Abstract
The development of oncolytic viruses has recently made great progress towards being available to cancer patients. With the breakthrough into clinics, it is crucial to analyze the existing clinical experience and use it as a basis for treatment improvements. Here, we report clinical data from 290 patients treated with oncolytic adenovirus. Using clinical variables and treatment characteristics, we constructed statistical models with regard to treatment response and overall survival (OS). Additionally, we investigated effects of neutralizing antibodies, tumor burden, and peripheral blood leucocyte counts on these outcomes. We found the absence of liver metastases to correlate with an improved rate of disease control (P = 0.021). In multivariate evaluation, patients treated with viruses coding for immunostimulatory granulocyte macrophage colony-stimulating factor were linked to better prognosis (hazard ratio (HR) 0.378, P < 0.001), as well as women with any cancer type (HR 0.694, P = 0.017). In multivariate analysis for imaging response, patients treated via intraperitoneal injection were more likely to achieve disease control (odds ratio (OR) 3.246, P = 0.027). Patients with low neutrophil-to-lymphocyte ratio before treatment had significantly longer OS (P < 0.001). These findings could explain some of the variation seen in treatment outcomes after virotherapy. Furthermore, the results offer hypotheses for treatment optimization and patient selection in oncolytic adenovirus immunotherapy.
Introduction
Oncolytic viruses are one modality of cancer immunotherapy, which has become an increasingly important part of treatment options for cancer in recent years.1,2 After years of development, first oncolytic viruses are finally entering clinical use due to recent recommendations and approvals in European Union and United States.3,4 In addition to Talimogene Laherparepvec (T-VEC; Imlygic™), a granulocyte macrophage colony-stimulating factor (GMCSF) coding oncolytic herpes simplex virus, numerous other viruses is being tested in clinical trials.5,6 The data thus far are promising with good safety across the spectrum of viruses used and with signs of treatment efficacy linked to several specific products.6,7 However, our current knowledge on factors that influence treatment outcomes of oncolytic viruses is still quite limited.8
Clinical trials have indicated a small number of parameters that are associated with better or worse treatment responses. In the phase 3 trial of T-VEC for advanced melanoma, disease stage and previous treatments had a clear effect on durable response rate, which was the primary objective of the trial.9 Patients with metastatic stage IVM1b or IVM1c disease (metastases in lungs or other organs) received little benefit from T-VEC, as opposed to patients staged as IVM1a, IIIC or IIIB (only lymph node or cutaneous metastases at the primary tumor or distant locations for stage III and IV, respectively). Treatment benefits were also smaller in patients receiving T-VEC as second-line or greater therapy. In a phase 2 trial of oncolytic vaccinia virus JX-594—also coding for GMCSF—for liver cancer, higher treatment dose was found to correlate with significantly longer overall survival (OS).10 Patients with multiple tumors were found to have poor survival compared to patients with a single tumor. On the other hand, presence or absence of neutralizing antibodies before treatment was not associated with length of survival.
Oncolytic adenoviruses have been used in clinical trials and patient access programs. Previous reports have indicated that preexisting systemic immune activation could be linked to inferior responses to oncolytic immunotherapy.11,12 In a case–control estimation of treatment efficacy, adenovirus-treated patients with good performance score had improved survival when compared to matched controls.13 In the same report, ovarian cancer patients seemed to receive significant benefit from treatment, with almost quadrupled median survival versus controls not treated with adenovirus.
The aim of this retrospective clinical–epidemiological analysis is to identify factors influencing outcomes of adenovirus-based immunotherapy. We measured pre and posttreatment neutralizing antibody titers from patients receiving their first oncolytic adenovirus treatment. We also evaluated the initial tumor burden of the patients by measuring primary tumors and metastases in different organs. Different clinical parameters from all of the 290 patients treated in Advanced Therapy Access Program were incorporated into a Cox regression model to estimate prognostic factors significant in the context of oncolytic adenovirus therapy. In addition, logistic regression was used to find independent factors predictive of disease control. Finally, peripheral white blood cell counts before and after treatment were collected.
Results
Absence of neutralizing antiviral antibodies at baseline correlates with longer survival after treatment with oncolytic adenovirus
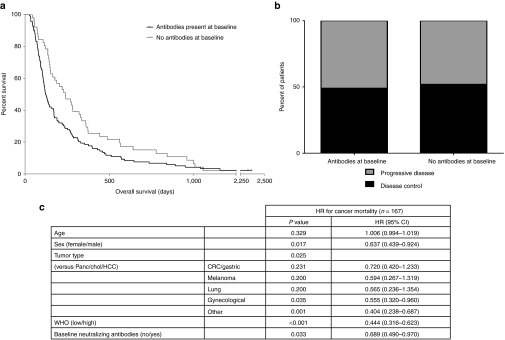
Neutralizing antibodies were measured in 170 patients, who were then—for statistical analysis—divided into two categories based on the presence of neutralizing antibodies. 119 patients did have neutralizing antibodies (any titer) before treatment, while 51 patients did not (titer = 0). Patients without neutralizing antibodies had significantly longer OS compared to patients with preexisting antibodies, medians 239 and 122 days, respectively (P = 0.022) (Figure 1a). This finding was also confirmed by multivariate Cox regression model using patient characteristics as confounding factors (P = 0.033) (Figure 1a). However, when comparing imaging responses instead of OS, no significant difference was seen (Figure 1b). Patients with baseline neutralizing antibodies were further divided into low and high groups. There was no difference in median survival between these two groups (120.5 and 126 days), although mean survival was longer in the latter group (219 and 318 days, not significant) (see Supplementary Figure S1).
Figure 1.
Overall survival (OS) and disease control rate in patients with or without baseline neutralizing antiviral antibodies. Patients with zero neutralizing antibody titer in pretreatment samples were considered antibody-negative. (a) Patients with no neutralizing antibodies had longer OS (median OS 239) compared to patients with preexisting neutralizing antibodies (median OS 122, P = 0.022). (b) Overall imaging disease control rate in patients with available neutralizing antibody samples was 50.4% (n = 115). No significant difference between patients with or without preexisting antibodies was observed. (c) Multivariate Cox regression analysis for prognostic value of baseline neutralizing antiviral antibodies. CRC/gastric, colorectal carcinoma and gastric cancer; gynecological, breast cancer, ovarian cancer, endometrial cancer and cervical cancer; HR, hazard ratio; panc/chol/HCC, pancreatic cancer, cholangiocarcinoma and hepatocellular carcinoma.
