Abstract
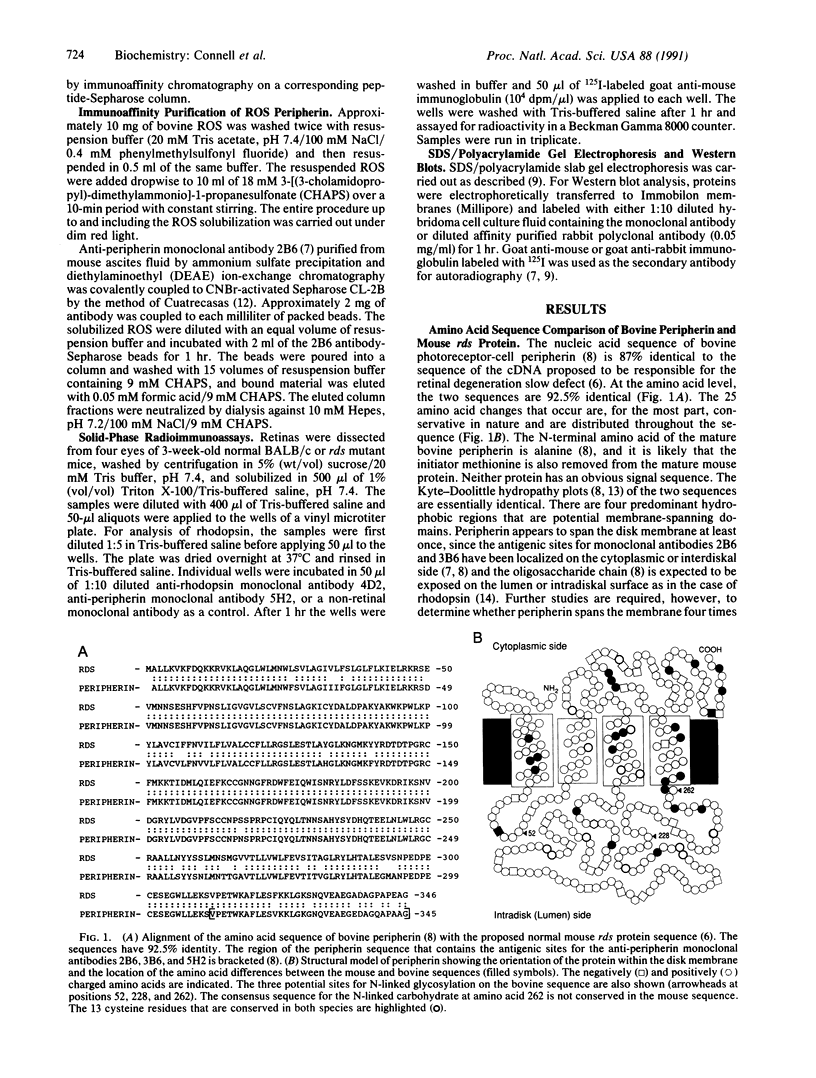
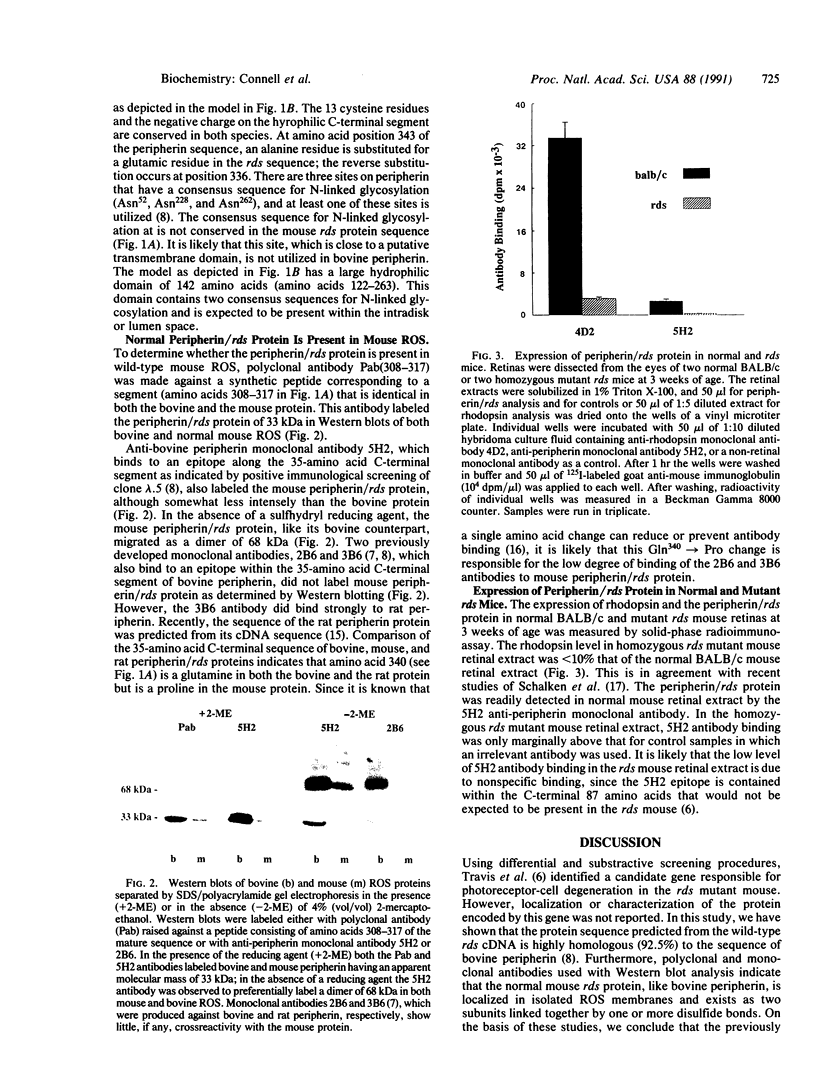
Retinal degeneration slow (rds) is a retinal disorder of an inbred strain of mice in which the outer segment of the photoreceptor cell fails to develop. A candidate gene has recently been described for the rds defect [Travis, G. H., Brennan, M. B., Danielson, P. E., Kozak, C. & Sutcliffe, J. G. (1989) Nature (London) 338, 70-73]. Neither the identity of the normal gene product nor its intracellular localization had been determined. We report here that the amino acid sequence of the bovine photoreceptor-cell protein peripherin, which was previously localized to the rim region of the photoreceptor disk membrane, is 92.5% identical to the sequence of the mouse protein encoded by the normal rds gene. The differences between the two sequences can be attributed to species variation. Monoclonal antibodies were used with Western blot analysis to localize the wild-type mouse peripherin/rds protein to isolated mouse rod outer segments and to show that it, like bovine peripherin, exists as two subunits linked by one or more disulfide bonds. The relative amounts of peripherin/rds protein and rhodopsin in retinal extracts of normal and rds mutant mice were also compared. Identification of peripherin as the protein encoded by the normal rds gene and its localization to membranes of rod outer segments will serve as a basis for studies directed toward defining the role of this protein in the morphogenesis and maintenance of the outer segment and toward understanding the mechanism by which the rds mutation causes retinal degeneration.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Begy C., Bridges C. D. Nucleotide and predicted protein sequence of rat retinal degeneration slow (rds). Nucleic Acids Res. 1990 May 25;18(10):3058–3058. doi: 10.1093/nar/18.10.3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson J., Drevin H., Axén R. Protein thiolation and reversible protein-protein