Abstract
Background.
Yolk sac tumour diagnosis should be considered for young age patients admitted to the hospital with non-specific complaints of widespread disease. Correct diagnosis and carefully planned treatment is the key to a successful outcome.
Methods and materials.
We present a rare case of a widespread yolk sack tumour of a uterine broad ligament. Our team directed a special attention towards the patient’s young age, advanced disease, and fertility sparing strategy of treatment.
Results and conclusions.
Stage IV yolk sac tumours of extragonadal origin are rarely reported in the literature. Hence, diagnosis and treatment often pose a challenge for emergency care unit doctors, gynaecologists, and oncologists. However, it can be a potentially curable disease. Moreover, patients’ fertility can also be preserved. We believe that further analysis of similar cases is necessary to study outcomes and evaluate patients’ responses to a sequence of medical decisions taken for this specific case.
Keywords: yolk sac tumour, metastases, fertility preservation, alpha-fetoprotein, BEP chemotherapy
Abstract
SĖKMINGAS VĖLYVOS STADIJOS EKSTRAGONADINĖS KILMĖS TRYNIO MAIŠO NAVIKO GYDYMAS: ATVEJO PRISTATYMAS IR LITERATŪROS APŽVALGA
Santrauka
Tikslas. Hospitalizuojant jauno amžiaus pacientes su nespecifiniais išplitusios onkologinės ligos simptomais turėtų būti atsižvelgta į galimą trynio maišo naviko diagnozę. Laiku nustatyta teisinga diagnozė ir atidžiai suplanuota gydymo taktika – esminiai veiksniai siekiant sėkmingos ligos baigties.
Medžiaga ir metodai. Straipsnyje aprašomas retas išplitusio trynio maišo naviko, esančio plačiajame gimdos raištyje, atvejis. Ypatingą dėmesį gydytojai skyrė jauno amžiaus pacientei, išplitusiai ligai ir vaisingumą išsaugančiai gydymo strategijai.
Rezultatai ir išvados. IV stadijos ekstragonadinės kilmės trynio maišo navikai retai aprašomi literatūroje, todėl jų diagnostika ir gydymas dažnai tampa iššūkiu priėmimo skyriaus gydytojams, ginekologams bei onkologams. Tačiau ši liga gali būti ne tik sėkmingai išgydoma, bet ir lieka galimybė išsaugoti pacienčių vaisingumą. Reikalinga tolimesnė panašių atvejų analizė siekiant įvertinti pasekmes ir pacienčių gydymo atsaką į tokių ligų pateikiamą gydymo metodiką.
Raktažodžiai: trynio maišo navikas, metastazės, vaisingumo išsaugojimas, alfafetoproteinas, BEP chemoterapija
INTRODUCTION
Yolk sac tumours (YST), or perhaps more correctly they should be named as endodermal primitive tumours, are rare and malignant germ cell neoplasms that usually occur in gonads (1, 2). YST usually presents in young patients. The median age at the time of diagnosis is 18 years (3). YST arising in the pelvis outside of the ovary are uncommon. We present a case of widely spread YST that originated from a broad uterine in a young female, that was diagnosed and successfully treated at the Vilnius University Hospital Santariskiu Clinics (Santariškių Klinikos).
CASE PRESENTATION
A 22 year-old woman was admitted to the emergency room with severe lower abdominal pain, nausea, diarrhea, and fever.
On physical examination, a bulky and painful, fixed to the left pelvic sidewall, tumour was palpated in the left lower quadrant. Bimanual examination revealed the presence of a bulky mass, consisting of the uterus and adnexa.
On ultrasound examination, a non-homogenous tumour of the pelvis, up to 9 × 6 cm in size, was seen.
Apart from the pelvic lesion, multiple liver foci were suspected as metastasis. About 1,000 ml of ascites was present.
Contrast enhanced CT scan revealed a non-homogenous pelvic mass, approximately 113 × 105 × 154 mm in size, displacing the uterus and bladder, deforming and possibly infiltrating the front wall of the rectum (Fig. 1).