After treatment, neutralizing antibodies increased in patients as expected.14 Interestingly, increase was not observed in five patients (see Supplementary Table S1). These patients had all different cancers (rectum, head and neck, ovarian, Wilms tumor, and sarcoma) and received different virus treatments. Imaging responses of these patients varied (1 partial response, 1 minor response, 1 stable disease, and 1 progressive disease), but the patients tended to have longer than average OS (170–890 days).
Presence of liver metastases is associated with worse response rate
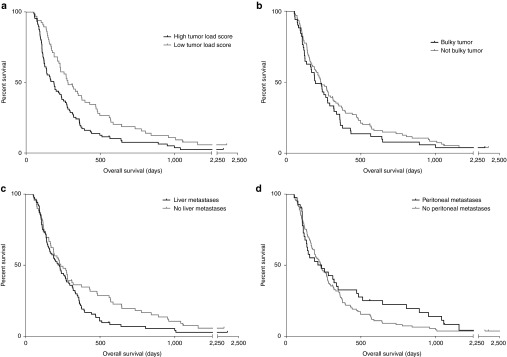
Tumor load was assessed in 154 patients. As a measure of initial tumor load, size of the largest tumor lesion was measured. Patients were divided into either having or not having large “bulky” according to size of the largest tumor. In addition, the presence of metastases was recorded for lymph nodes, liver, lungs, bones, peritoneal cavity, and other sites. Based on these data, a tumor load score was assigned to each patient to indicate disease burden. In survival analyses, patients with high tumor load score had significantly shorter OS (P = 0.003) (Figure 2a). Bulkiness of the largest tumor or presence of metastases did not correlate significantly with OS, although some trend for association was seen between liver metastases and worse survival (P = 0.065) (Figure 2b–d).
Figure 2.
Overall survival (OS) in different tumor load groups. (a–d) OS was compared between patients with high or low tumor load score and presence or absence of bulky disease, liver metastases, and peritoneal metastases. Patients with low tumor load score had significantly longer OS (P = 0.003), while no significant difference was found between bulky disease (P = 0.260), peritoneal (P = 0.272) or liver metastases (P = 0.065) groups.
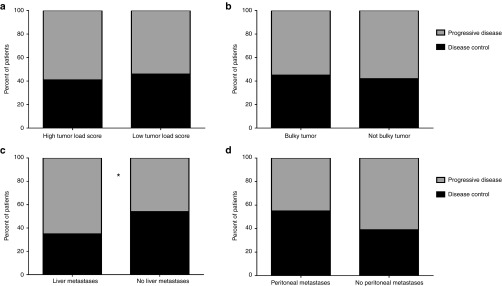
In predictive comparisons, patients with high and low tumor load score had no difference in response rates (Figure 3a). However, the absence of liver metastases indicated a significantly higher probability for imaging response (P = 0.021) (Figure 3c). Interestingly, peritoneal metastases seemed to be associated, albeit not significantly, with better response rates (Figure 3d).
Figure 3.
Imaging response rates in different tumor load groups. Asterisk indicates P < 0.05. Tumor load and imaging response were evaluable from 148 patients. (a,b) Patients with high or low combined tumor load score or presence or absence of bulky a tumor had no significant differences in disease control rates. (c) Absence of liver metastases before treatment was associated with higher response rate (P = 0.021). (d) Patients with peritoneal metastases had a higher proportion of imaging responses, but the difference was not considered significant (P = 0.094).
Tumor type and virus transgene are independent prognostic factors in patients receiving oncolytic adenovirus
Clinical variables from 290 patients (see Supplementary Table S2) were used to construct a Cox proportional hazards model. These included patient age, gender, tumor type, WHO performance status,15 viral transgene, capsid and promoter, administration route, concomitant treatments, and prior immunotherapy (Table 1). OS after the first virus treatment was deemed as dependent variable.
Table 1 . Results from Cox proportional hazards regression model for overall survival and logistic regression for imaging disease control.
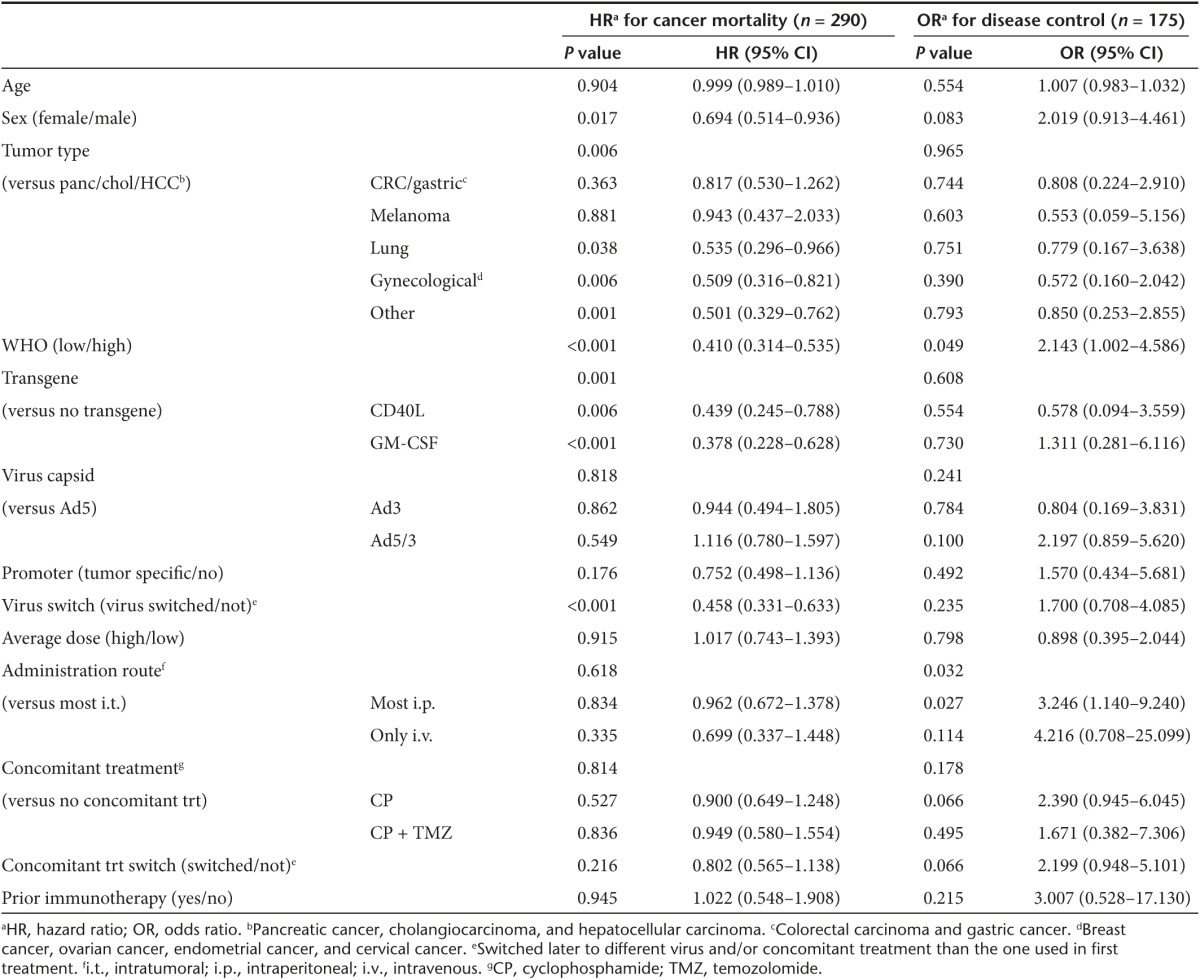
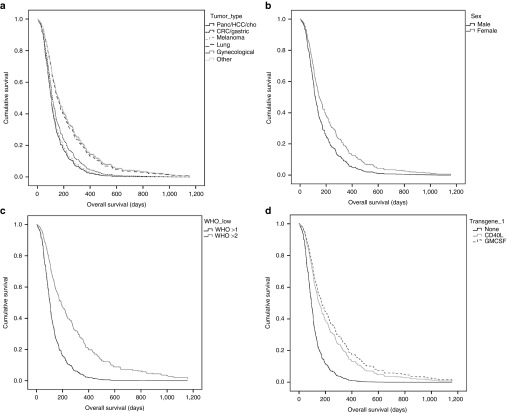
The Cox model provided several prognostic factors for patients receiving oncolytic adenovirus treatment. Patients with different tumor types had differences in OS (P = 0.006), and since this is known in oncology, it can be considered an internal test of validity of the model. Patients with pancreatic/biliary tract/hepatocellular cancer, melanoma, and colorectal/gastric cancer had worse survival compared to patients with lung cancer and gynecological cancers. More interestingly, other significant factors in the model included gender, WHO performance status, and virus switch (Figure 4). Of particular interest, also the use of a transgene in the treatment virus was associated with significantly (P = 0.001) better OS (Figure 4d).
