On 28 September 2015, the Nonhuman Primate (NHP) Induced Pluripotent Stem Cells (iPSCs) Workshop was held at the National Institutes of Health (NIH) campus in Bethesda, Maryland. The workshop was sponsored by the Office of Scientific Director of the National Heart, Lung, and Blood Institute (NHLBI).
Pluripotent stem cells (PSCs), including embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs), offer unique opportunities for cell-based therapies because of their capacity of unlimited self-renewal and the ability to generate all cell and tissue types. ESC-derived cells or tissues are now in clinical trials for therapy of several diseases, and the first clinical trial of iPSC-derived cells was recently initiated for age-related macular degeneration.1 However, several concerns have been raised, including tumorigenicity, immunogenicity, and cellular heterogeneity associated with this potential therapeutic approach.2,3,4 Efforts to investigate and optimize both the safety and efficacy of human PSC-based regenerative therapies have relied predominantly on in vitro cell-based and human–murine xenograft models. Although these experiments have provided important mechanistic and proof-of-principle information, their predictive value is limited, in particular for questions related to long-term safety, immunogenicity, and functional integration of PSC-derived cells and tissues.
NHPs are developmentally and physiologically closely related to humans, and their immune systems have been well studied.5 ESCs and iPSCs from NHPs, unlike their leukemia inhibitory factor–dependent murine counterparts, resemble human PSCs in terms of gene expression and supportive culture conditions.4,6 Furthermore, NHP models provide opportunities for not only human–NHP xenograft studies of organ integration but also NHP-NHP allogeneic or autologous transplantation of PSC-derived cell products, to carefully analyze inflammation, immune response, and intraspecies rather than interspecies tissue integration. Therefore, preclinical utilization of NHP models could provide invaluable information for moving PSC-derived therapies forward into clinical applications. The goal of this workshop was to discuss how and whether NHP models should be integrated into human PSC therapy development. In addition, the place for such studies in the regulatory framework for enabling investigational new drug applications.
Sharing their research and perspectives were 11 speakers from the NIH, the US Food and Drug Administration (FDA), academia, and industry. The first session on stem cell biology and pluripotency covered the latest scientific and technology developments regarding NHP PSC derivation, maintenance, and characterization. Don P. Wolf (representing the laboratory of Shoukhrat Mitalipov at the Oregon National Primate Research Center) outlined some of the challenges of NHP-based research, including lack of funding mechanisms to support critical NHP infrastructure development that is not usually provided by NIH R01 grants. The prolonged generation times of macaques also complicate studies involving gene targeting or modification. PSC investigators using NHPs must also develop or adapt derivation, propagation, differentiation, and characterization protocols for NHP PSCs, generally using human conditions as a starting point, but expecting some differences to exist between human and NHP protocols. In this regard, Mitalipov's group has published a study that involved molecular comparisons, in human, between iPSCs, nuclear transfer–ESCs, and in vitro fertilization–ESCs, asking whether all PSCs are equal.7 Residual epigenetic memory found in human iPSCs, inherited from somatic cells, indicated that some iPSC lines will probably be superior to others and suggested that the same approach could be used to identify the best NHP PSC lines.
Yoav Gilad (University of Chicago) extended the discussion regarding standardization of methods for establishing, culturing, and differentiating NHP iPSCs. He pointed out that the heterogeneity of iPSC methodology represents a major challenge to the use of these cells in biomedical research, in terms of reprogramming, cell origin, culture conditions, and differentiation protocols. To study functional comparative genomics in primates, his group has generated several fully characterized chimpanzee iPSC lines as well as human lines using a consistent protocol and cell type of origin. Using this panel, he observed much less within-species variation in iPSCs derived from different individuals than in somatic cells, indicating that the reprogramming process erases many interindividual differences. Also, Gilad's group has identified novel interspecies regulatory differences between human and NHP iPSCs.8
Cynthia Dunbar (NHLBI) shared her experience in developing autologous rhesus macaque models for evaluating the safety and regenerative capacity of iPSC-derived cells.9 She showed that undifferentiated autologous iPSCs indeed formed teratomas in a dose-dependent manner in immune-competent rhesus macaques. Interestingly, tumor formation was accompanied by an inflammatory reaction, even in the autologous setting. By contrast, iPSC-derived mesodermal stromal-like cells formed new bone in vivo without any evidence of teratoma formation or inflammation. These results suggested that the risk of adverse events such as tumorigenicity is calculable, and the immunogenicity of iPSCs may differ depending on their differentiation status or in vitro culture system.
