Abstract
Chronic systemic inflammation contributes to the development of adverse health conditions, yet the influence of fixed and modifiable risk factors on many serologic biomarkers of inflammation remains largely unknown. Serum concentrations of twenty-three biomarkers, including C-reactive protein (CRP), cytokines (CXCL11, CXCL8, CXCL10, CCL2, CCL13, CCL4, CCL17, CXCL13, IL-10, IL-12p70, IL-6, TNF-α, IL-2, IFN-γ, IL-1β, GM-CSF, BAFF), and soluble immune receptors (sCD14, sIL-2Rα, sCD27, sgp130, sTNF-R2) were measured longitudinally using multiplexed immunometric assays in 250 HIV-uninfected men followed in the Multicenter AIDS Cohort Study (1984–2009). Generalized gamma regression was used to determine the statistical significance of factors associated with each biomarker. After accounting for age, race, and education, and for analysis of multiple biomarkers, higher concentrations of specific individual biomarkers were significantly (P<0.002) associated with hypertension, obesity, hepatitis C infection, stimulant use, and diabetes and lower concentrations with hypercholesterolemia. These associations should be taken into account in epidemiological studies of these biomarkers, and may provide potential targets for disease prevention and treatment.
Keywords: immune activation, cytokines, chemokines, cytokine receptors, C-reactive protein, risk factors
1. Introduction
Chronic inflammation and immune activation, as measured by concentrations of circulating inflammatory biomarkers, such as cytokines and chemokines, are associated with increased risk of several chronic diseases, such as cancer, cardiovascular disease (CVD), diabetes, AIDS, kidney disease, and aging.1–8 Elevated levels of pro-inflammatory cytokines, such as IL-6 and TNF-α, and acute phase proteins such as C-reactive protein (CRP), are prognostic of CVD outcomes, including acute myocardial infarction, congestive heart failure, and death, with CRP now being recommended in global risk prediction models for CVD.9–11 Inflammation and dysregulated immune activation also have an etiologic role in carcinogenesis,12,13 for example, in hepatocellular carcinoma due to infection with hepatitis B or C virus.14 Elevated levels of B-cell stimulating cytokines, including IL-4 and IL-6, have been associated with AIDS-related non-Hodgkin lymphoma, possibly due to cytokine-mediated hyperstimulation of B-cell proliferation.2,15–17 Inflammation may also contribute to obesity-related insulin resistance and by promoting macrophage infiltration into pancreatic islets.18 Thus, understanding the host characteristics that influence inflammation is critical for understanding etiologic pathways, potential targets for preventive and therapeutic interventions, and risk stratification, and for the design of valid epidemiologic studies to define these.
Inflammatory pathways are complex and often involve overlapping immune processes. However, most studies have analyzed small numbers of inflammatory biomarkers, such as C-reactive protein (CRP), an acute phase reactant, and the pro-inflammatory cytokines IL-6 and TNF-α. This has greatly limited understanding of the relationships between inflammation and sociodemographics, risk behaviors, and morbidities. However, now that multiplex assay technologies are available that permit much more extensive characterizations using small quantities of serum,19 this situation is starting to improve. For example, a recent study of healthy women found that TNF-α, IL-1β, IL-2, sIL-2Rα, IL-10, and IL-12p40/70 were significantly associated with age, body mass index and reproductive factors.20
With the foregoing in mind, the objective of this study was to evaluate the association of sociodemographics, risk behaviors, and select morbidities, hypothesized a priori, with circulating concentrations of 23 markers including cytokines, chemokines, soluble immune receptors, and CRP. In addition to the morbidities noted above, obesity was analyzed because, as mentioned, it has been associated with inflammation.21,22 Behaviors analyzed included smoking, alcohol consumption, and use of recreational substances, including marijuana, amyl nitrates, and stimulants, that may stimulate the inflammatory system.20,23–32 For these analyses, we capitalized on the existence of specimens collected and stored with standardized methods in a long-term longitudinal study with well-characterized participants.
2. Materials and Methods
2.1. Study population and design
The Multicenter AIDS Cohort Study (MACS) is an ongoing prospective study of HIV-infected and HIV-uninfected men who have sex with men enrolled in Baltimore/Washington D.C., Chicago, Los Angeles, and Pittsburgh; 6,972 men were enrolled from 1984 through 2003. Standardized interviews and physical examinations were administered at semiannual study visits, including specimen collection for storage and testing in a national repository. A full description of study procedures has been published.33 Study documentation may be found at http://www.statepi.jhsph.edu/macs/macs.html. The institutional review board at each center approved the MACS protocols; informed consent was obtained from all participants.
We capitalized on the availability of archived serum obtained from the study visit to examine the effects of host factors on these biomarkers. Serum samples were processed within 6 hours of blood draw and stored at −80°C. Prior to testing, a previously unthawed stock vial was thawed for each study visit, aliquoted, and stored at −80°C until testing. The study was restricted to HIV-uninfected men to exclude the effect of HIV infection on these markers. We aimed to sample 4 longitudinal visits approximately 5 years apart for each of 250 HIV-uninfected participants, spanning the duration of the MACS (1984–2009 at the time); 90% (n=224) of these participants had samples available at all 4 visits, 9% (n=23) had 3 samples and the other 3 people had 1–2 samples. These men were randomly selected from 1,012 persistently HIV-uninfected men in the MACS with ≥4 longitudinal visits, with the exception that all HIV-uninfected men who had chronic hepatitis C infection were selected to obtain sufficient numbers of HCV-infected men to examine the effect of this infection on the markers studied.
2.2. Laboratory methods
Two electrochemiluminesence-based multiplex assay panels (Proinflammatory 9-plex and Chemokine 7-plex; Meso-Scale Diagnostics, LLC, Rockville, MD) were used to determine concentrations of IL-1β, IL-2, IL-6, IL-10, IL-12p70, GM-CSF, IFN-γ, TNF-α, CXCL8 (IL-8), CXCL10 (IP-10), CCL11 (eotaxin), CCL2 (MCP-1), CCL13 (MCP-4), CCL4 (MIP-1β), and CCL17 (TARC). All testing was done at a centralized laboratory. Analyte- and plate-specific lower limits of detection (LLOD) were calculated as concentrations 2.5 standard deviations above the background for each analyte on each plate. Concentrations of five soluble receptors (sCD14, sgp130, sIL-2Rα, sTNF-R2), a cytokine (BAFF), and a chemokine CXCL13 (BLC-BCA1), were measured in a single panel (Human Biomarker Custom Premix Kit A) using the fluorescent bead-based multiplexed Luminex xMAP system at a centralized laboratory (Fluorokine® MAP, R&D Systems, Minneapolis, MN), and a Bio-Plex 200 Luminex instrument and Bio-Plex software (Bio-Rad, Hercules, CA). A single assay lot was used. Finally, CRP was measured at Quest Diagnostics using a high-sensitivity nephelometric assay (Dade Behring, Inc., Newark, DE). All specimens for any given individual were run on the same plate.
2.3. Exposure variables
Sociodemographics included age at visit, race (non-black vs. black), and baseline educational level (four-year college degree or higher vs. less than college degree). Body-mass index (BMI = weight (kg)/height (m)2) was categorized into clinically meaningful categories: ≤24.9 (normal/underweight), 25–29.9 (overweight), and ≥30 (obese). Data from the visit closest to the blood draw (≤1.5 years) were used to define time-varying exposures. Detailed behavioral data were collected from participants at each visit using interviewer-administered and/or audio computer assisted self-interview (ACASI) format. Behavioral factors included any sexual activity, risky sex (≥2 partners [male or female]), smoking status (never, former, current), alcohol consumption (binge-heavy drinking [≥5 drinks/day for ≥1 month], moderate-heavy [3–4 drinks/day for >1 month, or ≥5 drinks/day per month], low-moderate [1–2 drinks/day or 3–4 drinks/day for ≤1 month], or none), and use of recreational drugs (marijuana, amyl nitrates, or any stimulant [cocaine, ecstasy, methamphetamines, or any other uppers]). Hepatitis C infection (HCV) was categorized as negative, cleared [antibody+ only], or chronic [RNA positive]. Sexually transmitted disease was present if any new diagnosis of herpes virus infection, syphilis, genital warts, or gonorrhea was reported. Presence of depressive symptoms was defined as ≥16 on the Center for Epidemiologic Studies Depression Scale (CES-D).34 An expanded categorization of depressive symptoms additionally included men who reported using medication for depression since the last visit, regardless of their CES-D score. Persistent diabetes (fasting glucose>126 mg/dl, hemoglobin A1c (HbA1c) ≥6.5%, or diabetic medication use) and persistent hypertension (systolic blood pressure (SBP) ≥140 mmHg, diastolic (DBP) ≥90 mmHg, or antihypertensive medication use) were defined if present at ≥2 visits prior to blood draw. Hypercholesterolemia was defined as fasting total serum cholesterol ≥200 mg/dl. Time of blood draw was P.M. versus A.M.
