects Prevention S Craniosynostosis and nutrient intake during pregnancy. Birth Defects Res A Clin Mol Teratol. 2010;88(12):1032–1039. doi: 10.1002/bdra.20717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chawla R, Alden TD, Bizhanova A, Kadakia R, Brickman W, Kopp PA. Squamosal Suture Craniosynostosis Due to Hyperthyroidism Caused by an Activating Thyrotropin Receptor Mutation (T632I) Thyroid. 2015;25(10):1167–1172. doi: 10.1089/thy.2014.0503. [DOI] [PubMed] [Google Scholar]
- Chen L, Li D, Li C, Engel A, Deng CX. A Ser252Trp [corrected] substitution in mouse fibroblast growth factor receptor 2 (Fgfr2) results in craniosynostosis. Bone. 2003;33(2):169–178. doi: 10.1016/s8756-3282(03)00222-9. [DOI] [PubMed] [Google Scholar]
- Cohen MM., Jr Craniosynostosis update 1987. Am J Med Genet Suppl. 1988;4:99–148. doi: 10.1002/ajmg.1320310514. [DOI] [PubMed] [Google Scholar]
- Cooper GM, Lensie EL, Cray JJ, Jr., Decesare GE, Smalley MA, Losee JE, Mooney MP. BMP-4 response in wild-type and craniosynostotic rabbit bone cells. Plast Reconstr Surg. 2010;125(5):1403–1411. doi: 10.1097/PRS.0b013e3181d62ad4. [DOI] [PubMed] [Google Scholar]
- Cory AH, Owen TC, Barltrop JA, Cory JG. Use of an aqueous soluble tetrazolium/formazan assay for cell growth assays in culture. Cancer Commun. 1991;3(7):207–212. doi: 10.3727/095535491820873191. [DOI] [PubMed] [Google Scholar]
- Coussens AK, Wilkinson CR, Hughes IP, Morris CP, van Daal A, Anderson PJ, Powell BC. Unravelling the molecular control of calvarial suture fusion in children with craniosynostosis. BMC Genomics. 2007;8:458. doi: 10.1186/1471-2164-8-458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane NJ, Morris MD, Ignelzi MA, Yu G. Raman imaging demonstrates FGF2-induced craniosynostosis in mouse calvaria. J Biomed Opt. 2005;10(3):031119. doi: 10.1117/1.1908057. [DOI] [PubMed] [Google Scholar]
- Cray JJ, Jr., Khaksarfard K, Weinberg SM, Elsalanty M, Yu JC. Effects of thyroxine exposure on osteogenesis in mouse calvarial pre-osteoblasts. PLoS One. 2013;8(7):e69067. doi: 10.1371/journal.pone.0069067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cray JJ, Jr., Weinberg SM, Parsons TE, Howie RN, Elsalanty M, Yu JC. Selective serotonin reuptake inhibitor exposure alters osteoblast gene expression and craniofacial development in mice. Birth Defects Res A Clin Mol Teratol. 2014;100(12):912–923. doi: 10.1002/bdra.23323. [DOI] [PubMed] [Google Scholar]
- de Lima MA, Oliveira LB, Paim N, Borges Mde F. Congenital hyperthyroidism: autopsy report. Rev Hosp Clin Fac Med Sao Paulo. 1999;54(3):103–106. doi: 10.1590/s0041-87811999000300007. [DOI] [PubMed] [Google Scholar]
- Delahyne S, Bernard JP, Renier D, Villw Y. Prenatal ultrasound diagnosis of fetal craniosynostosis. Ultrasound Obstet Gynecol. 2003;21:347–353. doi: 10.1002/uog.91. [DOI] [PubMed] [Google Scholar]
- Durham E, Jen S, Wang L, Nasworthy J, Elsalanty M, Weinberg S, Yu J, Cray J. Effects of Citalopram on Sutural and Calvarial Cell Processes. PLoS One. 2015;10(10):e0139719. doi: 10.1371/journal.pone.0139719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng W, Choi I, Clouthier DE, Niswander L, Williams T. The Ptch1(DL) mouse: a new model to study lambdoid craniosynostosis and basal cell nevus syndrome-associated skeletal defects. Genesis. 2013;51(10):677–689. doi: 10.1002/dvg.22416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong SG. The Fgfr2 W290R mouse model of Crouzon syndrome. Childs Nerv Syst. 2012;28(9):1495–1503. doi: 10.1007/s00381-012-1792-y. [DOI] [PubMed] [Google Scholar]