There are significant differences in reproductive physiology between rodents and primates,10 adding to the importance of using NHP preclinical models for reproductive and regenerative medicine. Erin Wolff (NHLBI/National Institute of Child Health and Human Development) explained the use of a rhesus macaque NHP model to demonstrate isolation11 and function of ovarian-derived stem cells (OSCs). She showed that OSCs could be cultured in vitro, genetically labeled, and transplanted into both healthy autologous and ovarian-injury rhesus macaque models to generate mature oocytes (unpublished). The transplantation model developed in the study could be useful to validate safety and functions of iPSC-derived germ cells as an alternative source for future human infertility therapy, and offers a means to test various forms of OSC germ-line editing for creation of disease models.
The second session of the workshop focused on more directly preclinical aspects of NHP PSC models. Humans and NHPs share similar hematopoietic stem cell dynamics, engraftment properties, and cytokine requirements, making NHPs a potentially very appropriate model for preclinical testing of iPSC-based therapies for blood diseases.12 However, all in vivo animal models developed to date, from mouse–mouse to human–mouse xenografts, have shown negligible engraftment of phenotypic hematopoietic stem/progenitor cells (HSPCs) differentiated from PSCs. It remains unclear whether poor engraftment is due to incomplete differentiation, embryonic or fetal HSPC characteristics that preclude marrow homing, or nonphysiological interactions with the marrow microenvironment. The workshop featured presentations from several investigators with the most encouraging data to date addressing these hurdles. Igor Slukvin (University of Wisconsin) demonstrated that GSK3β inhibition promoted mesoderm induction and increased the efficiency of multipotent hematopoietic progenitor cell production from various types of NHP iPSCs by at least 10-fold, compared to previous approaches.13 NHP iPSC-derived HSPCs were capable of differentiating into mature erythroid, myeloid, and lymphoid cells in the presence of cytokines and/or OP9 stromal coculture. Autologous cynomolgus monkey iPSC-derived CD34+CD45+ HSPCs were transplanted following nonmyeloablative conditioning, and were well tolerated with low-level engraftment detected for up to 6 months after infusion (unpublished).
Jennifer L. Gori (Fred Hutchinson Cancer Research Center) took another approach to improve HSPC production and engraftment by coculturing pigtail macaque iPSCs with an engineered endothelial cell (EC) supportive line, and demonstrated that this re-creation of a “vascular niche” promoted definitive hematopoiesis via the action of notch ligands including JAG1 and DLL4 expressed on ECs.14 She then demonstrated that iPSC-derived and EC-induced HSPCs were able to engraft and produce both myeloid and lymphoid progeny over the long term in immunodeficient mice, at levels higher than previously reported by any investigators utilizing human or NHP PSC-derived HSPCs, and similar to patterns seen with cord blood–derived HSPCs.
Penelope Hallett (Harvard Medical School) presented her research on autologous transplantation of iPSC-derived midbrain-like dopamine neurons in a NHP model of Parkinson's disease. Cynomolgus monkey iPSCs were differentiated into dopaminergic neurons in vitro, and transplanted into the putamen of donor parkinsonian monkeys, in which stable parkinsonism had been induced using systemic administration of low-dose 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Cell survival, function, and safety was monitored for up to 2 years.15 When at least 13,000 transplanted dopaminergic neurons survived for the follow-up period, they were sufficient to result in increased motor activity and functional motor improvement contralateral to the side of the brain injected with the dopaminergic iPSC-derived neurons, without the need for immunosuppression. This study represents the longest reported monitoring following transplantation of iPSC-derived cells in NHP models, and provides proof-of-concept demonstration of functional efficacy of iPSC-derived midbrain dopamine neuron transplantation in a NHP parkinsonian model. This study also highlights the potential advantages of autologous vs. allogeneic transplantation.
