Abstract
Previously, we constructed a bispecific NK-cell-engager (BiKE) bearing single-chain variable fragments (scFv) against CD16 on NK cells and EpCAM on tumor cells. This BiKE facilitated antigen-specific antibody-dependent cell-mediated cytotoxicity (ADCC) but did not induce NK cell expansion. We incorporated a modified interleukin-15 cross-linker to create a trispecific construct (TriKE) in order to improve activation, proliferation, and survival of NK cells. Synthesis and assembly of hybrid genes encoding the TriKE was accomplished using DNA-shuffling and DNA-ligation techniques. The TriKE was tested for specificity, efficacy, proliferative capability, and cytokine profile using functional assays. The molecular modifications improved yield without compromising binding to EpCAM+ HT-29 colorectal carcinoma cells. 51Chromium-release and degranulation assays showed better killing rates with TriKE compared to BiKE. TriKE was more active in a variety of different carcinoma cell lines. TriKE showed the ability to stimulate expansion of CD56+CD3- NK cells. BiKE and TriKE showed enhanced but not supraphysiologic levels of cytokine secretion. 1615EpCAM TriKE drives enhanced ADCC while significantly improving proliferation, activation, and survival of NK cell effectors. The TriKE provides a selectively delivered self-sustaining signal at the NK/tumor cell synapse. Targeted cytokine stimulation, rather than systemic cytokine administration, may impact toxicity in patients rendering the TriKE a promising new off-the-shelf carcinoma therapy.
Introduction
Epithelial cell adhesion molecule (EpCAM) is a transmembrane protein, normally expressed on epithelial tissue. Overexpression occurs in several cancer entities such as colon-, ovarian-, breast-, and prostate carcinoma,1,2,3,4 making it a valuable marker for cancer targeting. In neoplasia, EpCAM has relevant functions in regulation of cell processes such as signaling, proliferation, differentiation, and migration.5,6 There are growing lines of evidence indicating that EpCAM is connected to the Wnt/β-catenin pathways,7,8 known for relevant roles in regulation of self-renewal and differentiation of stem cells and cancer stem-cell (CSC). EpCAM expression has clinical impact by being predictive of cancer progression and survival.1,2,3,4 Thus, EpCAM has been chosen as a therapeutic target with some degree of success.
Catumaxomab9 and blinatumomab10 are among the immune engagers that have displayed clinical success. In these two drugs, which are already part of clinical routine, anti-CD3 is linked to a single chain variable fragment (scFv) targeting cancer in order to create an immune synapse between the T cell and cancer cell. This leads to effector-related stimulation and anticancer effect. However, activation of T cells can lead to harmful cytokine toxicity with consecutive significant disorders like cytokine release syndrome, disseminated intravascular coagulation, and nervous system events including encephalopathy and seizures (reviewed in ref. 11). Thus, we have been interested in selectively engaging natural killer (NK) cells instead of T cells to kill tumors, which when used for bispecific targeting showed excellent activity12,13 with diminished induction of inflammatory cytokines, necessary for cytokine storm.14
NK cells are large granular lymphocytes of the innate immune system capable of killing neoplastic-transformed cells. NK cells play a major role in tumor surveillance and have shown potential in a number of studies involving solid tumors and hematologic cancer.12,15,16 Therapeutic antibodies, such as Rituxan and Herceptin, can drive killing of bound tumors through NK-cell-mediated antibody-dependent cell-mediated cytotoxicity (ADCC). In a previous study, we engineered a bispecific NK engager (BiKE) by splicing a humanized scFv recognizing FCγRIII receptor (CD16) to a scFv recognizing EpCAM, resulting in a heterodimeric bispecific antibody capable of driving NK-cell-mediated ADCC.13
The immune stimulatory cytokine interleukin-15 (IL-15) is recognized as one of the most promising cancer cure drugs in an NIH-guided review and is currently in clinical trial alone or as an adjuvant for certain types of metastatic solid tumors. It primarily functions as an NK-cell regulator,17 interacting with the IL-15 receptor consisting of three subunits: IL-15 receptor-α (CD215), IL-2/15 receptor-β (CD122), and the common γ-chain (CD132). IL-15-mediated cytokine stimulation of NK cells leads to increased NK expansion, ADCC, lymphokine-activated killer activity, and production of other costimulatory mediators like interferon (IFN), tumor-necrosis factor (TNF), and granulocyte-macrophage colony-stimulating factor (GM-CSF).17,18,19,20,21
We engineered a fully humanized trispecific NK-cell engager (TriKE) by utilizing human IL-15 as a modified crosslinker between the anti-CD16 scFv and the anti-EpCAM scFv, thus combining ADCC capabilities with the ability to mediate NK expansion in the same therapeutic molecule. The IL-15 TriKE is specific and fully active against EpCAM bearing cancer cells, inducing selective NK cell degranulation. Additionally, the TriKE is functionally superior to the BiKE and capable of stimulating NK proliferation and expansion in a manner similar to exogenous IL-15 despite its intramolecular conformation.
