Abstract
Background.
Malnutrition (MN) is prevalent in cardiac surgery, but there are no specific preoperative risk factors of MN. The aim of this study is to assess the clinically relevant risk factors of MN for cardiac surgery patients.
Materials and methods.
The nutritional state of the patients was evaluated one day prior to surgery using a bioelectrical impedance analysis phase angle (PA). Two groups of patients were generated according to low PA: malnourished and well nourished. Risk factors of MN were divided into three clinically relevant groups: psychosocial and lifestyle factors, laboratory findings and disease-associated factors. Variables in each different group were entered into separate multivariate logistic regression models.
Results.
A total of 712 patients were included in the study. The majority of them were 65-year old men after a CABG procedure. Low PA was present in 22.9% (163) of patients. The analysis of disease-related factors of MN revealed the importance of heart functions (NYHA IV class OR: 3.073, CI95%: 1.416–6.668, p = 0.007), valve pathology (OR: 1.825, CI95%: 1.182–2.819, p = 0.007), renal insufficiency (OR: 4.091, CI95%: 1.995–8.389, p < 0.001) and body mass index (OR: 0.928, CI95%: 0.890–0.968, p < 0.001). Laboratory values related to MN were levels of haemoglobin (OR: 0.967, CI95%: 0.951–0.983, p < 0.001) and C-reactive protein (OR: 1.015, CI95%: 1.002–1.028, p = 0.0279). The lifestyle variables that qualified as risk factors concerned the intake of food (OR: 3.030, CI95%: 1.353–6.757, p = 0.007) and mobility (OR: 2.770, CI95%: 1.067–7.194, p = 0.036).
Conclusions.
MN risk factors comprise three different clinical groups: psychosocial and lifestyle factors, laboratory findings and disease-associated factors. The patients who are most likely to be malnourished are those with valve pathology, severe imparted heart function, insufficient renal function and high inflammatory markers. Also these patients have decreased mobility and food intake.
Keywords: malnutrition, cardiac surgery, phase angle, bioelectrical impedance analysis, post-operative outcomes
Abstract
PRIEŠOPERACINIO MITYBOS NEPAKANKAMUMO RIZIKOS VEIKSNIAI KARDIOCHIRURGINIAMS PACIENTAMS
Santrauka
Darbo tikslas. Nors mitybos nepakankamumo (MN) dažnis tarp kardiochirurginių pacientų yra didelis, nėra nustatyta specifinių ir kliniškai reikšmingų priešoperacinių MN rizikos veiksnių. Šio tyrimo tikslas – nustatyti priešoperacinius kardiochirurginių pacientų mitybos nepakankamumo rizikos veiksnius.
Metodika. Dieną prieš operaciją pacientų mitybos būklė įvertinta naudojant bioelektrinio impedanso analizės metu gautą fazės kampą (FK). Pacientai suskirstyti į dvi grupes atsižvelgiant į FK reikšmę: nepakankamos ir pakankamos mitybos. Priešoperacinio MN rizikos veiksniai buvo suskirstyti į tris grupes: psichosocialiniai ir gyvenimo būdo, laboratoriniai rodikliai ir su liga susiję priežastys. Į šias grupes patekę rizikos sukėlėjai išanalizuoti taikant tris atskiras daugiaveiksnes pažangesnes logistines regresines analizes, nustatyti kiekvienos grupės reikšmingi MN rizikos veiksniai.
Rezultatai. Tyrime dalyvavo 712 pacientų, dauguma jų buvo 65 metų vyrai, kuriems atlikta apeinamųjų koronarinių jungčių operacija. Žemas FK nustatytas 22,9 % (163) pacientų. Su liga susijusių MN rizikos svarbiausi veiksniai: NYHA IV klasės širdies nepakankamumas (OR: 3,073; CI95 %: 1,416–6,668; p = 0,007), vožtuvų patologija (OR: 1,976; CI95 %: 1,292–3,030; p = 0,002), inkstų funkcijos nepakankamumas (OR: 4,505; CI95 %: 2,232–9,091; p < 0,001) ir kūno masės indeksas (OR: 0,928; CI95 %: 0,890–0,968; p < 0,001). Laboratoriniai rodikliai, susiję su MN: hemoglobinas (OR: 0,967; CI95 %: 0,951–0,983; p < 0,001) ir C reaktyviojo baltymo koncentracijos (OR: 1,015; CI95 %: 1,002–1,0280; p = 0,028). Svarbiausi psichosocialinių ir gyvenimo būdo parametrai – sumažėjęs suvalgomo maisto kiekis (OR: 3,030; CI95 %: 1,353–6,757; p = 0,007) ir mobilumas (OR: 2,770; CI95 %: 1,067–7,1940; p = 0,036).
Išvados. MN rizikos veiksniai pasiskirsto į tris kliniškai svarbias grupes: psichosocialiniai ir gyvenimo būdo, laboratoriniai rodikliai ir su liga susiję priežastys. Pacientai, labiausiai linkę į mitybos nepakankamumą, turėjo širdies vožtuvų patologiją, sutrikusį širdies funkcinį pajėgumą (NYHA IV klasės), inkstų funkcijos nepakankamumą ir padidėjusias uždegiminių markerių koncentracijas. Taip pat buvo sumažėjęs šių pacientų mobilumas ir maisto kiekio suvartojimas.
