Abstract
Objective To manage patients with viral hepatitis, it is important to screen for hepatitis, conduct a comprehensive examination if such screening is positive, administer antiviral treatment, and conduct surveillance for hepatocellular carcinoma (HCC). The proper execution of this strategy is expected to effectively reduce the number of deaths from viral hepatitis. Such an “optimal” follow-up for HCC surveillance is therefore important. This study aimed to determine the benefits of performing an optimal follow-up of patients with viral hepatitis.
Methods The subjects were infected with the hepatitis virus and were initially diagnosed with or treated for HCC from 2004-2012. We retrospectively analyzed the history of a patient's current illness using the hospital discharge summary. To minimize any lead-time bias, we calculated the corrected survival for patients who received an optimal follow-up.
Results Of 333 patients, 107 (32.1%) did not receive an optimal follow-up and thus had low cumulative survival rates in comparison to those who did. The median corrected survival was 51.5 months for patients with an optimal follow-up compared with 31.4 months for those without (p=0.011). A multivariate analysis revealed that AFP <35 [odds ratio (OR), 2.054], Child-Pugh A (OR, 2.488), and an optimal follow-up (OR, 4.539) were independent factors associated with the detection of early-stage HCC. Age (OR, 0.939), tumor stage I/II (OR, 6.918), and an optimal follow-up (OR, 3.213) were found to be independent factors associated with receiving curative treatment.
Conclusion An optimal follow-up of patients with viral hepatitis independently increased the detection of early-stage HCC and the administration of curative treatment. Patients with an optimal follow-up survived longer than those without.
Keywords: viral hepatitis, hepatocellular carcinoma, follow-up, survival, surveillance
Introduction
Viral hepatitis is the most prevalent infection worldwide. The number of carriers of the hepatitis B virus is estimated to be 350 million worldwide and from 1.3-1.5 million in Japan, and the number of carriers of the hepatitis C virus is estimated to be 170 million worldwide and from 1.5-2.0 million in Japan (1). Hepatitis viruses B and C cause chronic liver disease that progresses to cirrhosis and liver cancer (1). In Japan, liver cancer is the fifth-leading cause of death with approximately 30,000 deaths each year (2,3). Ninety-four percent of primary liver cancers are hepatocellular carcinomas (HCCs), and approximately 80-90% of HCCs are caused by a hepatitis virus (4,5). Therefore, the treatment of viral hepatitis is extremely important to prevent HCCs and related deaths, and achieving this goal requires the implementation of a multistage management strategy comprising screening for hepatitis virus, comprehensive examinations of virus-positive patients, antiviral treatment, and surveillance for HCC. The number of deaths from HCC will decrease only when individuals with hepatitis virus infection undergo surveillance for HCC.
The aim of the present study was to determine whether an optimal follow-up of patients with viral hepatitis was associated with the detection of HCC, the selection of treatment, and the prognosis of survival.
Materials and Methods
The subjects consisted of consecutive patients from the Division of Hepatology, Diabetes, Metabolism, and Endocrinology of Saga University Hospital, Japan who had viral hepatitis and were initially diagnosed with or treated for HCC from 2004-2012. The 333 patients included 220 men and 113 women, and their median age was 70 years (range, 23-91 years). We conducted a retrospective analysis of their medical histories using hospital discharge summaries acquired from the hospital's electronic medical records database.
In the present study, achieving an optimal follow-up was fulfilled if the patients underwent diagnostic imaging for chronic hepatitis every 6 months or for cirrhosis every 3-4 months, according to the surveillance algorithm of the Consensus-Based Clinical Practice Guidelines for HCC published by the Japan Society of Hepatology (6). The tumor stages were determined according to the guidelines of the Liver Cancer Study Group of Japan (7). This method includes three conditions as follows: i) tumor diameter ≤20 mm, ii) single tumor, and iii) no vascular invasion. Tumors that met three, two, one, or none of the conditions were classified as stage I, II, III, or IV, respectively. Patient and tumor characteristics were extracted from the data acquired at the time HCC was diagnosed. We investigated whether patients diagnosed with HCC received an optimal follow-up and then attempted to identify any associated benefits.
A statistical analysis was performed using the SPSS Statistics software program ver. 21 (SPSS Japan, Tokyo, Japan). The chi-square test was used to perform qualitative intergroup comparisons. The Mann-Whitney, Kruskal-Wallis, and Steel-Dwass tests were used for quantitative intergroup comparisons. A logistic regression model was used to perform univariate and multivariate analyses. The Kaplan-Meier method was used to analyze cumulative survival; and the log-rank test was used to determine the statistical significance of differences between the data sets. In all analyses, a p value <0.001 was considered to be significant.