Results
1615EpCAM
In order to construct a self-sustaining hybrid immune engager, a 1615EpCAM TriKE (Figure 1a) was assembled through incorporation of a modified IL-15 into the EpCAM16 BiKE (Figure 1b). The TriKE construct contains DNA fragments encoding the VH and VL regions of an anti-CD16 scFv, spliced to IL-15 and then to the VH and VL regions of an anti-EpCAM scFv. The IL-15 DNA fragment is flanked on either side by a 20 amino acid (aa) segment and EASGGPE. Absorbance tracing for 1615EpCAM TriKE and EpCAM16 BiKE eluted from the Fast Flow Q (FFQ) ion exchange column as the first phase in drug purification using a three-step elution protocol are displayed in Figure 1c,d (respectively). The first peak eluted from the column represents the product of interest. When a similar quantity of inclusion bodies were refolded and purified, yield was unexpectedly improved with the addition of the IL-15 cross-linker. When compared to the EpCAM16 BiKE, absorbance nearly tripled in the 1615EpCAM TriKE, indicating superior yield. Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and Coomasie Blue staining show purity after both ion exchange and size exclusion column purifications (Figure 1e,f) resulting in a product that is over 90% pure with a size of about 68,860 Da. Thus, an added advantage of incorporating IL-15 directly into hybrid TriKE was superior purification properties in comparison to the EpCAM16 BiKE.
Figure 1.
1615EpCAM TriKE elicits superior purification properties over EpCAM16 BiKE. Diagrams (a) and (b) indicate gene placement of the various BiKE and TriKE moieties in pET expression vectors. (c) The absorbance tracing for 1615EpCAM TriKE eluted from the FFQ ion exchange column as the first phase in drug purification using a 3-step elution protocol. The first peak eluted from the column represents our product. (d) Similar quantity of inclusion bodies were refolded and purified for EpCAM16 BiKE, the absorbance of peak 1 is displayed. (e) The second step purification over a size exclusion column. (f) The SDS-PAGE gel and Coomasie Blue staining shows the purity and size.
EpCAM binding
In order to assess EpCAM expression on carcinoma cells, various types of carcinomas were analyzed. Expression was high on BT-474, PC-3, UMSCC-11B, and Calu-3, but was low on Daudi lymphoma and U87 glioma (Figure 2a). To assess selectivity and functional binding of 1615EpCAM, a blocking assay was performed in which HT-29 colon carcinoma cells were preincubated with the anti-EpCAM scFv prior to incubation with 1615EpCAM-FITC. 1615EpCAM-FITC (200 nmol/l) was blocked by 500 nmol/l anti-EpCAM scFv but not by negative control CD2219, a bispecific molecule consisting of anti-CD22 scFv spliced to anti-CD19 scFv (Figure 2b). Results were reproducible also with a lower amount of the 1615EpCAM (50 and 100 nmol/l), data not shown. CD19 and CD22 are not expressed on HT-29 cells. Thus, the experiment shows 1615EpCAM binds EpCAM selectively.
Figure 2.