Charles Murry (University of Washington), Joseph Wu (Stanford University), and Steven Kattman (Cellular Dynamics International, CDI) focused on cardiomyocyte differentiation from PSCs and preclinical transplantation testing of these cells in NHPs and other large-animal models. Dr Murry updated his group's published experience with the transplantation of human ESC-derived cardiomyocytes (hESC-CMs) into pigtail macaque hearts following induction of a myocardial infarction (MI).16 In their protocol, MI was induced via balloon occlusion of the left anterior descending coronary artery 2 weeks before cell delivery, which required very intensive immunosuppression, given the delivery of xenogenic human cells to macaques with normal immune systems. One billion hESC-CMs were injected into each infarcted macaque heart. Animals were sacrificed at 2 weeks, 4 weeks, and 3 months following injection, and hearts were harvested for histology and ex vivo functional assays. No macroscopic or microscopic evidence of teratomas or other tumors were detected in any animal. Immunostaining for the sarcomeric protein α-actinin indicated that up to 98% of engrafted human cells in the MI zone were CMs, and underwent progressive morphological maturation between the 2-week and 3-month time points, as evidenced by increased myofibril alignment, sarcomere registration, and CM diameter. Meanwhile, the hESC-CM grafts were perfused by host vessels and were shown to be electromechanically coupled to the host heart, as demonstrated via ex vivo electrophysiological studies on the explanted organ at study completion. Despite these encouraging findings, nonfatal but serious ventricular arrhythmias were detected by ECG recording in all monkeys after hESC-CM delivery. Dr Murry pointed out that these potentially serious side effects had not been detected during his group's prior work utilizing delivery of hESC-CMs to the hearts of smaller animals, perhaps because of the disparity in heart sizes and beating rates, and emphasizing the importance of NHP preclinical testing. He also discussed the limitations of his studies and ways forward, focusing on optimizing his group's model to incorporate larger infarcts to allow detection of functional improvements compared to controls with feasible group sizes, adapting hESC lines to defined acceptable clinical culture conditions under in-house current good manufacturing practice, as well as improving cell delivery methods.
Dr Wu set the stage for his work by summarizing the overall modest results in over a decade of multiple human clinical trials utilizing adult stem cells delivered to the hearts of patients with cardiovascular disease. He suggested that problems with production of well-characterized cell types, delivery, engraftment, and inappropriate end points all contributed to lack of progress.17 He then presented unpublished work in a porcine model for hESC-CM transplantation as an example of progress utilizing an alternative large-animal model. There are advantages and disadvantages of pig vs. NHP models, with the major advantages of the pig model being the similarity of heart size and physiology to those of humans, but their immunology has been much less well characterized compared with NHPs. Dr Wu's group delivered human ESC-CMs with or without cardiac patches into porcine hearts. Survival of transplanted cells in the heart was documented histologically using human-specific antibodies at 4 weeks following transplantation. No evidence of teratoma was found in other major organs including liver, spleen, kidney, lung, and brain at the same time point, suggesting evidence for safety.