To minimize any lead-time bias, which we defined as an increase in the survival time associated with the tumor diagnosis at an earlier stage determined from an optimal follow-up (8), we calculated the lead-time for patients with an optimal follow-up using the formula reported by Schwartz (9) as follows: T=3×DT×log (d1/d0)/log (2), where T is the lead-time in days, DT is the median tumor-volume doubling time (days), and d0 and d1 are the median tumor diameters of patients with and without an optimal follow-up, respectively. The corrected survival for patients with an optimal follow-up was calculated by subtracting the calculated lead-time from the length of the survival. If the value was negative, we assigned a corrected survival of 1 day.
The Clinical Research Ethics Review Committee of Saga University Hospital approved this study (approval number, 2012-03-14). An opt-out approach was used to obtain informed consent from the patients, and personal information was carefully protected during data extraction.
Results
Patient characteristics at the diagnosis of HCC
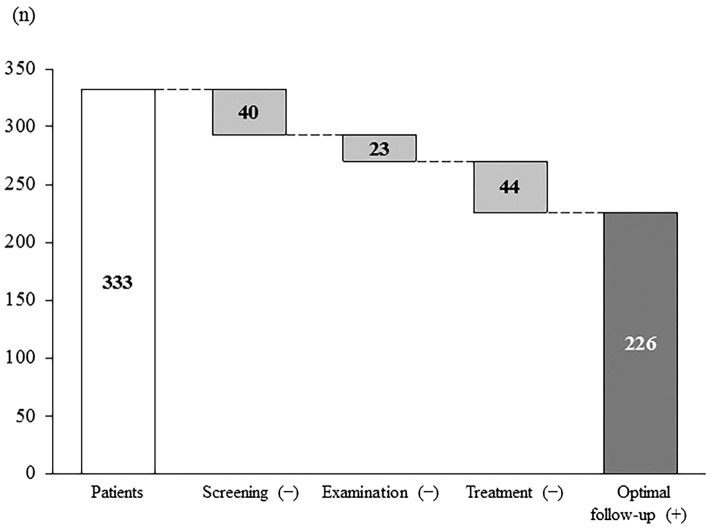
We enrolled 333 patients, median age 70 years (range, 23-91 years); and 220 (66.1%) were men. The patients' characteristics are summarized in Table 1. The underlying liver disease was hepatitis B in 52 patients (15.6%) and hepatitis C in 281 (84.4%). The hepatic functional reserve was Child-Pugh A in 253 patients (76%), B in 70 (21%), and C in 10 (3%). The tumor stage was I in 81 (24.3%), II in 110 (33%), III in 105 (31.5%), and IV in 37 (11.1%). The median tumor diameter was 24 mm. The first treatment was surgery for 92 (27.6%), radiofrequency ablation (RFA) for 126 (37.8%), transcatheter arterial chemoembolization for 70 (21.1%), transcatheter arterial infusion for 23 (6.9%), a molecularly targeted drug for 1 (0.3%), and palliative care for 21 (6.3%). The patients who did or did not receive an optimal follow-up numbered 226 (67.9%) and 107 (32.1%), respectively. Of the 107 patients who did not receive optimal follow-up, 40 learned about their hepatitis because of HCC (incomplete screening), 23 did not undergo comprehensive examinations (incomplete examination), and 44 did not undergo treatment (incomplete treatment) (Fig. 1). The last group of patients included those who discontinued hospital visits, those treated without follow-up imaging for surveillance despite the recommendation of the guidelines, and those who did not make all of their scheduled visits to the hospital.
Table 1.
Characteristics of Patients upon Diagnosis of Hepatocellular Carcinoma.
| Factors | n=333 |
|---|---|
| Sex, n (%) | |
| Female | 113 (33.9 %) |
| Male | 220 (66.1 %) |
| Age (years), median (range) | 70 (23-91) |
| PLT (×104/µL), median (range) | 10.8 (2.0-37.2) |
| PT (%), median (range) | 79.2 (10-119) |
| ALB (g/dL), median (range) | 3.6 (1.6-4.9) |
| T-BIL (mg/dL), median (range) | 1.0 (0.3-7.7) |
| ALT (IU/L), median (range) | 44 (10-421) |
| AFP (ng/mL), median (range) | 35.9 (1-1,320,000) |
| Tumor diameter (mm), median (range) | 24 (5-200) |
| Child-Pugh class, n (%) | |
| A | 253 (76.0%) |
| B | 70 (21.0%) |
| C | 10 (3.0%) |
| Tumor stage, n (%) | |
| I | 81 (24.3%) |
| II | 110 (33.0%) |
| III | 105 (31.5%) |
| IV | 37 (11.1%) |
| Treatment, n (%) | |
| Surgery | 92 (27.6%) |
| RFA | 126 (37.8%) |
| TACE/TAI | 93 (28.0%) |
| Others | 22 (6.6%) |
| Etiology, n (%) | |
| HBV | 52 (15.6%) |
| HCV | 281 (84.4%) |
| Optimal follow-up, n (%) | |
| With | 226 (67.9%) |
| Without | 107 (32.1%) |
Abbreviations: PLT: platelet count, PT: prothrombin activity, ALB: albumin, T-BIL: total bilirubin, ALT: alanine aminotransferase, AFP: α-fetoprotein, RFA: radiofrequency ablation, TACE: transcatheter arterial chemoembolization, TAI: transcatheter arterial infusion, HBV: hepatitis B virus, HCV: hepatitis C virus
Figure 1.