Raktažodžiai: mitybos nepakankamumas, kardiochirurgija, fazės kampas, bioelektrinio impedanso analizė, pooperacinės pasekmės
INTRODUCTION
A significant number of preoperatively hospitalised patients are malnourished. It ranges from 1.2 to 46.4% among cardiac surgery patients (1–12). Despite that, malnutrition is frequently neglected and not diagnosed in time. This leads to increased morbidity, mortality and impaired quality of life (12–15). These deleterious outcomes are responsible for increased hospital costs and a greater need for rehabilitation (16). A precise and timely nutritional state assessment would enable malnourished patients at a higher risk of post-operative complications to be identified. As a result, this could reduce both the incidence of adverse clinical outcomes and the treatment cost.
Bioelectrical impedance analysis (BIA) is a method of assessing body composition and nutritional status using impedance data of the tissues. Specific prediction equations for the estimation of the body compartments (e. g. fat mass (FM) and fat free mass (FFM)) have been developed and validated using gold standard techniques such as dual-energy X-ray absorbmetry (DEXA) for healthy populations (17). The point at issue is that predictive BIA equations might cause significant errors in some pathological states. Therefore the application of raw impedance data (e. g. reactance, resistance and phase angle (PA)) may have greater accuracy and potency in nutritional state evaluation (18). This assumption is grounded by measuring the phase angle, which has been reported to be an indicator of adverse clinical outcomes and malnutrition in various conditions (19, 20).
Cardiac surgery patients are in a poor nutritional state because the body composition is altered by the changes occurring in acute and chronic disease (21). Moreover, these patients are lacking in uptake or intake of nutrition and frequently have an impaired neurophysiological status or physical mobility (22, 23). Heart failure patients are not physically active because of the disease, and the disease progresses further because of the lack of exercise. Furthermore, a longer time spent in the hospital before surgery reduces physical activity (24). Hospitalised patients suffer from high levels of anxiety and depression, which adversely affect their nutritional habits before surgery (25). Thus, the nutritional state deteriorates further for preoperatively hospitalised patients.
Even though the conventional factors of malnutrition are well known and their use is well established in the European Society for Clinical Nutrition and Metabolism (ESPEN) recommended guidelines on malnutrition diagnostics (26), there are no studies that can provide specific preoperative malnutrition risk factors for cardiac surgery patients. Therefore, the aim of this study is to assess the early, specific and clinically relevant risk factors of malnutrition for cardiac surgery patients.
MATERIALS AND METHODS
Patients
An observational study was conducted in a tertiary referral university hospital between March 2013 and March 2014. Ethical approval for this study (Ethical Committee N°158200-12-561-162) was provided by the Vilnius Regional Biomedical Research Ethics Committee, Vilnius, Lithuania, on 29 November 2012.
During this period, data regarding patients undergoing elective cardiac surgery were gathered. The only patients excluded from the study were those who did not agree to participate or had impaired mental function and could not give an informed consent.
Nutritional state evaluation
The nutritional state of the patients was evaluated one day prior to surgery. BIA was performed using an InBody S10 (Biospace, Seoul, Korea) body composition analyser. The device was calibrated prior to the study and set to use a Caucasian population reference equation for the analysis. During the analysis the patients were in a supine position with arms abducted 15 degrees from the trunk and legs spread apart at the shoulder width and according to all other recommendations of the ESPEN and the manufacturers (27).
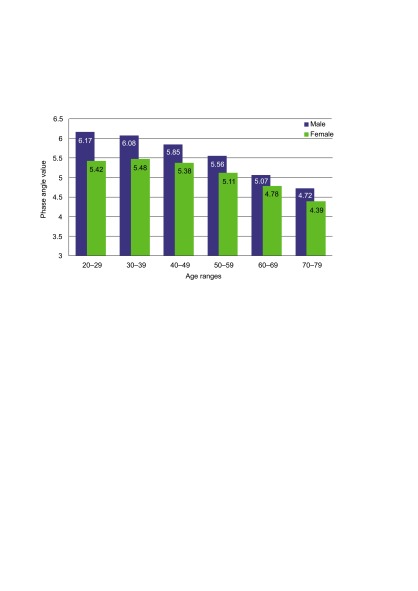
The measurement of the PA was obtained from BIA and was used to evaluate the nutritional state of the patients (28). The mean segmental PA of the 50 kHz frequency was used to classify patients into groups of the nutritional state according to the age and gender reference values of the healthy subjects (29). This step allowed the physiological changes of the body composition occurring in women and elderly patients, which result in lower but not pathological values of the PA, to be accounted for. The low PA margin was set at the 15th percentile of the mean population reference values, generating two groups of patients: malnourished and wellnourished (30). The age and gender adjusted cut-off values of the PA are presented in the Figure.
Assessment of risk factors
Preoperative variables expected to be associated with the development of preoperative malnutrition were obtained during a preoperative interview with the patients and using chart records. These variables included the questions asked in the ESPEN recommended nutrition risk screening questionnaires, Nutritional Risk Screening 2002 (NRS-2002), Malnutrition Universal Screening Tool (MUST) and Mini Nutritional Assessment (MNA), and various clinical parameters and co-morbidities presented in Table 1 (26). These variables were divided into three clinically relevant groups of malnutrition risk factors: psychosocial and lifestyle factors, laboratory findings and disease-associated factors (31). The psychosocial and lifestyle factors encompassed the intake of food and weight loss, the presence of depresssion and neurophysiological problems, the severity of the chronic disease, mood disorders and impaired mobility as defined in the latter questionnaires. The laboratory values tested were those indicating chronic inflammation, impaired haemopoiesis and other most clinically relevant parameters for cardiac surgery patients. The disease-associated factors group was generated by employing all of the most prevalent co-morbidities for the patients in question and by adding the hospitalisation and demographic parameters (32).
Table 1.