Finally, Dr Wu discussed the major challenges for implementation of ESC/iPSC clinical therapies, focusing on the need for further development of appropriate large-animal models, but the difficulties in obtaining funding to carry out expensive but necessary good laboratory practice–like studies that are often deemed not mechanistic by NIH study sections. For instance, development of a large-animal MI model with meaningful reduction in left ventricular ejection fraction results in some degree of surgical mortality and thus increased expense for animals and animal care. In addition, current delivery approaches appear to result in the majority of cells dying within a month of transplantation—an important clinical hurdle for cardiac stem cell therapy.18 All these issues and others must be addressed for preclinical application of ESC/iPSC-based therapies in cardiovascular disease.3
Dr Kattman presented an industry perspective on advancing PSC-derived CMs into clinical trials. He outlined and contrasted cell manufacturing requirements (purity, consistency, scale, phenotype, function, cryopreservation, and quality assurance) for human PSC-derived CMs (hPSC-CMs) depending on their utilization, whether in laboratories as research tools, in disease modeling, or as potential cell therapies. Typical manufacturing runs at CDI for cells used as research tools yield 1–6 billion hPSC-CMs per batch with >95% purity. For disease modeling, CDI has collaborated with the NHLBI to produce iPSC-CMs (>50 million cells per donor in 90% purity) from blood samples collected from 250 patients, including 100 informative families included in the Hypertension Genetic Epidemiology Network Cohort. Collaborating investigators are using these cells to study mechanisms of disease, such as the induction of a hypertrophy phenotype in vitro, and are attempting to correlate genome-wide association study findings with in vitro phenotypes.19 To work toward therapeutics, CDI has focused on the avoidance of immunogenicity in allogeneic transplantation, given the likely impossibility of using autologous cells in most cardiovascular clinical situations. CDI has generated two human iPSC cell lines under the current good manufacturing practices from “superdonors,” with common human leukocyte antigen (HLA) types predicted to be matched to up to 19% of the US population (unpublished). The HLA superdonor approach presents a partial HLA match that has been shown to be beneficial in organ transplants. By expansion to a larger number of lines, these workers plan to generate a master stem cell bank predicted to be compatible with 95% of the US population. It has been estimated that a tissue bank from 150 selected homozygous HLA-typed volunteers could match 93% of the UK population with a minimal requirement for immunosuppression.20 HLA superdonor cell lines will potentially provide a beneficial genetic match to large numbers of patients. Dr Kattman also discussed CDI's work in producing the cardiac progenitor cells that have the capacity to generate all cardiac lineages including CMs, endothelial cells, and vascular smooth muscle cells.
Alexander Bailey, a team leader in the Office of Cellular, Tissue, and Gene Therapies within the Center for Biologics Evaluation and Research at the FDA, discussed the preclinical regulatory considerations for PSC-based therapies. According to the Code of Federal Regulations Title 21, Part 312, adequate information derived from pharmacology and toxicology studies is needed to support a conclusion that the clinical trial is reasonably safe to conduct. Thus, data obtained from preclinical studies are used to help guide the design of early-phase clinical trials,21 including: (i) extrapolation of a safe starting dose, dose escalation scheme, and dosing schedule in human subjects, (ii) determination of a potential safe route of administration in subjects, (iii) identification of potential target tissues of toxicity and activity, (iv) selection of appropriate subject eligibility criteria, and (v) establishment of an adequate clinical monitoring plan. In addition, data generated from the preclinical studies may also support the scientific rationale for the use of the proposed investigational product in the specified clinical indication. The biological properties of stem cells make them attractive candidates for therapeutic development but also pose regulatory challenges from evaluation of associated risks.22 As such, Dr Bailey overviewed the current Center for Biologics Evaluation and Research's regulatory practices regarding the preclinical development of PSC-based products as well as the potential regulatory and scientific challenges in designing preclinical studies to enable investigational new drug applications.23
When designing a preclinical study, it is critical to select an appropriate animal model to ensure biological relevance. A detailed list of considerations for selecting an animal model for regenerative medicine product is available in a previously published article by Dr Bailey.23 In general, an animal species or model that is anatomically and pathophysiologically similar to humans should be selected for preclinical studies of the target disease or injury.23 Although there is no default regulatory requirement to use a specific animal species,23 small-animal models often present a challenge in meeting these criteria because of differences in organ size and architecture as well as differences in response to disease or injury compared with the human counterparts.