Analysis of EpCAM expression on cancer cell lines of various origins and blocking. (a) EpCAM expression was analyzed using EpCAM-FITC on BT-474, PC-3, UMSCC-11B, Calu-3, and negative control U87 glioma and Daudi lymphoma cell lines. (b) Flow cytometry based blocking assay testing the ability of anti-EpCAM scFv (EpCAM) to block the binding of FITC-labeled 1615EpCAM on EpCAM+ HT-29 cells. CD2219 BiKE was used as a negative control since HT-29 cells do not express CD22 and CD19.
Chromium-51 release
In order to determine the functional activity of 1615EpCAM, its killing ability was measured in standard 51chromium release assays (Figure 3). To determine the effect of incorporating IL-15 into the EpCAM16 scaffold, we evaluated NK-cell-mediated cytotoxicity in a wide range of donors with different NK-cell content. Freshly isolated peripheral blood mononuclear cells (PBMCs) were added to HT-29 cells at Effector (E):Target (T) ratios of 20:1, 6.6:1, and 2.2:1, generating cytolytic curves. The engineered reagents were added at the concentration of 30 nmol/l (maximum effective dose after titration experiments, data not shown). Donors with 3.8%, 6.4%, 15%, and enriched NK cells >80% (as determined by flow) showed that for the most part the IL-15 component improves upon the killing capabilities of the NK cells (Figure 3a–d respectively). In Figure 3e, only the donor curves for 1615EpCAM were graphed, emphasizing a direct correlation between increasing NK presence and cytolytic activity. Figure 3f shows that in the case of EpCAM16, no such correlation exists. Due to baseline variation, reproducibility was ensured by repeats with different donors. Together, the data indicate that the greater the presence of NK cells in the assay, the greater the effect.
Figure 3.
Evaluation of the activity of the 1615EpCAM TriKE in 51chromium release assays. Freshly isolated peripheral blood mononuclear cells were added to HT-29 target cells with the respective effector:target ratios in a chromium release assay to quantitate NK cell activity as indicated. Donors were chosen with naturally different levels of circulating NK cells; (a) 3.8%, (b) 6.4%, (c) 15%, or (d) > 80% enriched NK cells. Effectors and target cells were treated with no antibody (No Ab), anti-CD16 scFv (CD16), EpCAM16 BiKE, or 1615EpCAM TriKE. (e) Comparison of killing with TriKE amongst donors. (f) Comparison of killing with the EpCAM16 BiKE.
Lytic degranulation and IFN-γ expression against HT-29 target cells
In order to study lytic degranulation as a parameter of NK cell activity, CD107a expression was measured within the CD56+/CD3- NK cell population incubated with EpCAM expressing HT-29 targets. Though cells incubated with EpCAM16 BiKE showed elevated CD107a expression when compared with effectors alone, effectors plus targets without drug, or effectors plus targets with anti-EpCAM scFv, the 1615EpCAM TriKE had significantly more CD107a expression than the BiKE (Figure 4a). Addition of exogenous IL-15 to the EpCAM16 BiKE enhanced functionality compared to the BiKE alone, but still did not match CD107a expression on the TriKE indicating that the TriKE is inducing superior function perhaps through delivery of IL-15 to the NK/target synapse. The 1615EpCAM molecule also showed significantly elevated degranulation when cocultivated with HT-29 target cells (P < 0.001) when compared to an extensive panel of controls including E:T alone, E:T plus anti-EpCAM scFv devoid of 1615, E:T plus anti-CD16 scFv alone, CD2219 and E:T plus a combination of IL-12 and IL-18. The controls did not have any effect (Figure 4c). Two different sources of stand-alone IL-15 when combined with E:T also did not enhance lytic degranulation (IL-15 self, linker protein; IL-15 NCI, NCI derived). IFN-γ production was also enhanced in the 1615EpCAM TriKE treated NK cells when compared to the BiKE alone or the BiKE plus IL-15, indicating the biological ability of the IL-15 moiety within the TriKE to induce priming for cytokine secretion (Figure 4b). Overnight incubation with the TriKE further induced the IFN-γ priming (data not shown). As before, an extensive panel of controls was tested against the TriKE, in which only IL-12/IL-18 supraphysiologic stimulation outperformed the TriKE (Figure 4d).
Figure 4.