Baseline characteristics of the patients and comparison of low PA and normal PA groups
| Overall N = 712 | Low PA group N = 163 (22.9%) | Normal PA group N = 549 (77.1%) | P value | |
|---|---|---|---|---|
| Factor | n (%) or M [IQR] | Median [IQR] or mean ± SD | ||
| Patients | ||||
| Age, years | 65 [58–73] | 64.98 ± 12.35 | 64.39 ± 10.46 | 0.584 |
| Gender: Male (% within male) | 471 (66.2) | 96 (20.4) | 375 (79.6) | 0.026 |
| Female (% within female) | 241 (33.8) | 67 (27.8) | 174 (72.2) | |
| Hospitalisation length | ||||
| Before surgery, days | 6 [3–9] | 12.5 [3.75–19.75] | 7 [4–10] | 0.020 |
| Nutrition markers | ||||
| PA, ° | 5.5 [4.9–6.14] | 4.46 ± 0.60 | 5.84 ± 0.75 | <0.000 |
| FFMI, kg/m2 | 19.26 [17.43–20.86] | 17.99 ±2.27 | 19.66 ± 2.34 | <0.001 |
| Low FFMI | 61 (8.6) | 34 (20.9) | 27 (5.0) | <0.001 |
| BMI, kg/m2 | 28.23 [25.28–31.64] | 27.65 ± 5.46 | 29.14 ± 4.74 | 0.001 |
| Unintended weight loss > 5%, n (%) | 165 (23.2) | 50 (40.3) | 115 (27.1) | 0.005 |
| Co-morbidities | ||||
| Smoking, n (%) | 135 (19.0) | 34 (21.0) | 101 (18.5) | 0.495 |
| Hypertension, n (%) | 554 (78.1) | 118 (72.8) | 436 (79.7) | 0.063 |
| Peripheral artery disease, n (%) | 96 (13.6) | 23 (14.2) | 73 (13.4) | 0.787 |
| COPD, n (%) | 36 (5.1) | 9 (8.2) | 27 (27.8) | 0.689 |
| Renal failure, n (%) | 42 (5.9) | 19 (11.8) | 23 (4.2) | <0.001 |
| Oncologic disease, n (%) | 56 (7.9) | 16 (9.9) | 40 (7.3) | 0.288 |
| MI, n (%) | 255 (36.0) | 58 (35.8) | 197 (36.0) | 0.961 |
| MI < 30 days, n (%) | 84 (11.9) | 20 (12.3) | 64 (11.7) | 0.829 |
| Rhythm abnormalities, n (%) | 168 (23.7) | 47 (29.0) | 121 (22.1) | 0.070 |
| Diabetes, n (%) | 151 (21.2) | 40 (24.7) | 111 (20.3) | 0.230 |
| Stroke, n (%) | 69 (9.7) | 20 (12.3) | 49 (9.0) | 0.201 |
| Psychological disease, n (%) | 5(0.7) | 0 (0.0) | 5 (3.9) | 0.594 |
| Gastric erosions, n (%) | 170 (24.0) | 32 (19.8) | 138 (25.2) | 0.152 |
| NYHA class, n (%) | ||||
| I | 6 (0.9) | 3 (1.9) | 3 (0.6) | <0.001 |
| II | 77 (11.3) | 15 (9.6) | 62 (11.8) | |
| III | 562 (82.3) | 120 (76.4) | 442 (84.0) | |
| IV | 38 (5.6) | 19 (12.1) | 19 (3.6) | |
| Echocardiography | ||||
| LVEF | 50.43 [45–55] | 55 [40–55] | 55 (49–55] | 0.055 |
| LVEF < 30 mmHg, n (%) | 41 (6.0) | 17 (11.0) | 24 (4.6) | 0.006 |
| LVED diameter, cm | 5.3 [5.0–5.8] | 5.50 ± 0.89 | 5.40 ± 0.70 | 0.181 |
| Enlarged LV, n (%) | 195 (29.0) | 56 (36.6) | 139 (26.8) | 0.019 |
| AVI ≥ III°, n (%) | 35 (4.9) | 14 (8.6) | 21 (3.8) | 0.022 |
| AVS ≥ II°, n (%) | 94 (13.3) | 23 (14.1) | 71 (13.0) | 0.715 |
| MVI ≥ II°, n (%) | 156 (22.0) | 50 (30.7) | 106 (19.4) | 0.002 |
| TVI ≥ II°, n (%) | 82 (11.6) | 28 (17.2) | 54 (9.9) | 0.011 |
| Laboratory values | ||||
| Glucose, mmol/L | 5.83 [5.03–6.94] | 6.14 ± 1.29 | 6.34 ± 2.47 | 0.830 |
| Haemoglobin, g/L | 139 [128–149] | 130.26 ± 16.34 | 139.76 ± 15.38 | <0.001 |
| Haematocrit (ratio) | 0.41 [0.38–0.43] | 0.39 ± 0.47 | 0.41 ± 0.44 | <0.001 |
| Platelet count, 109/L | 206.0 [175.8–248.0] | 205 [193.8–324.3] | 211 [186–263] | 0.031 |
| WBC (109/L) | 7.18 [5.90–8.42] | 7.86 ± 3.35 | 7.29 ± 2.05 | 0.066 |
| Troponin level, ng/ml | 0.39 [0.01–1.90] | 1.44 [0.00–3.06] | 0.44 [0.01–1.40] | 0.143 |
| Creatinine level, pmol/l | 82 [72–97] | 95 [74.8–119.8] | 81 [70–106] | 0.805 |
| CRP, mg/L | 3.25 [1.3–9.4] | 10.2 [5.65–15.8] | 2.6 [1.58–9.2] | <0.000 |
| Operative risk | ||||
| Euroscore II | 1.77 [1.06–2.49] | 2.44 [1.12–6.60] | 2.00 [1.41–2.96] | <0.000 |
| Operative procedure | ||||
| STS CABG, n (%) | 423 (60.3) | 87 (53.4) | 336 (62.5) | 0.038 |
| STS valve, n (%) | 234 (33.4) | 59 (36.2) | 151 (28.1) | 0.051 |
| STS combined, n (%) | 24 (3.4) | 4 (2.5) | 20 (3.7) | 0.623 |
| STS other, n (%) | 44 (6.3) | 13 (8.0) | 31 (5.8) | 0.307 |
| Not operated, n (%) | 11 (1.5) | 0 (0.0) | 11 (2.0) | 0.078 |
Values are presented in absolute numbers and rates for categorical factors and for continuous factors by median (M) and interquartile range (IQR) in brackets.