2.4. Statistical methods
Multivariate generalized gamma regression models were used to assess independent associations of exposures with individual biomarkers. The generalized gamma distribution is a flexible three-parameter distribution and permitted us to avoid making strong assumptions regarding the distributions of different biomarkers.35 Biomarker concentrations were inversed so that measurements below the lower limit of detection could be handled as right-censored. In all models, only the location (β) parameter was allowed to vary by exposure category, while scale (σ) and shape (λ) were held constant. Relative percentiles (the exponential of the –β, to account for using the inverse value) in biomarker concentrations were calculated for each covariate category. Because σ and λ were held constant, the relative percentile comparing one exposure category to another is constant across all percentiles of each biomarker. Using the relative percentiles, we calculated the percent difference in biomarker concentrations between the exposed and unexposed [(relative percentile–1) × 100]. Robust standard errors adjusted for repeated measurements by using the vce(cluster) option in Stata. We also conducted logistic regression analyses accounting for correlated outcomes to determine associations between the factors and detectability of those cytokines with < 80% detectable values. All statistical tests were 2-sided. To account for multiple testing, a Bonferroni adjustment at an α-level of 0.05 [(0.05/23)=0.002] for statistical significance was employed. Results with 0.002<P≤0.05 were considered marginally significant.
Separate analyses were conducted for each biomarker, controlling first for age, study site, race, education, and time of blood draw. Subsequent models included modifiable factors: BMI, HCV, risk behaviors, and depressive symptoms. Because consistent ascertainment of morbidities (diabetes, hypertension, and hypercholesterolemia) began only in 2001, models examining these morbidities were restricted to data obtained from the period 2001–2009. For all models, age was centered at the median (46 years) across all 971 person-visits and associations per 10-years were assessed. The estimated differences are presented graphically, in the form of a heatmap, which permits examination of the associations of individual exposures with each biomarker as well as examining the biomarkers that are affected by the exposures. Color densities for the heatmap were standardized using the sample standard deviation of each exposure.
Analyses were conducted using SAS, version 9.3 (SAS Institute, Inc., Cary, North Carolina) and Stata, version 13 (College Station, Texas).
3. Results
The characteristics of the study population are described in Table 1. Of the 250 men, 52% (n=129) entered into the cohort before 2001, and 121 (48%) in 2001–3. Overall, the median time from first to last sample analyzed for an individual was 18.3 years (IQR: 5.5–19.8); the median interval between samples was 4.5 years (IQR: 2.6–12.8). The median age across all person-visits was 45.6 years (IQR: 39.7–52.5), and 55% of men were overweight or obese. Thirty percent reported having depressive symptoms, and 37% were classified as depressed when those using antidepressant medication were added to those with only depressive symptoms. Approximately 23% of the study group had chronic HCV infection, which reflects the original design of the larger cohort from which this group was drawn.
Table 1.
Characteristics of person-visits from 250 HIV-uninfected men from the Multicenter AIDS Cohort Study (MACS), 1984 – 2009
| Sociodemographics | Median (IQR) or %
|
|---|---|
| Overall (N = 971) | |
| Age at blood sampling (years), median (IQR) | 45.6 (39.7–52.5) |
| ≤30 | 7 |
| 30.1 ≤ 40 | 19 |
| 40.1 ≤ 50 | 41 |
| 50.1 ≤ 60 | 24 |
| 60.1 ≤ 70 | 8 |
| 70.1+ | 1 |
| Race | |
| Non-black | 61 |
| Black | 39 |
| Education (at baseline) | |
| Four-year college degree or higher | 47 |
| Less than college degree | 53 |
| Body mass index at blood sampling, kg/m2 | |
| Obese (≥30) | 19 |
| Overweight (25–29.9) | 37 |
| Normal/underweight (≤24.9) | 45 |
| Hepatitis C infection at blood sampling | |
| Chronic | 23 |
| Cleared (Antibody+ only) | 7 |
| Negative | 70 |
| CES-D Score, mean (SD)a | 11 |
| Depressive symptomsb | |
| Yes | 29 |
| No | 72 |
| Depressionc | |
| Yes | 37 |
| No | 63 |
| Study site | |
| Baltimore/Washington D.C. | 22 |
| Chicago | 24 |
| Pittsburgh | 35 |
| Los Angeles | 19 |
| Time of blood draw | |
| A.M. | 54 |
| P.M. | 47 |
| Behaviors | Median (IQR) or % Overall (N = 971) |
|---|---|
| Smoking status at blood sampling | |
| Current | 41 |
| Former | 35 |
| Never | 24 |
| Drinking classification at blood samplingd | |
| Binge | 11 |
| Moderate-heavy | 20 |
| Low-moderate | 50 |
| Non drinker | 19 |
| Marijuana use since last visit | |
| Yes | 31 |
| No | 69 |
| Amyl nitrates use since last visit | |
| Yes | 19 |
| No | 81 |
| Stimulant use since last visite | |
| Yes | 20 |
| No | 80 |
| Risky sex since last visitf | |
| Yes | 46 |
| No | 54 |
| Sexually transmitted infection since last visitg | |
| Yes | 14 |
| No | 86 |
|
| |
| Morbidities (2001 – 2009) | N = 720 |
|
| |
| Persistent diabetes mellitush | |
| Yes | 9 |
| No | 91 |
| Uncontrolled diabetesi | |
| Yes | 7 |
| No | 93 |
| Persistent hypertensionj | |
| Yes | 49 |
| No | 51 |
| Uncontrolled hypertensionk | |
| Yes | 30 |
| No | 70 |
| Total cholesterol, mg/dll | |
| Borderline-high (≥200) | 34 |
| Desirable (<200) | 66 |
| High density lipoprotein (mg/dl) | 49 (40 – 58) |
| Low density lipoprotein (mg/dl)m | 110 (88 – 137) |
Abbreviations: AIDS, acquired immunodeficiency syndrome; HIV, human immunodeficiency virus; IQR, interquartile range (25%, 75%).
Center for Epidemiologic Studies Depression Scale (CES-D) score 10-item version (possible range 0–30 points).
Presence of depressive symptoms was defined as CES-D score ≥16.
Depression was defined as presence of depressive symptoms (CES-D≥16) or use of depression medications.
Binge drinking was defined as ≥5 drinks/day for ≥ 1 month; moderate-heavy drinking was defined as 3–4 drinks/day for >1 month, or ≥5 drinks/day per month; low-moderate drinking was defined as 1–2 drinks/day or 3–4 drinks/day for ≤1 month.
Stimulants were defined as any use of cocaine, ecstasy, methamphetamines, or any other upper.
Risky sex was defined as ≥2 partners [male or female] in the past six months.
Sexually-transmitted infection was defined as any new diagnosis of herpes, syphilis, genital warts, or gonorrhea since the last visit.
Persistent diabetes was defined as having a history of diabetes (fasting glucose>126 mg/dL, Hemoglobin A1c≥6.5%, or use of diabetic medications) on at least 2 occasions prior to the blood sampling date.
Uncontrolled diabetes was defined as fasting glucose >126 mg/dL or Hemoglobin A1c≥ 6.5% at blood sampling.
