Abstract
Background. Herpes zoster, or shingles, is a common, painful reactivation of latent varicella zoster virus infection. Understanding host factors that predispose to herpes zoster may permit development of more effective prevention strategies. Our objective was to examine mitochondrial haplogroups as a potential host factor related to herpes zoster incidence.
Methods. Study participants were drawn from BioVU, a deoxyribonucleic acid (DNA) biobank connected to deidentified electronic medical records (EMRs) from Vanderbilt University Medical Center. Our study used 9691 Caucasian individuals with herpes zoster status determined by International Classification of Diseases, Ninth Revision codes 053–053.9. Cases and controls were matched on sex and date of birth within 5 years. Mitochondrial haplogroups were defined from mitochondrial DNA variants genotyped on the Illumina 660W or Illumina Infinium Human-Exome Beadchip. Sex and date of birth were extracted from the EMR.
Results. European mitochondrial haplogroup H had a protective association with herpes zoster status (odds ratio [OR] = .82; 95% confidence interval [CI], .71–.94; P = .005), whereas haplogroup clade IWX was a risk factor for herpes zoster status (OR = 1.38; 95% CI, 1.07–1.77; P = .01).
Conclusions. Mitochondrial haplogroup influences herpes zoster risk. Knowledge of a patient's mitochondrial haplogroup could allow for a precision approach to the management of herpes zoster risk through vaccination strategies and management of other modifiable risk factors.
Keywords: herpes zoster, immunosenescence, mitochondrial haplogroup, shingles
Herpes zoster, or shingles, is a common and frequently painful reactivation of latent varicella zoster virus (VZV) infection affecting nerve root ganglia and their associated dermatomes [1]. Herpes zoster risk increases with age, and it can occur both in apparently immunocompetent and immunocompromised hosts. Incidence is estimated to be 4–4.5 cases per 1000 person-years and has been increasing as the population ages [2]. Although rarely fatal in immunocompetent persons, chronic and frequently debilitating pain, termed postherpetic neuralgia (PHN), is a common sequela of herpes zoster, complicating 18%–33% of cases and increasing with age [2]. Antiviral drugs at the time of herpes zoster are modestly effective at reducing duration of symptoms but do not decrease risk of PHN [3]. Nonetheless, given the near ubiquity of VZV infection in the prevaricella vaccine population (born before 1995), the burden of disease due to herpes zoster is substantial [4, 5]. In 2006, a live, attenuated vaccine for prevention of herpes zoster was licensed for persons >60 years of age. Clinical trials demonstrated an overall vaccine efficacy against herpes zoster of 51% [6–8] and a decrease in PHN incidence of 67% [6, 7]. However, despite good efficacy, uptake of the vaccine has been low, with an estimated 3.9% coverage rate among eligible US Medicare beneficiaries between 2007 and 2009 [9].
The observation that not all aged persons with latent VZV infection develop herpes zoster suggests a role for other host factors that might contribute to risk of VZV reactivation, including genetic variation. Patients who have herpes zoster are more likely than controls to have a relative with a history of zoster, indicating a role for genetics [10, 11]. Genetic factors that influence immune function could explain variable rates of reactivation in persons of otherwise similar age and health status. Relatively few studies have attempted to identify genetic risk factors for herpes zoster, although one study in almost 23 000 US adults identified an association between a noncoding region in the major histocompatibility complex region and age of onset of herpes zoster in both combined and European ancestry groups [12].
Waning T-cell immunity with age likely is a critical component of herpes zoster pathogenesis. Varicella zoster virus is in the human herpesvirus (HHV) family together with herpes simplex virus, cytomegalovirus, and HHV-6, -7, and -8. Common features of HHV are ease of transmission, high rates of seropositivity by adulthood in most populations, and establishment of latent infection with relatively rare clinical manifestations in immunocompetent hosts [13]. Growing evidence suggests that chronic HHV infection may contribute to chronic immunosenescence [14, 15], and that diminished T-cell function with age may influence reactivation of HHV [16].
The role of mitochondrial function in T-cell immunobiology is increasingly recognized [17, 18]. The normal functioning of cell-mediated immunity requires that specific subpopulations of T cells exhibit particular metabolic programs that are coordinated at the level of the mitochondrion. Impairment of the required metabolic functions impairs efficient T-cell proliferation and activation. In addition to the known relationships between T-cell functioning and herpesvirus activity, the direct influence of mitochondrial function on HHV-related disease has been explored. Several HHVs have been shown to deliberately destroy host mitochondrial deoxyribonucleic acid (mtDNA) in infected cells, suggesting that impairment of mitochondrial function is advantageous to these viruses [19]. Cytomegalovirus has been shown to reprogram host cell metabolism of all of the major classes of carbon substrate (carbohydrates, amino acids, and fatty acids) through direct interactions with mitochondria [20, 21].
