Abstract
Background
Class III β-tubulin (βIII-tubulin) has been reported to express at the invasive margin of colorectal cancer. The present study aimed to investigate the clinical implication of βIII-tubulin expression at the invasive margin of colorectal cancer.
Material/Methods
We recruited 111 patients with surgically resected colorectal carcinoma for βIII-tubulin expression analysis. The cases with βIII-tubulin-positive tumor cells found only in the invasive front tumor area were assigned to the invasive front group, while the remaining cases were all assigned to the non-invasive front group. Clinical analysis of βIII-tubulin and other clinical data was performed.
Results
The positive staining rates and staining intensity of βIII-tubulin were significantly different between the invasive and non-invasive front groups (p=0.001 and p=0.006), and there was a significant difference in tumor differentiation between the 2 groups (p=0.032). In the non-invasive front group, staining intensity of βIII-tubulin was significantly associated with positive staining rates and lymphatic metastasis (p<0.001 and p=0.048).
Conclusions
Our data showed the tissue distribution of βIII-tubulin expression at invasive margin or diffuse distribution. Expression of βIII-tubulin was correlated with tumor differentiation and lymphatic metastasis, suggesting a potential role of βIII-tubulin in tumor differentiation and metastasis. This study may shed light on βIII-tubulin as a novel potential molecular target for a new anti-cancer drug.
MeSH Keywords: Colorectal Neoplasms, Lymphatic Metastasis, Tubulin
Background
As the third most common malignancy, colorectal carcinoma is the fourth leading cause of cancer-related death worldwide [1]. In China, colorectal carcinoma has been ranked as the fifth most common human malignant disease, accounting for 6.51% of mortality in urban areas and 4.64% in rural areas [2]. The etiology of colorectal cancers is known to be heterogeneous and various mechanisms have been associated with the increased risk for development and progression of colorectal carcinoma, as well as the aggressive behaviors of the tumors [3–5]. Tumors at the invasive front appear morphologically more poorly differentiated and show different molecular characteristics [6]. Previous studies of colorectal carcinoma have suggested the potential role of β-tubulin expression in rectal cancer development [7,8]. A study by Portyanko et al. found expression of class III β-tubulin (βIII-tubulin, also known as TUBB3) at the invasive margin of colorectal cancer [9]. However, the authors mainly focused on the immunoreactivity of βIII-tubulin in colorectal cancer patients, and the clinical implication of their findings has not been well investigated. Recently, Mariani et al. investigated the role of βIII-tubulin as a predictive biomarker [10]. The distribution of tubulin has also been reported in human leukemia cell line HL-60 [11]. However, the potential effect of βIII-tubulin expression at the invasive margin of colorectal cancer was not taken into consideration. Therefore, the present investigated the clinical implication of βIII-tubulin expression at the invasive margin of colorectal cancer.
Material and Methods
Patients and tissue samples
This was a retrospective cohort study. A total of 111 patients with colorectal carcinoma who underwent radical colorectal resection were recruited at Beijing Tongren Hospital from February 2012 to February 2015. We excluded patients with tumor history, familial polyposis colon cancer, Crohn’s disease, malignant transformation of ulcerative colitis, simultaneous multiple primary tumors, or incomplete clinical data. The formalin-fixed and paraffin-embedded tissue samples containing the invasive part of the tumors were obtained and stained with hematoxylin and eosin (HE).
Immunohistochemical assay
Immunohistochemical staining of βIII-tubulin was performed with the method of immunohistochemistry envision-two steps using MaxVision™2 (Fuzhou Maixin Biotech. Co., Ltd., Fujian, China) according to the manufacturer’s instructions. Briefly, the tissue specimen was cut into 4-μm-thick sections, and deparaffinized in xylene and later rehydrated in graded alcohol. Antigen was retrieved using citrate buffer (pH 6.0) in a microwave oven. After washing 3 times with Tris-buffered saline (TBS), the sections were processed with 3% hydrogen peroxide for 5–10 min to block endogenous peroxidase activity. Sections were then incubated with mouse anti-human antibody against βIII-tubulin (Clone TuJ-1, MAB-0636, Fuzhou Maixin Biotechnology Development Co., Ltd., Fuzhou, China) or cytokeratin (AE1/AE3, KIT-0009, Fuzhou Maixin Biotech Co., Ltd., Fujian, China) overnight at 4°C, followed by horseradish peroxidase-linked sheep anti-mouse secondary antibody (KIT-5930-1, Fuzhou Maixin Biotech Co., Ltd., Fujian, China) for 60 min. The reactions were visualized by 3, 3-diaminobenzidine (DAB). The slides were then counterstained with hematoxylin. The same procedure with the primary antibody omitted was used as a negative control. Sections of peripheral nerve tissue were used as a positive control.