Patients who did not receive the recommended management strategy. One hundred and seven (32.1%) of 333 patients diagnosed with hepatocellular carcinoma (HCC) deviated from the management strategy as follows: 40/107 patients did not undergo screening, 23/107 patients did not undergo examinations, and 44/107 patients did not undergo treatment. An optimal follow-up was thus achieved in 226 (67.9%) patients.
Characteristics of the patients at the diagnosis of HCC according to an optimal follow-up
A higher proportion of men did not receive an optimal follow-up (75.7% vs. 61.5%, p=0.011), had hepatitis B as the underlying liver disease (26.2% vs. 10.6%, p<0.001), and did not receive curative treatment, such as surgery or RFA (58.9% vs. 23.0%, p<0.001), compared with the patients who received an optimal follow-up. The former patients had advanced-stage HCC (p<0.001), higher Child-Pugh class (p=0.010), greater tumor diameters (p<0.001), higher platelet counts (p=0.002), and higher α-fetoprotein (AFP) levels (p=0.003). Imaging for surveillance was a combination of computed tomography (CT), magnetic resonance imaging (MRI), and ultrasonography (US) in 155 patients (68.6%) and only US in 71 (31.4%). The data are summarized in Table 2a. Stage I/II patients without an optimal follow-up had greater tumor diameters (p=0.003) and did not receive curative treatment (p=0.005) in comparison to stage I/II patients with an optimal follow-up (Table 2b).
Table 2.
Characteristics of Patients who Did Or Did Not Receive Optimal Follow-up at the Time of Diagnosis: (a) All Patients, (b) Patients with Stage I/II.
(a).
| Variables | Optimal follow-up n=226 |
No optimal follow-up n=107 |
p value |
|---|---|---|---|
| Sex, n (%) | 0.11 | ||
| Female | 87 (38.5 %) | 26 (24.3%) | |
| Male | 139 (61.5 %) | 81 (75.7%) | |
| Age (years), median (range) | 71 (33-87) | 69 (23-91) | 0.047 |
| PLT (×104/µL), median (range) | 10.3 (2.0-26.2) | 12.7 (3.5-37.2) | 0.002 |
| PT (%), median (range) | 79 (10-119) | 80 (19-116) | 0.817 |
| ALB (g/dL), median (range) | 3.6 (1.6-4.9) | 3.5 (2.0-4.9) | 0.129 |
| T-BIL (mg/dL), median (range) | 1.0 (0.3-5.3) | 0.9 (0.3-7.7) | 0.637 |
| ALT (IU/L), median (range) | 44 (10-360) | 45 (10-421) | 0.237 |
| AFP (ng/mL), median (range) | 28.6 (1-61,200) | 92.9 (1.8-1,320,000) | 0.003 |
| Tumor diameter (mm), median (range) | 21 (5-100) | 41 (7-200) | <0.001 |
| Child-Pugh class, n (%) | 0.010 | ||
| A | 182 (80.5%) | 71 (66.4%) | |
| B | 40 (17.7%) | 30 (28.0%) | |
| C | 4 (1.8%) | 6 (5.6%) | |
| Tumor stage, n (%) | <0.001 | ||
| I | 71 (31.4%) | 10 (9.3%) | |
| II | 85 (37.6%) | 25 (23.4%) | |
| III | 60 (26.5%) | 45 (42.1%) | |
| IV | 10 (4.4%) | 27 (25.2%) | |
| Treatment, n (%) | <0.001 | ||
| Surgery | 63 (27.9%) | 29 (27.1%) | |
| RFA | 111 (49.1%) | 15 (14.0%) | |
| TACE/TAI | 48 (21.2%) | 45 (42.1%) | |
| Others | 4 (1.8%) | 18 (16.8%) | |
| Etiology, n (%) | <0.001 | ||
| HBV | 24 (10.6%) | 28 (26.2%) | |
| HCV | 202 (89.4%) | 79 (73.8%) | |
| Imaging for surveillance, n (%) | |||
| Combination of CT, MRI, and US | 155 (68.6%) | - | |
| Only US | 71 (31.4%) | - | |
(b).