Combinations of single nucleotide polymorphisms (SNPs) in mtDNA define haplogroups associated with maternal ancestry and ancient migration patterns. Haplogroups and other mtDNA variants have been associated with differences in mitochondrial function [22, 23], and the clinical relevance of mtDNA variation for successful aging and disease risk is well described [24–26]. A deeper understanding of the role of mitochondrial function in immunosenescence is emerging, and with it, the field of mitochondrial immunology [27]. To the best of our knowledge, no studies have tested the impact of mitochondrial genetics on herpes zoster. Our objective was to examine mitochondrial haplogroups as a potential host factor related to herpes zoster incidence. We hypothesized that the mtDNA genetic variation represented by haplogroups influences susceptibility to herpes zoster in immunocompetent adults by conferring subtle differences in the aging immune system's ability to maintain herpes zoster in a state of latency. We tested this hypothesis utilizing BioVU, a unique DNA biorepository linked to data from the electronic medical record (EMR) [28].
METHODS
Study Design
The study is a matched case-control design with a longitudinal follow up. The case-control study had 1:10 matching based on sex and date of birth within 5 years. The longitudinal follow-up study used all records in an individual's EMR to investigate the time courses involved with zoster infection in our dataset.
Data Source and Study population
This study was approved by the Institutional Review Board at Vanderbilt University. This study used participants from the BioVU DNA repository [28, 29] at Vanderbilt University. This resource links leftover clinical blood samples to deidentified EMRs. All individuals used in this study were classified as “White” and had available genome-wide genotyping data from previous analyses. From within BioVU, we had 2 independent datasets: individuals genotyped on the Illumina 660W and individuals with genotyping on the Illumina Human Exome Bead Chip (Illumina, San Diego, CA). These populations were combined into a single dataset (Table 1).
Table 1.
Summary of Demographics in Whole Unmatched Dataset and by Mitochondrial Haplogroup
| Characteristic | All | H | IWX | J | T | Uk | Other | P Valuea |
|---|---|---|---|---|---|---|---|---|
| Total Unmatched Data | ||||||||
| Number of Individuals | 20 551 | 9798 | 1322 | 2123 | 2246 | 4577 | 485 | |
| Sex (%Male) | 9278 (45.1) | 4365 (44.5) | 575 (43.5) | 974 (45.9) | 1043 (46.4) | 2117 (46.3) | 204 (42.1) | .11 |
| Zoster (%Cases) | 881 (4.3) | 379 (3.9)* | 76 (5.7)* | 101 (4.8) | 98 (4.4) | 208 (4.5) | 19 (3.9) | .02 |
| Cases vaccinated (%) | 107 (0.5) | 49 (0.5) | 8 (0.6) | 7 (0.3) | 13 (0.6) | 27 (0.6) | 3 (0.6) | .79 |
| Median age first zoster (IQR) | 67 (57, 77) | 68 (56, 76) | 65 (57, 75) | 68 (59, 77) | 67 (57, 78) | 67 (56, 77) | 70 (52, 82) | .80 |
| Median age last record (IQR) | 63 (52, 76) | 63 (52, 76) | 64 (51, 76) | 63 (52, 75) | 63 (52, 76) | 64 (52, 76) | 62 (50, 75) | .59 |
Abbreviation: IQR, interquartile range.
a The significance across all haplogroups by χ2 test, Fisher test, or Kruskal–Wallis test.
*P < .01. Significance of each haplogroup vs all others column.
Phenotype Determination and Covariates
Case-control criteria are defined in Figure 1. Individuals with 2 or more human immunodeficiency virus (HIV) International Classification of Diseases, Ninth Revision (ICD-9) codes (042) were excluded from analysis, as most likely having HIV and being immunocompromised. Individuals who were not at least 20 years old at the last ICD-9 code in their record were also excluded. Case status was determined by the presence of 1 or more ICD-9 codes for herpes zoster (053–053.9; Supplementary Table 1) in an individual's medical record [12, 30]. A previous study determined that a single herpes zoster ICD-9 code could identify cases with high positive and negative predictive values of 90% and 99.3%, respectively [31]. Potential cases were excluded if they had the Current Procedural Terminology (CPT) code for the zoster vaccine (90 736) up to 1 month before their first herpes zoster code or had an ICD-9 code for herpes zoster before the age of 20. Controls were determined by the lack of any ICD-9 code for herpes zoster. Potential controls were excluded if they ever had the CPT code for the zoster vaccine. Controls were required to have at least 1 year of record.
Figure 1.