Figure 4.
Hazard functions obtained from the Cox proportional hazards model. (a) Tumor type had a significant effect on overall hazard for tumor mortality (P = 0.006). Hazard ratios were smaller for gynecological cancers, lung cancer and other cancer types, compared to melanoma, pancreatic/hepatocellular/biliary tract cancers and colorectal/gastric cancers. (b-c) WHO performance status and gender were significant factors in Cox model (P < 0.001 and P = 0.017, respectively). (d) Patients who received virus coding for immunostimulatory transgene as first treatment had smaller hazard ratios compared to other patients (P = 0.001).
Administration route is an independent predictive factor for disease control
The same clinical variables used in the Cox model were included in a predictive logistic regression model (Table 1) and 175 patients with available imaging response data were included in the analysis. The only clinical variables reaching significant odds ratio (OR) for disease control were WHO performance status (P = 0.049) and administration route of virus treatments (P = 0.032). Patients who received most of the treatments via intraperitoneal or intrapleural injection had on average over three times higher probability for disease control (OR = 3.246, P = 0.027), compared to patients treated mostly or exclusively with intratumoral injections. Patients treated with intravenous virus injection also had a high OR, which failed to reach statistical significance (P = 0.114) due to small sample size (n = 8).
Differences in white blood cell counts before and after treatment associate with OS and treatment response
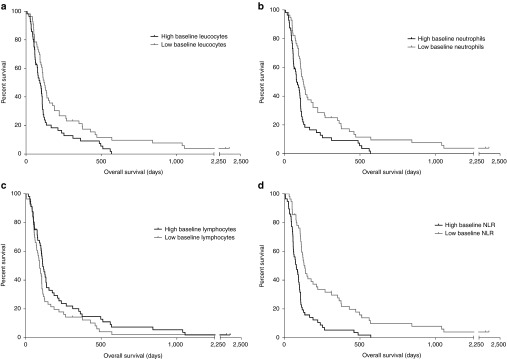
Although oncolytic viruses were initially developed as targeted tumor-lysing agents, recent data from patients suggest that an important proportion of the benefit associated with treatment might be attributable to lymphocyte activation against tumor.16,17,18,19 In contrast, some innate immune cells may dampen this immunotherapeutic efficacy, while some are also beneficial.12 Therefore, we assessed white blood cell counts in patients before and after therapy. Baseline white blood cell counts were available for 112 patients. OS was compared between patients with below or above median leucocyte, neutrophil and lymphocyte counts, and neutrophil-to-lymphocyte ratio (NLR).
Higher baseline leucocyte and neutrophil counts correlated with shorter OS (P = 0.008 and P = 0.002, respectively) (Figure 5a,b). There was also a trend, although not significant, for association of lymphocyte counts with longer OS (Figure 5c). Patients with low pretreatment NLR had significantly longer OS (P < 0.001), compared to patients with high NLR (Figure 5d). Additionally, this difference was confirmed by multivariate Cox model using patient characteristics as confounding factors (P < 0.001) (see Supplementary Table S3). Differences in treatment responses between the groups were not significant (see Supplementary Figure S2).
Figure 5.
Overall survival (OS) in patients with different baseline white blood cell counts. Baseline cell counts were measured 1 day before or on the same day of the first treatment. (a,b) Leucocyte and neutrophil counts that were below median before treatment were associated with significantly longer survival (P = 0.008 and P = 0.002, respectively). (c) No significant difference in OS between different baseline lymphocyte count groups was observed (P = 0.119). (d) Patients with lower than median neutrophil-to-lymphocyte ratio (NLR) had significantly longer OS compared to patients with high NLR (P < 0.001).
Cell counts showed clear changes in posttreatment samples (see Supplementary Figure S3). In general, at early time points, lymphocytes decreased following treatment while leucocytes increased. We compared relative cell count changes between patients who showed imaging response (disease control or better) after treatment and patients who had progressive disease. The magnitude of the changes was similar, although temporal patterns varied between the groups (see Supplementary Figure S3).