| Variables | Optimal follow-up n=156 | No optimal follow-up n=35 | p value |
|---|---|---|---|
| Sex, n (%) | 0.326 | ||
| Female | 93 (59.6 %) | 24 (68.6%) | |
| Male | 63 (40.4 %) | 11 (31.4%) | |
| Age (years), median (range) | 70 (33-87) | 72 (42-83) | 0.712 |
| PLT (×104/µL), median (range) | 9.9 (2.0-26.2) | 12.3 (3.5-19.8) | 0.114 |
| PT (%), median (range) | 79 (10-109) | 81 (44-102.1) | 0.721 |
| ALB (g/dL), median (range) | 3.6 (1.6-4.9) | 3.6 (2.0-4.6) | 0.332 |
| T-BIL (mg/dL), median (range) | 0.9 (0.3-5.3) | 0.9 (0.4-5.8) | 0.611 |
| ALT (IU/L), median (range) | 44 (10-360) | 45 (10-421) | 0.142 |
| AFP (ng/mL), median (range) | 45 (10-306) | 40 (12-109) | 0.960 |
| Tumor diameter (mm), median (range) | 17.5 (5-70) | 22 (7-120) | 0.003 |
| Child-Pugh class, n (%) | 0.188 | ||
| A | 130 (83.3%) | 26 (74.3%) | |
| B | 24 (15.4%) | 7 (20.0%) | |
| C | 2 (1.3%) | 2 (5.7%) | |
| Treatment, n (%) | 0.005 | ||
| Surgery | 40 (25.6%) | 13 (37.1%) | |
| RFA | 96 (61.5%) | 11 (31.4%) | |
| TACE/TAI | 18 (11.5%) | 9 (25.7%) | |
| Others | 2 (1.3%) | 2 (5.7%) | |
| Etiology, n (%) | 0.223 | ||
| HBV | 19 (12.2%) | 7 (20.0%) | |
| HCV | 137 (87.8%) | 29 (80.0%) | |
| Imaging for surveillance, n (%) | |||
| Combination of CT, MRI, and US | 108 (69.2%) | - | |
| Only US | 48 (30.8%) | - |
Abbreviations: PLT: platelet count, PT: prothrombin activity, ALB: albumin, T-BIL: total bilirubin, ALT: alanine aminotransferase, AFP: α-fetoprotein, RFA: radiofrequency ablation, TACE: transcatheter arterial chemoembolization, TAI: transcatheter arterial infusion, HBV: hepatitis B virus, HCV: hepatitis C virus, CT: computed tomography, MRI: magnetic resonance imaging, US: ultrasonography
Factors associated with the detection of early-stage HCC
A univariate analysis revealed the significant factors associated with the detection of early-stage (stage I/II) HCC to be a female sex, alanine aminotransferase ≤60, AFP ≤35, Child-Pugh class A, and receiving an optimal follow-up. A multivariate analysis revealed that AFP ≤35 [Odds ratio (OR), 2.054; 95% confidence interval (CI), 1.162-3.629; p=0.013], Child-Pugh A (OR, 2.488; 95% CI, 1.263-4.900; p=0.008), and optimal follow-up (OR, 4.539; 95% CI, 2.459-8.379; p<0.001) independently increased the opportunity of detecting early-stage HCC. The data are summarized in Table 3a. In patients with an optimal follow-up, there was no difference in imaging for surveillance between stage I/II patients and stage III/IV patients (p=0.755). A multivariate analysis revealed that imaging for surveillance was not a significant factor associated with the detection of early-stage HCC after adjusting for sex, ALT, AFP, and Child-Pugh class (Table 3b).
Table 3.
Independent Factors for the Detection of Early-stage HCC: (A) All Patients, (B) Patients with Optimal Follow-up.
(a).
| Variables | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| P value | OR | 95% CI | p value | |
| Sex | 0.032 | |||
| Male | 1 | Referent | ||
| Female | 1.568 | 0.857-2.869 | 0.145 | |
| Age (years), | 0.652 | |||
| PLT (×104/µL) | 0.089 | |||
| <15 | ||||
| ≥15 | ||||
| ALT (IU/L) | 0.042 | |||
| >60 | 1 | Referent | ||
| ≤60 | 1.095 | 0.586-2.046 | 0.776 | |
| AFP (ng/mL) | 0.001 | |||
| >35 | 1 | Referent | ||
| ≤35 | 2.054 | 1.162-3.629 | 0.013 | |
| Child-Pugh class | 0.005 | |||
| B/C | 1 | Referent | ||
| A | 2.488 | 1.263-4.900 | 0.008 | |
| Etiology | 0.243 | |||
| HBV | ||||
| HCV | ||||
| Optimal follow-up | <0.001 | |||
| Without | 1 | Referent | ||
| With | 4.539 | 2.459-8.379 | <0.001 | |
(b).