- Grova M, Lo DD, Montoro D, Hyun JS, Chung MT, Wan DC, Longaker MT. Models of cranial suture biology. J Craniofac Surg. 2012;23(7 Suppl 1):1954–1958. doi: 10.1097/SCS.0b013e318258ba53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashmi SS, Canfield MA, Marengo L, Moffitt KB, Belmont JW, Freedenberg D, Tanksley SM, Lupo PJ. The association between neonatal thyroxine and craniosynostosis, Texas, 2004–2007. Birth Defects Res A Clin Mol Teratol. 2012;94(12):1004–1009. doi: 10.1002/bdra.23077. [DOI] [PubMed] [Google Scholar]
- Hermann CD, Lee CS, Gadepalli S, Lawrence KA, Richards MA, Olivares-Navarrete R, Williams JK, Schwartz Z, Boyan BD. Interrelationship of cranial suture fusion, basicranial development, and resynostosis following suturectomy in twist1(+/−) mice, a murine model of Saethre-Chotzen syndrome. Calcif Tissue Int. 2012;91(4):255–266. doi: 10.1007/s00223-012-9632-3. [DOI] [PubMed] [Google Scholar]
- Hernandez A, Martinez ME, Fiering S, Galton VA, St Germain D. Type 3 deiodinase is critical for the maturation and function of the thyroid axis. J Clin Invest. 2006;116(2):476–484. doi: 10.1172/JCI26240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes G, Basilico C. Mesodermal expression of Fgfr2S252W is necessary and sufficient to induce craniosynostosis in a mouse model of Apert syndrome. Dev Biol. 2012;368(2):283–293. doi: 10.1016/j.ydbio.2012.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes G, Rothschild G, Roy UB, Deng CX, Mansukhani A, Basilico C. Early onset of craniosynostosis in an Apert mouse model reveals critical features of this pathology. Dev Biol. 2009;328(2):273–284. doi: 10.1016/j.ydbio.2009.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Meng T, Wang S, Zhang H, Mues G, Qin C, Feng JQ, D'Souza RN, Lu Y. Twist1- and Twist2-haploinsufficiency results in reduced bone formation. PLoS One. 2014;9(6):e99331. doi: 10.1371/journal.pone.0099331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson D, Wilkie AO. Craniosynostosis. Eur J Hum Genet. 2011;19(4):369–376. doi: 10.1038/ejhg.2010.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston MC, Bronsky PT. Prenatal craniofacial development: new insights on normal and abnormal mechanisms. Critical reviews in oral biology and medicine : an official publication of the American Association of Oral Biologists. 1995;6(4):368–422. doi: 10.1177/10454411950060040601. [DOI] [PubMed] [Google Scholar]
- Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol. 2010;8(6):e1000412. doi: 10.1371/journal.pbio.1000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause K, Weiner J, Hones S, Kloting N, Rijntjes E, Heiker JT, Gebhardt C, Kohrle J, Fuhrer D, Steinhoff K, Hesse S, Moeller LC, Tonjes A. The Effects of Thyroid Hormones on Gene Expression of Acyl-Coenzyme A Thioesterases in Adipose Tissue and Liver of Mice. Eur Thyroid J. 2015;4(Suppl 1):59–66. doi: 10.1159/000437304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronenberg H, Williams RH. Williams textbook of endocrinology. 11th ed. xix. Saunders/Elsevier; Philadelphia: 2008. p. 1911. [Google Scholar]