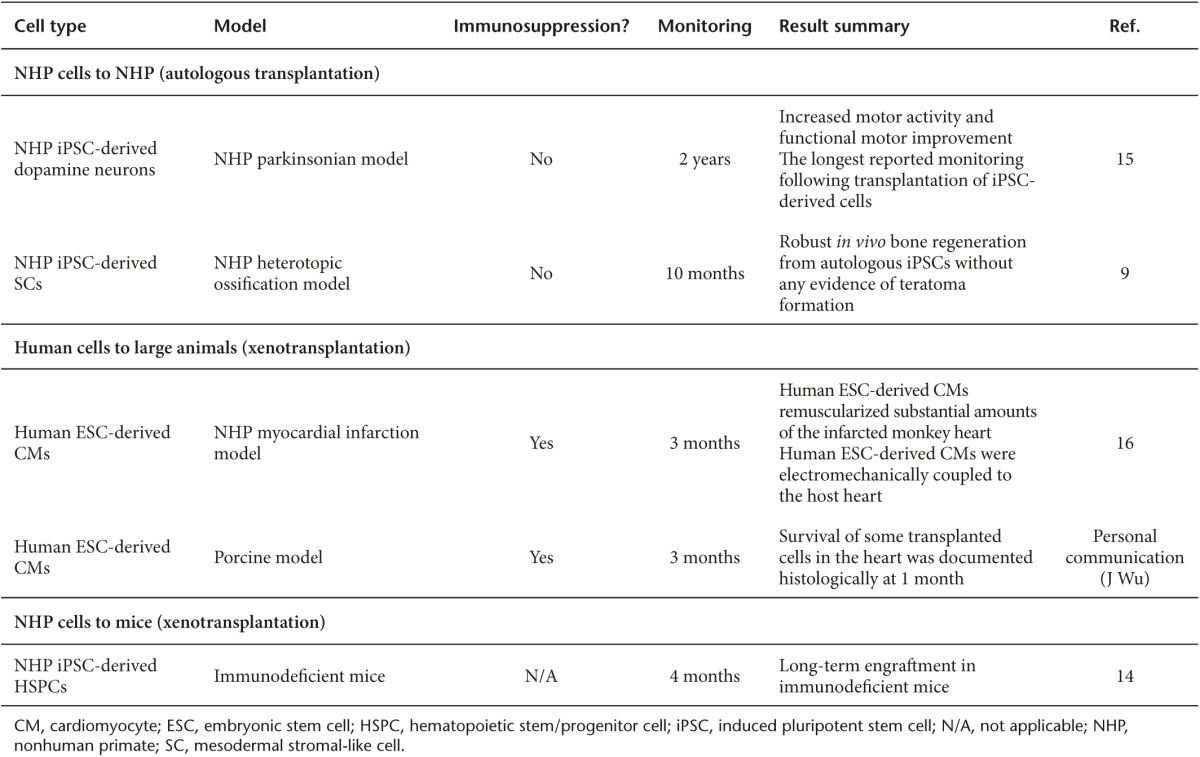
At the end of the workshop, the speakers and participants concluded that NHP models were a critical bridge to close the gap between human PSC studies in small animals and clinical trials in humans. Table 1 summarizes in vivo studies presented at the workshop. The particular strengths of these models were felt to be in evaluation of biodistribution, tumorigenicity, and immunogenicity during the development of human PSC-based cell therapies. NHP models were also noted to be particularly important for long-term safety studies. In the future, NHP models will provide a unique opportunity to test the degree of immunosuppression needed for engraftment and persistence of allogeneic therapies, a treatment approach impossible to study in immunodeficient mice. The obstacles that hinder broader use of NHP models for preclinical development of human PSC therapies were then summarized.
Table 1. Summary of in vivo studies presented at the NIH Nonhuman Primate (NHP) Induced Pluripotent Stem Cells (iPSCs) Workshop.
First, although NHP models are physiologically similar to humans, careful optimization is often necessary to maintain pluripotency of NHP PSCs in vitro. Whereas human PSCs have been broadly studied, resulting in the availability of highly optimized and established culture conditions, experience is much more limited in NHP PSC pluripotency maintenance and is not supported by access to commercial NHP-optimized culture media. Published NHP PSC maintenance methods vary by medium composition (using defined vs. mouse embryonic fibroblast-conditioned medium), dependence on extracellular matrix or feeder cells, passaging method (enzymatic, chemical, or manual), and even by NHP species. Some methods are labor intensive and plagued by batch-to-batch inconsistencies.