| Variables | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| P value | OR | 95% CI | p value | |
| Sex | 0.384 | |||
| Male | 1 | Referent | ||
| Female | 1.582 | 0.788-3.175 | 0.197 | |
| Age (years), | 0.255 | |||
| PLT (×104/µL) | 0.416 | |||
| <15 | ||||
| ≥15 | ||||
| ALT (IU/L) | 0.677 | |||
| >60 | 1 | Referent | ||
| ≤60 | 0.606 | 0.286-1.281 | 0.189 | |
| AFP (ng/mL) | 0.089 | |||
| >35 | 1 | Referent | ||
| ≤35 | 1.815 | 0.935-3.525 | 0.078 | |
| Child-Pugh class | 0.115 | |||
| B/C | 1 | Referent | ||
| A | 1.797 | 0.794-4.068 | 0.160 | |
| Etiology | 0.261 | |||
| HBV | ||||
| HCV | ||||
| Imaging for surveillance | 0.755 | |||
| Combination of CT, MRI, and US | 1 | Referent | ||
| Only US | 1.004 | 0.495-2.034 | 0.991 | |
Abbreviations: OR: odds ratio, CI: confidence interval, PLT: platelet count, ALT: alanine aminotransferase, AFP: α-fetoprotein, HBV: hepatitis B virus, HCV: hepatitis C virus, CT: computed tomography, MRI: magnetic resonance imaging, US: ultrasonography
Factors associated with receiving curative treatment
A univariate analysis revealed that the significant factors associated with receiving curative treatment, such as curative surgery or RFA were age, AFP ≤35, Child-Pugh A, tumor stage I/II, and an optimal follow-up. A multivariate analysis revealed that age (OR, 0.939; 95% CI, 0.906-0.974; p=0.001), tumor stage I/II (OR, 6.918; 95% CI, 3.550-13.484; p<0.001), and an optimal follow-up (OR, 3.213; 95% CI, 1.615-6.391; p=0.001) independently increased a patient's opportunity to receive curative treatment. The data are summarized in Table 4.
Table 4.
Independent Factors for the Choice of Curative Treatment.
| Variables | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| P value | OR | 95% CI | p value | |
| Sex | 0.622 | |||
| Male | ||||
| Female | ||||
| Age (years), | 0.041 | 0.939 | 0.906-0.974 | 0.001 |
| PLT (×104/µL) | 0.267 | |||
| <15 | ||||
| ≥15 | ||||
| ALT (IU/L) | 0.356 | |||
| >60 | ||||
| ≤60 | ||||
| AFP (ng/mL) | 0.003 | |||
| >35 | 1 | Referent | ||
| ≤35 | 1.584 | 0.840-2.985 | 0.155 | |
| Child-Pugh class | <0.001 | |||
| B/C | 1 | Referent | ||
| A | 1.899 | 0.917-3.933 | 0.084 | |
| Tumor stage | <0.001 | |||
| III/IV | 1 | Referent | ||
| I/II | 6.918 | 3.550-13.484 | <0.001 | |
| Etiology | 0.517 | |||
| HBV | ||||
| HCV | ||||
| Optimal follow-up | <0.001 | |||
| Without | 1 | Referent | ||
| With | 3.213 | 1.615-6.391 | 0.001 | |
Abbreviations: RFA: radiofrequency ablation, OR: odds ratio, CI: confidence interval, PLT: platelet count, ALT: alanine aminotransferase, AFP: α-fetoprotein, HBV: hepatitis B virus, HCV: hepatitis C virus
Survival
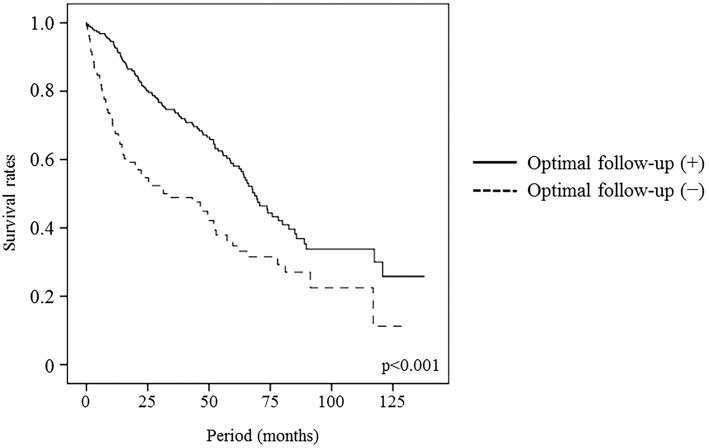
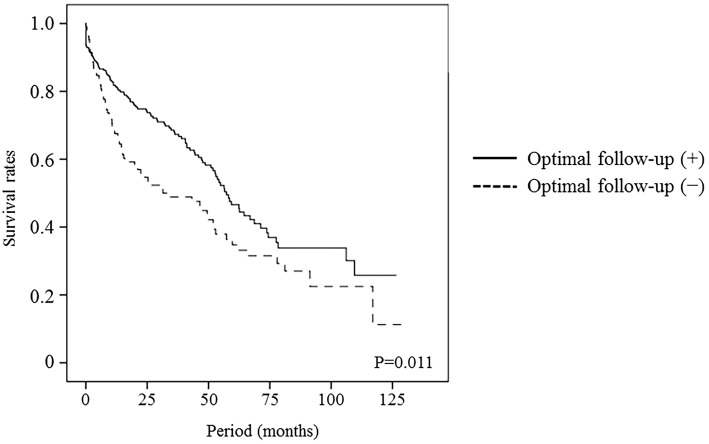
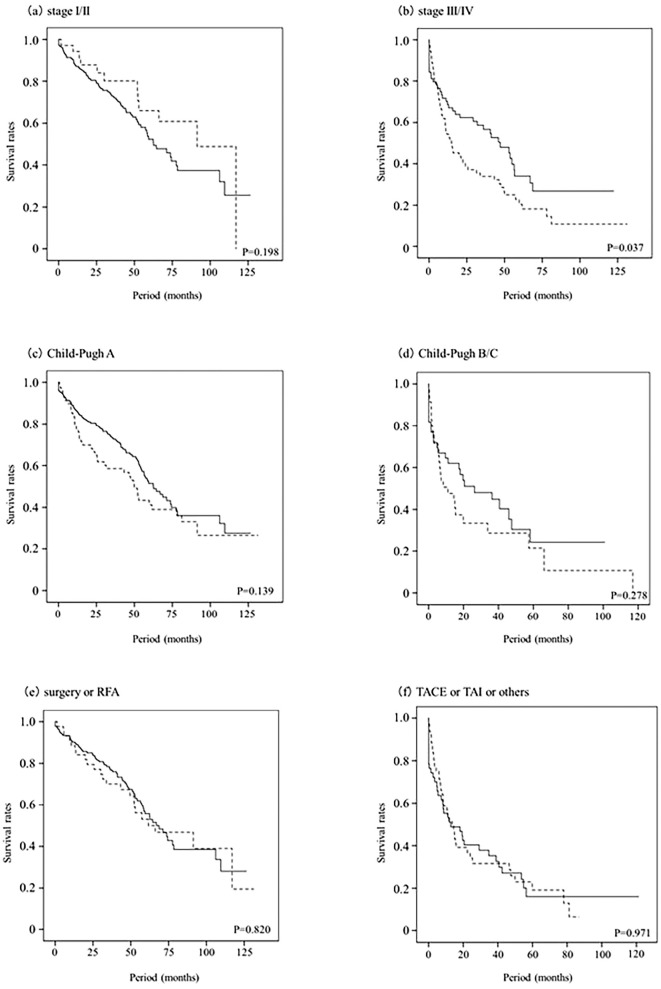