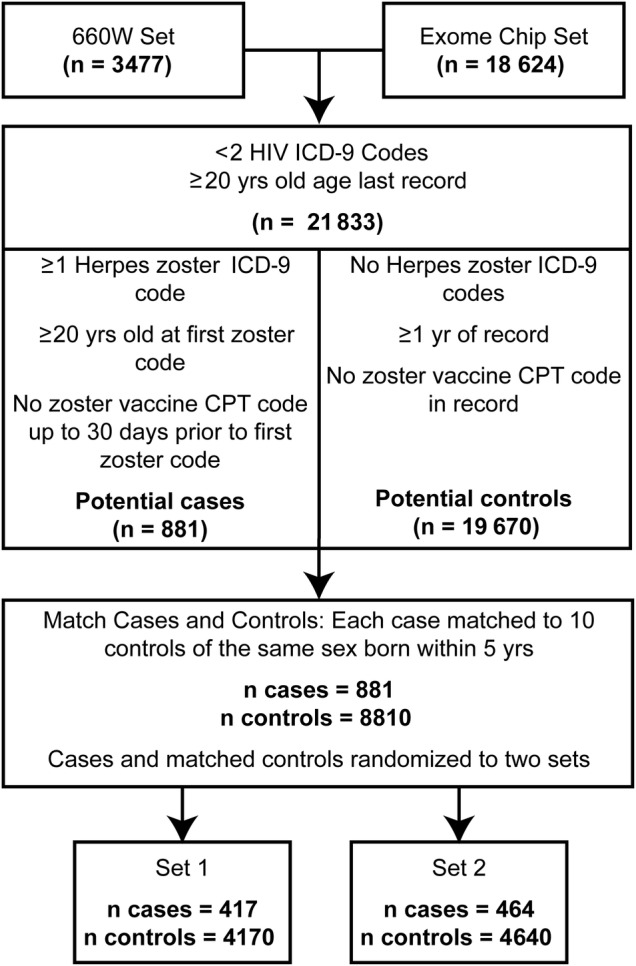
Criteria for defining and matching herpes zoster cases and controls. The flowchart describes the pipeline from initial genotyped sets through matching and splitting into analysis sets. Exclusionary criteria, and case and control definition along with the relative numbers that passed through each filtering step, are presented. A total of 881 cases were matched and randomly split into 2 sets of 417 and 464 cases. Abbreviations: CPT, Current Procedural Terminology; HIV, human immunodeficiency virus.
Date of birth and sex were abstracted from the medical records. For individuals who were herpes zoster cases, we calculated age at the first incidence of a herpes zoster ICD-9 code. For all individuals, we calculated the age at last record and the length of record as determined by ICD-9 code. As part of the deidentification process, all dates in the EMR have been shifted by an amount within 1 year (random between study individuals, constant within each individual [28]). Consequently, age-at-event values are accurate.
Case-Control Matching and Set Assignment
We matched herpes zoster cases to controls by sex and by age using date of birth. Cases were matched to 10 controls born within 5 years. Cases with the fewest potential matched controls in their age window were matched first. Cases and their matched controls were randomly assigned to the discovery (Set 1) and replication (Set 2) sets.
Genotyping and Haplogroup Assignment
Individuals had been previously genotyped on the Illumina 660W (3477 individuals) or the Illumina Infinium HumanExome BeadChip [32] (18 624 individuals) at the VANTAGE Core laboratory, and the genotyping data had been redeposited in BioVU for research purposes [28]. The Illumina 660W genotyping chip contains 138 SNPs from the mitochondrial genome, whereas the Exome chip contains 219 mtDNA SNPs. The mtDNA SNPs were used to generate a variant list for each BioVU study individual relative to the standard mtDNA reference sequence, the revised Cambridge Reference Sequence [33]. The mitochondrial haplogroup of each individual was determined from this variant list using HaploGrep [34].
Statistical Analyses
Before matching, Fisher or χ2 tests were performed to compare herpes zoster status, zoster vaccination status, and sex across haplogroups. Logistic regressions adjusting for age at last record and sex were used to test for the association of specific haplogroups with herpes zoster status. Mantel-Haentzel tests were used to analyze the frequency of haplogroup in cases compared with their matched controls. Five tests were done per dataset, one for each major European mitochondrial haplogroup. Among cases, Wilcoxon rank-sum tests were used in a pairwise manner to determine whether the age at first herpes zoster ICD-9 code differed by haplogroup.
For the longitudinal study, we carried out Cox proportional hazard tests in the unmatched dataset of 20 551 individuals. Receiving an ICD-9 code for herpes zoster was considered an event, and the age at that ICD-9 code was used as the time. Individuals whose records ended without ever including a herpes zoster ICD-9 code were censored at that time. No records before the age of 20 were included. Cox proportional hazard regressions were run both unadjusted and adjusted for sex.
All analyses were performed using R 3.1.3. Cox proportional hazards and Kaplan-Meier curves were done with the Survival package [35] version 2.38-1 for R.