We compared disease control rates in more detail between patients who had increase or decrease in leucocyte and neutrophil counts on day 1 posttreatment (see Supplementary Figure S4). Patients with increases in leucocytes and neutrophils had higher response rates, albeit the differences were not significant (P = 0.055 and P = 0.116, respectively).
Discussion
In parallel with other forms of immunotherapy, oncolytic virotherapy has been associated with a limited response rate, but many of the responses that are seen seem to be durable.9,20,21 Moreover, again in accord with other potent immunotherapies, survival could be affected more than tumor size.9,20,21 Thus, with existing treatment options, it is important to develop clinical criteria to select the right patients for therapy,17 as well as to find biomarkers11,22 to determine which patients are benefiting from the therapy. Clinical trials with oncolytic viruses have reported a small number of potential candidates for predictive and prognostic factors.9,10 Thus, the work towards establishing clear principles for evaluation of likelihood of treatment benefit from oncolytic virotherapy has only begun.
We have reported here the associations of several clinical variables and laboratory tests on treatment outcomes of patients receiving oncolytic immunotherapy with adenovirus. We found the presence of antiviral neutralizing antibodies before treatment as well as high overall tumor load to correlate significantly with shorter survival. Additionally, high pretreatment total leucocyte counts, neutrophil counts, and, especially, NLRs were linked to worse survival. In a prognostic regression model for OS, certain tumor types had significantly smaller hazards ratios, as well as treatments with viruses that were armed with GMCSF. A predictive model for imaging response indicated higher OR for disease control for patients who were treated with intraperitoneal virus injection.
Baseline neutralizing antibodies had a significant effect on OS. This finding is in contrast with a previous report from phase 2 trial of oncolytic vaccinia virus, where neutralizing antibodies had no effect on survival.10 The smaller number of patients in the vaccinia study (n = 30) could affect conclusions, but if there is a discrepancy, it might reflect a difference in the biology of the two viruses. Vaccinia is thought to be able to evade the immune system to some degree,23 and this could also translate to lower sensitivity to antibody-dependent inactivation, compared to adenovirus which lacks a cell-derived envelope and just has a protein capsid.
However, it is key to keep in mind that responses and/or long survival were seen across antibody categories. Thus, while antibodies are an interesting topic for scientific discussion, they are not useful as a biomarker for patient selection. This becomes apparent when considering that in almost all patients antibodies increase after treatment, regardless of treatment benefits, which may last even years in the presence of high antibody titers. Moreover, virus circulation is seen often for weeks or even months despite antibody titers.18,19,24 Accordingly, there was no difference in response rates in patients with or without antibodies at baseline. Further clarity would result from evaluation of neutralizing antibodies and survival in an even larger patient population.
While neutralizing antibodies can block transduction, they can also be conducive for immune responses through complement activation or antibody-dependent cellular cytotoxicity. Thus, in some patients antibodies can be beneficial while being harmful in others. One speculation is that the former have a smaller number of tumors which can be injected intratumorally while intravascular dissemination of virus is more important in the latter.25 However, again the picture is probably more complicated, since viruses may be able to escape neutralization by hiding inside cells, or they may simply overwhelm the capacity of the body to opsonize, given that huge amounts of virus are being produced by tumors for extended periods.25
There is one powerful example of the ability of oncolytic serotype 5/3 chimeric adenovirus to reach distant tumors despite antibodies. Patient N60 was treated with an intravenous injection and the treatment virus could be grown out of brain metastases.25 His baseline anti-adenovirus 5/3 antibody titer was 16 (=medium), reported here for the first time. Thus, virus was able to reach distant tumors through the blood stream, replicate, and new infectious virions were produced despite neutralizing antibodies present in blood. It is tantalizing that in our patient series, if we focus on patients with neutralizing antibodies present at baseline, those patients who had high titers seemed to do better than those with medium titers (mean survival was 94 days longer), which would be compatible with antibody-dependent direction of immune reactions as described above.
Five patients in this study did not develop increases in neutralizing antibodies after treatments, although all had preexisting neutralizing antibodies at baseline. It can be postulated that one explanation for this might be poor virus replication, but all five patients had virus present in their blood as measured by quantitative polymerase chain reaction after treatment.24,26,27 This suggests that, for some reason, the patients were not able to produce additional antibodies against the virus, perhaps because their B cells were depleted by previous chemotherapies and radiation. Alternatively, immunosuppression generated by the tumor could impact antibody responses and obviously these scenarios are not mutually exclusive. Previously, immune suppression has been proposed linked to worse responses with oncolytic virotherapy,8,12,28 but these five patients had good performance scores, treatment resulted in relatively good survival and some had also measurable imaging responses. Thus, there was nothing to suggest that they would have been particularly immunosuppressed on a systemic level. Nevertheless, if the immunosuppression would preferentially affect antibody formation in B cells, it might result in enhanced adenovirus replication and consequently a stronger direct oncolytic effect on the tumor.29 Nonetheless, this phenomenon is seemingly rare, since it was seen in only five patients, and thus difficult to study more extensively.
Overall, we think that some patients may benefit from “pure oncolysis”, while others from “pure oncolytic immunotherapy” (where the ability of the virus to lyse tumor cells has little relevance), although the majority fall in between these extremes, keeping in mind that oncolysis is an incredibly powerful way to induce antitumor immunity.30,31 All treatments described herein utilized replication competent adenoviruses with oncolytic activity.
High overall tumor load was associated with shorter survival, as expected, because it could indicate more advanced disease where the patient is closer to death. However, no significant correlation was seen with survival and disease bulkiness or presence of liver or peritoneal metastases. It was interesting that treatment response rates were not correlated with tumor load, but there were significantly less responses in patients with liver metastases. Thus, two conclusions can be drawn: (i) patients may benefit regardless of tumor size, (ii) the organ where metastases are present may be critical, probably because of immunological reasons. The liver and peritoneal cavity represent opposite ends of the spectrum when it comes to microenvironments favorable to immune responses as will be discussed.