Staining evaluation
Expression of βIII-tubulin was analyzed under an Olympus BX51 microscope (Olympus, Tokyo, Japan) by 2 senior professional physicians who were blinded to the clinical data of patients. The proportion of positive tumor cells was scored from 5 randomly selected slides and the results were expressed as the average of data of the 2 physicians. The cytoplasmatic staining intensity was graded as: negative (−, no staining), weak positive (+, pale yellow), moderate positive (++, claybank), and strong positive (+++, sepia).
Study group and follow-up
According to the distribution of βIII-tubulin in the tumor tissue, patients were assigned to either the invasive front group or the non-invasive front group. The invasive front group consisted of cases with βIII-tubulin-positive tumor cells found only in the invasive front tumor area, while the non-invasive front group consisted of the remaining cases. The invasive front was defined as the advancing edge of the tumor. We recorded clinical data on age, sex, tumor size, differentiation status, TNM stage, and lymphatic metastasis. Enrolled patients were followed up from the time of disease diagnosis to the time of death of patients or study deadline (May 2015). This study was approved by the Ethics Committee of Beijing Tongren Hospital of the Capital Medical University and informed consent was obtained from all patients.
Statistical analysis
Qualitative data are expressed as proportion and analyzed by χ2 or Fisher’s exact test. Normally distributed quantitative data are expressed as mean ±SD and analyzed by ANOVA. Data with abnormal distribution are expressed as interquartile range and were analyzed by Kruskal-Wallis test. Correlations analysis was performed by Spearman’s or Pearson’s test. P <0.05 was considered statistically significant.
Results
Clinical data
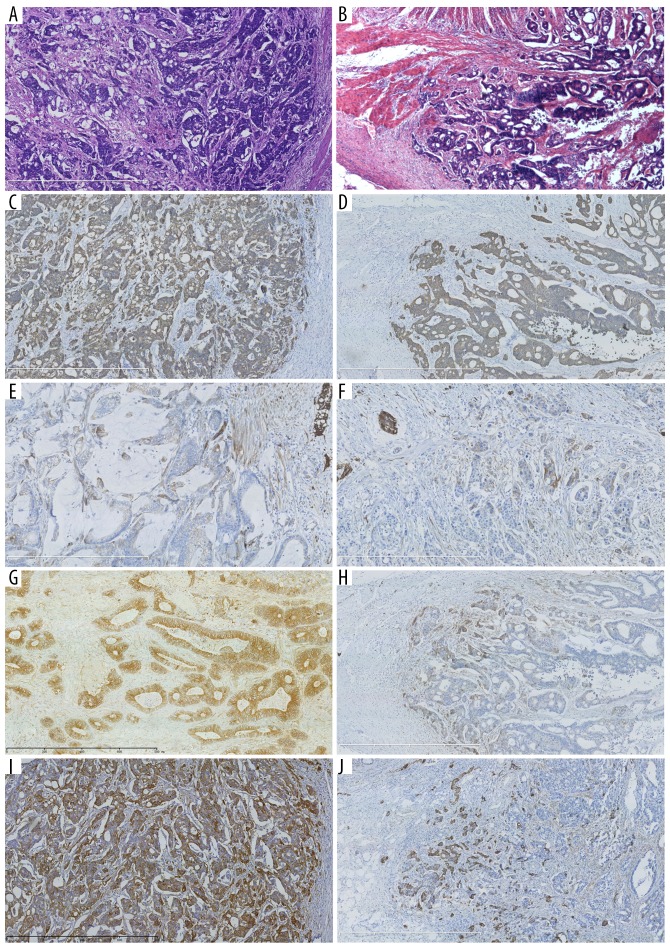
A total of 111 patients who underwent radical colorectal resection were included in this study. There were 55 males and 56 females. Mean age of the patients was 66.41±12.64 years (median: 68.00). Clinical data are summarized in Table 1. βIII-tubulin was positively stained in 0.31±0.27% of the tumor cells. The staining intensity was negative in 6 cases (5.41%), weakly positive in 10 cases (9.01%), moderately positive in 65 cases (58.56%), and strongly positive in 30 cases (27.03%). The invasive front group had 72 patients with βIII-tubulin expressed only in the invasive front of tumor tissue, and the non-invasive front group had the remaining 39 patients. HE staining results of colorectal cancer in the non-invasive front group and the invasive front group are shown in Figure 1A and 1B. Immunohistochemical analysis showed positive staining of cytokeratin (AE1/AE3) in the non-invasive front group (Figure 1C) and the invasive front group (Figure 1D). Immunohistochemical analysis of βIII-tubulin showed heterogeneous labeling in both the non-invasive front group and invasive front group, with weakly, moderately, and strongly stained areas observed (Figure 1E–1J).
Table 1.
