Abstract
We herein report three ischemic stroke patients who underwent emergency carotid artery stenting after receiving intravenous tissue plasminogen activator (t-PA) treatment. All patients received antiplatelet medications immediately before stent placement for loading as well as dual antiplatelet therapy after stenting. Under high-dose and dual antiplatelet therapy, none of the three patients showed symptomatic intracranial hemorrhaging. However, one case showed reocclusion of the placed stent after acute thrombosis. As a result, new treatment strategies for the use of antiplatelet agents during emergency stent placement must be developed, particularly for patients who have received intravenous t-PA therapy.
Keywords: acute ischemic stroke, recombinant tissue plasminogen activator, carotid artery stenting, antiplatelet therapy
Introduction
In recent years, the efficacy of endovascular treatment after the administration of intravenous recombinant tissue plasminogen activator (rt-PA) has been demonstrated in several studies (1-5). In fact, the number of patients undergoing endovascular treatment after intravenous rt-PA administration has been increasing. In addition, carotid artery stent placement during endovascular treatment is performed in selected patients in clinical practice. However, strong evidence that supports the effectiveness of emergent stenting treatment is unavailable. Patients who undergo emergency stent placement are recommended to receive antiplatelet therapy to prevent in-stent thrombosis (6), but only a few studies have reported the use of these drugs after carotid artery stenting and intravenous rt-PA administration (7,8). We herein report our experience with acute antiplatelet therapy in three stroke patients who underwent emergency carotid artery stent placement after intravenous rt-PA administration.
Case Reports
Case 1
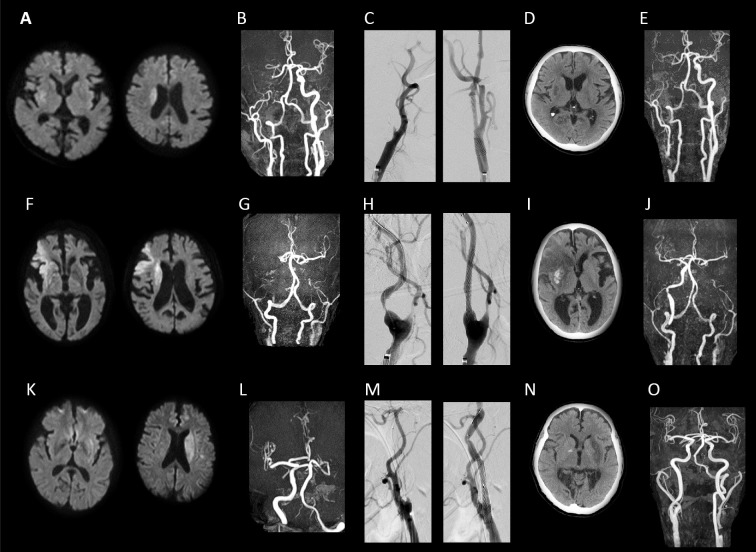
A 71-year-old man was brought to our hospital with the chief complaint of acute left hemiplegia. He had a history of hypertension and non-valvular atrial fibrillation (NVAF) and had been receiving oral administration of rivaroxaban (15 mg/day). On arrival, the patient's National Institute of Health Stroke Scale (NIHSS) score was 14. Head magnetic resonance imaging (MRI) revealed acute cerebral infarction in the right basal ganglia extending to the corona radiata [diffusion-weighted imaging (DWI)-Alberta Stroke Program Early Computed Tomography Score (ASPECTS): 9] (Figure A). Magnetic resonance angiography (MRA) revealed a right internal carotid artery occlusion (Figure B). At 110 minutes after the onset of stroke, rt-PA (time from the last administration of rivaroxaban to rt-PA: 590 minutes) and cerebral endovascular treatment were administered. The thrombus was aspirated using the PenumbraⓇ system (Penumbra, Alameda, USA). Although balloon percutaneous transluminal angioplasty (PTA) was performed for the residual right internal carotid artery stenotic lesion, elastic recoil occurred. Therefore, clopidogrel and aspirin were administered at loading doses of 300 and 200 mg, respectively, followed by emergency carotid artery stenting (Carotid WALLSTENT, Boston Scientific, Natick, USA) (Figure C). At 300 minutes after onset (time from the last administration of rivaroxaban to recanalization: 780 minutes), complete recanalization was achieved. After treatment, antiplatelet therapy (clopidogrel 75 mg + aspirin 100 mg) was continued. The patient's systolic blood pressure (SBP) was maintained at 100 to 130 mmHg by a continuous intravenous infusion of nicardipine. During the follow-up period, the patient remained free of concurrent symptomatic intracranial hemorrhaging (sICH) (Figure D). Although no neurologic changes were noted, follow-up MRA performed on hospital day 4 showed reocclusion, likely resulting from in-stent thrombosis (Figure E). On hospital day 35, the patient's modified Rankin Scale (mRS) score was 2, and he was transferred to another hospital.