Fig 1.
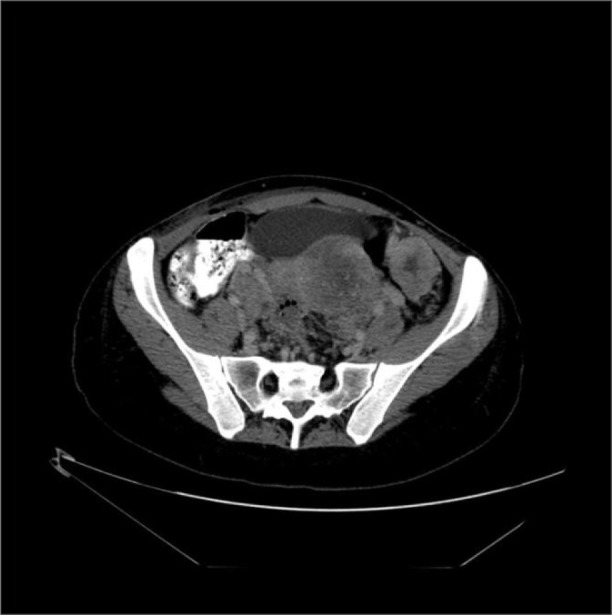
Contrast enhanced CT scan revealed non-homogenous pelvic mass, approximately 113 × 105 × 154 mm in size
Multiple liver metastases were observed (Fig. 2).
Fig 2.
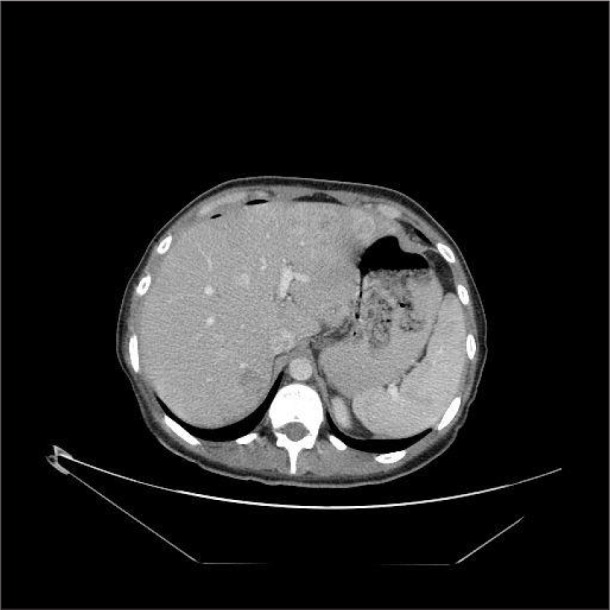
scan revealed multiple liver metastases
Blood test showed a high level of alpha-fetoprotein (AFP) 13,675 kU/l, serum hCG level was 2.75 U/l, LDH 356 U/l, CRP 69.9 mg/l, WBC 10.78*10e9/l (without shift to the left).
Diagnostic laparoscopy was performed in order to verify the tumour’s histology. The intraoperative findings revealed that the uterus was normal in appearance, displaced by the left broad ligament tumour to the right. Ovaries and fallopian tubes were normal. The rectum was fixed in the rectouterine excavation. Multiple peritoneal and bulky intrahepatic liver metastases were observed. Biopsies from a bulky tumour, peritoneal lesions, and liver metastasis were conducted.
Microscopic examination revealed tubulo-papillary structures, covered by cuboid epithelium with slightly irregular nuclei with nucleoli, positive for placental alkaline phosphatase and alpha-fetoprotein (Fig. 3), hence the diagnosis of yolk sac tumour was made.
Fig 3.