The median follow-up period after the HCC diagnosis was 40.3 months (range, 0.03-137.73 months), and 105 (45.5%) and 66 (62.3%) of patients who did or did not receive optimal follow-up, respectively, died during follow-up. Of the 281 patients with HCV, 44 (15.7%) received anti-HCV therapy after HCC treatment, and 27 (13.4%) and 3 (3.8%) of patients who did or did not receive optimal follow-up, respectively, had a sustained virological response (SVR). Thirty patients with SVR all survived during the observation period and 14 patients with non-SVR had a good survival compared to the patients without anti-HCV therapy history. The median survival was higher for the patients who received an optimal follow-up compared with those who did not (68.7 vs. 31.4 months, p<0.001). The estimated survival rates for the patients who did or did not receive optimal follow up at 1, 3, and 5 years were 92.6% vs. 67.6%, 74.1% vs. 48.9%, and 58.1% vs. 34.7%, respectively (Fig. 2). The calculated lead-time was 339 days using the median tumor-volume doubling time (117 days) proposed by Sheu et al. (10). The median survival of the patients who received an optimal follow-up corrected for this lead-time was 56.5 months, and the corrected survival rates at 1, 3, 5 years were 81.8%, 67.9%, and 46.6%. The median corrected survival in the patients who received an optimal follow-up was longer than the median survival of the patients who did not (56.5 vs. 31.4 months, p=0.011, Fig. 3). Fig. 4 shows the corrected cumulative survival rates of the patients who did or did not receive optimal follow-up according to tumor stage, Child-Pugh class, and treatment. Although stage I/II patients with an optimal follow-up did not have a good corrected survival compared to those without (p=0.198, Fig. 4a), stage III/IV patients did (p=0.037, Fig. 4b). There were no differences in the survival between the optimal follow-up group and non-optimal follow-up group according to Child-Pugh class and treatment (Fig. 4c-f).
Figure 2.
Survival of patients who did or did not receive an optimal follow-up. Patients who received an optimal follow-up had a higher cumulative survival rate in comparison to those who did not.
Figure 3.
Corrected survival of patients who received an optimal follow-up vs. the observed survival of patients who did not. An optimal follow-up significantly increased survival, even after adjusting for lead-time.
Figure 4.
Corrected survival of the patients who received an optimal follow-up vs. the observed survival of the patients who did not according to tumor stage (a, b), Child-Pugh class (c, d), and treatment (e, f). Full line (—), optimal follow-up group; dotted line (- - -), non-optimal follow-up group. Stage III/IV patients with an optimal follow-up had a higher cumulative survival rate compared to those without (p=0.037, b).