- Lammer EJ, Chen DT, Hoar RM, Agnish ND, Benke PJ, Braun JT, Curry CJ, Fernhoff PM, Grix AW, Jr., Lott IT, et al. Retinoic acid embryopathy. N Engl J Med. 1985;313(14):837–841. doi: 10.1056/NEJM198510033131401. [DOI] [PubMed] [Google Scholar]
- Lau EC, Li ZQ. Protection of mice from teratogen-induced cleft palate by exogenous methionine. Proc Soc Exp Biol Med. 1995;209(2):141–145. doi: 10.3181/00379727-209-43887. [DOI] [PubMed] [Google Scholar]
- Lenton KA, Nacamuli RP, Wan DC, Helms JA, Longaker MT. Cranial suture biology. Curr Top Dev Biol. 2005;66:287–328. doi: 10.1016/S0070-2153(05)66009-7. [DOI] [PubMed] [Google Scholar]
- Levi B, Wan DC, Wong VW, Nelson E, Hyun J, Longaker MT. Cranial suture biology: from pathways to patient care. J Craniofac Surg. 2012;23(1):13–19. doi: 10.1097/SCS.0b013e318240c6c0. [DOI] [PubMed] [Google Scholar]
- Liu B, Yu HM, Hsu W. Craniosynostosis caused by Axin2 deficiency is mediated through distinct functions of beta-catenin in proliferation and differentiation. Dev Biol. 2007;301(1):298–308. doi: 10.1016/j.physletb.2003.10.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long F. Building strong bones: molecular regulation of the osteoblast lineage. Nat Rev Mol Cell Biol. 2012;13(1):27–38. doi: 10.1038/nrm3254. [DOI] [PubMed] [Google Scholar]
- Marini JC, Blissett AR. New genes in bone development: what's new in osteogenesis imperfecta. J Clin Endocrinol Metab. 2013;98(8):3095–3103. doi: 10.1210/jc.2013-1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNab T, Ginsberg J. Use of anti-thyroid drugs in euthyroid pregnant women with previous Graves' disease. Clin Invest Med. 2005;28(3):127–131. [PubMed] [Google Scholar]
- Miraoui H, Marie PJ. Pivotal role of Twist in skeletal biology and pathology. Gene. 2010;468(1–2):1–7. doi: 10.1016/j.gene.2010.07.013. [DOI] [PubMed] [Google Scholar]
- Mooney MP, Losken HW, Tschakaloff A, Siegel MI, Losken A, Lalikos JF. Congenital bilateral coronal suture synostosis in a rabbit and craniofacial growth comparisons with experimental models. Cleft Palate Craniofac J. 1993;30(2):121–128. doi: 10.1597/1545-1569_1993_030_0121_cbcssi_2.3.co_2. [DOI] [PubMed] [Google Scholar]
- Mooney MP, Siegel MI, Burrows AM, Smith TD, Losken HW, Dechant J, Cooper G, Fellows-Mayle W, Kapucu MR, Kapucu LO. A rabbit model of human familial, nonsyndromic unicoronal suture synostosis. II. Intracranial contents, intracranial volume, and intracranial pressure. Childs Nerv Syst. 1998a;14(6):247–255. doi: 10.1007/s003810050220. [DOI] [PubMed] [Google Scholar]
- Mooney MP, Siegel MI, Burrows AM, Smith TD, Losken HW, Dechant J, Cooper G, Kapucu MR. A rabbit model of human familial, nonsyndromic unicoronal suture synostosis. I. Synostotic onset, pathology, and sutural growth patterns. Childs Nerv Syst. 1998b;14(6):236–246. doi: 10.1007/s003810050219. [DOI] [PubMed] [Google Scholar]