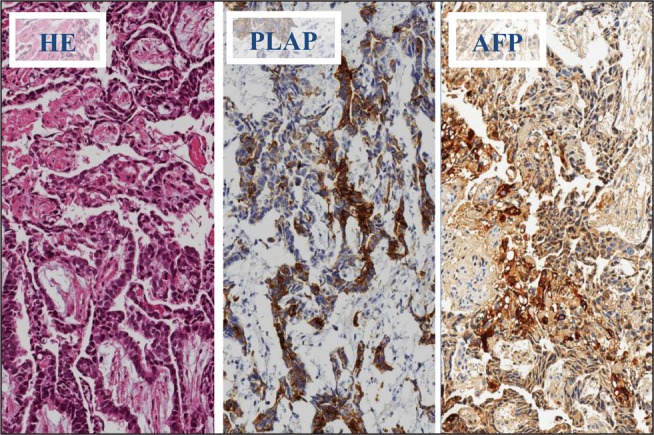
Primary biopsy: HE (left) and immunohistochemical stains, PLAP (placental alkaline phosphatase) (in between) and AFP (alpha-fetoprotein); magnification 200x: tubulopapillary structures with focal positivity to PLAP and AFP
The multidisciplinary team decision was to start chemotherapy followed by debulking surgery. A standard BEP chemotherapeutic regimen has been chosen: bleomycin (30 mg/day) given on days 2, 9 and 16; etoposide (100 mg/m2/day) given on days 1–5; and cisplatin (20 mg/m2/day) given on days 1–5 for 4 cycles. The cycles were repeated every 3 weeks. After the fourth cycle, the serum AFP level reduced to 6.05 kU/l.
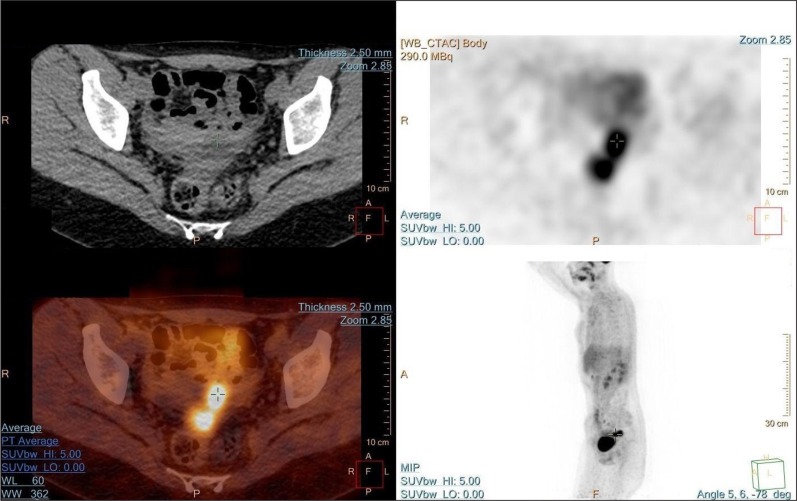
On the fourth month following the treatment, a PET CT scan was performed. Two metabolically active lesions in the pouch of Douglas and omentum were detected (Fig. 4).
A laparoscopic removal of the above-mentioned lesions was performed. After the surgery, the serum AFP level decreased to 0.575 kU/l. According to the histologic analysis, there was the same YST with 90% of tumour necrosis. The surgical margins were free from a tumour but not from the signs of necrosis.
Four months after the laparoscopy, the AFP level raised up to 12.7 kU/l. PET CT was performed and a metabolically active lesion in the area of the pouch of Douglas was observed.
The multidisciplinary team decision was to perform a laparotomy and completely resect the residual tumour. If extensive tumour deposits were found, a high dose chemotherapy with bone marrow transplantation would be considered as postoperative treatment.
During the surgery, the residual tumour was seen at the area of the rectovaginal septum and in the area of the left mesovarium. Excision of the rectovaginal area with a part of the posterior uterine wall and anterior resection of the rectum with left salpingectomy were performed, and end-to-end rectum anastomosis was conducted. The postoperative period was uneventful.