Clinical data of patients with colorectal carcinoma.
| Colorectal carcinoma patients (n=111) | |
|---|---|
| Age (years) | 66.41±12.64 |
| Gender n (%) | |
| Male | 55 |
| Female | 56 |
| Lymphatic metastasis n (%) | |
| No | 54 (48.65) |
| Yes | 57 (51.35) |
| Tumor differentiation n (%) | |
| Well | 18 (16.22) |
| Moderate | 68 (61.26) |
| Poor | 25 (22.52) |
| TNM stage n (%) | |
| I | 18 (16.22) |
| II | 35 (31.53) |
| III | 46 (41.44) |
| IV | 12 (10.81) |
| Local recurrence n (%) | |
| No | 109 (98.20%) |
| Yes | 2 (1.80) |
| Distant metastasis n (%) | |
| No | 97 (87.39%) |
| Yes | 14 (12.61) |
| Mortality n (%) | |
| No | 102 (91.89) |
| Yes | 9 (8.11) |
| Positive staining of βIII-tubulin (%) | 0.31±0.27 |
| Staining intensity of βIII-tubulin n (%) | |
| − | 6 (5.41) |
| + | 10 (9.01) |
| ++ | 65 (58.56) |
| +++ | 30 (27.03) |
| Tissue distribution of βIII-tubulin n (%) | |
| Invasive front group | 72 (64.87) |
| Non-invasive front group | 39 (35.13) |
Figure 1.
Histological and immunohistochemical analysis of colorectal cancer. HE staining of tumor tissue in the non-invasive front group (A, ×50) and invasive front group (B, ×50). Immunohistochemical staining of cytokeratin AE1/AE3 in the non-invasive front group (C, ×50) and invasive front group (D, ×50). Immunohistochemical analysis of βIII-tubulin with different positive intensities in the non-invasive front group (E: +; G: ++; I: +++; E, ×100, G and I, ×50) and invasive front group (F: +; H: ++; J: +++) (E, F, ×100, strong positivity of βIII-tubulin in nervous tissue as internal control; G–J, ×50).
Positive staining and staining intensity of βIII-tubulin in colorectal carcinoma patients
The rate of positive staining of βIII-tubulin in colorectal carcinoma patients was significantly correlated with staining intensity and tissue distribution of βIII-tubulin expression (p=0.018 and p=0.001, respectively, Table 2). The positive staining rate of βIII-tubulin was significantly higher in tumor tissues with higher staining intensity (p<0.05). The rate of βIII-tubulin positive staining in the non-invasive front group was also significantly higher when compared with the invasive front group. The other data (age, sex, lymphatic metastasis, TNM stage, local recurrence, distant metastasis, and mortality) were not found to be significantly associated with positive staining of βIII-tubulin. There was no significant difference in the clinical data among the groups with different βIII-tubulin staining intensity (Table 3)
Table 2.
Clinical analysis of positive staining of βIII-tubulin in colorectal carcinoma patients.
| Case (n) | Positive staining of bIII-tubulin (%) | t | P | |
|---|---|---|---|---|
| Age | 111 | – | −0.02 | 0.836 |
| Gender | ||||
| Male | 55 | 0.32±0.27 | 0.17 | 0.869 |
| Female | 56 | 0.31±0.27 | ||
| Lymphatic metastasis | ||||
| No | 54 | 0.32±0.25 | 0.15 | 0.878 |
| Yes | 57 | 0.31±0.29 | ||
| Tumor differentiation | ||||
| Well | 18 | 0.40±0.30 | 1.76 | 0.177 |
| Moderate | 68 | 0.28±0.25 | ||
| Poor | 25 | 0.34±0.29 | ||
| TNM stage | ||||
| I | 18 | 0.44±0.31a | 1.62 | 0.189 |
| II | 35 | 0.28±0.21a | ||
| III | 46 | 0.30±0.29a | ||
| IV | 12 | 0.28±0.26a | ||
| Local recurrence | ||||
| No | 109 | 0.31±0.27 | −0.35 | 0.783 |
| Yes | 2 | 0.47±0.62 | ||
| Distant metastasis | ||||
| No | 97 | 0.32±0.27 | 0.16 | 0.875 |
| Yes | 14 | 0.30±0.30 | ||
| Mortality | ||||
| No | 102 | 0.31±0.27 | −0.58 | 0.566 |
| Yes | 9 | 0.36±0.33 | ||
| Staining intensity of βIII-tubulin | ||||
| − | 6 | 0.00±0.00b | 3.49 | 0.018 |
| + | 10 | 0.24±0.31a | ||
| ++ | 65 | 0.34±0.26a | ||
| +++ | 30 | 0.34±0.28a | ||
| Tissue distribution of βIII-tubulin | ||||
| Non-invasive front group | 39 | 0.44±0.33 | 3.52 | 0.001 |
| Invasive front group | 72 | 0.24±0.20 | ||
Table 3.