Persistent hypertension was defined as having a history of hypertension (systolic blood pressure ≥140 mmHg, diastolic blood pressure ≥90 mmHg, or use of anti-hypertensive medications) on at least two previous occasions from the blood sampling date.
Uncontrolled hypertension was defined as systolic blood pressure ≥140 mmHg or diastolic blood pressure ≥90 mmHg at blood sampling.
Serum cholesterol measures were obtained after fasting.
491 person-visits had available low density lipoprotein available.
Table 2 shows the detectability and distributions of each biomarker across all person-visits. The chemokines, soluble receptors, IL-6, TNF-α, and BAFF were detected in all, or nearly all, samples. In contrast, GM-CSF, IL-2, IL-1β, and IFN-γ were detectable in <80% of samples.
Table 2.
Proportion detectable and median (IQR) of detectable biomarker concentrations, number of person-visits (N) = 971, Multicenter AIDS Cohort Study (MACS), 1984 – 2009
| Biomarkera
| ||
|---|---|---|
| % detectable | Median (IQRb) | |
| Chemokines
| ||
| CCL11 | 100 | 1,696 (1,219 – 2,419) |
| CXCL8 | 100 | 13.3 (8.9 – 22.9) |
| CXCL10 | 100 | 139 (92 – 234) |
| CCL2 | 100 | 508 (362 – 657) |
| CCL13 | 100 | 749 (580 – 991) |
| CCL4 | 99.6 | 145 (98 – 205) |
| CCL17 | 99.9 | 531 (368 – 836) |
| CXCL13 | 98.9 | 298 (246 – 349) |
|
Cytokines | ||
| Interleukin-10 (IL-10) | 99.8 | 3.2 (2.1 – 6.3) |
| Interleukin-12p70 (IL-12p70) | 93.5 | 2.6 (1.4 – 7.4) |
| Interleukin-6 (IL-6) | 99.9 | 1.0 (0.7 – 1.5) |
| Tumor Necrosis Factor-α (TNF-α) | 99.9 | 8.5 (6.9 – 10.6) |
| B-cell Activating Factor (BAFF) | 100 | 1,970 (1,731 – 2,263) |
| Interferon-γ (IFN-γ) | 60.5 | 1.3 (0.9 – 2.1) |
| Granulocyte/Macrophage-Colony Stimulating Factor (GM-CSF) | 63.9 | 1.1 (0.7 – 2.1) |
| Interleukin-2 (IL-2) | 71.8 | 0.7 (0.5 – 1.4) |
| Interleukin-1β (IL-1β) | 55.7 | 0.5 (0.3 – 0.9) |
|
Soluble receptors | ||
| Soluble CD14 (sCD14), ng/mL | 99.9 | 2,100 (1,800 – 2,500) |
| Soluble gp130 (sgp130), ng/mL | 100 | 250 (230 – 290) |
| Soluble Interleukin 2-receptor-α (sIL2-Rα) | 100 | 1,382 (1,138 – 1,739) |
| Soluble CD27 (sCD27) | 100 | 9,067 (7,491 – 11,441) |
| Soluble TNF-receptor 2 (sTNF-R2) | 100 | 2,303 (1,867 – 2,910) |
| C-reactive protein (CRP), mg/L | 89.7 | 1.1 (0.5 – 2.4) |
Units are pg/mL unless otherwise indicated.
25th–75th percentiles.
Associations between each host factor and each biomarker, adjusting only for age, MACS study center, and time of day of blood draw are provided in Figure 1 in McKay.36 Of the exposures studied, all were associated with at least one biomarker, with the exception of sexually transmitted disease and risky sex. Therefore, the multivariable models only included age, race, education, time of blood draw, BMI, HCV, smoking status, alcohol consumption, recreational drug use, and depressive symptoms using the entire sample (i.e., 1984–2009) [Model 1], and also diabetes, hypertension and hypercholesterolemia using the restricted sample (i.e., 2001–2009) [Model 2]. In these models, which are summarized in Figure 1, 16 of the 23 biomarkers were significantly associated with at least one of the examined host characteristics at the P<0.002 level, and the remaining 7 markers had associations at the P<0.05 level. The estimates in Figure 1 reflect percent differences in biomarker concentrations between the exposed and unexposed for each covariate presented. For example, chronic HCV was associated with 54% higher concentrations of IL-10 (P<0.002) (i.e., the overall distribution of IL-10 was 54% higher in those with chronic HCV vs. those who were HCV negative, controlling for age, race, education, time of blood draw, BMI, smoking status, alcohol consumption, recreational drug use, and depressive symptoms) and 54% lower concentrations of C-reactive protein (CRP) (P<0.002). The directionality of differences in detectability for the four cytokines with < 80% detectable values were similar to the results obtained from generalized gamma regression models.
Figure 1.
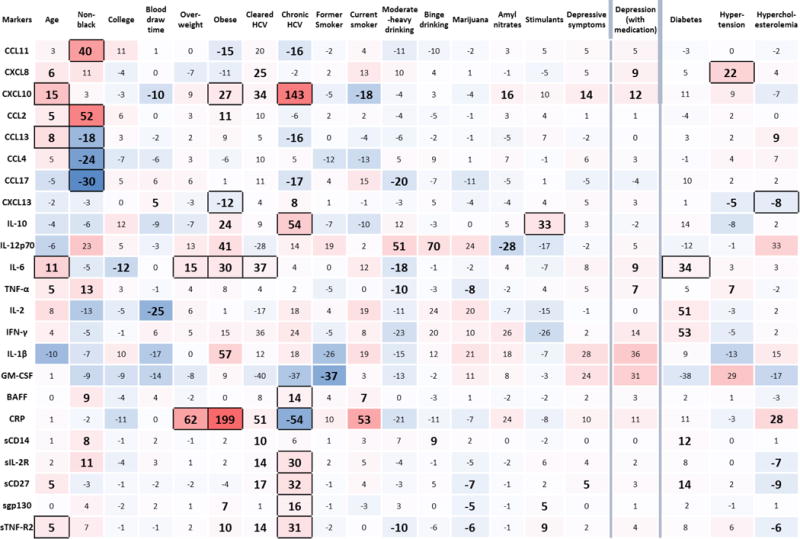
Percent differences from multivariate generalized gamma regression models examining the associations of age, non-black race, college education at baseline, blood draw time (P.M. versus A.M.), being overweight, obesity, cleared (antibody positive only) hepatitis C infection (HCV), chronic hepatitis C infection, former smoking, current smoking, moderate-heavy alcohol consumption, binge alcohol consumption, use of marijuana, use of amyl nitrates, use of stimulants, the presence of depressive symptoms, and depression including those taking depression medication (column headings) with individual biomarkers (row headings), using the full sample (1984–2009). Percent differences in biomarker concentrations associated with persistent diabetes, persistent hypertension, and hypercholesterolemia are adjusted for age, study site, race, baseline education, blood draw time, body mass index, depressive symptoms, hepatitis C infection (HCV), smoking status, alcohol consumption, and recreational drug use, using the restricted sample (2001–2009). Large bold text with black-bordered cells indicates significance at the P<0.002 level; large bold text without borders indicates marginal significance (0.002<P≤0.05). The color gradient of each cell illustrates the magnitude of the percent difference in biomarker concentration (darker red indicating stronger positive percent difference and darker blue indicating stronger negative percent difference). For example, chronic HCV is associated with 54% higher concentrations of IL-10 (P<0.002) and 54% lower concentrations of C-reactive protein (CRP) (P<0.002).
3.1. Relationships of biomarkers with fixed host characteristics
Several of the biomarkers were influenced by fixed host characteristics. Older age was significantly (P<0.002) associated with higher concentrations of chemokines, cytokines and soluble receptors, specifically CXCL10, CCL13, IL-6, sCD27, and sTNF-R2. Race primarily influenced chemokine levels, in that being non-black was associated significantly with higher levels of CCL11 and CCL2 and lower levels of CCL13, CCL4, and CCL17, and marginally (0.002<P≤0.05) with higher levels of TNF-α, BAFF, sCD14 and sIL-2Rα. IL-6 was the only biomarker associated with higher education. Compared to morning blood draws, specimens drawn in the afternoon/evening had significantly lower concentrations of IL-2; their lower CXCL10 and higher CXCL13 levels were of marginal significance.