Abbreviations: PA, phase angle; Low PA, low phase angle; SD, standard deviation; BMI, body mass index; CI, confidence interval; CAD, coronary artery disease; OP, operation; LVEF, left ventricle ejection fraction; LV, left ventricle; AVI, aortic valve insufficiency; MVI, mitral valve insufficiency; TVI, tricuspid valve insufficiency; WBC, white blood count; CRP, C-reactive protein; NRS 2002, Nutritional Risk Screening 2002; MUST, Malnutrition Universal Screening Tool; MNA, Mini Nutritional Assessment; Q, question; STS, Society of Thoracic Surgeons.
Statistical analysis
Descriptive statistics were used to describe the baseline characteristics. The data is presented using the median and interquartile range [IQR] of the sample.
The distribution of low PA predictors between the two independent normally and non-normally distributed data sets was evaluated using Student-t and Mann–Whitney U tests, respectively. The differences between independent two qualitative data groups were evaluated by the Chi-squared test.
Variables in each different group of low PA risk factors were entered into a univariate logistic regression model. Each group’s factors found to be significant in the univariate logistic regression analysis were entered into three different multivariate logistic regression models with the forward model selection process.
A two-tailed p-value of less than 0.05 was considered to be significant. Statistical analysis was performed using the Statistical Analysis System (SAS) package version 9.2 and the statistical tools package IBM SPSS Statistics v21.
RESULTS
Baseline characteristics of the patients
Seven hundred and twelve patients were included in the study. The majority of the patients were men with low operative risk. The median age of the study group was 65 years. Two-thirds of the hospitalised patients were scheduled to have CABG surgery. The most common co-morbidities in the study population were hypertension, myocardial infarction, rhythm abnormalities and diabetes. Almost all of the patients had second- or third-class cardiac congestion symptoms defined by the NYHA. These and other baseline characteristics are presented in Table 1.
Figure.
Phase angle population reference values of the 15th percentile for men and women
Nutritional state evaluation
Low PA was present in 163 (22.9%) patients. In this group the rates of low FFMI and unintended weight loss were higher. The incidence of low PA was higher within women and renal failure patients. Furthermore, higher rates of low PA were present in patients with cardiac congestion and enlarged ventricles and in those with valve pathology. Also, the median values of the inflammation and anaemia markers were noted to be higher in the low PA group (Table 1). These and other possible risk factors of malnutrition were entered into the regression analysis of the low PA risk factors.
Risk factors of malnutrition
The univariate and multivariate logistic regression analysis of low PA risk factors is presented in Table 2.
Table 2.
Univariate and multivariate logistic regression analysis of low PA risk factors
| Variable | Odds ratio | P value | Odds ratio | P value | ||||
|---|---|---|---|---|---|---|---|---|
| Estimate | Lower 95% CI | Upper 95% CI | Estimate | Lower 95% CI | Upper 95% CI | |||
| Univariate | Multivariate | |||||||
| Disease-associated factors | ||||||||
| Gender (male) | 0.665 | 0.464 | 0953 | 0.0263 | n. s. | |||
| BMI, kg/m2 | 0.936 | 0.901 | 0.973 | 0.0008 | 0.928 | 0.890 | 0.968 | <0.001 |
| CAD | 0.643 | 0.446 | 0.927 | 0.0179 | n. s. | |||
| Renal failure | 3.049 | 1.616 | 5.747 | 0.0006 | 4.091 | 1.995 | 8.389 | <.0001 |
| Days prior to OP | 1.037 | 1.003 | 1.073 | 0.0316 | n. s. | |||
| NYHA IV class | 3.681 | 1.898 | 7.136 | <0.001 | 3.073 | 1.416 | 6.668 | 0.005 |
| LVEF < 30% | 2.564 | 1.340 | 4.902 | 0.0044 | n. s. | |||
| Enlarged LV | 1.577 | 1.078 | 2.315 | 0.0192 | n. s. | |||
| AVI ≥ III° | 2.353 | 1.168 | 4.739 | 0.0166 | n. s. | |||
| MVI ≥ II° | 1.842 | 1.241 | 2.732 | 0.0024 | 1.825 | 1.182 | 2.819 | 0.007 |
| TVI ≥ II° | 1.887 | 1.149 | 3.096 | 0.0119 | n. s. | |||
| Laboratory values | ||||||||
| Haemoglobin, g/L | 0.964 | 0.951 | 0.976 | <.0001 | 0.967 | 0.951 | 0.983 | <.0001 |
| Platelet count 109/L | 1.004 | 1.001 | 1.007 | 0.0060 | 1.004 | 1.000 | 1.008 | 0.0338 |
| WBC 109/L | 1.092 | 1.013 | 1.178 | 0.0218 | n. s. | |||
| CRP, mg/L | 1.020 | 1.008 | 1.032 | 0.0007 | 1.015 | 1.002 | 1.028 | 0.0279 |
| Lifestyle and psychosocial factors | ||||||||
| NRS 2002 reduced dietary intake in the last week | 1.669 | 1.038 | 2.681 | 0.0343 | n. s. | |||
| NRS 2002 impaired nutritional status: | ||||||||
| Mild (score 1) | 1.001 | 0.001 | 1.667 | 0.9978 | n. s. | |||
| Moderate (score 2) | 3.077 | 1.603 | 5.882 | 0.0007 | n. s. | |||