RESULTS
Cohort Characteristics
Our total initial dataset consisted of 20 551 individuals (Table 1). Before age matching and removal of controls with zoster vaccination, 4.3% of individuals in our set were zoster cases, and, of these, 0.5% had been vaccinated for zoster at some point. As described in the Methods section, the cases were then matched to 10 unique controls by sex and birth date, dropping the total cohort size to 9691 (881 cases and 8810 controls) (Table 2). The cases were then randomly split into a discovery set and a replication set. Matched controls for each case were also brought into each set. The 2 sets are used to test for reproducibility of the results. The discovery set (Set 1) contained 4587 individuals, and the replication set (Set 2) contained 5104 individuals (Table 3). The proportion of males in cases and controls combined was statistically different between the discovery and replication set in all individuals by a χ2 test. However, this is misleading because only the cases were randomly distributed to the 2 sets. When only cases were considered, the proportion of males was not statistically different between the 2 groups (P = .35).
Table 2.
Summary of Demographics for All Individuals and by Mitochondrial Haplogroup in the Matched Dataset Before Randomization
| Characteristic | All | H | IWX | J | T | Uk | Other | P Valuea |
|---|---|---|---|---|---|---|---|---|
| Total Matched Dataset (Set 1 + Set 2) | ||||||||
| Number of Individuals | 9691 | 4602 | 641 | 1015 | 1061 | 2153 | 219 | |
| Sex (%Male) | 3608 (37.2) | 1665 (36.2)** | 231 (36.0) | 405 (39.9) | 414 (39.0) | 825 (38.3) | 68 (31.1) | .04 |
| Zoster (%Cases) | 881 (9.1) | 379 (8.2)* | 76 (11.9)** | 101 (10.0) | 98 (9.2) | 208 (9.7) | 190 (8.7) | .04 |
| Cases vaccinated (%) | 107 (1.1) | 49 (1.1) | 8 (1.2) | 7 (0.7) | 13 (1.2) | 27 (1.3) | 3 (1.4) | .71 |
| Median age first zoster (IQR) | 67 (57, 77) | 68 (56, 76) | 65 (57, 75) | 68 (59, 77) | 67 (57, 78) | 67 (56, 77) | 70 (52, 82) | .80 |
Abbreviation: IQR, interquartile range.
a The significance across all haplogroups by χ2 test, Fisher test, or Kruskal–Wallis test.
*P < .01. Significance of each haplogroup vs all others column.
**P < .05. Significance of each haplogroup vs all others column.
Table 3.
Summary of Demographics in the Randomly Defined Discovery and Replication Datasets
| Characteristic | All | H | IWX | J | T | Uk | Other | P Valuea |
|---|---|---|---|---|---|---|---|---|
| Discovery Set (Set 1) | ||||||||
| Number of Individuals | 4587 | 2199 | 296 | 481 | 505 | 991 | 115 | |
| Sex (%Male) | 1628 (35.5)b | 745 (33.9)** | 106 (35.8) | 172 (35.8) | 198 (39.2) | 372 (37.5) | 35 (30.4) | .12 |
| Zoster (%Cases) | 417 (9.1) | 180 (8.2)** | 40 (13.5)* | 47 (9.8) | 51 (10.1) | 85 (8.6) | 14 (12.2) | .04 |
| Cases vaccinated (%) | 47 (1.0) | 18 (0.8) | 4 (1.4) | 4 (0.8) | 7 (1.4) | 12 (1.2) | 2 (1.7) | .53 |
| Median age first zoster (IQR) | 68 (56,77) | 67 (54,76) | 67 (59, 77) | 67 (59, 78) | 71 (58, 82) | 69 (56, 77) | 70 (58, 81) | .60 |
| Replication Set (Set 2) | ||||||||
| Number of Individuals | 5104 | 2403 | 345 | 534 | 556 | 1162 | 104 | |
| Sex (%Male) | 1980 (38.8)b | 920 (38.3) | 125 (36.2) | 233 (43.6)** | 216 (38.8) | 453 (40.0) | 33 (31.7) | .12 |
| Zoster (%Cases) | 464 (9.1) | 199 (8.3) | 36 (10.4) | 54 (10.1) | 47 (8.5) | 123 (10.6) | 5 (4.8) | .11 |
| Cases vaccinated (%) | 60 (1.2) | 31 (1.3) | 4 (1.2) | 3 (0.6) | 6 (1.1) | 15 (1.3) | 1 (0.96) | .82 |
| Median age first zoster (IQR) | 67 (57, 76) | 68 (58, 77) | 61 (57, 74) | 68 (59, 77) | 65 (55, 74) | 66 (56, 76) | 70 (35, 80) | .69 |
Abbreviation: IQR, interquartile range.
a The significance across all haplogroups by χ2 test, Fisher test, or Kruskal–Wallis test.
b Discovery set is statistically different than replication set in all individuals.