3.2. Relationships of biomarkers with modifiable host characteristics
A number of modifiable characteristics were strongly associated with several of the biomarkers. Men with chronic HCV infection had significantly (P<0.002) higher concentrations of CXCL10, IL-10, BAFF, sIL-2Rα, sCD27, sgp130, and sTNF-R2, and lower concentrations of CRP than uninfected men, and marginally significantly lower levels of CCL11, CCL13, and CCL17. Men with cleared HCV infection had significantly higher concentrations of IL-6 than both uninfected men and men with chronic HCV infection.
Individuals who were overweight or obese had significantly higher IL-6 and CRP. Obesity was further associated with significantly higher levels of CXCL10 and lower levels of CXCL13, and marginally with other biomarkers.
Associations between behaviors and biomarkers were mostly of marginal significance (0.002<P≤0.05). The only significant association was between recreational stimulant use and higher levels of IL-10. Marijuana use was marginally associated with lower concentrations of TNF-α, sCD27, sgp130, and sTNF-R2, while the use of amyl nitrates was associated with higher concentrations of CXCL10 and lower concentrations of IL-12p70. Compared to non-smokers, current smokers had lower concentrations of CXCL10, and higher concentrations of BAFF and CRP, with the latter association approaching statistical significance (P = 0.003). Compared to non-drinkers, those who reported moderate-heavy drinking had lower CCL17, IL-6, TNF-α, and sTNF-R2 and higher concentrations IL-12p70 and sCD14. High values of IL-12p70 and sCD14 were also associated with binge drinking.
Men with depressive symptoms defined by CES-D>16 had higher CXCL10 and sCD27, but these associations were of marginal significance. The addition of men taking antidepressants to the group with depressive symptoms attenuated the association with sCD27 and revealed marginally significant associations of depression with higher values of CXCL8, IL-6, and TNF-α.
3.3. Relationships of biomarkers with morbidities
The last three columns of Figure 1 show the results from Model 2, which included, in addition to the factors in Model 1, three morbidities: persistent diabetes, persistent hypertension, and hypercholesterolemia. This analysis included only data from 2001–09. The associations found in Model 1 were essentially unchanged (see Figure 2 in McKay36), except that current smoking was associated with higher CXCL8 (PD = 23; P = 0.01) and IL-6 and CXCL10 were no longer associated with age after accounting for these morbid conditions. Men with persistent diabetes had significantly higher concentrations of IL-6 than those without diabetes; IL-2, IFN-γ, sCD14, and sCD27 levels were marginally significantly higher. These associations did not change when restricting the analysis to those with uncontrolled diabetes. Concentrations of CXCL8 were significantly higher in the presence of controlled or uncontrolled hypertension. However, IL-6 levels were only significantly higher among those with uncontrolled hypertension compared to normotensive persons. Levels of CXCL13 and IL-10 were lower in those with uncontrolled hypertension. Finally, hypercholesterolemia was associated with significantly lower concentrations of CXCL13 and sCD27, marginally significantly lower levels of sIL-2Rα and sTNF-R2, and higher levels of CCL13 and CRP. These associations were mostly due to changes in HDL versus LDL (Table 3).
Table 3.
Percent differences in biomarker concentrations for changes in high density and low density lipoprotein levels, Multicenter AIDS Cohort Study (MACS), 2001 – 2009
| Biomarker | High density lipoprotein levels
|
Low density lipoprotein levels
|
||
|---|---|---|---|---|
| Percent difference1 | p-value | Percent difference1 | p-value | |
| CCL11 | 0.6 | (0.809) | −1.2 | (0.611) |
| CXCL10 | −4.8 | (0.114) | −3.4 | (0.216) |
| CXCL8 | −0.9 | (0.717) | −2.0 | (0.499) |
| CCL2 | 2.4 | (0.214) | −3.1 | (0.143) |
| CCL13 | 7.3 | (0.009) | 0.5 | (0.841) |
| CCL4 | −4.2 | (0.288) | 2.9 | (0.303) |
| CXCL13 | 4.0 | (0.004) | −3.4 | (0.007) |
| IL-10 | −6.7 | (0.136) | 4.4 | (0.211) |
| IL-6 | −2.6 | (0.368) | 2.1 | (0.447) |
| IL-12p70 | 2.4 | (0.771) | −3.4 | (0.718) |
| TARC | −0.7 | (0.862) | 3.5 | (0.324) |
| TNF-α | −1.7 | (0.278) | −1.2 | (0.470) |
| IL-2 | 10.3 | (0.081) | −4.2 | (0.513) |
| IFN-γ | −10.0 | (0.074) | 3.9 | (0.588) |
| IL-1β | 2.7 | (0.763) | −14.4 | (0.167) |
| GM-CSF | 12.1 | (0.248) | −22.2 | (0.061) |
| BAFF | −1.3 | (0.266) | −0.6 | (0.588) |
| sCD14 | −1.4 | (0.448) | 0.7 | (0.649) |
| sIL-2Rα | −5.8 | (0.001) | −1.7 | (0.300) |
| sCD27 | −3.2 | (0.030) | −2.6 | (0.087) |
| sgp130 | 2.4 | (0.018) | 1.0 | (0.482) |
| sTNF-R2 | −6.4 | (0.000) | −3.0 | (0.067) |
| CRP | −15.1 | (0.010) | 20.8 | (0.002) |
Percent difference for a one standard deviation change in lipoprotein levels.
4. Discussion
Our results demonstrate that the inflammatory biomarkers investigated here were affected by sociodemographic and behavioral risk factors and by select morbidities. These findings are highly relevant for researchers investigating the role of these biomarkers in disease pathogenesis. Further, identifying modifiable risk factors that are associated with changes in these biomarkers may facilitate the development of clinical and behavioral interventions for inflammation-associated conditions. Finally, the observed associations between biomarkers and fixed characteristics indicate variables that need to be considered and controlled for when examining these biomarkers in epidemiological studies. To our knowledge, this is one of the largest studies to date to examine the relationships between host characteristics on a broad panel of inflammatory biomarkers.
The factor that affected these biomarkers the most was chronic HCV infection. Chronic HCV infection is a major risk factor for hepatocellular carcinoma (HCC), with evidence suggesting that HCV-induced inflammatory pathways are the primary mechanisms through which hepatocellular carcinogenesis is initiated.37 The identification of novel biomarkers associated with HCV may aid in the development of therapeutic targets for modifying inflammatory mediators. In our study, HCV infection was associated with higher CXCL10, IL-10, BAFF, sIL-2Rα, sCD27, sgp130, and sTNF-R2 and depressed CRP. The biomarkers that were higher in men with chronic HCV are consistent with the activation of immune cells by HCV. CXCL10, which is secreted by hepatocytes and is a chemoattractant for monocytes/macrophages, natural killer cells, T cells, and dendritic cells,38,39 has been used as a marker of HCV treatment outcome, with higher pre-treatment concentrations associated with greater risk of non-response.40 Our observation that chronic HCV was associated with CXCL10 levels that were 143 percent higher than those without HCV infection is consistent with the known effects of HCV on CXCL10.40 In the present study, chronic HCV was also associated with higher levels of the immune activation markers sTNF-R2, sIL-2Rα, and sCD27. This degree of difference in sIL-2Rα approaches that associated with a change in the International Prognostic Index risk group from Good to Very Good for patients with diffuse large B-cell lymphoma.41 Similarly, the percent difference in sCD27 associated with chronic HCV in this study approaches the levels that distinguished between non-Hodgkin lymphoma cases and controls observed in an earlier MACS study.42 These findings together with others support a role for inflammation in carcinogenesis.14,43,44 Another study recently showed that sTNF-R2 levels were associated with prognosis among those with chronic kidney disease, whereby the percent difference in baseline median values was smaller than the differences by HCV infection in the present study.45 sTNF-R2 and sIL-2Rα play a role in T cell activation, growth, and proliferation,46,47 and sCD27 interacts with IL-2 to promote CD8+ T cell effector function.48 sIL-2Rα, an essential component of IL-2 signaling, has been used as a measure of T cell activation and a marker of memory B cells.49 BAFF, expressed by macrophages, dendritic cells, and neutrophils, is a potent B cell activator.50 Interestingly, IL-6 was significantly associated with cleared HCV infection, but not chronic (i.e., active) HCV infection. This finding could be explained by the fact that sgp130, which is elevated in chronic HCV infection, is an inhibitor of IL-6;51 thus, it could be that normalization of sgp130 levels after HCV clearance unmasks elevations in IL-6. The decreased CRP levels in HCV chronically-infected persons has also been shown by others,52,53 providing external validity to these results.