| Severe (score 3) | 1.795 | 0.001 | 4.310 | 0.1883 | n. s. | |||
| NRS 2002 severity of disease: | ||||||||
| Mild (score 1) | 2.105 | 1.294 | 3.165 | 0.0020 | n. s. | |||
| Moderate (score 2) | 1.520 | 1.010 | 4.386 | 0.0469 | n. s. | |||
| Severe (score 3) | 10.753 | 0.000 | 14.925 | 0.7187 | n.s | |||
| MUST acute disease effect | 2.169 | 1.107 | 100.000 | 0.0406 | n. s. | |||
| MNA QA (intake of food): | ||||||||
| Moderate decrease | 3.690 | 1.280 | 3.676 | 0.0040 | 1.848 | 1.068 | 3.205 | 0.0280 |
| Severe decrease | 2.169 | 1.692 | 8.065 | 0.0010 | 3.030 | 1.353 | 6.757 | 0.0070 |
| MNA QC (mobility): | ||||||||
| Able to get out of bed/chair | 3.717 | 1.393 | 3.378 | 0.0006 | 1.802 | 1.131 | 2.874 | 0.0133 |
| Bed or chair bound | 1.821 | 1.486 | 9.259 | 0.0050 | 2.770 | 1.067 | 7.194 | 0.0364 |
| MNA QD (psychological stress) | 1.996 | 1.217 | 2.732 | 0.0036 | n. s. | |||
| MNA QE (neuropsychological problems): | ||||||||
| Mild dementia | 1.272 | 1.149 | 3.460 | 0.0141 | n. s. | |||
| Severe dementia or depression | 1.001 | 0.338 | 4.785 | 0.7219 | n. s. | |||
n. s. is not significant with P value below 0.05.
n. s., not significant with P value below 0.05; CI, confidence interval; BMI, body mass index; CAD, coronary artery disease; OP, operation; LVEF, left ventricle ejection fraction; LV, left ventricle; AVI, aortic valve insufficiency; MVI, mitral valve insufficiency; TVI, tricuspid valve insufficiency; WBC, white blood count; CRP, C-reactive protein; NRS 2002, Nutritional Risk Screening 2002; MUST, Malnutrition Universal Screening Tool; MNA, Mini Nutritional Assessment; Q, question.
Analysis of various co-morbidities and disease- related factors of malnutrition revealed the importance of heart functions and valve pathology in the development of cardiac cachexia. NYHA IV class heart insufficiency, mitral valve insufficiency and renal failure were revealed as the most potent predictors of low PA, increasing the risk of malnutrition from two to four times. Moreover, the preoperative body mass index was also indicated as a predictor of malnutrition.
The laboratory values related to low PA were blood serum levels of haemoglobin (Hgb), C-reactive protein (CRP) and platelet count. Decreased Hgb was strongly related to low PA, increasing the risk of malnutrition twofold for every 30 g/L decrease of its value. Whilst the reported effect of platelets on malnutrition was minor, the effect of CRP was unquestionable, increasing the risk of low PA by 50% for every 33 mg/L increase of its value.
Univariate analysis of lifestyle and psychosocial factors represented as questions from the malnutrition screening questionnaires revealed all three of these tools as potent predictors of low PA. However, the multivariate analysis results favoured the answers from the MNA questionnaire. The questions qualified as risk factors of malnutrition concerned the intake of food and the mobility of the patient. The answers to these questions resulted in an overall increase of malnutrition risk from two to three times, depending on the severity of the patients’ answers.
DISCUSSION
This study investigated the possible risk factors of preoperative MN in a group of cardiac surgery patients. Due to a vast variety of variables, they were divided into three groups: the co-morbidity and disease-related group, laboratory values and psychosocial and lifestyle factors as described in the current research on malnutrition development (32). All three groups were comprised of variables, which were found to be statistically significant in the univariate regression analysis of low PA risk factors. Further analysis of the compounds of each group revealed the main risk factors of MN for cardiac surgery and, most importantly, provided the basis for early MN recognition and prevention for these patients. The results are concordant with the main mechanisms responsible for the development of MN and are discussed in literature: metabolic dysfunction and malabsorption, insufficient diet and the loss of nutrients via the digestive or urinary tracts (33).
Amongst the co-morbidities and other disease- related factors, the most potent predictors of MN were renal and heart failure (34, 35). Both of these conditions can be either a cause or consequence of MN. Malnourished patients often have a decreased cardiac muscle mass, which results in a decrease of cardiac output and reduced renal perfusion. Furthermore, energy, micronutrient and electrolyte deficiencies cause changes in cytokines, glucocorticoids, insulin and insulin-like growth factors, which also result in decreased cardiac function. Congestive heart failure further reduces dietary intake and appetite sensation, creating the pathophysiological circulus vitiosus (36).