The post-surgical specimen was free from a tumour macroscopically and microscopically at the margins; hence, no chemotherapy was administered. Two years after the treatment, a PET-CT scan showed no signs of the primary tumour or metastases. Serum AFP levels were normal.
The menstrual cycle came back 4 months following the last cycle of chemotherapy.
DISCUSSION
Primary germ cell tumours of extragonadal origin are rare and the exact incidence of this type of cancer is unknown. They have been estimated to represent 3–5% of all adult germ cell malignancies (4). The most common extragonadal site is the vagina, where extraovarian yolk sac tumours arise in young children (5). Rare cases have been reported in the vulva (6), the cervix and the endometrium (7). Yolk sac tumours arising in the pelvis outside of the ovary are distinctly uncommon (1, 8).
The pathogenesis of YST is not known. The classical theory suggests that it arises from local transformation of misplaced primordial germ cells. From the fourth to the sixth week of embryogenesis, germ cells migrate through the midline dorsal mesentery; a remnant of tissue anywhere along the migration course can be a site of GCT in the future (1). The histopathological features of YST are distinctive, with proliferation of tubular and papillary structures and sinusoidal formation from fibrovascular cores lined by tumour cells (Schiller–Duval bodies) with frequent mitotic figures (9).
Fig 4.
PET CT scan showed metabolically active lesions in the rectouterine pouch and large omentum
The majority of adults with YST present with advanced local disease and distant metastases; therefore, the complete local excision is rarely feasible. This tumour most commonly spreads to the regional lymph nodes, lung, liver, and bone. Extragonadal germ cell tumours may reach a large size with no or relatively few symptoms (10). The most common presenting symptoms for patients with endodermal primitive tumours are rapidly enlarging pelvic mass and pain (11). In the presented case the patient’s major concern was fever lasting for two months. According to the literature, fever is present in 10–25% of YST cases (12). During further investigation, our patient was also diagnosed with high value of alpha-fetoprotein (AFP 13,675 ng/mL) that significantly decreased during the treatment the patient received: after 4 cycles of BEP chemotherapy the AFP value was 6.05 ng/mL and 0.575 ng/mL after the laparoscopic tumour debulking surgery, respectively. The decreasing ratio of post-operative serum AFP is an effective indicator to determine whether residual disease remains after surgery (13). Response to chemotherapy can also be evaluated by normalization of AFP [14]. According to the literature, in order to detect a relapse, the AFP is more sensitive than the CT scan (15). Yet despite its significance, the prognostic value of high levels of AFP in yolk sac tumours at the time of diagnosis remains controversial. In two studies AFP > 1,000 kU/L was associated with a higher risk of relapse after treatment (14, 16). Two other studies found that pre-operative serum AFP levels before initial surgery had no significant correlation with prognosis (17).
In order to confirm diagnosis morphologically, laparoscopic surgery has been made in our case. In the past after the laparoscopic diagnosis of YST, conversion to laparotomy was recommended in order to achieve an optimal staging and to avoid an uncertain tumour cell spread. Today, advancement in technologies enabled us to achieve the same or even better surgical prognosis and a potentially faster recovery with less traumatic minimally invasive laparoscopic surgery compared to a conventional laparotomy (18, 19).
Extragonadal germ cell tumours have been managed under the same principle as their primary gonadal counterparts, using the treatment comprised of systemic chemotherapy together with local treatment, including surgery and radiotherapy (20–24). The aim of surgery is removing the primary tumour without excessive morbidity. Neoadjuvant chemotherapy can be considered for patients with extensive intra-abdominal disease when initial debulking surgery is not an option (25). In our case neoadjuvant chemotherapy with 4 BEP cycles was chosen as an option to reduce the tumour size and also to minimize the extent of surgical procedure causing as minimal as possible impact on the patient’s fertility. Before the introduction of effective chemotherapy, the prognosis for patients with YST was poor, with a 3-year survival rate of 13% for malignant endodermal primitive tumours. The establishment of BEP chemotherapy for malignant germ cell tumours has significantly improved outcomes. BEP chemotherapy is regarded to be a gold-standard regimen for the first-line treatment of germ cell tumours at all stages of disease (26). As our experience showed, in stage IV of YST neoadjuvant chemotherapy with following surgery is a considerably better choice for the patient as our patient had her menstrual cycle restored; hence, it is likely that the fertility of our patient is also preserved.