- Morita J, Nakamura M, Kobayashi Y, Deng CX, Funato N, Moriyama K. Soluble form of FGFR2 with S252W partially prevents craniosynostosis of the apert mouse model. Dev Dyn. 2014;243(4):560–567. doi: 10.1002/dvdy.24099. [DOI] [PubMed] [Google Scholar]
- Morriss-Kay GM, Wilkie AO. Growth of the normal skull vault and its alteration in craniosynostosis: insights from human genetics and experimental studies. J Anat. 2005;207(5):637–653. doi: 10.1111/j.1469-7580.2005.00475.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss M, Young R. A functional approach to craniology. Am J Phys Anthropol. 1960;18:281–292. doi: 10.1002/ajpa.1330180406. [DOI] [PubMed] [Google Scholar]
- Motch Perrine SM, Cole TM, 3rd, Martinez-Abadias N, Aldridge K, Jabs EW, Richtsmeier JT. Craniofacial divergence by distinct prenatal growth patterns in Fgfr2 mutant mice. BMC Dev Biol. 2014;14:8. doi: 10.1186/1471-213X-14-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motomura K, Brent GA. Mechanisms of thyroid hormone action. Implications for the clinical manifestation of thyrotoxicosis. Endocrinol Metab Clin North Am. 1998;27(1):1–23. doi: 10.1016/s0889-8529(05)70294-2. [DOI] [PubMed] [Google Scholar]
- Nah HD, Koyama E, Agochukwu NB, Bartlett SP, Muenke M. Phenotype profile of a genetic mouse model for Muenke syndrome. Childs Nerv Syst. 2012;28(9):1483–1493. doi: 10.1007/s00381-012-1778-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Shea PJ, Bassett JH, Sriskantharajah S, Ying H, Cheng SY, Williams GR. Contrasting skeletal phenotypes in mice with an identical mutation targeted to thyroid hormone receptor alpha1 or beta. Mol Endocrinol. 2005;19(12):3045–3059. doi: 10.1210/me.2005-0224. [DOI] [PubMed] [Google Scholar]
- Parikh RP, Farber SJ, Nguyen D, Skolnick GB, Patel K, Woo AS. Risk Factors for Postoperative Complications After Surgical Correction of Craniosynostosis: A Nationwide Analysis of 1357 Intracranial Procedures. Plast Reconstr Surg. 2015;136(4 Suppl):40. [Google Scholar]
- Parsons TE, Weinberg SM, Khaksarfard K, Howie RN, Elsalanty M, Yu JC, Cray JJ., Jr Craniofacial shape variation in Twist1+/− mutant mice. Anat Rec (Hoboken) 2014;297(5):826–833. doi: 10.1002/ar.22899. [DOI] [PubMed] [Google Scholar]
- Penfold JL, Simpson DA. Premature craniosynostosis-a complication of thyroid replacement therapy. J Pediatr. 1975;86(3):360–363. doi: 10.1016/s0022-3476(75)80963-2. [DOI] [PubMed] [Google Scholar]
- Perlyn CA, DeLeon VB, Babbs C, Govier D, Burell L, Darvann T, Kreiborg S, Morriss-Kay G. The craniofacial phenotype of the Crouzon mouse: analysis of a model for syndromic craniosynostosis using three-dimensional MicroCT. Cleft Palate Craniofac J. 2006;43(6):740–748. doi: 10.1597/05-212. [DOI] [PubMed] [Google Scholar]
- Radetti G, Zavallone A, Gentili L, Beck-Peccoz P, Bona G. Foetal and neonatal thyroid disorders. Minerva Pediatr. 2002;54(5):383–400. [PubMed] [Google Scholar]
- Rasmussen SA, Yazdy MM, Carmichael SL, Jamieson DJ, Canfield MA, Honein MA. Maternal thyroid disease as a risk factor for craniosynostosis. Obstet Gynecol. 2007;110(2 Pt 1):369–377. doi: 10.1097/01.AOG.0000270157.88896.76. [DOI] [PubMed] [Google Scholar]
- Rawlins JT, Opperman LA. Tgf-beta regulation of suture morphogenesis and growth. Front Oral Biol. 2008;12:178–196. doi: 10.1159/000115038. [DOI] [PubMed] [Google Scholar]