Obesity was also strongly associated with biomarkers of inflammation, specifically with higher IL-6, CRP, CXCL10 and lower CXCL13. Obesity has been implicated in the development of a host of chronic conditions, including cardiovascular disease, diabetes, and several cancers, primarily through inflammatory-mediated pathways originating from adipose tissue.54 Adipose tissue produces large amounts of inflammatory molecules, including IL-6 and CXCL10.21 In the present study, levels of both IL-6 and CXCL10 were nearly 30% higher in obese men than in those with normal BMI.21 These findings are consistent with a recent report of higher IL-6, CRP and TNF-α associated with increasing BMI among women.20 The size of this effect on IL-6 is clinically meaningful; for example, in one study, increases in IL-6 levels of 40% were associated with 38% elevated risk of myocardial infarction in otherwise healthy men.55 An association between obesity and lower levels of CXCL13, a B-cell chemoattractant, has also been reported in women.56
Risk behaviors also affected specific biomarkers. For example, CRP levels were 53% higher in current smokers than in nonsmokers, consistent with a proinflammatory role of smoking in carcinogenesis and atherogenesis. Current smoking was also associated with lower CXCL10 concentrations, suggesting that smoking may suppress anti-tumor activity by effector T-cells. Otherwise, smoking was not significantly associated with the other biomarkers studied. Reports vary widely on the effect of smoking on inflammatory biomarkers.20,23,24,57 This variation may be due to heterogeneous populations, residual confounding (e.g., the cumulative effects of exposure using units of pack-years vs. using the current categorizations), and not controlling for BMI which, as discussed above, is correlated with concentrations of circulating pro-inflammatory biomarkers.
Regarding the immunomodulatory effects of recreational drug use, marijuana use was associated with lower sCD27, sTNF-R2, sgp130, and TNF-α concentrations, although the effect sizes were all <10%. Nevertheless, this is consistent with a growing body of research that suggests cannabinoids inhibit the co-stimulatory activity of macrophages and suppress the release of pro-inflammatory mediators.58,59 The therapeutic potential of cannabinoids as anti-inflammatory agents has been examined in clinical studies of multiple sclerosis, arthritis, and traumatic brain injury.28 Further evaluation of the putative anti-inflammatory effects of cannabinoids should be examined to characterize their therapeutic utility. Interestingly, stimulant use was associated with significantly elevated concentrations of IL-10, an anti-inflammatory, B cell-stimulatory cytokine secreted by T-regulatory cells, suggesting an immunosuppressive response. The increased IL-10 in stimulant users provides additional support for a pathway between use of stimulants and increased NHL risk, as reported previously.16,60–62 In a recent meta-analysis, higher IL-10 was associated with adverse survival in many types of cancer, suggesting the potential clinical utility of IL-10 antagonists in cancer treatment.63 We did not observe an elevation in pro-inflammatory cytokines or CRP with stimulant use, as described by others.32,64 This discrepancy could be due to our summarization of any stimulant use, which may have masked heterogeneity associated with different drugs. Alternatively, a threshold may exist below which the immune system is not significantly influenced.
Although these participants were generally free of morbidity, observed associations were consonant with known relationships. The inverse association between hypercholesterolemia with sIL-2Rα, sCD27 and sTNF-R2 was likely due to the anti-inflammatory nature of HDL.65 CRP was the only biomarker directly associated with LDL, consistent with our knowledge of atherogenesis.66 Although we observed a strong association of diabetes with the pro-inflammatory marker IL-6, as shown by others,4,67,68 we did not find a significant association with TNF-α or CRP. One possible explanation is the low prevalence of diabetes in our study population, thus limiting our power to detect associations with small effects. Concentrations of CRP were generally low in our population, thus it is likely that these men were at lower risk for many morbidities. The association of uncontrolled hypertension with significantly higher CXCL8 and IL-6 is consistent with evidence implicating T cell cytokines in vascular disease.69 Interestingly, when adjusting for these co-morbidities, most of the associations between markers of immune activation with age persisted, but the associations with inflammatory markers IL-6, CXCL8, and CXCL10 attenuated and became non-significant. This suggests that that the excess inflammatory burden observed with age may be explained by age-related morbidities. However, other pathways leading to immune activation with increasing age should be considered.
Depressive symptoms, as defined by a CES-D score >16, was associated with higher levels of CXCL8, CXCL10, IL-6, and TNF-α when we included men who reported use of antidepressant medications, possibly because use of these medications may reflect clinically-defined depression and improve the classification. These findings are consistent with those published by others.70,71 While depression may promote a dysregulated immune response through multiple pathways (e.g., adiposity),72,73 it is also proposed to have an inflammatory pathogenesis. Additional prospective examination of the relationship between inflammation and depression is needed to establish the temporal relationship. It is a limitation of this study that the medication class and the adequacy of disease control were both unknown. Further research might clarify biomarker-mediated pathways.
As noted above, increasing evidence suggests that differences in inflammatory biomarkers are clinically informative. CRP has perhaps been the most frequently investigated, with elevated levels associated with 2-fold increased risk of cardiovascular disease even after controlling for traditional cardiovascular risk factors such as age, family history, and smoking.74 A doubling of CRP levels has also been implicated in increased risk of hepatocellular carcinoma75 and cognitive impairment.76 In the present study, the median CRP level for men with normal BMI was 0.1 mg/L and was more than doubled for men with overweight and obese BMI (1.1 mg/L, and 2.2 mg/L, respectively). Current smoking and hypercholesterolemia were also associated with elevated CRP levels (PD = 53% and 28%, respectively).
Emerging evidence also suggests that inflammatory biomarker levels are associated with risk of certain cancers in addition to non-Hodgkin lymphoma. For example, a doubling of IL-6 has been associated with an increased risk of epithelial ovarian cancer, hepatocellular carcinoma, and lung cancer.1,57,75,77 In the present study, differences of 15–34% in levels of IL-6 were associated with increasing BMI, cleared HCV, and persistent diabetes, while smaller increases were observed with increasing age and depression. It also is important to consider that relatively small effect sizes may contribute to risk in a synergistic manner. Determination of the clinical meaningfulness of these inflammatory biomarkers will require standardized laboratory measurements and additional studies that consistently find that the same cutoffs predict disease, as was done for CRP.
The study had some limitations. Biomarkers could have degraded in storage of the specimens studied. However, non-differential degradation will bias toward the null and differential degradation by exposure is unlikely given the random selection of person-visits studied. Thus, the estimates presented are likely to be conservative. Further, restricting the analysis to samples collected from 2001–2009 did not change the results, which also argues against a problem with early specimens. Some potentially important mediators or confounders, e.g., use of anti-inflammatory medications and physical activity, could not be studied. In addition, the possibility of acute illness at the time of blood draw was not accounted for in our analysis. However, we would not expect that the prevalence of such acute illnesses would differ by the exposure categories examined here. This non-differential effect would bias results toward the null; therefore, the results of this study would be conservative. Although generalizability to other populations, such as women or children, may not be appropriate, studies performed in women have shown similar associations with shared biomarkers.20,27 Because reference ranges have not been established for most of the studied biomarkers, inferences from this study must be limited to relative distributions, rather than absolute cutoffs. Finally, our study involved a considerable number of statistical comparisons. Although adjustment for multiple comparisons was conducted using a Bonferroni correction, which is considered conservative, it remains possible that some associations were significant solely due to chance. The observed associations should therefore be interpreted with caution and be further evaluated in independent populations.