The possibility of a residual tumour and its management is a subject of consideration in IV stage disease. Patients who have a residual tumour after treatment with chemotherapy ought to undergo secondary surgery so that all residual tumour lesions would be excised (27). In the presented case, surgical management of the residual tumour in the front rectum wall and the rectovaginal portion of the peritoneum after initial laparoscopic debulking was chosen as a treatment option and rectum resection was made. High-dose chemotherapy (HDCT) along with an autologous stem cell transplant (ASCT) is also a way to manage a residual germ cell tumour and must be taken into consideration if a residual tumour is present (28). Despite risks of chemotherapy concerning the reproductive function of female patients, studies have shown that most of the women can anticipate a normal menstrual and reproductive function after treatment (29–31).
After the treatment a follow-up is required involving an abdominal/pelvic exam, CT, AFP levels and CXR as an option (32). Two studies reported specifically on the prognostic factors of ovarian yolk sac tumours. In the study of Nawa et al., the 5-year survival rate was 95.2, 75, 30, and 25% for patients with stage I, II, III, and IV, respectively. The respective numbers in the study by Kawai et al. were 92, 44, and 29% for stage I, II, and III. According to these two studies, other than the stage of ovarian YST significant prognostic factors were the presence and quantity of ascites before initial surgery and the amount of residual disease after surgery (16, 32). In contrast to the study by Murugaesu et al. (33), there was no correlation between the pre-operative serum AFP levels and prognosis. In the study by Mitchell et al. (14) the amount of residual disease after initial surgery and the extent of initial surgery did not significantly affect the outcome.
CONCLUSIONS
Our case illustrates that even at stage IV of YST, positive treatment results can be achieved through neoadjuvant chemotherapy and laparoscopic surgery. In order to achieve the results reported in our case, patients with late stage YST must be admitted to tertiary care hospitals where highly specialized medical treatment facilities can be provided. Highly qualified specialists who employ multidisciplinary approaches from the very beginning can be of crucial importance in order to avoid more complex surgical procedures. Another important aspect to successful treatment is an early preoperative histological verification of malignant pathology. Our patient has been disease free for a 24-month period after the latest surgical intervention.
Vilius Rudaitis, Ugnius Mickys, Justina Katinaitė, Justyna Dulko
References
- Dede M, Pabuccu R, Yagci G, Yenen MC, Goktolga U, Gunhan O. Extragonadal yolk sac tumor in pelvic localization. A case report and literature review. Gynecol Oncol. 2004. March; 92(3): 989–91. [DOI] [PubMed] [Google Scholar]
- Umezu T, Kajiyama H, Terauchi M, Shibata K, Ino K, Nawa A, et al. Long-term outcome and prognostic factors for yolk sac tumor of the ovary. Nagoya J Med Sci. 2008. March; 70(1–2): 29–34. [PubMed] [Google Scholar]
- McBee WC, Brainard J, Sawady J, Rose PG. Yolk sac tumor of the ovary associated with endometrioid carcinoma with metastasis to the vagina: a case report. Gynecol Oncol. 2007. April; 105(1): 244–7. [DOI] [PubMed] [Google Scholar]