Testing the presence of CD107a positivity as an indicator for lytic degranulation and the presence of IFN-γ positive cells using flow cytometry. Freshly isolated PBMCs containing NK effectors (E) were added to target cells (T). (a) CD107a and (b) IFN-γ expression on CD56+CD3- NK cells was measured after incubation with or without HT-29 targets and anti-EpCAM scFv, EpCAM16, 1615EpCAM, or EpCAM16 plus IL-15 (n = 3). (c) CD107a expression and (d) IFN-γ on NK cells after incubation with EpCAM expressing HT-29 targets and 1615EpCAM or controls: anti-EpCAM scFv (EpCAM), anti-CD16 scFv (CD16), interleukin-15 (IL-15) cloned in our laboratory (self) or obtained from NCI (NCI), CD22CD19 bispecific scFv (CD2219), or IL-12/IL-18 control without targets. Significance was observed with 1615EpCAM treatment compared to controls (marked with *), (n = 3). (e) CD107a and (f) IFN-γ expression after incubation with EpCAM- HL60 target cells (n = 3). P values were estimated with one-way analysis of variance and presented with SD.
In Figure 4e, no CD107a expression was observed when NK cells were incubated with EpCAM negative HL-60 myeloid leukemia cells. There was elevation of IFN-γ producing NK cells with HL-60 targets, but only control cells treated with IL-12/IL-18 showed robust expression of IFN-γ (Figure 4f).
Lytic degranulation and IFN-γ expression against various cell lines
To determine whether other EpCAM expressing target cell lines induced similar 1615EpCAM TriKE-mediated NK-cell activation as the HT-29 target line, NK-cell function was tested on a variety of targets in conjunction to different drug treatments. Breast cancer (Figure 5a,b), prostate cancer (Figure 5c,d), head and neck cancer (Figure 5e,f), and ovarian cancer cell lines (Figure 5g) were studied. All EpCAM+ carcinoma lines treated with 1615EpCAM induced significantly elevated NK-cell degranulation (P < 0.001) when compared to E:T alone, E:T plus IL-15, E:T plus CD16CD133 (an irrelevant BiKE), and E plus IL-12/IL-18. As expected, the EpCAM16 BiKE also had marked percentages of cells expressing CD107a, but in all cases the values observed were significantly less than values observed for 1615EpCAM (P < 0.001).
Figure 5.
Lytic degranulation in different target cancer cell lines. 1615EpCAM was studied for its ability to induce lytic degranulation in various EpCAM+ cell lines. Effector peripheral blood mononuclear cells were incubated with different EpCAM bearing target cell lines (n = 3) including BT-474 (a), SK-BR-3 (b), PC-3 (c), DU145 (d), UMSCC-11B (e), NA (f), and SKOV-1 (g). 107a expressing cells were evaluated within the gated CD56+/CD3- NK-cell population. TriKE added to effector (E) and target (T) cells induced significantly higher percentages of CD107a expression compared to controls (Effectors + Targets; E+T+ interleukin-15 (IL-15), E+T+CD16CD133 and E+IL-12/IL-18) (marked with *)). The TriKE showed also enhanced degranulation compared to the BiKE EpCAM16 (marked with # or ##). P values were estimated with one-way analysis of variance and presented with SD.
In Figure 6a–f, these same effectors and targets were analyzed for IFN-γ expression. All lines demonstrated measureable elevations, but only elevations in BT-474, SK-BR-3 (Figure 6a,b), and PC-3 (Figure 6c) were significant (P < 0.001). None of the values approached those observed for the positive controls treated with IL-12/IL-18. Together, these findings indicated that the 1615EpCAM enhances the NK-cell-mediated killing of EpCAM expressing tumor cell lines. Although IFN-γ can be elevated during activation, it does not approach levels seen when supraphysiologic cytokine stimulation occurs.
Figure 6.