*P < .01. Significance of each haplogroup vs all others column.
**P < .05. Significance of each haplogroup vs all others column.
Case Control Study
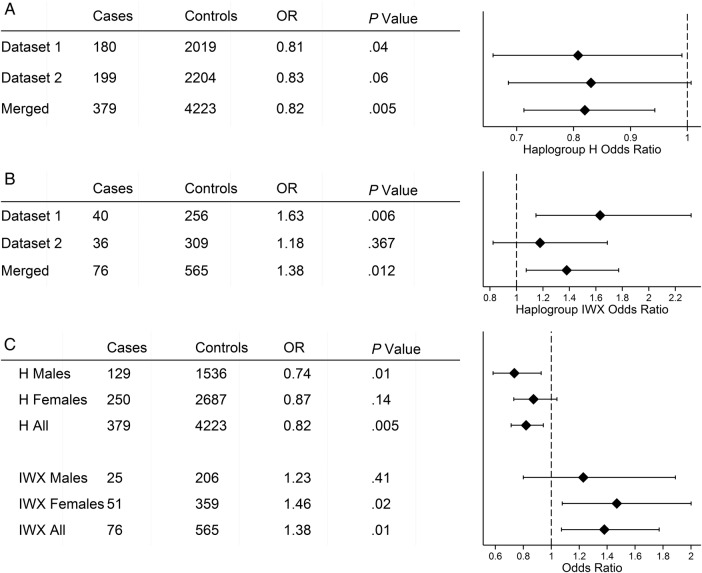
The common European haplogroups J, T, and Uk had no significant associations with herpes zoster in our analysis. In Set 1, haplogroup H was protective for herpes zoster (odds ratio [OR] = .81; 95% confidence interval [CI], .66–.99; P = .04), whereas in Set 2 haplogroup H was trending towards protective for herpes zoster (OR = .83; 95% CI, .69–1.00; P = .06). In the merged set, the protective effect for haplogroup H was highly significant (OR = .82; 95% CI, .71–.94; P = .005) (Figure 2A). The haplogroup clade IWX, combining the 3 rare haplogroups I, W, and X, which are neighboring in the mitochondrial phylogenetic tree, was a risk factor for herpes zoster (OR = 1.63; 95% CI, 1.15–2.32; P = .006) in Set 1. However, in Set 2, haplogroup clade IWX was not significant as a risk factor for herpes zoster (OR = 1.18; 95% CI, .82–1.69; P = .37). In the merged dataset, haplogroup clade IWX was significant at the P = .05 level (OR = 1.38; 95% CI, 1.07–1.77; P = .01) (Figure 2B).
Figure 2.
Association of haplogroups H and IWX with herpes zoster by Mantel-Haentsel tests. Forest plots for (A) haplogroup H and (B) clade IWX with case and control numbers, odds ratio (OR), and P value. The merged group has greater power for group H, whereas for IWX a significant association was only seen in Dataset 1. (C) Stratification by sex. In H the protective effect is stronger in males, and the risk effect of IWX is stronger in females.
In the initial dataset with unmatched controls, female sex was a significant predictor of zoster risk (OR = 1.41; 95% CI, 1.23–1.62; P = 1.6e-6), consistent with published literature [36]. Because sex also significantly differed (P = .04) across haplogroups in the total matched dataset by χ2 test (Table 1), we performed an analysis stratified by sex in this set. The association of haplogroup H with zoster had a significant protective effect in males (OR = .74; 95% CI, .58–.93; P = .01), but a weaker effect in females that did not reach significance (Figure 2C). Haplogroup clade IWX had the reverse sex pattern and was not statistically associated with herpes zoster in males, but it was significantly associated in females (OR = 1.47; 95% CI, 1.08–2.00; P = .02).