- Reefhuis J, Honein MA, Schieve LA, Rasmussen SA, National Birth Defects Prevention S Use of clomiphene citrate and birth defects, National Birth Defects Prevention Study, 1997–2005. Hum Reprod. 2011;26(2):451–457. doi: 10.1093/humrep/deq313. [DOI] [PubMed] [Google Scholar]
- Riesenfeld A. Biodynamics of Head Form and Cranio-Facial Relationships. Homo. 1967;18:133–251. [Google Scholar]
- Robin NH. Molecular genetic advances in understanding craniosynostosis. Plast Reconstr Surg. 1999;103(3):1060–1070. [PubMed] [Google Scholar]
- Roy WA, Iorio RJ, Meyer GA. Craniosynostosis in vitamin D-resistant rickets. A mouse model. J Neurosurg. 1981;55(2):265–271. doi: 10.3171/jns.1981.55.2.0265. [DOI] [PubMed] [Google Scholar]
- Sardi M, Ventrice F, Ramirez Rozzi F. Allometrics throughout the late prenatal and early postnatal human craniofacial ontogeny. Anatomical record. 2007;290:1112–1120. doi: 10.1002/ar.20581. [DOI] [PubMed] [Google Scholar]
- Schaller BJ, Filis A, Merten HA, Buchfelder M. Premature craniosynostosis--the role of skull base surgery in its correction. A surgical and radiological experience of 172 operated infants/children. J Craniomaxillofac Surg. 2012;40(3):195–200. doi: 10.1016/j.jcms.2011.04.003. [DOI] [PubMed] [Google Scholar]
- Seto ML, Hing AV, Chang J, Hu M, Kapp-Simon KA, Patel PK, Burton BK, Kane AA, Smyth MD, Hopper R, Ellenbogen RG, Stevenson K, Speltz ML, Cunningham ML. Isolated sagittal and coronal craniosynostosis associated with TWIST box mutations. Am J Med Genet A. 2007;143A(7):678–686. doi: 10.1002/ajmg.a.31630. [DOI] [PubMed] [Google Scholar]
- Shukla V, Coumoul X, Wang RH, Kim HS, Deng CX. RNA interference and inhibition of MEK-ERK signaling prevent abnormal skeletal phenotypes in a mouse model of craniosynostosis. Nat Genet. 2007;39(9):1145–1150. doi: 10.1038/ng2096. [DOI] [PubMed] [Google Scholar]
- Simpson A, Wong AL, Bezuhly M. Surgical Correction of Nonsyndromic Sagittal Craniosynostosis: Concepts and Controversies. Ann Plast Surg. 2016 doi: 10.1097/SAP.0000000000000713. [DOI] [PubMed] [Google Scholar]
- Spyropoulou A, Karamesinis K, Basdra EK. Mechanotransduction pathways in bone pathobiology. Biochim Biophys Acta. 2015;1852(9):1700–1708. doi: 10.1016/j.bbadis.2015.05.010. [DOI] [PubMed] [Google Scholar]
- Sun Z, Lee E, Herring SW. Cranial sutures and bones: growth and fusion in relation to masticatory strain. Anat Rec A Discov Mol Cell Evol Biol. 2004;276(2):150–161. doi: 10.1002/ar.a.20002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabery J. Biometric and developmental gene-environment interactions: looking back, moving forward. Dev Psychopathol. 2007;19(4):961–976. doi: 10.1017/S0954579407000478. [DOI] [PubMed] [Google Scholar]
- Thordarson G, Fielder P, Lee C, Hom YK, Robleto D, Ogren L, Talamantes F. Mammary gland differentiation in hypophysectomized, pregnant mice treated with corticosterone and thyroxine. Biol Reprod. 1992;47(4):676–682. doi: 10.1095/biolreprod47.4.676. [DOI] [PubMed] [Google Scholar]
- Tiaden AN, Richards PJ. The emerging roles of HTRA1 in musculoskeletal disease. Am J Pathol. 2013;182(5):1482–1488. doi: 10.1016/j.ajpath.2013.02.003. [DOI] [PubMed] [Google Scholar]