Second, the protocols to differentiate NHP PSCs toward multiple relevant cell lineages and tissues are limited by many of the same types of problems in comparison with human PSCs. To accelerate preclinical investigation of NHP PSC-derived cell products, efforts to develop efficient and large-scale differentiation protocols should be pursued, preferably via open collaboration between the major groups working on NHP PSC models.
Third, the costs of housing large animals and the costs and technical difficulties associated with optimization of surgical injury and delivery models remain significant, especially in the current NIH funding climate.
Fourth, it can be challenging to receive NIH funding for preclinical studies in NHPs or other large-animal models, as these experiments often are criticized as “lacking mechanistic insights,” and reviewers may have poor understanding of these models if their own scientific experience is limited to in vitro or small-model organisms.
Finally, the ongoing major public relations effort by animal welfare organizations to further restrict the utilization of NHPs in research, following the recent discontinuation of NIH-funded chimpanzee studies, is a potential looming issue, and potential new guidelines will be discussed by a NIH panel in the summer and fall of 2016. All participants agreed that these considerations make it very important to publicize the value of these models to the clinical development of PSC therapies, and to improve the efficiency and cost-effectiveness of experimental design, potentially by more extensive collaborations both between academic labs and with industry. The NIH-funded National Primate Research Centers and the intramural NIH primate programs have already played a central role in US PSC efforts, and offer critical resources for further progress as centers of excellence and expertise. Regular workshops to exchange information will be helpful to guide future directions and to interact with representatives from both funding and regulatory agencies.
Acknowledgments
This work was supported by funding from the Division of Intramural Research at NHLBI at the NIH. We thank all speakers, including Don P. Wolf (Oregon Health & Science University), Erin F. Wolff (National Institute of Child Health and Human Development), Yoav Gilad (University of Chicago), Igor Slukvin (University of Wisconsin), Jennifer L. Gori (Fred Hutchinson Cancer Research Center), Penelope J. Hallett (Harvard Medical School), Charles E. Murry (University of Washington), Joseph C. Wu (Stanford University), Steven Kattman (CDI), and Alexander M. Bailey (FDA) for participating in the workshop and helping us prepare this meeting report. We thank Manfred Boehm (NHLBI) for support and helpful discussions.
References
- Trounson, A and DeWitt, ND (2016). Pluripotent stem cells progressing to the clinic. Nat Rev Mol Cell Biol 17: 194–200. [DOI] [PubMed] [Google Scholar]
- Lee, AS, Tang, C, Rao, MS, Weissman, IL and Wu, JC (2013). Tumorigenicity as a clinical hurdle for pluripotent stem cell therapies. Nat Med 19: 998–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neofytou, E, O'Brien, CG, Couture, LA and Wu, JC (2015). Hurdles to clinical translation of human induced pluripotent stem cells. J Clin Invest 125: 2551–2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong, SG, Dunbar, CE and Winkler, T (2013). Assessing the risks of genotoxicity in the therapeutic development of induced pluripotent stem cells. Mol Ther 21: 272–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daadi, MM, Barberi, T, Shi, Q and Lanford, RE (2014). Nonhuman primate models in translational regenerative medicine. Stem Cells Dev 23 (Suppl. 1): 83–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, H, Zhu, F, Yong, J, Zhang, P, Hou, P, Li, Het al. (2008). Generation of induced pluripotent stem cells from adult rhesus monkey fibroblasts. Cell Stem Cell 3: 587–590. [DOI] [PubMed] [Google Scholar]