Clinical analysis of staining intensity of βIII-tubulin in colorectal carcinoma patients.
| Staining intensity of βIII-tubulin | χ2 | P | ||||
|---|---|---|---|---|---|---|
| − (n=6) | + (n=10) | ++ (n=65) | +++ (n=30) | |||
| Age (years) | 59.83±21.64 | 66.90±12.01 | 66.65±11.37 | 67.03±13.62 | 0.57* | 0.635 |
| Gender n (%) | ||||||
| Male | 3 (50.00) | 6 (60.00) | 33 (50.77) | 13 (43.33) | 0.01 | 0.829 |
| Female | 3 (50.00) | 4 (40.00) | 32 (49.23) | 17 (56.67) | ||
| Lymphatic metastasis | ||||||
| No | 5 (83.33) | 4 (40.00) | 31 (47.69) | 14 (46.67) | 0.00 | 0.383 |
| Yes | 1 (16.67) | 6 (60.00) | 34 (52.31) | 16 (53.33) | ||
| Tumor differentiation n (%) | ||||||
| Well | 3 (50.00) | 1 (10.00) | 12 (18.46) | 2 (6.67) | 0.00 | 0.078 |
| Moderate | 1 (16.67) | 6 (60.00) | 42 (64.62) | 19 (63.33) | ||
| Poor | 2 (33.33) | 3 (30.00) | 11 (16.92) | 9 (30.00) | ||
| TNM stage | ||||||
| I | 1 (16.67) | 3 (30.00) | 10 (15.38) | 4 (13.33) | 0.00 | 0.597 |
| II | 4 (66.67) | 1 (10.00) | 22 (33.85) | 8 (26.67) | ||
| III | 1 (16.67) | 5 (50.00) | 26 (40.00) | 14 (46.67) | ||
| IV | 0 (0.00) | 1 (10.00) | 7 (10.77) | 4 (13.33) | ||
| Local recurrence | ||||||
| No | 6 (100.0) | 9 (90.00) | 65 (100.0) | 29 (96.67) | 0.05 | 0.100 |
| Yes | 0 (0.00) | 1 (10.00) | 0 (0.00) | 1 (3.33) | ||
| Distant metastasis | ||||||
| No | 5 (83.33) | 9 (90.00) | 58 (89.23) | 25 (83.33) | 0.03 | 0.785 |
| Yes | 1 (16.67) | 1 (10.00) | 7 (10.77) | 5 (16.67) | ||
| Mortality | ||||||
| No | 6 (100.0) | 9 (90.00) | 60 (92.31) | 27 (90.00) | 0.07 | 0.932 |
| Yes | 0 (0.00) | 1 (10.00) | 5 (7.69) | 3 (10.00) | ||
| Positive staining of βIII-tubulin (%) | 0.00±0.00 | 0.24±0.31* | 0.34±0.26* | 0.34±0.28* | 3.49 | 0.018 |
p<0.05 vs. the βIII-tubulin negative (−) group.
Tissue distribution of βIII-tubulin in colorectal carcinoma patients
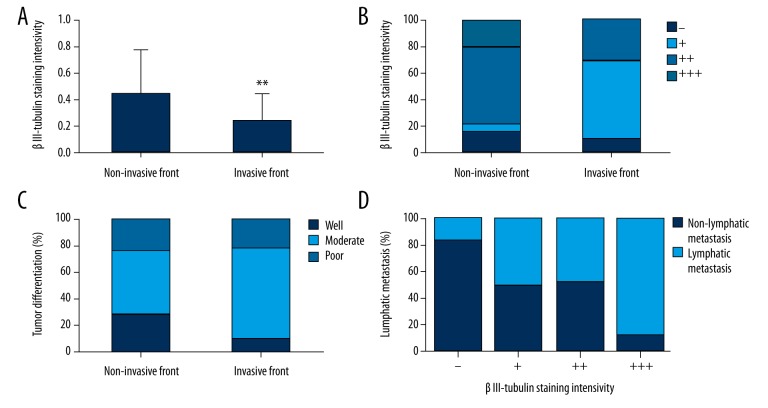
The patients were further divided into invasive front and non-invasive front groups according to the tissue distribution of βIII-tubulin in colorectal carcinoma. The rate of positive staining and staining intensity of βIII-tubulin were significantly different between the invasive and non-invasive front groups (p=0.001 and p=0.006, respectively, Table 4, Figure 2A, 2B), and there was a significant difference in tumor differentiation between the 2 groups (p=0.032, Figure 2C). However, the differences in other data between the invasive and non-invasive front groups did not reach statistical significance.
Table 4.
Clinical analysis of patients with βIII-tubulin distributed in invasive front and non-invasive front area.