- Twigg SR, Healy C, Babbs C, Sharpe JA, Wood WG, Sharpe PT, Morriss-Kay GM, Wilkie AO. Skeletal analysis of the Fgfr3(P244R) mouse, a genetic model for the Muenke craniosynostosis syndrome. Dev Dyn. 2009;238(2):331–342. doi: 10.1002/dvdy.21790. [DOI] [PubMed] [Google Scholar]
- Twigg SR, Wilkie AO. A Genetic-Pathophysiological Framework for Craniosynostosis. Am J Hum Genet. 2015;97(3):359–377. doi: 10.1016/j.ajhg.2015.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veistinen L, Takatalo M, Tanimoto Y, Kesper DA, Vortkamp A, Rice DP. Loss-of-Function of Gli3 in Mice Causes Abnormal Frontal Bone Morphology and Premature Synostosis of the Interfrontal Suture. Front Physiol. 2012;3:121. doi: 10.3389/fphys.2012.00121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang E, Nam HK, Liu J, Hatch NE. The effects of tissue-non-specific alkaline phosphatase gene therapy on craniosynostosis and craniofacial morphology in the FGFR2C342Y/+ mouse model of Crouzon craniosynostosis. Orthod Craniofac Res. 2015;18(Suppl 1):196–206. doi: 10.1111/ocr.12080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkie AO. Craniosynostosis: genes and mechanisms. Hum Mol Genet. 1997;6(10):1647–1656. doi: 10.1093/hmg/6.10.1647. [DOI] [PubMed] [Google Scholar]
- Wilkie AO. Epidemiology and genetics of craniosynostosis. Am J Med Genet. 2000;90(1):82–84. doi: 10.1002/(sici)1096-8628(20000103)90:1<82::aid-ajmg15>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Wilkie AO, Bochukova EG, Hansen RM, Taylor IB, Rannan-Eliya SV, Byren JC, Wall SA, Ramos L, Venancio M, Hurst JA, O'Rourke AW, Williams LJ, Seller A, Lester T. Clinical dividends from the molecular genetic diagnosis of craniosynostosis. Am J Med Genet A. 2007;143A(16):1941–1949. doi: 10.1002/ajmg.a.31905. [DOI] [PubMed] [Google Scholar]
- Wilkie AO, Wall SA. Craniosynostosis: novel insights into pathogenesis and treatment. Curr Opin Neurol. 1996;9(2):146–152. [PubMed] [Google Scholar]
- Yasuda Y, Okamoto M, Konishi H, Matsuo T, Kihara T, Tanimura T. Developmental anomalies induced by all-trans retinoic acid in fetal mice: I. Macroscopic findings. Teratology. 1986;34(1):37–49. doi: 10.1002/tera.1420340106. [DOI] [PubMed] [Google Scholar]
- Yeh E, Fanganiello RD, Sunaga DY, Zhou X, Holmes G, Rocha KM, Alonso N, Matushita H, Wang Y, Jabs EW, Passos-Bueno MR. Novel molecular pathways elicited by mutant FGFR2 may account for brain abnormalities in Apert syndrome. PLoS One. 2013;8(4):e60439. doi: 10.1371/journal.pone.0060439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan JS, Reed A, Chen F, Stewart CN., Jr Statistical analysis of real-time PCR data. BMC bioinformatics. 2006;7:85. doi: 10.1186/1471-2105-7-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakhary GM, Montes DM, Woerner JE, Notarianni C, Ghali GE. Surgical correction of craniosynostosis. A review of 100 cases. J Craniomaxillofac Surg. 2014;42(8):1684–1691. doi: 10.1016/j.jcms.2014.05.014. [DOI] [PubMed] [Google Scholar]
- Zhao H, Feng J, Ho TV, Grimes W, Urata M, Chai Y. The suture provides a niche for mesenchymal stem cells of craniofacial bones. Nat Cell Biol. 2015;17(4):386–396. doi: 10.1038/ncb3139. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.