Discussion
The major findings of the present study are as follows: i) approximately 68% of HCC patients with viral hepatitis received an optimal follow-up, ii) an optimal follow-up was associated with the detection of early-stage HCC and the administration of curative treatment, and iii) patients who received an optimal follow-up survived longer than those who did not. We therefore conclude that an optimal follow-up improved survival because of the increased opportunity to detect early-stage HCC and thus administer curative treatment.
In this study, 31.0% and 67.3% of the patients with or without an optimal follow-up, respectively, had stage III/IV HCC. In the patients who received an optimal follow-up, there were no significant differences in sex, age, etiology, and imaging for surveillance between stage I/II patients and stage III/IV. In stage III/IV patients, patients with an optimal follow-up had smaller tumor diameters than did patients without an optimal follow-up [33 mm (range, 21-100 mm) vs. 50 mm (range, 21-200 mm), p<0.001] and this suggested that stage III patients with an optimal follow-up contained some patients similar to stage II. We estimated that this was one of the reasons why 31% of the patients with an optimal follow-up, not a low percentage, had stage III/IV HCC. Age was a factor associated with curative treatment. We estimated that an age-related poor performance status caused the selection of non-curative treatment. Additionally, a Child-Pugh A status was an independent factor associated with the detection of early-stage HCC for some unknown reason. Abdominal US revealed that the liver in patients with a Child-Pugh A status showed a homogeneous parenchymal echo pattern. We estimated that it was easy to detect HCC in patients with a Child-Pugh A status compared to those with a Child-Pugh B/C status due to parenchymal echo patterns.
The above-mentioned findings of the present study are consistent with those of previous studies that found surveillance for viral hepatitis to be beneficial. For example, two prospective studies revealed the benefits of surveillance for survival of viral hepatitis (11,12). Retrospective studies have found that surveillance increased survival when corrected for lead-time (13-16) and increased the opportunity for patients to receive curative treatment (11,12,17). The Japanese report of Tanaka et al. (16) indicated that the proportion of patients with small size HCC, solitary HCC, and local therapy (hepatectomy or RFA), respectively, was significantly higher in the surveillance group than in the non-surveillance group, and surveillance increased survival in patients with Child-Pugh A after adjusting for the lead-time using 120 days or less as the doubling time. Our study mainly contained two differences relative to their report. The first was that surveillance was associated with the detection of early-stage HCC and the second was that surveillance increased the opportunity to administer curative treatment to these patients. We confirmed these benefits of an optimal follow-up using a multivariate regression analysis.
Our present findings are similar to previous studies (11-17) related to detecting early-stage HCC, thereby increasing the opportunity to administer curative treatment, and thus improve survival. A unique and significant aspect of the present study is that we assessed the benefits of surveillance regarding these three factors at a time using a regression analysis after adjusting for lead-time.
Our study is associated with several limitations because of its retrospective cohort design. The first is the existence of lead-time bias. We used 117 days (lead-time of 339 days) as the tumor-volume doubling time and minimized any lead-time bias by calculating the corrected survival of patients who received an optimal follow-up. Further, the corrected survival lacks accuracy, because we could not calculate the respective tumor-volume doubling time using computed tomography, magnetic resonance imaging or ultrasonography. Another limitation is the existence of selection bias. The subjects consisted of consecutive patients treated at a single center. The reliability of the assumption that patients received an optimal follow-up is another limitation. For example, many patients were referred by their primary care physician who informed us in an introductory letter about whether, in their opinion, the patient had received an optimal follow-up. In addition, some patients provided a self-evaluation of follow-up which means a less reliable degree of accuracy.
To overcome these limitations, we must minimize any lead-time bias by calculating the tumor-volume doubling time of each patient, conducting a multicenter study, and determining with certainty whether or not appropriate surveillance occurred. To these ends, cooperation between community medical organizations and their affiliated hospitals will be required to improve the quality of surveillance practiced by each.
It has been found to be impossible to completely clear HBV from the body (18) and even patients who become HBV surface antigen negative remain at risk for developing HCC (19). HCC in patients with SVR has been reported (20). All physicians should therefore recognize that patients with a history of viral hepatitis need to receive a continued optimal follow-up regardless of the antiviral treatment history.
In conclusion, we herein showed an optimal follow-up to be an independent factor associated with increased opportunities for detecting early-stage HCC and for patients to receive curative treatment. Moreover, when we adjusted for lead-time, the data showed that patients with HCC who received an optimal follow-up survived longer in comparison to those who did not.
The authors state that they have no Conflict of Interest (COI).
Financial Support
This study was supported by the Health Labour Sciences Research Grants (H26).
Acknowledgement
The authors thank the physicians and nurses at Saga University Hospital for their assistance in informing us about the administration of an optimal follow-up.