Longitudinal Analysis
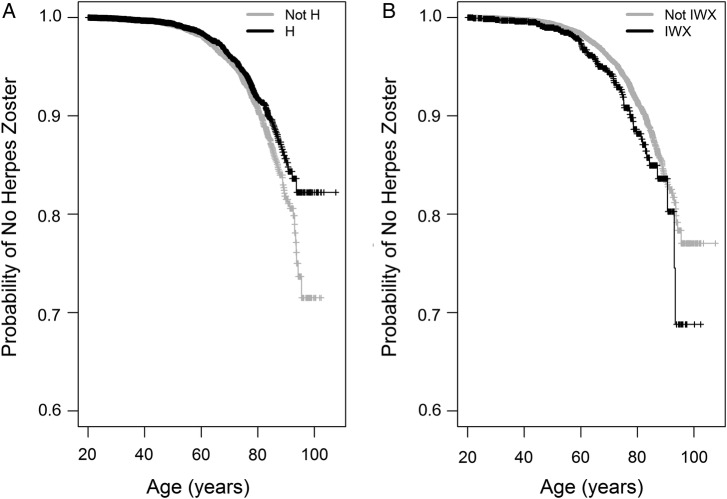
For the longitudinal analysis, we returned to the initial unmatched dataset of 20 551 individuals (Table 1). As described under the Methods, we tested for the age at occurrence of the first herpes zoster ICD-9 code through the course of the EMR. In an unadjusted Cox proportional hazards model, haplogroup H showed a protective effect from having a zoster ICD-9 code (hazard ratio [HR] = .82; 95% CI, .71–.93; P = .003), compared with all subjects from other haplogroups (Figure 3A). In contrast, haplogroup clade IWX had a risk effect (HR = 1.36; 95% CI, 1.08–1.73; P = .009) (Figure 3B). The IWX and not IWX populations began to deviate in the probability of not having a herpes zoster ICD-9 code at approximately the age of 60, whereas the divergence of the H and not-H groups developed more gradually over time. Results from the Cox proportional hazard tests are very similar to those from the simpler Mantel-Haenszel tests. In Cox proportional hazard models accounting for sex (Supplementary Table 2), sex was a significant predictor in both models, but the inclusion of sex did not remove the effect of haplogroup H and clade IWX on probability of having a herpes zoster ICD-9 code in the medical record.
Figure 3.
Kaplan-Meier curves showing unadjusted probability of not having a herpes zoster International Classification of Diseases, Ninth Revision (ICD-9) code over time. Data analyzed were age at first herpes zoster ICD-9 for the cases and age at last medical record for the controls. Incidence of a herpes zoster ICD-9 code was considered an event. We compared (A) haplogroup H subjects to all subjects not in haplogroup H and (B) IWX haplogroup clade subjects vs all others. Censored values from the end of the medical record are denoted by vertical lines.
DISCUSSION
In the present study, we have demonstrated a previously unknown association between mitochondrial haplogroups and the reactivation of VZV in humans. Haplogroup H, the most recent of the Caucasian haplogroups in evolutionary time, is associated with a lower likelihood of herpes zoster. In contrast, haplogroup clade IWX, which is evolutionarily much older, is significantly associated with an approximately 40% increase in the likelihood of developing clinically apparent herpes zoster. These results are consistent with previous data from others for phenotypes involving the immune system showing protective associations with haplogroup H and/or increased risk associations with haplogroup clade IWX. Conditions previously reported to be impacted in these ways include age-related macular degeneration and severe sepsis [37, 38]. Haplogroup H has been reported to associate with an increased maximal mitochondrial functional capacity in humans [39]. The effect of sex seen in our study was consistent with females being at increased risk of zoster compared with males [2, 10]. In haplogroup clade IWX, the significant risk affect was driven by the females, whereas in males IWX was not a risk factor. In contrast, haplogroup H was not a statistically significant protective factor in females, whereas it was in males.
In a previous study of herpes zoster in an EMR dataset, Crosslin et al [12] found several SNPs in the human leukocyte antigen (HLA) region that were associated with protection from herpes zoster. We were unable to test these HLA variants in our dataset because these SNPs were not on the Human Exome BeadChip. Further exploration of genetic variation known to impact immune response including the HLA region is necessary to fully understand the impact of mitochondrial haplogroup on herpes zoster. Because haplogroup-defining mtDNA variants have been linked to altered expression of apoptosis, inflammation, and complement genes, suggesting that mitochondrial-nuclear interactions play a role in immune regulation [40], it is possible that some interaction between nuclear and mitochondrial biology could influence herpes zoster.
The limitations of our study include that some individuals we classified as cases may be immunocompromised. Although we excluded exceedingly young zoster cases and individuals with HIV, we did not exclude individuals with a history of transplant or those with a history of blood cancers. In addition, although we required a record of 1 year for controls, it is possible that some individuals we included as controls had herpes zoster at some point that did not appear in their Vanderbilt EMR. The dataset was taken from a hospital-derived cohort, so it is likely that the vast majority of both cases and controls for this study have other medical comorbidities. We did not control for, or ascertain, all published confounders, including medications. We also did not manually review and verify all records.
We believe that the matching protocol used was overly conservative. Matching on sex and date of birth allowed us to minimize the impact of sex on our results. Matching by date of birth paired cases and controls who had risk of herpes zoster due to age but did not account for factors such as general health over a lifetime and other diseases or comorbidities. The matching procedure also removed a small number of cases from analysis, primarily those that were on the ends of the age spectrum, due to lack of a sufficient number of matching controls, reducing our power slightly. Despite this, our study still found associations after a matching protocol designed to reduce the effect of other known risk factors. More importantly, we saw the same associations in our dataset both before and after matching.