| Colorectal carcinoma patients | χ2 | P | ||
|---|---|---|---|---|
| Non-invasive front group (n=39) | Invasive front group | |||
| Age (years) | 64.79±14.40 | 67.28±11.59 | −0.99 | 0.325 |
| Gender n (%) | ||||
| Male | 22 (56.41) | 33 (45.83) | 1.13 | 0.287 |
| Female | 17 (43.59) | 39 (54.17) | ||
| Lymphatic metastasis | ||||
| No | 19 (48.72) | 35 (48.61) | 0.00 | 0.991 |
| Yes | 20 (51.28) | 37 (51.39) | ||
| Tumor differentiation n (%) | ||||
| Well | 11 (28.21) | 7 (9.72) | 6.88 | 0.032 |
| Moderate | 19 (48.72) | 49 (68.06) | ||
| Poor | 9 (23.08) | 16 (22.22) | ||
| TNM stage | ||||
| I | 8 (20.51) | 10 (13.89) | 3.01 | 0.390 |
| II | 11 (28.21) | 24 (33.33) | ||
| III | 18 (46.15) | 28 (38.89) | ||
| IV | 2 (5.13) | 10 (13.89) | ||
| Local recurrence | ||||
| No | 38 (97.44) | 71 (98.61) | 0.46 | 1.000 |
| Yes | 1 (2.56) | 1 (1.39) | ||
| Distant metastasis | ||||
| No | 35 (89.74) | 62 (86.11) | 0.21 | 0.767 |
| Yes | 4 (10.26) | 10 (13.89) | ||
| Mortality | ||||
| No | 35 (89.74) | 67 (93.06) | 0.23 | 0.717 |
| Yes | 4 (10.26) | 5 (6.94) | ||
| Staining intensity of βIII-tubulin | ||||
| − | 6 (15.38) | 0 (0.00) | 0.00 | 0.006 |
| + | 2 (5.13) | 8 (11.11) | ||
| ++ | 23 (58.97) | 42 (58.33) | ||
| +++ | 8 (20.51) | 22 (30.56) | ||
| Positive staining of βIII-tubulin (%) | 0.44±0.33 | 0.24±0.20 | 3.52 | 0.001 |
Figure 2.
Positive staining (A) and staining intensity of βIII-tubulin (B) and tumor differentiation (C) were significantly different in patients in the invasive and non-invasive front groups. Staining intensity of βIII-tubulin was significantly associated with lymphatic metastasis in the non-invasive front group (D). ** p<0.01 vs. the non-invasive front group.
Clinical analysis of βIII-tubulin staining intensity in invasive and non-invasive front groups
Staining intensity of βIII-tubulin in patients in the invasive and non-invasive front groups was further analyzed. As shown in Table 5, in the non-invasive front group, staining intensity of βIII-tubulin was significantly associated with the rates of βIII-tubulin-positive staining and lymphatic metastasis of tumors (p<0.001 and p=0.048, respectively, Figure 2D), but there were no significant differences in any of the clinical data with respect to the βIII-tubulin staining intensity in patients in the invasive front group.
Table 5.
clinical analysis of βIII-tubulin staining intensity in patients of invasive and non-invasive front groups.
| Colorectal carcinoma patients | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Non-invasive front group | χ2 | P | Invasive front group | χ2 | P | ||||||
| − | + | ++ | +++ | + | ++ | +++ | |||||
| (n=6) | (n=2) | (n=23) | (n=8) | (n=8) | (n=42) | (n=22) | |||||
| Age (years) | 59.83±21.64 | 63.50±7.78 | 64.74±11.63 | 69.00±17.78 | 0.45 | 0.719 | 67.75±13.14 | 67.69±11.23 | 66.32±12.19 | 0.11 | 0.9 |
| Gender n (%) | |||||||||||
| Male | 3 (50.00) | 1 (50.00) | 13 (56.52) | 5 (62.50) | 0.05 | 1 | 5 (62.50) | 20 (47.62) | 8 (36.36) | 0.03 | 0.422 |
| Female | 3 (50.00) | 1 (50.00) | 10 (43.48) | 3 (37.50) | 3 (37.50) | 22 (52.38) | 14 (63.64) | ||||
| Lymphatic metastasis | |||||||||||
| No | 5 (83.33) | 1 (50.00) | 12 (52.17) | 1 (12.50) | 0 | 0.048 | 3 (37.50) | 19 (45.24) | 13 (59.09) | 0.03 | 0.515 |
| Yes | 1 (16.67) | 1 (50.00) | 11 (47.83) | 7 (87.50) | 5 (62.50) | 23 (54.76) | 9 (40.91) | ||||
| Tumor differentiation n (%) | |||||||||||
| Well | 3 (50.00) | 1 (50.00) | 7 (30.43) | 0 (0.00) | 0 | 0.185 | 0 (0.00) | 5 (11.90) | 2 (9.09) | 0 | 0.633 |
| Moderate | 1 (16.67) | 1 (50.00) | 12 (52.17) | 5 (62.50) | 5 (62.50) | 30 (71.43) | 14 (63.64) | ||||
| Poor | 2 (33.33) | 0 (0.00) | 4 (17.39) | 3 (37.50) | 3 (37.50) | 7 (16.67) | 6 (27.27) | ||||
| TNM stage | |||||||||||
| I | 1 (16.67) | 1 (50.00) | 5 (21.74) | 1 (12.50) | 0 | 0.169 | 2 (25.00) | 5 (11.90) | 3 (13.64) | 0 | 0.876 |
| II | 4 (66.67) | 0 (0.00) | 7 (30.43) | 0 (0.00) | 1 (12.50) | 15 (35.71) | 8 (36.36) | ||||
| III | 1 (16.67) | 1 (50.00) | 10 (43.48) | 6 (75.00) | 4 (50.00) | 16 (38.10) | 8 (36.36) | ||||
| IV | 0 (0.00) | 0 (0.00) | 1 (4.35) | 1 (12.50) | 1 (12.50) | 6 (14.29) | 3 (13.64) | ||||
| Local recurrence | |||||||||||
| No | 6 (100.0) | 2 (100.0) | 23 (100.0) | 7 (87.50) | 0.21 | 0.414 | 7 (87.50) | 42 (100.0) | 22 (100.0) | 0.11 | 0.110 |
| Yes | 0 (0.00) | 0 (0.00) | 0 (0.00) | 1 (12.50) | 1 (12.50) | 0 (0.00) | 0 (0.00) | ||||
| Distant metastasis | |||||||||||
| No | 5 (83.33) | 2 (100.0) | 22 (95.65) | 6 (75.00) | 0.05 | 0.302 | 7 (87.50) | 36 (85.71) | 19 (86.36) | 0.12 | 1.000 |
| Yes | 1 (16.67) | 0 (0.00) | 1 (4.35) | 2 (25.00) | 1 (12.50) | 6 (14.29) | 3 (13.64) | ||||
| Mortality | |||||||||||
| No | 6 (100.0) | 2 (100.0) | 21 (91.30) | 6 (75.00) | 0.09 | 0.449 | 7 (87.50) | 39 (92.86) | 21 (95.45) | 0.14 | 0.640 |