conjugation. N-Succinimidyl 3-(2-pyridyldithio)propionate, a new heterobifunctional reagent. Biochem J. 1978 Sep 1;173(3):723–737. doi: 10.1042/bj1730723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark S. P., Molday R. S. Orientation of membrane glycoproteins in sealed rod outer segment disks. Biochemistry. 1979 Dec 25;18(26):5868–5873. doi: 10.1021/bi00593a017. [DOI] [PubMed] [Google Scholar]
- Cohen A. I. Some cytological and initial biochemical observations on photoreceptors in retinas of rds mice. Invest Ophthalmol Vis Sci. 1983 Jul;24(7):832–843. [PubMed] [Google Scholar]
- Connell G. J., Molday R. S. Molecular cloning, primary structure, and orientation of the vertebrate photoreceptor cell protein peripherin in the rod outer segment disk membrane. Biochemistry. 1990 May 15;29(19):4691–4698. doi: 10.1021/bi00471a025. [DOI] [PubMed] [Google Scholar]
- Cuatrecasas P. Protein purification by affinity chromatography. Derivatizations of agarose and polyacrylamide beads. J Biol Chem. 1970 Jun;245(12):3059–3065. [PubMed] [Google Scholar]
- Démant P., Iványi D., van Nie R. The map position of the rds gene on the 17th chromosome of the mouse. Tissue Antigens. 1979 Jan;13(1):53–55. doi: 10.1111/j.1399-0039.1979.tb01136.x. [DOI] [PubMed] [Google Scholar]
- Fliesler S. J., Rapp L. M., Hollyfield J. G. Photoreceptor-specific degeneration caused by tunicamycin. Nature. 1984 Oct 11;311(5986):575–577. doi: 10.1038/311575a0. [DOI] [PubMed] [Google Scholar]
- Hicks D., Molday R. S. Differential immunogold-dextran labeling of bovine and frog rod and cone cells using monoclonal antibodies against bovine rhodopsin. Exp Eye Res. 1986 Jan;42(1):55–71. doi: 10.1016/0014-4835(86)90017-5. [DOI] [PubMed] [Google Scholar]
- Hodges R. S., Heaton R. J., Parker J. M., Molday L., Molday R. S. Antigen-antibody interaction. Synthetic peptides define linear antigenic determinants recognized by monoclonal antibodies directed to the cytoplasmic carboxyl terminus of rhodopsin. J Biol Chem. 1988 Aug 25;263(24):11768–11775. [PubMed] [Google Scholar]