Our data are in line with the phase 3 T-VEC trial,9 where melanoma patients having deep visceral metastases (liver being the most common such organ) were not likely to benefit from treatment. There are two reasons why liver metastases might be poor targets for oncolytic immunotherapy: (i) liver is probably one of the most immunosuppressive of all human organs,32 and (ii) the liver is an important site of virus clearance.8 With regard to the latter, the liver might act as a local “sink” for virions being produced by the tumor. Alternatively, the opposite could be hypothesized. Metastases might interfere with the normal processing of viral particles by hepatic Kupffer cells, thus hindering the development of antiviral immunity. This might then inhibit immunostimulatory effects of viruses at the tumor site, in contrast to the lytic effect discussed earlier. For these speculations, it should be noted that no patients with clear liver dysfunction were accepted in the treatment program and thus no patient had extensive liver damage.
In the Cox regression model, low WHO performance score and female gender were significantly associated with better OS. Effect of low performance score is evident, as it is linked to less advanced disease, and these patients might have lived longer than high WHO patients, even without any treatment. However, it could be relevant that performance score is also linked to immune competence. Many forms of immunotherapy work better in patients in better condition, probably because when the tumor affects the condition of the patient more, there is also more immunosuppression.
The suggestion that women benefit more from oncolytic immunotherapy is intriguing but in line with previous speculations. Lower survival rates for males have been described earlier for several cancer types, and various factors, such as differences in lead time and comorbidity, have been proposed to be underlying causes for this phenomenon.33 Thus, if men had more advanced disease, it could mean that there was more immunosuppression. However, this may not be the full explanation since women have a higher prevalence of autoimmune diseases,34 which is interesting in the context of immunotherapy. This could potentially indicate that women have a more active immune system, which in turn could mean higher responsiveness to immunotherapeutic interventions. Tumor types for male and female patients were partly different, but this effect was taken into account as a confounding factor in the multivariate analysis.
Tumor types had different hazard ratios, which for some part reflect the typical courses of these diseases. However, different immunogenicity35 and stromal composition of the tumors36 could also directly affect the efficacy of virotherapy. In contrast to previous findings with oncolytic vaccinia virus,10 we found no significant correlations between treatment dose and OS, which is logical since adenovirus replicates exponentially, and the input dose should, thus, have a limited relevance.
Interestingly, patients receiving GM-CSF coding virus as first-line oncolytic therapy (168/290 patients, 58% total) had significantly better OS. This supports the widely accepted principle of arming viruses with immonomodulatory cytokines for enhanced efficacy.17,37 Adenoviruses coding for GM-CSF have been previously shown to induce antitumor T-cell responses,16,18 which is likely to contribute to the improved outcomes seen.
In logistic regression analysis predictive of imaging results, low (=better) WHO performance scores and the intraperitoneal administration route were significantly correlated with higher OR for disease control. High OR in low WHO score patients could indicate that patients with earlier stage disease respond more frequently to adenovirus therapy than patients with more advanced cancer. As already mentioned, probably the most important mechanism by which tumors could escape the effects of adenovirus is immunosuppression, which is more common in advanced tumors.38,39
Higher OR in intraperitoneally treated patients compared to intratumorally treated has two important implications for oncolytic immunotherapy. First, locoregional delivery may be an optimal scenario because much of the virus ends up in the vicinity of tumors. When virus replicates in metastases, e.g., in the liver, many of the daughter virions probably enter the blood stream and are lost to nontarget organs. In the peritoneum, newly replicated virus may be able to again disseminate locally. Importantly, peritoneal tumor masses are often nonbulky, presenting as carcinomatosis, resulting in a favorable surface area to volume ratio which could be useful for both viral transduction and the recruitment and activity of lymphocytes. Second, the peritoneal cavity can be seen as one large immune organ,40,41 and thus intraperitoneal treatment could result in more effective activation of antitumor immunity, e.g., by activating the infiltrating NK cell and T-cell subsets.42,43 Further, in contrast to blood, which is the usual location for circulating neutralizing antibodies, the presence of antibodies in the peritoneum depends on the accumulation of ascites and if it was drained prior to the intraperitoneal virus injection.44
Tumor size assessments are difficult in oncolytic immunotherapy, because of the tremendous amount of inflammation caused by virus replication, and subsequent swelling of tumors.45 Nevertheless, in our patient series, the association of intraperitoneal disease and treatment benefits is supported by both imaging data and OS data. Of course, many patients with intraperitoneal disease and receiving locoregional virus injection are in fact ovarian cancer patients, since peritoneal carcinomatosis is their typical clinical situation. Thus, tumor type-specific reasons could have some impact on these data.
Baseline leucocyte counts could offer interesting clues about the immune system before treatment. Neutrophil counts and NLRs have both been previously found to be prognostic in several cancers.46,47 We also saw this in our data, and especially high NLR was remarkably associated with worse OS. We have previously reported evidence of a link between poor prognosis and baseline activation of innate immunity.12 The association of high NLR and shorter survival is well in accord with this data. To our knowledge, this is the first study demonstrating the prognostic potential of NLR in the context of oncolytic virotherapy, and this could be a variable with possible practical utility. However, the exact phenotype of the neutrophils48 and existence of tumor infiltrating neutrophils, possibly in comparison to tumor infiltrating lymphocytes, should be evaluated in future studies to better understand the role of neutrophils in this setting.
In summary, we have investigated the role of neutralizing antibodies, tumor load, white blood cell counts, and several other clinical factors in the context of OS and treatment response in a comprehensive cohort of patients treated with oncolytic adenoviruses. In prognostic analyses, the presence of neutralizing antibodies and high neutrophil or NLR values before treatment were indicative of poor survival. Patients treated with GMCSF coding viruses were more likely to survive longer compared to viruses not coding for immunostimulatory cytokines. Predictive analyses suggested that performance score and the location of metastases, especially liver versus peritoneum, were significantly associated with treatment response. These results have implications for designing treatments with oncolytic adenovirus. Additionally, the findings offer basis for further development of tools for clinical decision-making in the context of oncolytic virotherapy. However, to be translated to practice on the field of personalized oncology, these concepts require prospective investigation in clinical trials.
Materials and Methods
Patients. All patients reported here were treated with oncolytic adenovirus in the Advanced Therapy Access Program, which was a personalized therapy program ongoing 2007–201249. Before treatment in Advanced Therapy Access Program, patients had solid tumors that were refractory to standard treatments and no major organ dysfunctions were present. Other exclusion criteria were as previously reported.50 A written informed consent was received from all of the patients participating in the program. Analysis of the data reported here was approved by the Helsinki University Central Hospital Operative Ethics Committee (HUS 62/13/03/02/2013).