| Yes | 0 (0.00) | 0 (0.00) | 2 (8.70) | 2 (25.00) | 1 (12.50) | 3 (7.14) | 1 (4.55) | ||||
| Positive staining of βIII-tubulin (%) | 0.00±0.00 | 0.80±0.00* | 0.46±0.28* | 0.64±0.27* | 8.91 | <0.001 | 0.10±0.10 | 0.27±0.21 | 0.24±0.20 | 2.47 | 0.092 |
p<0.05 vs. the βIII-tubulin negative (−) group.
Discussion
βIII-tubulin is one of 9 β-isoforms that participate in formation of microtubules; it is known to play a critical role in cell growth, division, motility, signaling development, and cell shape maintenance [12]. Overexpression of βIII-tubulin was originally identified to be a prominent factor contributing to resistance to taxane drugs [13–18]. Down-regulation of βIII-tubulin expression has been reported to contribute to favorable clinical outcome following anti-tubulin therapy [14]. Recently, however, increasing studies have shown that βIII-tubulin is involved more in tumor development and progression than as a predictor of response to chemotherapy in various types of tumors [18–23]. βIII-tubulin has been reported to be expressed in a variety of tumors, especially in those with aggressive behavior that are likely to metastasize [24–26]. In the present study, the tissue distribution of class III β-tubulin expression at the invasive margin or diffuse distribution was investigated in colorectal cancer. Our study showed that βIII-tubulin was expressed at the invasive margin in 64.78% of our colorectal cancer cases. The expression of βIII-tubulin may be involved with tumor differentiation and lymphatic metastasis, suggesting a potential role of βIII-tubulin in tumor differentiation and metastasis.
In colorectal cancer, aberrant βIII-tubulin expression has been suggested to be involved with tumor development and poor survival of the patients [8,10]. However, a previous study of βIII-tubulin expression in colorectal cancer indicated that expression of βIII-tubulin was relatively uncommon in colorectal carcinomas [7]. A study by Portyanko et al. showed the preferential localization of βIII-tubulin at the invasive margin in colorectal cancer, suggesting a potential role of βIII-tubulin in modulation of the invading activity of cancer cells [9]. However, those studies had relatively small sample sizes and the clinical implications of βIII-tubulin at the invasive margin in colorectal cancer had not yet been defined. Therefore, in the present study, 111 patients with surgically resected colorectal cancer were recruited, and the expression of βIII-tubulin at the invasive margin was investigated. The findings of our study showed that βIII-tubulin was expressed at the invasive margin in 72 of 111 cases of colorectal cancer, accounting for 64.87% of all cases. These data dramatically differed from the results reported by Portyanko et al., which showed βIII-tubulin expression at the invasive margin in 28 of 29 cases of colorectal cancer [9]. These conflicting results can be partially explained by differences in race and number of patients recruited, as well as the lack of consistency in study criteria. However, despite these differences, our study showed a significant difference in tumor differentiation between the invasive front and non-invasive front groups, with many more poorly and moderately differentiated tumors identified in the invasive front group. This finding was partially corroborated with the results of a previous study reporting more βIII-tubulin immunoreactivity in poorly differentiated colorectal carcinomas [7]. Sex has been recently suggested to play a role in the ability of βIII-tubulin to predict poor outcome in colorectal cancer [10]. In our study, however, no such sex-related difference was observed. The upregulated expression of βIII-tubulin has been reported in several types of cancers and was associated with aggressive behavior. A previous papillary thyroid carcinoma study showed strong staining of βIII-tubulin in widely infiltrating PTCs, particularly at the invasive margin, or in moderately differentiated PTCs, and most cases with lymphatic metastasis showed strong βIII-tubulin immunoreactivity [27]. In an esophageal squamous cell carcinoma study, Yu et al. found that βIII-tubulin expression was associated with the lymphatic metastasis of tumors [28]. The results of the present study showed that the staining intensity of βIII-tubulin was associated with the lymphatic metastasis in the non-invasive front group, suggesting the potential role of βIII-tubulin in tumor invasion or metastasis. However, our study showed no significant association of βIII-tubulin with tumor recurrence, metastasis, or survival. Further research is needed to clarify the potential role of βIII-tubulin in colorectal cancer and patient prognosis.