- Jansen H. G., Sanyal S. Development and degeneration of retina in rds mutant mice: electron microscopy. J Comp Neurol. 1984 Mar 20;224(1):71–84. doi: 10.1002/cne.902240107. [DOI] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Molday R. S., Hicks D., Molday L. Peripherin. A rim-specific membrane protein of rod outer segment discs. Invest Ophthalmol Vis Sci. 1987 Jan;28(1):50–61. [PubMed] [Google Scholar]
- Molday R. S., Molday L. L. Differences in the protein composition of bovine retinal rod outer segment disk and plasma membranes isolated by a ricin-gold-dextran density perturbation method. J Cell Biol. 1987 Dec;105(6 Pt 1):2589–2601. doi: 10.1083/jcb.105.6.2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roof D. J., Heuser J. E. Surfaces of rod photoreceptor disk membranes: integral membrane components. J Cell Biol. 1982 Nov;95(2 Pt 1):487–500. doi: 10.1083/jcb.95.2.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanyal S., Jansen H. G. Absence of receptor outer segments in the retina of rds mutant mice. Neurosci Lett. 1981 Jan 1;21(1):23–26. doi: 10.1016/0304-3940(81)90051-3. [DOI] [PubMed] [Google Scholar]
- Schalken J. J., Janssen J. J., Sanyal S., Hawkins R. K., de Grip W. J. Development and degeneration of retina in rds mutant mice: immunoassay of the rod visual pigment rhodopsin. Biochim Biophys Acta. 1990 Jan 29;1033(1):103–109. doi: 10.1016/0304-4165(90)90201-7. [DOI] [PubMed] [Google Scholar]
- Travis G. H., Brennan M. B., Danielson P. E., Kozak C. A., Sutcliffe J. G. Identification of a photoreceptor-specific mRNA encoded by the gene responsible for retinal degeneration slow (rds). Nature. 1989 Mar 2;338(6210):70–73. doi: 10.1038/338070a0. [DOI] [PubMed] [Google Scholar]
- van Nie R., Iványi D., Démant P. A new H-2-linked mutation, rds, causing retinal degeneration in the mouse. Tissue Antigens. 1978 Aug;12(2):106–108. doi: 10.1111/j.1399-0039.1978.tb01305.x. [DOI] [PubMed] [Google Scholar]