Oncolytic viruses and treatments. Viruses that were used in the treatments have been previously published.14,18,19,24,26,37,51,52,53 All of the viruses, such as Ad5-d24-GMCSF, are based on the serotype 5 adenovirus, excluding Ad3-hTERT-E1, which is based on the serotype 3 adenovirus. Ad5-d24-RGD, Ad5-RGD-d24-GMCSF, and ICOVIR-7 have a RGD modification in the fiber region. Some viruses, such as Ad5/3-d24-GMCSF, Ad5/3-Cox2L-d24, and Ad5/3-E2F-d24-GMCSF have a chimeric 5/3 capsid, where the Ad5 knob is replaced by the serotype 3 knob. Additionally, several viruses are armed with immunostimulatory transgene GMCSF, whereas Ad5/3-hTERT-CD40L codes for CD40 ligand and Ad5/3-d24-hNIS expresses a sodium iodide symporter protein. Viruses were made tumor selective by introducing a 24-bp deletion in the retinoblastoma binding site of E1A and/or by inserting a tumor-specific promoter, such as E2F, hTERT or Cox2L.
Viruses were administered intratumorally, using ultrasound or CT guidance when needed, or by intraperitoneal/intrapleural or intravenous injection. If no contraindications were present, patients received also low-dose cyclophosphamide to reduce the number of regulatory T cells.54 A subset of patients received also oral temozolomide to induce autophagy in combination with the virus treatment.55
Response evaluation. Treatment responses were evaluated by changes of tumor size and/or metabolic activity in pre and posttreatment imaging, performed by computer tomography (CT) or positron emission tomography with CT (F18-FDG-PET-CT). Modified RECIST 1.1 criteria16 were used for evaluating CT results, and custom PET criteria45 were used to interpret PET-CT imaging results. Magnetic resonance imaging was used rarely.56 Responses were graded into following categories: progressive disease or progressive metabolic disease (PD/PMD), stable disease or stable metabolic disease (SD/SMD), minor response or minor metabolic response (MR/MMR) and complete response or complete metabolic response (CR/CMR). Posttreatment imaging was typically done 2–3 months after the treatment45 (range 1.0–4.7 months, median 2.3 months). The duration of the responses was not assessed. OS was calculated from the date of the first viral treatment.
Neutralizing antibodies. Neutralizing antiviral antibody titer was obtained by measuring transgene activity from cells infected with luciferase-coding adenovirus after incubation with different dilutions of patient serum, as described previously.26,52 Serotype of the virus used in the assay was always selected to match exactly the capsid of the virus that was used in the treatment of a given patient.
Tumor load. Tumor load score evaluation was based on the pretreatment CT and/or PET-CT scan images. Largest tumor diameter values were measured and organs with metastases were identified. Metastases in lymph nodes, liver, lungs, bones, peritoneal cavity, and other sites were graded with score from 0 to 3 (zero indicating no detectable metastasis). If the largest axial tumor diameter exceeded 64 mm, the tumor was considered bulky and graded with a score of 3 (otherwise zero). Scores from metastases and tumor bulkiness were combined to form a total score for disease burden (0–21 points).
Peripheral blood cell counts. Baseline peripheral blood samples were obtained from patients on the day of the treatment or 1 day before. Posttreatment samples were obtained on varying dates during days 1–30 after treatment. Samples were immediately analyzed by the laboratory of the treating hospital using standard techniques. Neutrophil count was derived by subtracting the lymphocyte count from total leucocyte count. Neutrophil to lymphocyte ratio was calculated as the quotient from the division of neutrophil and lymphocyte counts.
Statistical analyses. Statistical analyses were performed using SPSS Statistics v23 (International Business Machines Corporation, Armonk, NY), Microsoft Excel (Microsoft Corporation, Redmond, WA), and GraphPad Prism (GraphPad Software, La Jolla, CA). Log-rank test was used to compare OS between different neutralizing antibody, tumor load or blood cell count groups. Differences in treatment responses between groups were analyzed with Fisher's exact test. Hazard ratios for clinical variables were calculated using Cox proportional hazards regression model. ORs for disease control were obtained using logistic regression. P values of less than 0.05 were considered statistically significant.
SUPPLEMENTARY MATERIAL Figure S1. Overall survival in different baseline neutralizing antibody groups. Figure S2. Response rates in different baseline cell count groups. Figure S3. White blood cell counts after treatment. Figure S4. Response rates based on day 1 cell count change. Table S1. Characteristics of patients without post-treatment neutralizing antibodies. Table S2. Patient characteristics. Table S3. Multivariate analysis for prognostic value of baseline neutrophil-to-lymphocyte ratio.
Acknowledgments
We thank Tiina Hakonen for expert assistance. This research was supported by Targovax ASA (formerly Oncos Therapeutics), ASCO Foundation, HUCH Research Funds (EVO), Sigrid Juselius Foundation, Biocentrum Helsinki, Biocenter Finland, Finnish Cancer Organizations, Finnish Medical Foundation, Paulo Foundation, University of Helsinki. A.H. is Jane and Aatos Erkko Professor of Oncology at the University of Helsinki. A.H. is shareholder in Targovax ASA. A.H. is employee and shareholder in TILT Biotherapeutics.