This study has some limitations. The number of patients recruited in was comparatively small. Therefore, only the patients with βIII-tubulin expressed in the invasive front of tumor tissue were included in the invasive front group, and all remaining patients were included in the non-invasive front group. However, despite these limitations, our study did show the association of βIII-tubulin expression with tumor differentiation and lymphatic metastasis in colorectal cancer. Studies with larger samples are needed to further clarify the clinical implications of βIII-tubulin expression at the invasive margin in colorectal cancer.
Conclusions
Our data showed the tissue distribution of class III β-tubulin expression at the invasive margin or diffuse distribution, and that βIII-tubulin expression may be involved in tumor differentiation and metastasis. This study may shed light on βIII-tubulin as a novel potential molecular target for new anti-cancer drugs.
Footnotes
Conflict of Interest
None.
Source of support: This work was supported by the National Natural Science Foundation of China (Grant no. 81360361)
References
- 1.Haggar FA, Boushey RP. Colorectal cancer epidemiology: Incidence, mortality, survival, and risk factors. Clin Colon Rectal Surg. 2009;22(4):191–97. doi: 10.1055/s-0029-1242458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhao P, Dai M, Chen W, Li N. Cancer trends in China. Jpn J Clin Oncol. 2010;40(4):281–85. doi: 10.1093/jjco/hyp187. [DOI] [PubMed] [Google Scholar]
- 3.Yang X, Li J, Cai W, et al. Adiponectin gene polymorphisms are associated with increased risk of colorectal cancer. Med Sci Monit. 2015;21:2595–606. doi: 10.12659/MSM.893472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Süren D, Yıldırım M, Kaya V, et al. Loss of tight junction proteins (Claudin 1, 4, and 7) correlates with aggressive behavior in colorectal carcinoma. Med Sci Monit. 2014;20:1255–62. doi: 10.12659/MSM.890598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Süren D, Yıldırım M, Demirpençe Ö, et al. The role of high mobility group box 1 (HMGB1) in colorectal cancer. Med Sci Monit. 2014;20:530–37. doi: 10.12659/MSM.890531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chan C, Jankova L, Fung CL, et al. Fascin expression predicts survival after potentially curative resection of node-positive colon cancer. Am J Surg Pathol. 2010;34(5):656–66. doi: 10.1097/PAS.0b013e3181db36c0. [DOI] [PubMed] [Google Scholar]
- 7.Jirásek T, Cipro S, Musilova A, et al. Expression of class III beta-tubulin in colorectal carcinomas: An immunohistochemical study using TU-20 & TuJ-1 antibody. Indian J Med Res. 2009;129(1):89–94. [PubMed] [Google Scholar]
- 8.Giarnieri E, De Francesco GP, Carico E, et al. α-and β-tubulin expression in rectal cancer development. Anticancer Res. 2005;25(5):3237–41. [PubMed] [Google Scholar]
- 9.Portyanko A, Kovalev P, Gorgun J, Cherstvoy E. βIII-tubulin at the invasive margin of colorectal cancer: possible link to invasion. Virchows Arch. 2009;454(5):541–48. doi: 10.1007/s00428-009-0764-4. [DOI] [PubMed] [Google Scholar]
- 10.Mariani M, Zannoni GF, Sioletic S, et al. Gender influences the class III and V β-tubulin ability to predict poor outcome in colorectal cancer. Clin Cancer Res. 2012;18(10):2964–75. doi: 10.1158/1078-0432.CCR-11-2318. [DOI] [PubMed] [Google Scholar]
- 11.Grzanka A, Grzanka D. The influence of etoposide on the distribution of tubulin in human leukemia cell line HL-60. Med Sci Monit. 2003;9(1):BR66–69. [PubMed] [Google Scholar]
- 12.Mitchison T, Kirschner M. Dynamic instability of microtubule growth. Nature. 1984;312(5991):237–42. doi: 10.1038/312237a0. [DOI] [PubMed] [Google Scholar]
- 13.Verdier-Pinard P, Wang F, Martello L, et al. Analysis of tubulin isotypes and mutations from taxol-resistant cells by combined isoelectrofocusing and mass spectrometry. Biochemistry. 2003;42(18):5349–57. doi: 10.1021/bi027293o. [DOI] [PubMed] [Google Scholar]