Figure.
Imaging findings of the three cases. Case 1: A-E. A: Diffusion-weighted imaging (DWI) on arrival showed acute cerebral infarction in the right basal ganglia extending to the corona radiata, B: Magnetic resonance angiography (MRA) on arrival showed an occlusion of the right internal carotid artery, C: Before and after stent placement, D: Computed tomography (CT) at 24hours after t-PA showed no hemorrhagic change, E: MRA on the 4th hospital day showed reocclusion due to in-stent thrombosis. Case 2: F-J. F: DWI on arrival showed acute cerebral infarction in the right frontal temporal lobe and corona radiata, G: MRA on arrival showed a bilateral occlusion of the internal carotid artery, H: Before and after stent placement, I: CT at 24 hours after t-PA showed a hemorrhagic change in the right basal ganglia, J: MRA on the 5th hospital day. The carotid stent was patent. Case 3: K-O. K: DWI on arrival showed acute cerebral infarction in the left basal ganglia extending to the corona radiata, L: MRA on arrival showed an occlusion of the left internal carotid artery, M: Before and after stent placement, N: CT at 24 hours after t-PA showed no hemorrhagic change, O: MRA on the 14th hospital day. The carotid stent was patent.
Case 2
An 85-year-old man was brought to our hospital with the chief complaints of left hemiplegia and dysarthria. He had a history of hypertension, old cerebral infarction, and NVAF and had been receiving oral administration of aspirin (100 mg/day). On arrival, the patient's NIHSS score was 14. Head MRI revealed acute cerebral infarction in the right frontal temporal lobe and corona radiata (DWI-ASPECTS: 6) (Figure F). MRA revealed a bilateral internal carotid artery occlusion (Figure G). At 240 minutes after onset, rt-PA was administered, and cerebral endovascular treatment was additionally administered. Because stenosis at the origin of the right internal carotid artery was so severe that a therapeutic catheter could not be crossed over the lesion, clopidogrel was administered at a loading dose of 300 mg, followed by emergency carotid artery stenting (WALLSTENT) (Figure H). Then, aspiration with the PenumbraⓇ system was performed for a right middle cerebral artery occlusion (M1 segment), and complete recanalization was achieved at 323 minutes after onset. After treatment, antiplatelet therapy (clopidogrel 50 mg + aspirin 100 mg) was continued. SBP was maintained at 100 to 130 mmHg by a continuous intravenous infusion of nicardipine. Head computed tomography performed 24 hours later revealed asymptomatic hemorrhagic changes (Figure I). The course of rehabilitation therapy was uneventful, however, aortic dissection occurred on hospital day 38. The patient was therefore transferred to the department of cardiovascular medicine.
Case 3
A 72-year-old man with aphasia and a history of diabetes mellitus and dyslipidemia was brought to a previous hospital with a chief complaint of right hemiplegia. The patient's NIHSS score was 24. Head MRI revealed acute cerebral infarction in the left basal ganglia extending to the corona radiata (DWI-ASPECTS: 9) (Figure K). MRA revealed a left internal carotid artery occlusion (Figure L). After the administration of rt-PA at 130 minutes after onset, he was transferred to our hospital, where cerebral endovascular treatment was added. To remove an occlusion at the origin of the left middle cerebral artery, the thrombus was retrieved using the PenumbraⓇ system and Solitaire FRⓇ (Covidien, Irvine, USA). Although PTA for residual stenosis in the left internal carotid artery was performed, elastic recoil occurred. Therefore, clopidogrel and aspirin were administered at loading doses of 300 and 200 mg, respectively, followed by emergency carotid artery stenting (WALLSTENT) (Figure M). Complete recanalization was achieved at 366 minutes after onset. Antiplatelet therapy (clopidogrel 50 mg + aspirin 100 mg) was subsequently continued. The patient's SBP was maintained at 100 to 130 mmHg by a continuous intravenous infusion of nicardipine. During the follow-up period, he remained free of concurrent sICH (Figure N). On hospital day 25, the patient's mRS score was 2, and he was transferred to another hospital.
The use of antiplatelet drugs within 24 hours after intravenous rt-PA administration for patients undergoing emergency stent placement was approved by the ethical committee of our hospital (application no. 10-054).
Discussion
Carotid artery stent placement during endovascular treatment for patients who are unresponsive to intravenous rt-PA administration has become a popular treatment in clinical practice. The Multicenter Randomized Clinical Trial of Endovascular Treatment for Acute Ischemic Stroke in the Netherlands reported that acute carotid artery stent placement was performed in 12.9% of patients (1). We have performed immediate endovascular treatment after intravenous rt-PA administration for 29 patients in our hospital since September 2012; 23 of these patients had a stroke in the area of anterior circulation. In particular, carotid artery stent placement was performed in three (13%) of 23 patients. The leading complication of carotid artery stent placement after intravenous rt-PA administration is considered to be sICH. In the present study, all three patients received loading doses of antiplatelet drugs during surgery and subsequently received dual antiplatelet therapy, without showing sICH.