This study also has several strengths. The MACS is a large, longstanding prospective cohort study of men followed for more than 25 years with in-depth standardized data collection and specimen collection, processing and storage. This permitted the evaluation of diverse correlates of immune activation with a precision generally not possible in smaller studies. Person-visits were selected to reflect the entire period of cohort follow-up (1984–2009) and to contain important subgroups (e.g., African-Americans and those with chronic HCV). Multiplex testing allowed for simultaneous quantitation of a broad spectrum of biomarkers using small sample volumes, reducing cost and improving the efficient use of valuable stored specimens. Testing of previously unthawed specimens was conducted in single laboratories for given analytes, with all longitudinal specimens per individual run on the same plate to minimize assay variability. Finally, innovative statistical methods, i.e., generalized gamma regression, were utilized to incorporate observations below limits of detection. Other methods, e.g., assigning undetectable levels with ½ LLOD, may result in estimates with overly narrow confidence intervals, yielding incorrect inferences. Adjustment for multiple testing was used to reduce the likelihood of false-positive findings. Finally, data on risk behaviors, including self-reported drug use, have been captured through an ACASI software system, a standardized method for data collection that safeguards privacy and has been shown in numerous populations to increase the accurate and unbiased reporting of behaviors that may be socially sensitive.78–80
Our study has both analytic and clinical implications. Fixed (e.g., race) and modifiable (e.g., obesity, HCV infection) host characteristics suggest potential confounders for future etiologic studies of inflammation and potential targets for risk prediction, prevention, and therapeutic interventions. These findings will inform both our understanding of cellular interactions in the immune system and potential inflammatory pathways in chronic disease development.
Highlights.
Body mass index was associated with higher IL-6, CRP, and CXCL10 and lower CXCL13.
Hepatitis C was associated with higher CXCL10, IL-10, BAFF, sIL-2Rα, sCD27, sgp130, sTNF-R2 and lower CRP.
Levels of IL-10 were higher in stimulant users than non-users.
Depressive symptoms were associated with higher CXCL10.
Diabetes and hypertension were associated with higher IL-6 and CXCL8, respectively.
Acknowledgments
Samples and data in this manuscript were collected by the Multicenter AIDS Cohort Study (MACS) with support from an American Recovery and Reinvestment Act (ARRA) supplement with centers (Principal Investigators) at: Johns Hopkins University Bloomberg School of Public Health (Joseph Margolick), U01-AI35042; Northwestern University (Steven Wolinsky), U01-AI35039; University of California, Los Angeles (Roger Detels, Otoniel Martinez-Maza), U01-AI35040; University of Pittsburgh (Charles Rinaldo), U01-AI35041; the Center for Analysis and Management of MACS, Johns Hopkins University Bloomberg School of Public Health (Lisa Jacobson), UM1-AI35043. The MACS is funded primarily by the National Institute of Allergy and Infectious Diseases (NIAID), with additional co-funding from the National Cancer Institute (NCI). This work was supported by the HIV Prevention Trials Network (HPTN) sponsored by the National Institute of Allergy and Infectious Diseases (NIAID), National Institute on Drug Abuse, National Institute of Mental Health, and Office of AIDS Research, of the NIH, DHHS (UM1 AI068613). Website located at http://www.statepi.jhsph.edu/macs/macs.html. The contents of this publication are solely the responsibility of the authors and do not represent the official views of the National Institutes of Health (NIH).
Abbreviations
- ACASI
audio computer assisted self-interview
- BAFF
B-cell activating factor
- BMI
body mass index
- CRP
C-reactive protein
- CI
confidence interval
- CCL11
C-C motif ligand 11
- CES-D
Center for Epidemiologic Studies Depression Scale
- CXCL8
C-X-C motif ligand 8
- CVD
cardiovascular disease
- GM-CSF
granulocyte-macrophage colony-stimulating factor
- HCC
hepatocellular carcinoma
- HCV
hepatitis C virus
- HDL
high-density lipoprotein
- HIV
human immunodeficiency virus
- IFN
interferon
- IL
interleukin
- sIL-2Rα
soluble IL-2 receptor alpha
- LDL
low-density lipoprotein
- MACS
Multicenter AIDS Cohort Study
- NHL
non-Hodgkin lymphoma
- sCD14
soluble cluster of differentiation 14
- sgp130
soluble glycoprotein 130
- TNF-α
tumor necrosis factor-alpha
- sTNF-R2
soluble tumor necrosis factor-receptor 2
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no conflict of interest to report.
References
- 1.Clendenen TV, et al. Circulating inflammation markers and risk of epithelial ovarian cancer. Cancer Epidemiol Biomarkers Prev. 2011;20:799–810. doi: 10.1158/1055-9965.EPI-10-1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gu Y, et al. Circulating cytokines and risk of B-cell non-Hodgkin lymphoma: A prospective study. Cancer Causes Control. 2010;21:1323–1333. doi: 10.1007/s10552-010-9560-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Il’yasova D, et al. Circulating levels of inflammatory markers and cancer risk in the health aging and body composition cohort. Cancer Epidemiol Biomarkers Prev. 2005;14:2413–2418. doi: 10.1158/1055-9965.EPI-05-0316. [DOI] [PubMed] [Google Scholar]
- 4.Duncan BB, et al. Low-Grade Systemic Inflammation and the Development of Type 2 Diabetes: The Atherosclerosis Risk in Communities Study. Diabetes. 2003;52:1799–1805. doi: 10.2337/diabetes.52.7.1799. [DOI] [PubMed] [Google Scholar]
- 5.Appay V, Sauce D. Immune activation and inflammation in HIV-1 infection: Causes and consequences. Journal of Pathology. 2008;214:231–241. doi: 10.1002/path.2276. [DOI] [PubMed] [Google Scholar]
- 6.Deeks SG. HIV infection, inflammation, immunosenescence, and aging. Annu Rev Med. 2011;62:141–155. doi: 10.1146/annurev-med-042909-093756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tonelli M, Sacks F, Pfeffer M, Jhangri GS, Curhan G. Biomarkers of inflammation and progression of chronic kidney disease. Kidney Int. 2005;68:237–245. doi: 10.1111/j.1523-1755.2005.00398.x. [DOI] [PubMed] [Google Scholar]
- 8.Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352:1685–1695. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- 9.Libby P, Ridker PM, Hansson GK. Inflammation in Atherosclerosis. From Pathophysiology to Practice. Journal of the American College of Cardiology. 2009;54:2129–2138. doi: 10.1016/j.jacc.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stoner L, et al. Inflammatory biomarkers for predicting cardiovascular disease. Clinical Biochemistry. 2013;46:1353–1371. doi: 10.1016/j.clinbiochem.2013.05.070. [DOI] [PubMed] [Google Scholar]
- 11.Goff DC, et al. 2013 ACC/AHA Guideline on the Assessment of Cardiovascular Risk: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:S49–73. doi: 10.1161/01.cir.0000437741.48606.98. [DOI] [PubMed] [Google Scholar]
- 12.Balkwill F, Coussens LM. Cancer: an inflammatory link. Nature. 2004;431:405–406. doi: 10.1038/431405a. [DOI] [PubMed] [Google Scholar]