References
- 1. IARC Working Group on the Evaluation of Carcinogenic Risks to Humans Hepatitis viruses. IARC Monogr Eval Carcinog Risks Hum 59: 1-255, 1994. [PMC free article] [PubMed] [Google Scholar]
- 2. Cancer Information Service, National Cancer Center, Japan. Vital Statistics Japan (Ministry of Health, Labour and Welfare), 2011 (in Japanese) .
- 3. Ministry of Health, Labour and Welfare. Vital Statistics in Japan, 2011 .
- 4. Ikai I, Kudo M, Arii S, et al. . Report of the 18th follow-up survey of primary liver cancer in Japan. Hepatol Res 40: 1043-1059, 2010. [DOI] [PubMed] [Google Scholar]
- 5. Parkin DM. Global cancer statistics in the year 2000. Lancet Oncol 2: 533-543, 2001. [DOI] [PubMed] [Google Scholar]
- 6. Kudo M, Izumi N, Kokudo N, et al. . Management of hepatocellular carcinoma in Japan: Consensus-Based Clinical Practice Guidelines proposed by the Japan Society of Hepatology (JSH) 2010 updated version. Dig Dis 29: 339-364, 2011. [DOI] [PubMed] [Google Scholar]
- 7. Kudo M, Izumi N, Kokudo N, et al. . Management Liver Cancer Study Group of Japan. In: The general rules for the clinical and pathological study of primary liver cancer 5th ed. Kanehara & Co., Ltd, 2008: . [Google Scholar]
- 8. Adams PC, Arthur MJ, Boyer TD, et al. . Screening in liver disease: report of an AASLD clinical workshop. Hepatology 39: 1204-1212, 2004. [DOI] [PubMed] [Google Scholar]
- 9. Schwartz M. A biomathematical approach to clinical tumor growth. Cancer 14: 1272-1294, 1961. [DOI] [PubMed] [Google Scholar]
- 10. Sheu JC, Sung JL, Chen DS, et al. . Growth rate of asymptomatic hepatocellular carcinoma and its clinical implications. Gastroenterology 89: 259-266, 1985. [DOI] [PubMed] [Google Scholar]
- 11. Zhang BH, Yang BH, Tang ZY. Randomized controlled trial of screening for hepatocellular carcinoma. J Cancer Res Clin Oncol 130: 417-422, 2004. [DOI] [PubMed] [Google Scholar]
- 12. Bolondi L, Sofia S, Siringo S, et al. . Surveillance programme of cirrhotic patients for early diagnosis and treatment of hepatocellular carcinoma: a cost effectiveness analysis. Gut 48: 251-259, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Santi V, Trevisani F, Gramenzi A, et al. . Italian Liver Cancer (ITA.LI.CA) Group. Semiannual surveillance is superior to annual surveillance for the detection of early hepatocellular carcinoma and patient survival. J Hepatol 53: 291-297, 2010. [DOI] [PubMed] [Google Scholar]
- 14. Tong MJ, Sun HE, Hsien C, Lu DS. Surveillance for hepatocellular carcinoma improves survival in Asian-American patients with hepatitis B: results from a community-based clinic. Dig Dis Sci 55: 826-835, 2010. [DOI] [PubMed] [Google Scholar]
- 15. Wong GL, Wong VW, Tan GM, et al. . Surveillance programme for hepatocellular carcinoma improves the survival of patients with chronic viral hepatitis. Liver Int 28: 79-87, 2008. [DOI] [PubMed] [Google Scholar]
- 16. Tanaka H, Nouso K, Kobashi H, et al. . Surveillance of hepatocellular carcinoma in patients with hepatitis C virus infection may improve patient survival. Liver Int 26: 543-551, 2006. [DOI] [PubMed] [Google Scholar]
- 17. Trevisani F, Cantarini MC, Labate AM, et al. . Italian Liver Cancer (ITALICA) group. Surveillance for hepatocellular carcinoma in elderly Italian patients with cirrhosis: effects on cancer staging and patient survival. Am J Gastroenterol 99: 1470-1476, 2004. [DOI] [PubMed] [Google Scholar]
- 18. Pollicino T, Saitta C, Raimondo G. Hepatocellular carcinoma: the point of view of the hepatitis B virus. Carcinogenesis 32: 1122-1132, 2011. [DOI] [PubMed] [Google Scholar]
- 19. Ahn SH, Park YN, Park JY, et al. . Long-term clinical and histological outcomes in patients with spontaneous hepatitis B surface antigen seroclearance. J Hepatol 42: 188-194, 2005. [DOI] [PubMed] [Google Scholar]
- 20. Asahina Y, Tsuchiya K, Tamaki N, et al. . Effect of aging on risk for hepatocellular carcinoma in chronic hepatitis C virus infection. Hepatology 52: 518-527, 2010. [DOI] [PubMed] [Google Scholar]