Future steps to understand the influence of mitochondrial haplogroup on herpes zoster status should include a secondary validation in an independent dataset. Further analysis of the influence of mitochondrial haplogroup on herpes zoster in African Americans will also be important, especially because previous literature has shown differences in mitochondrial function in European and African haplogroups [40]. The African American population available in the BioVU database was too small for reasonably powered analyses. Functional studies to understand how variants in the H and IWX haplogroups affect mitochondrial function would help in understanding the mechanisms of the association.
CONCLUSIONS
In summary, we explore mitochondrial haplogroups as a risk factor for herpes zoster. We identified that haplogroup H was a protective factor, whereas haplogroup clade IWX was a risk factor for herpes zoster. Considering that 45% of people of European ancestry are in haplogroup H and 6% are in the IWX clade, these factors are common and are important in the general population for zoster risk.
Supplementary Data
Supplementary material is available online at Open Forum Infectious Diseases online (http://OpenForumInfectiousDiseases.oxfordjournals.org/).
Acknowledgments
Financial support. The datasets used for the analyses described were obtained from Vanderbilt University Medical Center's BioVU, which is supported by institutional funding and by Vanderbilt Clinical and Translational Science Award (grant ULTR000445) from the National Center for Advancing Translational Sciences/National Institutes of Health. This work was supported by the National Institutes of Health (grants 5T32GM080178-09 [to R. T. L.] and K08 HL121174 [to J. P. F.]) and the Parker B. Francis Foundation (to J. P. F.).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. Cohen JI. Herpes zoster. N Engl J Med 2013; 369:1766–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Yawn BP, Gilden D. The global epidemiology of herpes zoster. Neurology 2013; 81:928–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chen N, Li Q, Yang J et al. . Antiviral treatment for preventing postherpetic neuralgia. Cochrane Database Syst Rev 2014; 2:CD006866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Panatto D, Bragazzi NL, Rizzitelli E et al. . Evaluation of the economic burden of herpes zoster (HZ) infection. Hum Vaccin Immunother 2015; 11:245–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gater A, Uhart M, McCool R, Preaud E. The humanistic, economic and societal burden of Herpes Zoster in Europe: a critical review. BMC Public Health 2015; 15:193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Levin MJ, Oxman MN, Zhang JH et al. . Varicella-zoster virus-specific immune responses in elderly recipients of a herpes zoster vaccine. J Infect Dis 2008; 197:825–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Oxman MN, Levin MJ, Johnson GR et al. . A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med 2005; 352:2271–84. [DOI] [PubMed] [Google Scholar]
- 8. Schmader KE, Oxman MN, Levin MJ et al. . Persistence of the efficacy of zoster vaccine in the shingles prevention study and the short-term persistence substudy. Clin Infect Dis 2012; 55:1320–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Langan SM, Smeeth L, Margolis DJ, Thomas SL. Herpes zoster vaccine effectiveness against incident herpes zoster and post-herpetic neuralgia in an older US population: a cohort study. PLoS Med 2013; 10:e1001420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gershon AA, Gershon MD, Breuer J et al. . Advances in the understanding of the pathogenesis and epidemiology of herpes zoster. J Clin Virol 2010; 48 (suppl 1):S2–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hicks LD, Cook-Norris RH, Mendoza N et al. . Family history as a risk factor for herpes zoster: a case-control study. Arch Dermatol 2008; 144:603–8. [DOI] [PubMed] [Google Scholar]
- 12. Crosslin DR, Carrell DS, Burt A et al. . Genetic variation in the HLA region is associated with susceptibility to herpes zoster. Genes Immun 2015; 16:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bennett JE, Dolin R, Blaser MJ. Principles and Practice of Infectious Diseases. Philadelphia, PA: Elsevier Health Sciences; 2014. [Google Scholar]
- 14. Badowski M, Shultz CL, Eason Y et al. . The influence of intrinsic and extrinsic factors on immune system aging. Immunobiology 2014; 219:482–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sansoni P, Vescovini R, Fagnoni FF et al. . New advances in CMV and immunosenescence. Exp Gerontol 2014; 55:54–62. [DOI] [PubMed] [Google Scholar]
- 16. Levin MJ. Immune senescence and vaccines to prevent herpes zoster in older persons. Curr Opin Immunol 2012; 24:494–500. [DOI] [PubMed] [Google Scholar]