There is an increasing evidence that neurohormonal and immune abnormalities play a crucial role in cardiac cachexia (33). Studies have shown that this may be linked to raised plasma levels of inflammatory cytokines, such as tumour necrosis factor alpha, a possible result of cell hypoxia in heart failure (37). Laboratory values related to MN were blood serum levels of haemoglobin, CRP and platelet count. These relationships reflect the multifactorial genesis of malnutrition (38). The shortage of nutrients is reflected by the suppression of erythropoesis and thrombopoesis (39). The increase of CRP levels is related to the chronic inflammation state of the undernourished tissues. All of these findings are concordant with the research on the origins of MN published in the ASPEN recommendations (38). Therefore, it is important to account for these factors whilst preparing the patient for cardiac surgery and adjust the nutrition care, which has been shown to increase the PA (40).
Psychosocial and lifestyle alterations add up to the complex nature of MN. In our study, psychological stress and neuropsychological problems, such as dementia or depression, are reported as risk factors of low PA (41). These findings are in line with conventional risk factors of MN (42). However, these variables are not independent from the mobility and food intake of the patient (43). This emphasizes the strength of the relationship between these conditions and implies a need for proper psychological screening of patients before surgery.
Possible limitations of our study include general limitations of BIA and the specific nature of cardiac surgery patients. The regression equations used in BIA devices are based on measurements of healthy populations but are not adapted for unhealthy cardiac surgery populations. Moreover, the specificity of the studied population also contributes to the limited applicability of the results. These results may only be applied to cardiac surgery patients and must be further evaluated in alternative setting.
CONCLUSIONS
It is clear that MN leads to adverse outcomes in cardiac surgery patients, therefore it is crucial to identify the modifiable risk factors at an early stage of preoperative management. MN risk factors comprise three different clinical groups: psychosocial and lifestyle factors, laboratory findings and disease-associated factors. The patients who are most likely to be malnourished are those with severe heart failure, valve pathology, insufficient renal function and high inflammatory markers. Also these patients have decreased mobility and food intake before surgery.
ACKNOWLEDGEMENTS
The authors are indebted to all patients who participated in the study.
Donata Ringaitienė, Dalia Gineitytė, Vaidas Vicka, Tadas Žvirblis, Jūratė Šipylaitė, Algimantas Irnius, Juozas Ivaškevičius
References
- van Straten AH, Bramer S, Soliman Hamad MA, van Zundert AA, Martens EJ, Schönberger JP, de Wolf AM. Effect of body mass index on early and late mortality after coronary artery bypass grafting. Ann Thorac Surg [Internet]. 2010; 89(1): 30–7. Available from: http://www.ncbi.nlm.nih.gov/pubmed/20103201 [DOI] [PubMed] [Google Scholar]
- van Venrooij LM, de Vos R, Borgmeijer-Hoelen MM, Haaring C, de Mol BA. Preoperative unintended weight loss and low body mass index in relation to complications and length of stay after cardiac surgery. Am J Clin Nutr [Internet]. 2008; 87(6): 1656–61. Available from: http://www.ncbi.nlm.nih.gov/pubmed/18541553 [DOI] [PubMed] [Google Scholar]
- van Venrooij LM, de Vos R, Zijlstra E, Borgmeijer-Hoelen MM, van Leeuwen PA, de Mol BA. The impact of low preoperative fat-free body mass on infections and length of stay after cardiac surgery: a prospective cohort study. J Thorac Cardiovasc Surg [Internet]. 2011; 142(5): 1263–9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21855896 [DOI] [PubMed] [Google Scholar]
- van Venrooij LM, Verberne HJ, de Vos R, Borgmeijer-Hoelen MM, van Leeuwen PA, de Mol BA. Postoperative loss of skeletal muscle mass, complications and quality of life in patients undergoing cardiac surgery. Nutrition [Internet]. 2012. January [cited 2016 Feb 28]; 28(1): 40–5. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21621393 [DOI] [PubMed] [Google Scholar]
- Yu P-J, Cassiere HA, Dellis SL, Manetta F, Kohn N, Hartman AR. Impact of preoperative prealbumin on outcomes after cardiac surgery. JPEN J Parenter Enteral Nutr [Internet]. 2015. September [cited 2016 Feb 28]; 39(7): 870–4. Available from: http://www. ncbi.nlm.nih.gov/pubmed/24898210 [DOI] [PubMed] [Google Scholar]
- Lomivorotov VV, Efremov SM, Boboshko VA, Nikolaev DA, Vedernikov PE, Deryagin MN, et al. Prognostic value of nutritional screening tools for patients scheduled for cardiac surgery. Interact Cardiovasc Thorac Surg. 2013; 16(5): 612–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trufa DI, Arhire LI, Nita O, Gherasim A, Nita G, Graur M. The evaluation of preoperative nutritional status in patients undergoing thoracic surgery. Rev Med Chir Soc Med Nat Iasi [Internet]. 2014. Apr-Jun [cited 2016 Feb 28]; 118(2): 514–9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25076724 [PubMed] [Google Scholar]