- Garnick MB, Canellos GP, Richie JP. Treatment and surgical staging of testicular and primary extragonadal germ cell cancer. JAMA. 1983. October 7; 250(13): 1733–41. [PubMed] [Google Scholar]
- Young RH, Scully RE. Endodermal sinus tumor of the vagina: a report of nine cases and review of the literature. Gynecol Oncol. 1984. July; 18(3): 380–92. [DOI] [PubMed] [Google Scholar]
- Traen K, Logghe H, Maertens J, Mattelaere C, Moerman P, Vergote I. Endodermal sinus tumor of the vulva: successfully treated with high-dose chemotherapy. Int J Gynecol Cancer. 2004. October; 14(5): 998–1003. [DOI] [PubMed] [Google Scholar]
- Sánchez Lihon J, Casavilca Zambrano S, Garcia Madrid J, Klinge Castro G. Tumor del seno endodermal extragonadal en la infancia. reporte de dos casos. Rev Medica Hered. 2003. April; 14(2): 99–103. [Google Scholar]
- Clement PB, Young RH, Scully RE. Extraovarian pelvic yolk sac tumors. Cancer. 1988. August 1; 62(3): 620–6. [DOI] [PubMed] [Google Scholar]
- Alkatan HM, Al-Kofide A, Al-Hussain H. Yolk sac tumor: histopathologic report of 2 cases. Can J Ophthalmol. 2008. February; 43(1): 125–6. [DOI] [PubMed] [Google Scholar]
- Bokemeyer C, Droz JP, Horwich A, Gerl A, Fossa SD, Beyer J, et al. Extragonadal seminoma: an international multicenter analysis of prognostic factors and long term treatment outcome. Cancer. 2001. April 1; 91(7): 1394–401. [PubMed] [Google Scholar]
- Bailey J, Church D. Management of germ cell tumours of the ovary. Rev Gynaecol Pract. 2005. December; 5(4): 201–6. [Google Scholar]
- Tewari K, Cappuccini F, Disaia PJ, Berman ML, Manetta A, Kohler MF. Malignant germ cell tumors of the ovary. Obstet Gynecol. 2000. January; 95(1): 128–33. [DOI] [PubMed] [Google Scholar]
- Sell A, Sogaard H, Norgaard-Pedersen B. Serum alpha-fetoprotein as a marker for the effect of post-operative radiation therapy and/or chemotherapy in eight cases of ovarian endodermal sinus tumour. Int J Cancer. 1976. November 15; 18(5): 574–80. [DOI] [PubMed] [Google Scholar]
- Mitchell PL, Al-Nasiri N, A’Hern R, Fisher C, Horwich A, Pinkerton CR, et al. Treatment of nondysgerminomatous ovarian germ cell tumors: an analysis of 69 cases. Cancer. 1999. May 15; 85(10): 2232–44. [DOI] [PubMed] [Google Scholar]
- Davidoff AM, Hebra A, Bunin N, Shochat SJ, Schnaufer L. Endodermal sinus tumor in children. J Pediatr Surg. 1996. August; 31(8): 1075–8; discussion 1078–9. [DOI] [PubMed] [Google Scholar]
- Nawa A, Obata N, Kikkawa F, Kawai M, Nagasaka T, Goto S, et al. Prognostic factors of patients with yolk sac tumors of the ovary. Am J Obstet Gynecol. 2001. May; 184(6): 1182–8. [DOI] [PubMed] [Google Scholar]
- Mayordomo JI, Paz-Ares L, Diaz-Puente MT, Lianes P, Garcia-Prats MD, Cortes-Funes H. Prognostic factors for women with ovarian germ cell tumors. J Clin Oncol. 1994. August; 12(8): 1737–8. [DOI] [PubMed] [Google Scholar]
- Tozzi R, Köhler C, Ferrara A, Schneider A. Laparoscopic treatment of early ovarian cancer: surgical and survival outcomes. Gynecol Oncol. 2004. April; 93(1): 199–203. [DOI] [PubMed] [Google Scholar]
- Khoo SK, Buntine DW, Massey PF, Jones ISC. Endodermal sinus tumour of the ovary: the place of alphafetoprotein detection, surgery and chemotherapy. Aust N Z J Obstet Gynaecol. 1981. November 1; 21(4): 217–25. [DOI] [PubMed] [Google Scholar]