Supplementary Material
References
- Maude, SL, Frey, N, Shaw, PA, Aplenc, R, Barrett, DM, Bunin, NJ et al. (2014). Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med 371: 1507–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postow, MA, Chesney, J, Pavlick, AC, Robert, C, Grossmann, K, McDermott, D et al. (2015). Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med 372: 2006–2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EMA (2015). Summary of opinion (initial authorisation): Imlygic. European Medicines Agency: London, UK. Report no. EMA/690530/2015. [Google Scholar]
- FDA (2015). FDA Approves First-of-Its-Kind Product for the Treatment of Melanoma (press release). US Food and Drug Administration: Silver Springs, Maryland, United States. [Google Scholar]
- Pol, J, Bloy, N, Obrist, F, Eggermont, A, Galon, J, Cremer, I et al. (2014). Trial Watch:: oncolytic viruses for cancer therapy. Oncoimmunology 3: e28694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamarin, D and Pesonen, S (2015). Replication-competent viruses as cancer immunotherapeutics: emerging clinical data. Hum Gene Ther 26: 538–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichty, BD, Breitbach, CJ, Stojdl, DF and Bell, JC (2014). Going viral with cancer immunotherapy. Nat Rev Cancer 14: 559–567. [DOI] [PubMed] [Google Scholar]
- Russell, SJ, Peng, KW and Bell, JC (2012). Oncolytic virotherapy. Nat Biotechnol 30: 658–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andtbacka, RH, Kaufman, HL, Collichio, F, Amatruda, T, Senzer, N, Chesney, J et al. (2015). Talimogene laherparepvec improves durable response rate in patients with advanced melanoma. J Clin Oncol 33: 2780–2788. [DOI] [PubMed] [Google Scholar]
- Heo, J, Reid, T, Ruo, L, Breitbach, CJ, Rose, S, Bloomston, M et al. (2013). Randomized dose-finding clinical trial of oncolytic immunotherapeutic vaccinia JX-594 in liver cancer. Nat Med 19: 329–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liikanen, I, Koski, A, Merisalo-Soikkeli, M, Hemminki, O, Oksanen, M, Kairemo, K et al. (2015). Serum HMGB1 is a predictive and prognostic biomarker for oncolytic immunotherapy. Oncoimmunology 4: e989771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taipale, K, Liikanen, I, Juhila, J, Turkki, R, Tähtinen, S, Kankainen, M et al. (2016). Chronic activation of innate immunity correlates with poor prognosis in cancer patients treated with oncolytic adenovirus. Mol Ther 24: 175–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanerva, A, Koski, A, Liikanen, I, Oksanen, M, Joensuu, T, Hemminki, O et al. (2015). Case–control estimation of the impact of oncolytic adenovirus on the survival of patients with refractory solid tumors. Mol Ther 23: 321–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koski, A, Kangasniemi, L, Escutenaire, S, Pesonen, S, Cerullo, V, Diaconu, I et al. (2010). Treatment of cancer patients with a serotype 5/3 chimeric oncolytic adenovirus expressing GMCSF. Mol Ther 18: 1874–1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oken, MM, Creech, RH, Tormey, DC, Horton, J, Davis, TE, McFadden, ET et al. (1982). Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 5: 649–655. [PubMed] [Google Scholar]
- Kanerva, A, Nokisalmi, P, Diaconu, I, Koski, A, Cerullo, V, Liikanen, I et al. (2013). Antiviral and antitumor T-cell immunity in patients treated with GM-CSF-coding oncolytic adenovirus. Clin Cancer Res 19: 2734–2744. [DOI] [PubMed] [Google Scholar]
- Kaufman, HL, Kohlhapp, FJ and Zloza, A (2015). Oncolytic viruses: a new class of immunotherapy drugs. Nat Rev Drug Discov 14: 642–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerullo, V, Pesonen, S, Diaconu, I, Escutenaire, S, Arstila, PT, Ugolini, M et al. (2010). Oncolytic adenovirus coding for granulocyte macrophage colony-stimulating factor induces antitumoral immunity in cancer patients. Cancer Res 70: 4297–4309. [DOI] [PubMed] [Google Scholar]
- Pesonen, S, Diaconu, I, Kangasniemi, L, Ranki, T, Kanerva, A, Pesonen, SK et al. (2012). Oncolytic immunotherapy of advanced solid tumors with a CD40L-expressing replicating adenovirus: assessment of safety and immunologic responses in patients. Cancer Res 72: 1621–1631. [DOI] [PubMed] [Google Scholar]
- Kantoff, PW, Higano, CS, Shore, ND, Berger, ER, Small, EJ, Penson, DF et al.; IMPACT Study Investigators. (2010). Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med 363: 411–422. [DOI] [PubMed] [Google Scholar]
- Hodi, FS, O'Day, SJ, McDermott, DF, Weber, RW, Sosman, JA, Haanen, JB et al. (2010). Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 363: 711–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zloza, A, Kim, DW, Kim-Schulze, S, Jagoda, MC, Monsurro, V, Marincola, FM et al. (2014). Immunoglobulin-like transcript 2 (ILT2) is a biomarker of therapeutic response to oncolytic immunotherapy with vaccinia viruses. J Immunother Cancer 2: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahar, MW, Graham, SC, Chen, RA, Cooray, S, Smith, GL, Stuart, DI et al. (2011). How vaccinia virus has evolved to subvert the host immune response. J Struct Biol 175: 127–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemminki, O, Parviainen, S, Juhila, J, Turkki, R, Linder, N, Lundin, J et al. (2015). Immunological data from cancer patients treated with Ad5/3-E2F-Δ24-GMCSF suggests utility for tumor immunotherapy. Oncotarget 6: 4467–4481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koski, A, Bramante, S, Kipar, A, Oksanen, M, Juhila, J, Vassilev, L et al. (2015). Biodistribution analysis of oncolytic adenoviruses in patient autopsy samples reveals vascular transduction of noninjected tumors and tissues. Mol Ther 23: 1641–1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nokisalmi, P, Pesonen, S, Escutenaire, S, Särkioja, M, Raki, M, Cerullo, V et al. (2010). Oncolytic adenovirus ICOVIR-7 in patients with advanced and refractory solid tumors. Clin Cancer Res 16: 3035–3043. [DOI] [PubMed] [Google Scholar]
- Bramante, S, Koski, A, Kipar, A, Diaconu, I, Liikanen, I, Hemminki, O et al. (2014). Serotype chimeric oncolytic adenovirus coding for GM-CSF for treatment of sarcoma in rodents and humans. Int J Cancer 135: 720–730. [DOI] [PubMed] [Google Scholar]
- Kirn, DH and Thorne, SH (2009). Targeted and armed oncolytic poxviruses: a novel multi-mechanistic therapeutic class for cancer. Nat Rev Cancer 9: 64–71. [DOI] [PubMed] [Google Scholar]