- Visser M, van Venrooij LM, Wanders DC, de Vos R, Wisselink W, van Leeuwen PA, de Mol BA. The bioelectrical impedance phase angle as an indicator of undernutrition and adverse clinical outcome in cardiac surgical patients. Clin Nutr. 2012. December; 31(6): 981–6. [DOI] [PubMed] [Google Scholar]
- Thourani VH, Keeling WB, Kilgo PD, Puskas JD, Lattouf OM, Chen EP, et al. The impact of body mass index on morbidity and short- and long-term mortality in cardiac valvular surgery. J Thorac Cardiovasc Surg [Internet]. 2011. November [cited 2016 Feb 28]; 142(5): 1052–61. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21450310 [DOI] [PubMed] [Google Scholar]
- Rady MY, Ryan T, Starr NJ. Clinical characteristics of preoperative hypoalbuminemia predict outcome of cardiovascular surgery. JPEN J Parenter Enteral Nutr [Internet]. 1997. Mar-Apr [cited 2016 Feb 28]; 21(2): 81–90. Available from: http://www.ncbi.nlm.nih.gov/pubmed/9084010 [DOI] [PubMed] [Google Scholar]
- Lomivorotov VV, Efremov SM, Boboshko VA, Nikolaev DA, Vedernikov PE, Lomivorotov VN, et al. Evaluation of nutritional screening tools for patients scheduled for cardiac surgery. Nutrition [Internet]. 2013. February [cited 2016 Feb 28]; 29(2): 436–42. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23200301 [DOI] [PubMed] [Google Scholar]
- Engelman DT, Adams DH, Byrne JG, Aranki SF, Collins JJ, Couper GS, et al. Impact of body mass index and albumin on morbidity and mortality after cardiac surgery. J Thorac Cardiovasc Surg [Internet]. 1999. November [cited 2016 Feb 28]; 118(5): 866–73. Available from: http://www.ncbi.nlm.nih.gov/pubmed/10534692 [DOI] [PubMed] [Google Scholar]
- Nakagomi A, Kohashi K, Morisawa T, Kosugi M, Endoh I, Kusama Y, et al. Nutritional status is associated with inflammation and predicts a poor outcome in patients with chronic heart failure. J Atheroscler Thromb [Internet]. 2016. January 18 [cited 2016 Feb 27] Available from: https://www.jstage.jst.go.jp/article/jat/advpub/0/advpub_31526/_article [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyle UG, Soundar EP, Genton L, Pichard C. Can phase angle determined by bioelectrical impedance analysis assess nutritional risk? A comparison between healthy and hospitalized subjects. Clin Nutr [Internet]. 2012; 31(6): 875–81. Available from: http://dx.doi.org/10.1016/j.clnu.2012.04.002 [DOI] [PubMed] [Google Scholar]
- Lim SL, Ong KCB, Chan YH, Loke WC, Ferguson M, Daniels L. Malnutrition and its impact on cost of hospitalization, length of stay, readmission and 3-year mortality. Clin Nutr [Internet]. 2012; 31(3): 345–50. Available from: http://linkinghub.elsevier.com/retrieve/pii/S0261561411001993 [DOI] [PubMed] [Google Scholar]
- Voss AC, Tootell M, Gussler JD. Malnutrition: A hidden cost in health care. Columbus: Abbott Laboratories; 2006. [Google Scholar]
- Kyle U. Bioelectrical impedance analysis? Part I: review of principles and methods. Clin Nutr [Internet]. 2004; 23(5): 1226–43. Available from: http://linkinghub.elsevier.com/retrieve/pii/S0261561404000937 [DOI] [PubMed] [Google Scholar]
- Paiva SI, Borges LR, Halpern-Silveira D, Assunção MCF, Barros AJD, Gonzalez MC. Standardized phase angle from bioelectrical impedance analysis as prognostic factor for survival in patients with cancer. Support Care Cancer [Internet]. 2010. February [cited 2016 Feb 28]; 19(2): 187–92. Available from: http://www.ncbi.nlm.nih.gov/pubmed/20039074 [DOI] [PubMed] [Google Scholar]
- Urbain P, Birlinger J, Ihorst G, Biesalski H-K, Finke J, Bertz H. Body mass index and bioelectrical impedance phase angle as potentially modifiable nutritional markers are independent risk factors for outcome in allogeneic hematopoietic cell transplantation. Ann Hematol [Internet]. 2013. January [cited 2016 Feb 28]; 92(1): 111–9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22968661 [DOI] [PubMed] [Google Scholar]
- Beberashvili I, Azar A, Sinuani I, Shapiro G, Feldman L, Stav K, et al. Bioimpedance phase angle predicts muscle function, quality of life and clinical outcome in maintenance hemodialysis patients. Eur J Clin Nutr [Internet]. 2014. June [cited 2016 Feb 6]; 68(6): 683–9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24736681 [DOI] [PubMed] [Google Scholar]
- Slee A, Birch D, Stokoe D. A comparison of the malnutrition screening tools, MUST, MNA and bioelectrical impedance assessment in frail older hospital patients. Clin Nutr [Internet]. 2015. April [cited 2016 Jan 28]; 34(2): 296–301. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24835154 [DOI] [PubMed] [Google Scholar]
- Girish M, Bhattad S, Ughade S, Mujawar N, Gaikwad K. Physical activity as a clinical tool in the assessment of malnutrition. Indian Pediatr [Internet]. 2014. June [cited 2016 Feb 28]; 51(6): 478–80. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24986285 [DOI] [PubMed] [Google Scholar]