- Ma, H, Morey, R, O'Neil, RC, He, Y, Daughtry, B, Schultz, MDet al. (2014). Abnormalities in human pluripotent cells due to reprogramming mechanisms. Nature 511: 177–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallego Romero, I, Pavlovic, BJ, Hernando-Herraez, I, Zhou, X, Ward, MC, Banovich, NEet al. (2015). A panel of induced pluripotent stem cells from chimpanzees: a resource for comparative functional genomics. Elife 4: e07103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong, SG, Winkler, T, Wu, C, Guo, V, Pittaluga, S, Nicolae, Aet al. (2014). Path to the clinic: assessment of iPSC-based cell therapies in vivo in a nonhuman primate model. Cell Rep 7: 1298–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinbauer, GF, Niehoff, M, Niehaus, M, Srivastav, S, Fuchs, A, Van Esch, Eet al. (2008). Physiology and endocrinology of the ovarian cycle in macaques. Toxicol Pathol 36: 7S–23S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez, SF, Vahidi, NA, Park, S, Weitzel, RP, Tisdale, J, Rueda, BRet al. (2015). Characterization of extracellular DDX4- or Ddx4-positive ovarian cells. Nat Med 21: 1114–1116. [DOI] [PubMed] [Google Scholar]
- Donahue, RE and Dunbar, CE (2001). Update on the use of nonhuman primate models for preclinical testing of gene therapy approaches targeting hematopoietic cells. Hum Gene Ther 12: 607–617. [DOI] [PubMed] [Google Scholar]
- D'Souza, SS, Maufort, J, Kumar, A, Zhang, J, Smuga-Otto, K, Thomson, JAet al. (2016). GSK3beta inhibition promotes efficient myeloid and lymphoid hematopoiesis from non-human primate-induced pluripotent stem cells. Stem Cell Rep 6: 243–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gori, JL, Butler, JM, Chan, YY, Chandrasekaran, D, Poulos, MG, Ginsberg, Met al. (2015). Vascular niche promotes hematopoietic multipotent progenitor formation from pluripotent stem cells. J Clin Invest 125: 1243–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallett, PJ, Deleidi, M, Astradsson, A, Smith, GA, Cooper, O, Osborn, TMet al. (2015). Successful function of autologous iPSC-derived dopamine neurons following transplantation in a non-human primate model of Parkinson's disease. Cell Stem Cell 16: 269–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong, JJ, Yang, X, Don, CW, Minami, E, Liu, YW, Weyers, JJet al. (2014). Human embryonic-stem-cell-derived cardiomyocytes regenerate non-human primate hearts. Nature 510: 273–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher, SA, Doree, C, Mathur, A and Martin-Rendon, E (2015). Meta-analysis of cell therapy trials for patients with heart failure. Circ Res 116: 1361–1377. [DOI] [PubMed] [Google Scholar]
- Nguyen, PK, Riegler, J and Wu, JC (2014). Stem cell imaging: from bench to bedside. Cell Stem Cell 14: 431–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aggarwal, P, Turner, A, Matter, A, Kattman, SJ, Stoddard, A, Lorier, Ret al. (2014). RNA expression profiling of human iPSC-derived cardiomyocytes in a cardiac hypertrophy model. PloS One 9: e108051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor, CJ, Peacock, S, Chaudhry, AN, Bradley, JA and Bolton, EM (2012). Generating an iPSC bank for HLA-matched tissue transplantation based on known donor and recipient HLA types. Cell Stem Cell 11: 147–152. [DOI] [PubMed] [Google Scholar]
- US Food and Drug Administration, Center for Biologics Evaluation and Research (2013). Guidance for Industry: Preclinical Assessment of Investigational Cellular and Gene Therapy Products <http://www.fda.gov/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/CellularandGeneTherapy/ucm376136.htm> (November 2013).
- Bailey, AM (2012). Balancing tissue and tumor formation in regenerative medicine. Sci Trans Med 4: 147fs128. [DOI] [PubMed] [Google Scholar]
- Bailey, AM, Mendicino, M and Au, P (2014). An FDA perspective on preclinical development of cell-based regenerative medicine products. Nat Biotechnol 32: 721–723. [DOI] [PubMed] [Google Scholar]