In our hospital, the imaging indications for endovascular treatment after intravenous rt-PA administration are based on the MRA-DWI mismatch status (9). In this regard, DWI-ASPECTS ≥6 and a major artery occlusion [internal carotid artery or middle cerebral artery (M1 segment)] were considered to indicate MRA-DWI mismatch and therefore provided support for the use of additional endovascular treatment. Selecting patients according to their MRI findings (e.g., MRA-DWI mismatch) appears to be important to prevent sICH. In our department, the goal of BP control in patients undergoing carotid artery stenting after intravenous rt-PA therapy is set at SBP ≤130 mmHg. Strict BP control is important to prevent hyperperfusion syndrome.
In addition, patients with a vulnerable plaque are at an increased risk for artery-to-artery embolism and acute in-stent thrombosis, as shown in case 1. In this study, one of the patients experienced thrombus formation in the placed stent without any worsening of the neurological symptoms. Symptomatic in-stent thrombosis is rare and is reported to be between 0.04-2% (10,11). However, because in-stent thrombosis can result in serious consequences, emergency surgical treatment (e.g., PTA, stent-in-stent placement, or thrombectomy) should be considered for such cases.
There is no evidence of emergency stent placement for acute cerebral infarction and there are no clear guidelines for specific medications or loading dosage of antiplatelet agents at the time of stent placement (6). In each of the presented cases, the antiplatelet drug types and doses for both pre-stent loading and after therapy were decided by the attending physician according to the patient's background variables, including age and pre-onset history of antiplatelet drug use. Therapeutic strategies for the use of antiplatelet drugs in patients undergoing emergency stent placement should be established, with a particular focus on the type and dosing of antiplatelet drugs for patients that have received intravenous rt-PA therapy.
The authors state that they have no Conflict of Interest (COI).
References
- 1. Berkhemer OA, Fransen PS, Beumer D, et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med 372: 11-20, 2015. [DOI] [PubMed] [Google Scholar]
- 2. Goyal M, Demchuk AM, Menon BK, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med 372: 1019-1030, 2015. [DOI] [PubMed] [Google Scholar]
- 3. Campbell BC, Mitchell PJ, Kleinig TJ, et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med 372: 1009-1018, 2015. [DOI] [PubMed] [Google Scholar]
- 4. Saver JL, Goyal M, Bonafe A, et al. Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med 372: 2285-2295, 2015. [DOI] [PubMed] [Google Scholar]
- 5. Powers WJ, Derdeyn CP, Biller J, et al. 2015 American Heart Association/American Stroke Association Focused Update of the 2013 Guidelines for the Early Management of Patients With Acute Ischemic Stroke Regarding Endovascular Treatment: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke 46: 3020-3035, 2015. [DOI] [PubMed] [Google Scholar]
- 6. Papanagiotou P, Roth C, Walter S, et al. Carotid artery stenting in acute stroke. J Am Coll Cardiol 58: 2363-2369, 2011. [DOI] [PubMed] [Google Scholar]
- 7. Sallustio F, Koch G, Rocco A, et al. Safety of early carotid artery stenting after systemic thrombolysis: a single center experience. Stroke Res Treat 2012: 904575, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sorkin GC, Dumont TM, Mokin M, et al. Hyperacute carotid stent thrombosis during emergent revascularization treated with intraarterial eptifibatide after systemic administration of recombinant tissue plasminogen activator. J Vasc Interv Neurol 8: 50-55, 2015. [PMC free article] [PubMed] [Google Scholar]
- 9. Deguchi I, Dembo T, Yoshimura S, et al. Relationship between magnetic resonance angiography-diffusion-weighted imaging mismatch and clinical outcome in endovascular treatment for acute ischemic stroke: subgroup analysis of the Recovery by Endovascular Salvage for Cerebral Ultra-acute Embolism-Japan Registry. J Stroke Cerebrovasc Dis 23: 1471-1476, 2014. [DOI] [PubMed] [Google Scholar]
- 10. Jongen LM, Hendrikse J, Waaijer A, et al. Frequency and consequences of early in-stent lesions after carotid artery stent placement. J Vasc Interv Radiol 20: 573-579, 2009. [DOI] [PubMed] [Google Scholar]
- 11. Watarai H, Kaku Y, Yamada M, et al. Follow-up study on in-stent thrombosis after carotid stenting using multidetector CT angiography. Neuroradiology 51: 243-251, 2009. [DOI] [PubMed] [Google Scholar]