- 13.Balkwill F, Mantovani A. Inflammation and cancer: Back to Virchow? Lancet. 2001;357:539–545. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 14.Grivennikov SI, Greten FR, Karin M. Immunity, Inflammation, and Cancer. Cell. 2010;140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rabkin CS, et al. Circulating cytokine levels, Epstein-Barr viremia, and risk of acquired immunodeficiency syndrome-related non-Hodgkin lymphoma. Am J Hematol. 2011;86:875–8. doi: 10.1002/ajh.22119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nolen BM, et al. Circulating mediators of inflammation and immune activation in AIDS-related non-Hodgkin lymphoma. PLoS One. 2014;9 doi: 10.1371/journal.pone.0099144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Breen EC, et al. Non-Hodgkin’s B cell lymphoma in persons with acquired immunodeficiency syndrome is associated with increased serum levels of IL10, or the IL10 promoter −592 C/C genotype. Clin Immunol. 2003;109:119–129. doi: 10.1016/s1521-6616(03)00214-6. [DOI] [PubMed] [Google Scholar]
- 18.Eguchi K, Manabe I. Macrophages and islet inflammation in type 2 diabetes. Diabetes, Obesity and Metabolism. 2013;15:152–158. doi: 10.1111/dom.12168. [DOI] [PubMed] [Google Scholar]
- 19.Chaturvedi AK, Moore SC, Hildesheim A. Invited commentary: circulating inflammation markers and cancer risk–implications for epidemiologic studies. Am J Epidemiol. 2013;177:14–19. doi: 10.1093/aje/kws357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clendenen TV, et al. Factors associated with inflammation markers, a cross-sectional analysis. Cytokine. 2011;56:769–78. doi: 10.1016/j.cyto.2011.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gregor MF, Hotamisligil GS. Inflammatory mechanisms in obesity. Annu Rev Immunol. 2011;29:415–445. doi: 10.1146/annurev-immunol-031210-101322. [DOI] [PubMed] [Google Scholar]
- 22.Visser M, Bouter LM, McQuillan GM, Wener MH, Harris TB. Elevated C-reactive protein levels in overweight and obese adults. Jama. 1999;282:2131–2135. doi: 10.1001/jama.282.22.2131. [DOI] [PubMed] [Google Scholar]
- 23.Ginns LC, De Hoyos A, Brown MC, Gaumond BR. Elevated concentration of soluble interleukin-2 receptors in serum of smokers and patients with lung cancer. Correlation with clinical activity. Am Rev Respir Dis. 1990;142:398–402. doi: 10.1164/ajrccm/142.2.398. [DOI] [PubMed] [Google Scholar]
- 24.Arnson Y, Shoenfeld Y, Amital H. Effects of tobacco smoke on immunity, inflammation and autoimmunity. J Autoimmun. 2010;34:J258–J265. doi: 10.1016/j.jaut.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 25.Levitzky YS, et al. Relation of smoking status to a panel of inflammatory markers: the framingham offspring. Atherosclerosis. 2008;201:217–24. doi: 10.1016/j.atherosclerosis.2007.12.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bermudez EA, Rifai N, Buring J, Manson JE, Ridker PM. Interrelationships among circulating interleukin-6, C-reactive protein, and traditional cardiovascular risk factors in women. Arterioscler Thromb Vasc Biol. 2002;22:1668–1673. doi: 10.1161/01.atv.0000029781.31325.66. [DOI] [PubMed] [Google Scholar]
- 27.Welsh P, Woodward M, Rumley A, Lowe G. Associations of plasma pro-inflammatory cytokines, fibrinogen, viscosity and C-reactive protein with cardiovascular risk factors and social deprivation: the fourth Glasgow MONICA study. Br J Haematol. 2008;141:852–61. doi: 10.1111/j.1365-2141.2008.07133.x. [DOI] [PubMed] [Google Scholar]
- 28.Klein TW. Cannabinoid-based drugs as anti-inflammatory therapeutics. Nat Rev Immunol. 2005;5:400–11. doi: 10.1038/nri1602. [DOI] [PubMed] [Google Scholar]
- 29.Imhof A, et al. Effect of alcohol consumption on systemic markers of inflammation. Lancet. 2001;357:763–7. doi: 10.1016/S0140-6736(00)04170-2. [DOI] [PubMed] [Google Scholar]
- 30.Pai JK, et al. Moderate alcohol consumption and lower levels of inflammatory markers in US men and women. Atherosclerosis. 2006;186:113–120. doi: 10.1016/j.atherosclerosis.2005.06.037. [DOI] [PubMed] [Google Scholar]
- 31.Nagarkatti P, Pandey R, Rieder SA, Hegde VL, Nagarkatti M. Cannabinoids as novel anti-inflammatory drugs. Future Med Chem. 2009;1:1333–49. doi: 10.4155/fmc.09.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tai H, Lai H, Jani J, Lai S, Kickler TS. HIV infection and cocaine use induce endothelial damage and dysfunction in African Americans. Int J Cardiol. 2012;161:83–87. doi: 10.1016/j.ijcard.2011.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dudley J, et al. The Multicenter AIDS Cohort Study: retention after 9 1/2 years. Am J Epidemiol. 1995;142:323–330. doi: 10.1093/oxfordjournals.aje.a117638. [DOI] [PubMed] [Google Scholar]
- 34.Radloff LS. The CES-D Scale: A Self-Report Depression Scale for Research in the General Population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- 35.Cox C, Chu H, Schneider MF, Muñoz A. Parametic survival analysis and taxonomy of hazard functions for the generalized gamma distribution. Stat Med. 2007;26:4352–4374. doi: 10.1002/sim.2836. [DOI] [PubMed] [Google Scholar]
- 36.McKay Heather, Bream Jay H, Margolick Joseph B, Martínez-Maza Otoniel, Phair John P, Rinaldo Charles R, Abraham Alison G, Jacobson LP. Data on serologic inflammatory biomarkers assessed using multiplex assays and host characteristics in the Multicenter AIDS Cohort Study (MACS) Cytokine Data Br. doi: 10.1016/j.dib.2016.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.de Oliveria Andrade LJ, et al. Association between hepatitis C and hepatocellular carcinoma. J Glob Infect Dis. 2009;1:33–7. doi: 10.4103/0974-777X.52979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Taub DD. Recombinant human interferon-inducible protein 10 is a chemoattractant for human monocytes and T lymphocytes and promotes T cell adhesion to endothelial cells. J Exp Med. 1993;177:1809–1814. doi: 10.1084/jem.177.6.1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Patzwahl R, Meier V, Ramadori G, Mihm S. Enhanced expression of interferon-regulated genes in the liver of patients with chronic hepatitis C virus infection: detection by suppression-subtractive hybridization. J Virol. 2001;75:1332–8. doi: 10.1128/JVI.75.3.1332-1338.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zeremski M, Petrovic LM, Talal AH. The role of chemokines as inflammatory mediators in chronic hepatitis C virus infection. J Viral Hepat. 2007;14:675–87. doi: 10.1111/j.1365-2893.2006.00838.x. [DOI] [PubMed] [Google Scholar]
- 41.Morito T, et al. Serum soluble interleukin-2 receptor level and immunophenotype are prognostic factors for patients with diffuse large B-cell lymphoma. Cancer Sci. 2009;100:1255–60. doi: 10.1111/j.1349-7006.2009.01167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Breen EC, et al. B-cell stimulatory cytokines and markers of immune activation are elevated several years prior to the diagnosis of systemic AIDS-associated non-Hodgkin B-cell lymphoma. Cancer Epidemiol Biomarkers Prev. 2011;20:1303–14. doi: 10.1158/1055-9965.EPI-11-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Balkwill F, Charles KA, Mantovani A. Smoldering and polarized inflammation in the initiation and promotion of malignant disease. Cancer Cell. 2005;7:211–7. doi: 10.1016/j.ccr.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 44.Zambirinis CP, Pushalkar S, Saxena D, Miller G. Pancreatic cancer, inflammation, and microbiome. Cancer J. 20:195–202. doi: 10.1097/PPO.0000000000000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Neirynck N, Glorieux G, Schepers E, Verbeke F, Vanholder R. Soluble tumor necrosis factor receptor 1 and 2 predict outcomes in advanced chronic kidney disease: a prospective cohort study. PLoS One. 2015;10:e0122073. doi: 10.1371/journal.pone.0122073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tartaglia LA, Goeddel DV. Two TNF receptors. Immunol Today. 1992;13:151–3. doi: 10.1016/0167-5699(92)90116-O. [DOI] [PubMed] [Google Scholar]