- Barker L, Gout B, Crowe T. Hospital malnutrition: prevalence, identification and impact on patients and the healthcare system. Int J Environ Res Public Health [Internet]. 2011; 8(12): 514–27. Available from: http://www.mdpi.com/1660–4601/8/2/514/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beveridge C, Knutson K, Spampinato L, Flores A, Meltzer DO, Van Cauter E, et al. Daytime physical activity and sleep in hospitalized older adults: association with demographic characteristics and disease severity. J Am Geriatr Soc [Internet]. 2015. July [cited 2016 Feb 28]; 63(7): 1391–400. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=4752005&tool=pmcentrez&rendertype=abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polikandrioti M, Goudevenos J, Michalis LK, Koutelekos J, Kyristi H, Tzialas D, et al. Factors associated with depression and anxiety of hospitalized patients with heart failure. Hellenic J Cardiol. [Internet]. 2015. Jan-Feb [cited 2016 Feb 28]; 56(1): 26–35. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25701969 [PubMed] [Google Scholar]
- Cederholm T, Bosaeus I, Barazzoni R, Bauer J, Van Gossum A, Klek S, et al. Diagnostic criteria for malnutrition: An ESPEN Consensus Statement. Clin Nutr. 2015; 34(3): 335–40. doi: 10.1016/j.clnu.2015.03.001. Epub 2015 Mar 9. [DOI] [PubMed] [Google Scholar]
- Kylea UG, Bosaeusb I, De Lorenzoc AD, Deurenbergd P, Eliae M, Go’mezf JM, et al. ESPEN guidelines: bioelectrical impedance analysis – part II: utilization in clinical practice. Clin Nutr. 2004; 23: 1430–53. [DOI] [PubMed] [Google Scholar]
- Wieskotten S, Heinke S, Wabel P, Moissl U, Becker J, Pirlich M, et al. Bioimpedance-based identification of malnutrition using fuzzy logic. Physiol Meas [Internet]. 2008; 29(5): 639–54. Available from: http://www.ncbi.nlm.nih.gov/pubmed/18460765 [DOI] [PubMed] [Google Scholar]
- Bosy-Westphal A Danielzik S Dörhöfer R-P, Later W Wiese S Müller MJ. Phase angle from bioelectrical impedance analysis: population reference values by age, sex, and body mass index. JPEN J Parenter Enteral Nutr. 2006; 30(4): 309–16. [DOI] [PubMed] [Google Scholar]
- Ringaitiene D, Gineityte D, Vicka V, Zvirblis T, Norkiene I, Sipylaite J, et al. Malnutrition assessed by phase angle determines outcomes in low-risk cardiac surgery patients. Clin Nutr. 2016. February. doi: 10.1016/j.clnu.2016.02.010. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Saunders J, Smith T, Stroud M. Malnutrition and undernutrition. Medicine. 2011. January; 39(1): 45–50. [Google Scholar]
- Saunders J, Smith T. Malnutrition: causes and consequences. Clin Med. 2010. December; 10(6): 624–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Haehling S, Lainscak M, Springer J, Anker SD. Cardiac cachexia: a systematic overview. Pharmacol Ther [Internet]. 2009; 121(3): 227–52. Available from: http://dx.doi.org/10.1016/j.pharmthera.2008.09.009 [DOI] [PubMed] [Google Scholar]
- Tevik K, Thürmer H, Husby MI, de Soysa AK, Helvik A-S. Nutritional risk screening in hospitalized patients with heart failure. Clin Nutr. 2015. April; 34(2): 257–64. [DOI] [PubMed] [Google Scholar]
- Dumler F, Kilates C. Prospective nutritional surveillance using bioelectrical impedance in chronic kidney disease patients. J Ren Nutr. 2005. January; 15(1): 148–51. [DOI] [PubMed] [Google Scholar]
- Cicoira M, Anker SD, Ronco C. Cardio-renal cachexia syndromes (CRCS): pathophysiological foundations of a vicious pathological circle. J Cachexia Sarcopenia Muscle. 2011. September; 2(3): 135–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasper D, Hummel M, Kleber FX, Reindl I, Volk HD. Systemic inflammation in patients with heart failure. Eur Heart J [Internet]. 1998. May [cited 2016 Mar 1]; 19(5): 761–5. Available from: http://www.ncbi.nlm.nih.gov/pubmed/9717010 [DOI] [PubMed] [Google Scholar]
- Jensen GL, Mirtallo J, Compher C, Dhaliwal R, Forbes A, Grijalba RF, et al. Adult starvation and disease-related malnutrition: a proposal for etiology- based diagnosis in the clinical practice setting from the International Consensus Guideline Committee. Clin Nutr. 2010. April; 29(2): 151–3. [DOI] [PubMed] [Google Scholar]
- Thakur N, Chandra J, Pemde H, Singh V. Anemia in severe acute malnutrition. Nutrition. 2014. April; 30(4): 440–2. [DOI] [PubMed] [Google Scholar]
- Richter E, Denecke A, Klapdor S, Klapdor R. Parenteral nutrition support for patients with pancreatic cancer: improvement of the nutritional status and the therapeutic outcome. Anticancer Res. 2012. May; 32(5): 2111–8. [PubMed] [Google Scholar]
- Ringaitienė D, Gineitytė D, Vicka V, Žvirblis T, Šipylaitė J, Irnius A, et al. Impact of malnutrition on postoperative delirium development after on pump coronary artery bypass grafting. J Cardiothorac Surg. 2015. January; 10: 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkert D, Chourdakis M, Faxen-Irving G, Frühwald T, Landi F, Suominen MH, et al. ESPEN guidelines on nutrition in dementia. Clin Nutr. 2015. September; 34(6): 1052–73. [DOI] [PubMed] [Google Scholar]
- Meijers JMM, Schols JMGA, Halfens RJG. Malnutrition in care home residents with dementia. J Nutr Health Aging. 2014. January; 18(6): 595–600. [DOI] [PubMed] [Google Scholar]
- Weimann A, Braga M, Harsanyi L, Laviano A, Ljungqvist O, Soeters P, et al. ESPEN guidelines on enteral nutrition: surgery including organ transplantation. Clin Nutr. 2006. April; 25(2): 224–44. [DOI] [PubMed] [Google Scholar]