- De Corti F, Sarnacki S, Patte C, Mosseri V, Baranzelli MC, Martelli H, et al. Prognosis of malignant sacrococcygeal germ cell tumours according to their natural history and surgical management. Surg Oncol. 2012. June; 21(2): e31–7. [DOI] [PubMed] [Google Scholar]
- Pliarchopoulou K, Pectasides D. First-line chemotherapy of non-seminomatous germ cell tumors (NSGCTs). Cancer Treat Rev. 2009. November; 35(7): 563–9. [DOI] [PubMed] [Google Scholar]
- de La Motte Rouge T, Pautier P, Duvillard P, Rey A, Morice P, Haie-Meder C, et al. Survival and reproductive function of 52 women treated with surgery and bleomycin, etoposide, cisplatin (BEP) chemotherapy for ovarian yolk sac tumor. Ann Oncol. 2008. August; 19(8): 1435–41. [DOI] [PubMed] [Google Scholar]
- Bokemeyer C, Hartmann JT, Fossa SD, Droz J-P, Schmol H-J, Horwich A, et al. Extragonadal germ cell tumors: relation to testicular neoplasia and management options. APMIS. 2003. January; 111(1): 49–59; discussion 59–63. [DOI] [PubMed] [Google Scholar]
- Kim SW, Park JH, Lim MC, Park JY, Yoo CW, Park SY. Primary yolk sac tumor of the omentum: a case report and review of the literature. Arch Gynecol Obstet. 2009. February; 279(2): 189–92. [DOI] [PubMed] [Google Scholar]
- National Guideline Clearinghouse | Ovarian germ cell tumours [Internet]. [cited 2016 May 3]. Available from: https://www.guideline.gov/content.aspx?id=47845 [Google Scholar]
- Williams S, Blessing JA, Liao SY, Ball H, Hanjani P. Adjuvant therapy of ovarian germ cell tumors with cisplatin, etoposide, and bleomycin: a trial of the Gynecologic Oncology Group. J Clin Oncol. 1994. April; 12(4): 701–6. [DOI] [PubMed] [Google Scholar]
- WHO | Medicines for treatment of the following cancers – review – EML and EMLc [Internet]. [cited 2016 May 3]. Available from: http://www.who.int/selection_medicines/committees/expert/20/ applications/cancer/en/ [Google Scholar]
- Feldman DR, Powles T. Salvage high-dose chemotherapy for germ cell tumors. Urol Oncol. 2015. August 1; 33(8): 355–62. [DOI] [PubMed] [Google Scholar]
- Zanetta G, Bonazzi C, Cantù M, Binidagger S, Locatelli A, Bratina G, et al. Survival and reproductive function after treatment of malignant germ cell ovarian tumors. J Clin Oncol. 2001. February 15; 19(4): 1015–20. [DOI] [PubMed] [Google Scholar]
- Byrne J, Mulvihill JJ, Myers MH, Connelly RR, Naughton MD, Krauss MR, et al. Effects of treatment on fertility in long-term survivors of childhood or adolescent cancer. N Engl J Med. 1987. November 19; 317(21): 1315–21. [DOI] [PubMed] [Google Scholar]
- Nicosia SV, Matus-Ridley M, Meadows AT. Gonadal effects of cancer therapy in girls. Cancer. 1985. May 15; 55(10): 2364–72. [DOI] [PubMed] [Google Scholar]
- Kawai M, Kano T, Furuhashi Y, Mizuno K, Nakashima N, Hattori SE, et al. Prognostic factors in yolk sac tumors of the ovary. A clinicopathologic analysis of 29 cases. Cancer. 1991. January 1; 67(1): 184–92. [DOI] [PubMed] [Google Scholar]
- Murugaesu N, Schmid P, Dancey G, Agarwal R, Holden L, McNeish I, et al. Malignant ovarian germ cell tumors: identification of novel prognostic markers and long-term outcome after multimodality treatment. J Clin Oncol. 2006. October 20; 24(30): 4862–6. [DOI] [PubMed] [Google Scholar]