- 14.Zhang H-L, Ruan L, Zheng L-M, et al. Association between class III β-tubulin expression and response to paclitaxel/vinorebine-based chemotherapy for non-small cell lung cancer: A meta-analysis. Lung Cancer. 2012;77(1):9–15. doi: 10.1016/j.lungcan.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 15.Hetland TE, Hellesylt E, Flørenes VA, et al. Class III β-tubulin expression in advanced-stage serous ovarian carcinoma effusions is associated with poor survival and primary chemoresistance. Hum Pathol. 2011;42(7):1019–26. doi: 10.1016/j.humpath.2010.10.025. [DOI] [PubMed] [Google Scholar]
- 16.Chen X, Wu J, Lu H, et al. Measuring β-tubulin III, Bcl-2, and ERCC1 improves pathological complete remission predictive accuracy in breast cancer. Cancer Sci. 2012;103(2):262–68. doi: 10.1111/j.1349-7006.2011.02135.x. [DOI] [PubMed] [Google Scholar]
- 17.Zheng W, Chen H, Yuan S, et al. Overexpression of βIII-tubulin and survivin associated with drug resistance to docetaxel-based chemotherapy in advanced gastric cancer. J BUON. 2011;17(2):284–90. [PubMed] [Google Scholar]
- 18.McCarroll JA, Sharbeen G, Liu J, et al. βIII-Tubulin: A novel mediator of chemoresistance and metastases in pancreatic cancer. Oncotarget. 2015;6(4):2235–49. doi: 10.18632/oncotarget.2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karki R, Mariani M, Andreoli M, et al. beta III-Tubulin: biomarker of taxane resistance or drug target? Expert Opin Ther Targets. 2013;17(4):461–72. doi: 10.1517/14728222.2013.766170. [DOI] [PubMed] [Google Scholar]
- 20.Levallet G, Bergot E, Antoine M, et al. High TUBB3 expression, an independent prognostic marker in patients with early non-small cell lung cancer treated by preoperative chemotherapy, is regulated by K-Ras signaling pathway. Mol Cancer Ther. 2012;11(5):1203–13. doi: 10.1158/1535-7163.MCT-11-0899. [DOI] [PubMed] [Google Scholar]
- 21.Raspaglio G, Petrillo M, Martinelli E, et al. Sox9 and Hif-2α regulate TUBB3 gene expression and affect ovarian cancer aggressiveness. Gene. 2014;542(2):173–81. doi: 10.1016/j.gene.2014.03.037. [DOI] [PubMed] [Google Scholar]
- 22.McCarroll JA, Gan PP, Erlich RB, et al. TUBB3/βIII-tubulin acts through the PTEN/AKT signaling axis to promote tumorigenesis and anoikis resistance in non-small cell lung cancer. Cancer Res. 2015;75(2):415–25. doi: 10.1158/0008-5472.CAN-14-2740. [DOI] [PubMed] [Google Scholar]
- 23.Tsourlakis MC, Weigand P, Grupp K, et al. βIII-tubulin overexpression is an independent predictor of prostate cancer progression tightly linked to ERG fusion status and PTEN deletion. Am J Pathol. 2014;184(3):609–17. doi: 10.1016/j.ajpath.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 24.Katsetos CD, Luis Del Valle M, Geddes JF, et al. Aberrant localization of the neuronal class III β-tubulin in astrocytomas. Arch Pathol Lab Med. 2001;125:613–24. doi: 10.5858/2001-125-0613-ALOTNC. [DOI] [PubMed] [Google Scholar]
- 25.Katsetos CD, Herman MM, Mörk SJ. Class III β-tubulin in human development and cancer. Cell Motil Cytoskeleton. 2003;55(2):77–96. doi: 10.1002/cm.10116. [DOI] [PubMed] [Google Scholar]
- 26.Katsetos CD, Del Valle L, Geddes JF, et al. Localization of the neuronal class III β-tubulin in oligodendrogliomas: Comparison with Ki-67 proliferative index and 1p/19q status. J Neuropathol Exp Neurol. 2002;61(4):307–20. doi: 10.1093/jnen/61.4.307. [DOI] [PubMed] [Google Scholar]
- 27.Colato C, Monzani F, Brazzarola P, et al. Immunohistochemical expression of [beta] III-tubulin and cell-cell adhesion proteins in papillary thyroid carcinoma: A preliminary report. Endocrine Abstracts. 2012;29:P1788. [Google Scholar]
- 28.Yu Y, Ding S, Liang Y, et al. Expression of ERCC1, TYMS, TUBB3, RRM1 and TOP2A in patients with esophageal squamous cell carcinoma: A hierarchical clustering analysis. Exp Ther Med. 2014;7(6):1578–82. doi: 10.3892/etm.2014.1659. [DOI] [PMC free article] [PubMed] [Google Scholar]