- 47.Waldmann TA. Minireview The Interleukin-2 Receptor. 1991:25–27. [Google Scholar]
- 48.Huang J, et al. Modulation by IL-2 of CD70 and CD27 Expression on CD8+ T Cells: Importance for the Therapeutic Effectiveness of Cell Transfer Immunotherapy. J Immunol. 2006;176:7726–7735. doi: 10.4049/jimmunol.176.12.7726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang Z-Z, et al. Soluble IL-2Rα facilitates IL-2-mediated immune responses and predicts reduced survival in follicular B-cell non-Hodgkin lymphoma. Blood. 2011;118:2809–20. doi: 10.1182/blood-2011-03-340885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schneider P, et al. BAFF, a novel ligand of the tumor necrosis factor family, stimulates B cell growth. J Exp Med. 1999;189:1747–56. doi: 10.1084/jem.189.11.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Garbers C, et al. Inhibition of classic signaling is a novel function of soluble glycoprotein 130 (sgp130), which is controlled by the ratio of interleukin 6 and soluble interleukin 6 receptor. J Biol Chem. 2011;286:42959–70. doi: 10.1074/jbc.M111.295758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Salter ML, et al. Correlates of elevated interleukin-6 and C-reactive protein in persons with or at high risk for HCV and HIV infections. J Acquir Immune Defic Syndr. 2013;64:488–495. doi: 10.1097/QAI.0b013e3182a7ee2e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Castello G, Scala S, Palmieri G, Curley Sa, Izzo F. HCV-related hepatocellular carcinoma: From chronic inflammation to cancer. Clin Immunol. 2010;134:237–50. doi: 10.1016/j.clim.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 54.Zeyda M, Stulnig TM. Obesity, inflammation, and insulin resistance–a mini-review. Gerontology. 2009;55:379–86. doi: 10.1159/000212758. [DOI] [PubMed] [Google Scholar]
- 55.Ridker PM, Rifai N, Stampfer MJ, Hennekens CH. Plasma Concentration of Interleukin-6 and the Risk of Future Myocardial Infarction Among Apparently Healthy Men. Circulation. 2000;101:1767–1772. doi: 10.1161/01.cir.101.15.1767. [DOI] [PubMed] [Google Scholar]
- 56.De Roos AJ, et al. Markers of B-cell activation in relation to risk of non-Hodgkin lymphoma. Cancer Res. 2012;72:4733–43. doi: 10.1158/0008-5472.CAN-12-1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pine SR, et al. Increased levels of circulating interleukin 6, interleukin 8, c-reactive protein, and risk of lung cancer. J Natl Cancer Inst. 2011;103:1112–1122. doi: 10.1093/jnci/djr216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Naftali T, Lev LB, Yablekovitz D, Half E, Konikoff FM. Treatment of Crohn’s disease with cannabis: an observational study. Isr Med Assoc J. 2011;13:455–458. [PubMed] [Google Scholar]
- 59.Chuchawankul S, Shima M, Buckley NE, Hartmann CB, McCoy KL. Role of cannabinoid receptors in inhibiting macrophage costimulatory activity. Int Immunopharmacol. 2004;4:265–278. doi: 10.1016/j.intimp.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 60.Chao C, et al. Recreational amphetamine use and risk of HIV-related non-Hodgkin lymphoma. Cancer Causes Control. 2009;20:509–516. doi: 10.1007/s10552-008-9258-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nelson RA, Levine AM, Marks G, Bernstein L. Alcohol, tobacco and recreational drug use and the risk of non-Hodgkin’s lymphoma. Br J Cancer. 1997;76:1532–1537. doi: 10.1038/bjc.1997.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Doody MM, et al. Risks of non-Hodgkin’s lymphoma, multiple myeloma, and leukemia associated with common medications. Epidemiology. 1996;7:131–139. doi: 10.1097/00001648-199603000-00005. [DOI] [PubMed] [Google Scholar]
- 63.Zhao S, Wu D, Wu P, Wang Z, Huang J. Serum IL-10 Predicts Worse Outcome in Cancer Patients: A Meta-Analysis. PLoS One. 2015;10:e0139598. doi: 10.1371/journal.pone.0139598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Siegel AJ, et al. Cocaine-induced erythrocytosis and increase in von Willebrand factor: evidence for drug-related blood doping and prothrombotic effects. Arch Intern Med. 1999;159:1925–1929. doi: 10.1001/archinte.159.16.1925. [DOI] [PubMed] [Google Scholar]
- 65.Säemann MD, et al. The versatility of HDL: A crucial anti-inflammatory regulator. European Journal of Clinical Investigation. 2010;40:1131–1143. doi: 10.1111/j.1365-2362.2010.02361.x. [DOI] [PubMed] [Google Scholar]
- 66.Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA: the journal of the American Medical Association. 2001;286 doi: 10.1001/jama.286.3.327. [DOI] [PubMed] [Google Scholar]
- 67.Mirza S, et al. Type 2-diabetes is associated with elevated levels of TNF-alpha, IL-6 and adiponectin and low levels of leptin in a population of Mexican Americans: a cross-sectional study. Cytokine. 2012;57:136–42. doi: 10.1016/j.cyto.2011.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Marques-Vidal P, et al. Association between circulating cytokine levels, diabetes and insulin resistance in a population-based sample (CoLaus study) Clin Endocrinol (Oxf) 2013;78:232–41. doi: 10.1111/j.1365-2265.2012.04384.x. [DOI] [PubMed] [Google Scholar]
- 69.Harrison DG, et al. Inflammation, immunity, and hypertension. Hypertension. 2011;57:132–140. doi: 10.1161/HYPERTENSIONAHA.110.163576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Berk M, et al. So depression is an inflammatory disease, but where does the inflammation come from. BMC Med. 2013;11:200. doi: 10.1186/1741-7015-11-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Felger JC, Lotrich FE. Inflammatory cytokines in depression: neurobiological mechanisms and therapeutic implications. Neuroscience. 2013;246:199–229. doi: 10.1016/j.neuroscience.2013.04.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shelton RC, Miller AH. Inflammation in depression: Is adiposity a cause? Dialogues Clin Neurosci. 2011;13:41–54. doi: 10.31887/DCNS.2011.13.1/rshelton. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dowlati Y, et al. A Meta-Analysis of Cytokines in Major Depression. Biol Psychiatry. 2010;67:446–457. doi: 10.1016/j.biopsych.2009.09.033. [DOI] [PubMed] [Google Scholar]
- 74.Ridker PM, Rifai N, Rose L, Buring JE, Cook NR. Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. N Engl J Med. 2002;347:1557–65. doi: 10.1056/NEJMoa021993. [DOI] [PubMed] [Google Scholar]
- 75.Aleksandrova K, et al. Inflammatory and metabolic biomarkers and risk of liver and biliary tract cancer. Hepatology. 2014;60:858–71. doi: 10.1002/hep.27016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wichmann MA, et al. Long-term systemic inflammation and cognitive impairment in a population-based cohort. J Am Geriatr Soc. 2014;62:1683–91. doi: 10.1111/jgs.12994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ohishi W, et al. Serum interleukin-6 associated with hepatocellular carcinoma risk: a nested case-control study. Int J cancer. 2014;134:154–63. doi: 10.1002/ijc.28337. [DOI] [PubMed] [Google Scholar]
- 78.Pluhar E, et al. Implementation of audio computer-assisted interviewing software in HIV/AIDS research. J Assoc Nurses AIDS Care. 18:51–63. doi: 10.1016/j.jana.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kim J, Dubowitz H, Hudson-Martin E, Lane W. Comparison of 3 data collection methods for gathering sensitive and less sensitive information. Ambul Pediatr. 8:255–60. doi: 10.1016/j.ambp.2008.03.033. [DOI] [PubMed] [Google Scholar]
- 80.Ghanem K, Hutton H. Audio computer assisted self interview and face to face interview modes in assessing response bias among STD clinic patients. Sex Transm. 2005 doi: 10.1136/sti.2004.013193. at < http://sti.bmj.com/content/81/5/421.short>. [DOI] [PMC free article] [PubMed]