- 17. Ron-Harel N, Sharpe AH, Haigis MC. Mitochondrial metabolism in T cell activation and senescence: a mini-review. Gerontology 2015; 61:131–8. [DOI] [PubMed] [Google Scholar]
- 18. Quintana A, Hoth M. Mitochondrial dynamics and their impact on T cell function. Cell Calcium 2012; 52:57–63. [DOI] [PubMed] [Google Scholar]
- 19. Duguay BA, Saffran HA, Ponomarev A et al. . Elimination of mitochondrial DNA is not required for herpes simplex virus 1 replication. J Virol 2014; 88:2967–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Seo JY, Cresswell P. Viperin regulates cellular lipid metabolism during human cytomegalovirus infection. PLoS Pathog 2013; 9:e1003497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yu Y, Clippinger AJ, Alwine JC. Viral effects on metabolism: changes in glucose and glutamine utilization during human cytomegalovirus infection. Trends Microbiol 2011; 19:360–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Brown MD, Trounce IA, Jun AS et al. . Functional analysis of lymphoblast and cybrid mitochondria containing the 3460, 11778, or 14484 Leber's hereditary optic neuropathy mitochondrial DNA mutation. J Biol Chem 2000; 275:39831–6. [DOI] [PubMed] [Google Scholar]
- 23. Gomez-Duran A, Pacheu-Grau D, Lopez-Gallardo E et al. . Unmasking the causes of multifactorial disorders: OXPHOS differences between mitochondrial haplogroups. Hum Mol Genet 2010; 19:3343–53. [DOI] [PubMed] [Google Scholar]
- 24. Mancuso M, Filosto M, Orsucci D, Siciliano G. Mitochondrial DNA sequence variation and neurodegeneration. Hum Genomics 2008; 3:71–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nishigaki Y, Fuku N, Tanaka M. Mitochondrial haplogroups associated with lifestyle-related diseases and longevity in the Japanese population. Geriatr Gerontol Int 2010; 10 (suppl 1):S221–35. [DOI] [PubMed] [Google Scholar]
- 26. Raule N, Sevini F, Santoro A et al. . Association studies on human mitochondrial DNA: methodological aspects and results in the most common age-related diseases. Mitochondrion 2007; 7:29–38. [DOI] [PubMed] [Google Scholar]
- 27. Gvozdjáková A. Mitochondrial Medicine: Mitochondrial Metabolism, Diseases, Diagnosis and Therapy. Dordrecht, London: Springer; 2008. [Google Scholar]
- 28. Roden DM, Pulley JM, Basford MA et al. . Development of a large-scale de-identified DNA biobank to enable personalized medicine. Clin Pharmacol Ther 2008; 84:362–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pulley J, Clayton E, Bernard GR et al. . Principles of human subjects protections applied in an opt-out, de-identified biobank. Clin Transl Sci 2010; 3:42–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Insinga RP, Itzler RF, Pellissier JM et al. . The incidence of herpes zoster in a United States administrative database. J Gen Intern Med 2005; 20:748–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. McDonald JR, Zeringue AL, Caplan L et al. . Herpes zoster risk factors in a national cohort of veterans with rheumatoid arthritis. Clin Infect Dis 2009; 48:1364–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Guo Y, He J, Zhao S et al. . Illumina human exome genotyping array clustering and quality control. Nat Protoc 2014; 9:2643–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Andrews RM, Kubacka I, Chinnery PF et al. . Reanalysis and revision of the Cambridge reference sequence for human mitochondrial DNA. Nat Genet 1999; 23:147. [DOI] [PubMed] [Google Scholar]
- 34. Kloss-Brandstatter A, Pacher D, Schonherr S et al. . HaploGrep: a fast and reliable algorithm for automatic classification of mitochondrial DNA haplogroups. Hum Mutat 2011; 32:25–32. [DOI] [PubMed] [Google Scholar]
- 35. Therneau TM, Grambsch PM. Modeling Survival Data: Extending the Cox Model. New York: Springer; 2000. [Google Scholar]
- 36. Opstelten W, Van Essen GA, Schellevis F et al. . Gender as an independent risk factor for herpes zoster: a population-based prospective study. Ann Epidemiol 2006; 16:692–5. [DOI] [PubMed] [Google Scholar]
- 37. Mueller EE, Schaier E, Brunner SM et al. . Mitochondrial haplogroups and control region polymorphisms in age-related macular degeneration: a case-control study. PLoS One 2012; 7:e30874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Baudouin SV, Saunders D, Tiangyou W et al. . Mitochondrial DNA and survival after sepsis: a prospective study. Lancet 2005; 366:2118–21. [DOI] [PubMed] [Google Scholar]
- 39. Larsen S, Diez-Sanchez C, Rabol R et al. . Increased intrinsic mitochondrial function in humans with mitochondrial haplogroup H. Biochim Biophys Acta 2014; 1837:226–31. [DOI] [PubMed] [Google Scholar]
- 40. Kenney MC, Chwa M, Atilano SR et al. . Molecular and bioenergetic differences between cells with African versus European inherited mitochondrial DNA haplogroups: implications for population susceptibility to diseases. Biochim Biophys Acta 2014; 1842:208–19. [DOI] [PMC free article] [PubMed] [Google Scholar]