- Young, BA, Spencer, JF, Ying, B, Tollefson, AE, Toth, K and Wold, WS (2013). The role of cyclophosphamide in enhancing antitumor efficacy of an adenovirus oncolytic vector in subcutaneous Syrian hamster tumors. Cancer Gene Ther 20: 521–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong, AW, Senzer, N, Cerullo, V, Templeton, NS, Hemminki, A and Nemunaitis, J (2012). Oncolytic viruses for induction of anti-tumor immunity. Curr Pharm Biotechnol 13: 1750–1760. [DOI] [PubMed] [Google Scholar]
- Cerullo, V, Vähä-Koskela, M and Hemminki, A (2012). Oncolytic adenoviruses: a potent form of tumor immunovirotherapy. Oncoimmunology 1: 979–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crispe, IN (2009). The liver as a lymphoid organ. Annu Rev Immunol 27: 147–163. [DOI] [PubMed] [Google Scholar]
- Cook, MB, McGlynn, KA, Devesa, SS, Freedman, ND and Anderson, WF (2011). Sex disparities in cancer mortality and survival. Cancer Epidemiol Biomarkers Prev 20: 1629–1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairweather, D, Frisancho-Kiss, S and Rose, NR (2008). Sex differences in autoimmune disease from a pathological perspective. Am J Pathol 173: 600–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexandrov, LB, Nik-Zainal, S, Wedge, DC, Aparicio, SA, Behjati, S, Biankin, AV et al.; Australian Pancreatic Cancer Genome Initiative; ICGC Breast Cancer Consortium; ICGC MMML-Seq Consortium; ICGC PedBrain (2013). Signatures of mutational processes in human cancer. Nature 500: 415–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junttila, MR and de Sauvage, FJ (2013). Influence of tumour micro-environment heterogeneity on therapeutic response. Nature 501: 346–354. [DOI] [PubMed] [Google Scholar]
- Pesonen, S, Diaconu, I, Cerullo, V, Escutenaire, S, Raki, M, Kangasniemi, L et al. (2012). Integrin targeted oncolytic adenoviruses Ad5-D24-RGD and Ad5-RGD-D24-GMCSF for treatment of patients with advanced chemotherapy refractory solid tumors. Int J Cancer 130: 1937–1947. [DOI] [PubMed] [Google Scholar]
- Vieweg, J, Su, Z, Dahm, P and Kusmartsev, S (2007). Reversal of tumor-mediated immunosuppression. Clin Cancer Res 13: 727s–732s. [DOI] [PubMed] [Google Scholar]
- Motz, GT and Coukos, G (2013). Deciphering and reversing tumor immune suppression. Immunity 39: 61–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melichar, B and Freedman, RS (2002). Immunology of the peritoneal cavity: relevance for host-tumor relation. Int J Gynecol Cancer 12: 3–17. [DOI] [PubMed] [Google Scholar]
- Wertel, I, Nowicka, A, Rogala, E and Kotarski, J (2011). Peritoneal immune system in patients with advance epithelial ovarian cancer. Int Rev Immunol 30: 87–101. [DOI] [PubMed] [Google Scholar]
- Wang, X, Deavers, M, Patenia, R, Bassett, RL Jr, Mueller, P, Ma, Q et al. (2006). Monocyte/macrophage and T-cell infiltrates in peritoneum of patients with ovarian cancer or benign pelvic disease. J Transl Med 4: 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi, I, Amsalem, H, Nissan, A, Darash-Yahana, M, Peretz, T, Mandelboim, O et al. (2015). Characterization of tumor infiltrating natural killer cell subset. Oncotarget 6: 13835–13843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemminki, A, Wang, M, Desmond, RA, Strong, TV, Alvarez, RD and Curiel, DT (2002). Serum and ascites neutralizing antibodies in ovarian cancer patients treated with intraperitoneal adenoviral gene therapy. Hum Gene Ther 13: 1505–1514. [DOI] [PubMed] [Google Scholar]
- Koski, A, Ahtinen, H, Liljenback, H, Roivainen, A, Koskela, A, Oksanen, M et al. (2013). [(18)F]-fluorodeoxyglucose positron emission tomography and computed tomography in response evaluation of oncolytic adenovirus treatments of patients with advanced cancer. Hum Gene Ther 24: 1029–1041. [DOI] [PubMed] [Google Scholar]
- Templeton, AJ, McNamara, MG, Šeruga, B, Vera-Badillo, FE, Aneja, P, Ocaña, A et al. (2014). Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst 106: dju124. [DOI] [PubMed] [Google Scholar]
- Bahig, H, Taussky, D, Delouya, G, Nadiri, A, Gagnon-Jacques, A, Bodson-Clermont, P et al. (2015). Neutrophil count is associated with survival in localized prostate cancer. BMC Cancer 15: 594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridlender, ZG, Sun, J, Kim, S, Kapoor, V, Cheng, G, Ling, L et al. (2009). Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN. Cancer Cell 16: 183–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemminki, A, Oksanen, M and Merisalo-Soikkeli, M (2013). Oncolytic virotherapy trials–letter. Clin Cancer Res 19: 4541–4542. [DOI] [PubMed] [Google Scholar]
- Koski, A, Raki, M, Nokisalmi, P, Liikanen, I, Kangasniemi, L, Joensuu, T et al. (2012). Verapamil results in increased blood levels of oncolytic adenovirus in treatment of patients with advanced cancer. Mol Ther 20: 221–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesonen, S, Nokisalmi, P, Escutenaire, S, Särkioja, M, Raki, M, Cerullo, V et al. (2010). Prolonged systemic circulation of chimeric oncolytic adenovirus Ad5/3-Cox2L-D24 in patients with metastatic and refractory solid tumors. Gene Ther 17: 892–904. [DOI] [PubMed] [Google Scholar]
- Hemminki, O, Diaconu, I, Cerullo, V, Pesonen, SK, Kanerva, A, Joensuu, T et al. (2012). Ad3-hTERT-E1A, a fully serotype 3 oncolytic adenovirus, in patients with chemotherapy refractory cancer. Mol Ther 20: 1821–1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajecki, M, Sarparanta, M, Hakkarainen, T, Tenhunen, M, Diaconu, I, Kuhmonen, V et al. (2012). SPECT/CT imaging of hNIS-expression after intravenous delivery of an oncolytic adenovirus and 131I. PLoS One 7: e32871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerullo, V, Diaconu, I, Kangasniemi, L, Rajecki, M, Escutenaire, S, Koski, A et al. (2011). Immunological effects of low-dose cyclophosphamide in cancer patients treated with oncolytic adenovirus. Mol Ther 19: 1737–1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liikanen, I, Ahtiainen, L, Hirvinen, ML, Bramante, S, Cerullo, V, Nokisalmi, P et al. (2013). Oncolytic adenovirus with temozolomide induces autophagy and antitumor immune responses in cancer patients. Mol Ther 21: 1212–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemminki, O, Immonen, R, Närväinen, J, Kipar, A, Paasonen, J, Jokivarsi, KT et al. (2014). In vivo magnetic resonance imaging and spectroscopy identifies oncolytic adenovirus responders. Int J Cancer 134: 2878–2890. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.