Interferon-γ (IFN-γ) production in different cancer cell lines. In the same flow cytometry experiment shown in Figure 5, 1615EpCAM was evaluated for IFN-γ production in different EpCAM bearing target cell lines (n = 3) including BT-474 (a), SK-BR-3 (b), PC-3 (c), DU145 (d), UMSCC-11B (e), NA (f), and SKOV-1 (g). The percentage of IFN-γ expressing cells were enhanced by 1615EpCAM treatment as compared to controls in all cell lines (Effectors + Targets (+) interleukin-15 (IL-15), CD16CD133 and IL-12/IL-18) (marked with *) except of the supraphysiologic IL-12/IL-18 control. 1615EpCAM expression was significantly higher in BT-474 and SK-BR-3 cancer cell lines (marked with #) compared to the BiKE EpCAM16. P-values were estimated with one-way analysis of variance and presented with SD.
Cytokine production with BiKE and TriKE stimulation
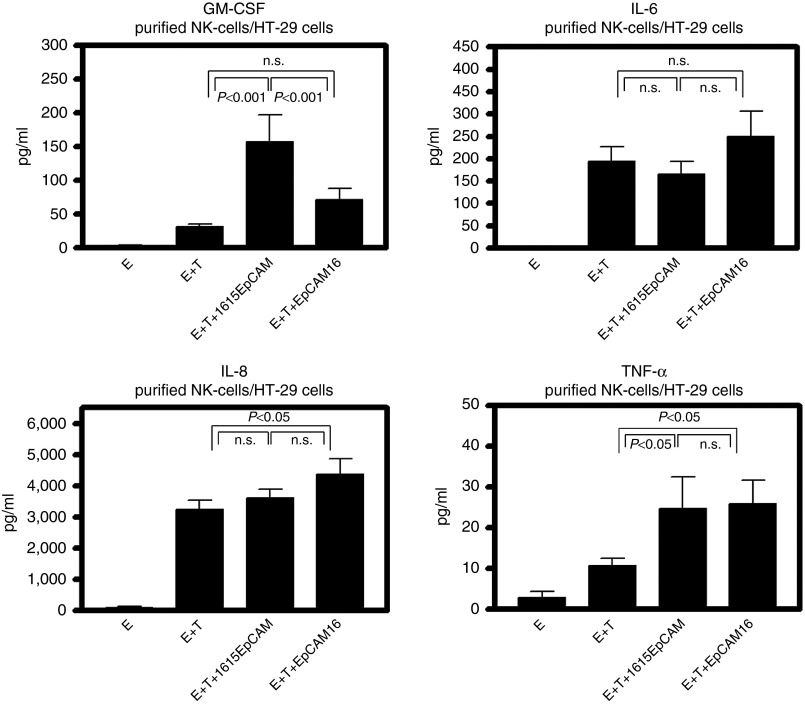
Besides IFN-γ, we wanted to evaluate a broader panel of cytokines that could influence anticancer activity and toxicity in the patients. Therefore, enriched NK cells were coincubated with HT-29 targets under different drug treatment conditions and supernatants were collected after 24 hours to perform multiplex-cytokine Luminex analysis. Granulocyte macrophage colony-stimulating factor (GM-CSF), was secreted at significantly higher levels when the TriKE was added to the coculture compared to the BiKE and to effectors plus targets alone (Figure 7a). Potentially toxic IL-6 and chemotactic IL-8 showed no significant differences between BiKE and TriKE samples, with only a mild difference between the TriKE and the E+T control with IL-8 (Figure 7b,c). TNF-α, known to inhibit tumorogenesis, was significantly higher when the BiKE or the TriKE was added. However, no difference between both drugs was obvious (Figure 7d). Taken together, the cytokine studies show that the BiKE and TriKE might induce components of the immune response necessary to tumor control without inducing potentially toxic IL-6.
Figure 7.
Cytokine profile of treatment with 1615EpCAM TriKE and EpCAM16 BiKE. Freshly isolated peripheral blood mononuclear cells were NK cell enriched from normal donor (n = 6) using magnetic beads. NK cells were cocultured with highly EpCAM positive HT-29 colon carcinoma cells for 24 hours. Supernatant was collected after incubation and assessed for GM-CSF, IL-6, IL-8, and TNF-α using a Luminex based multiplex assay. (a) GM-CSF secretion was significantly higher in the 1615EpCAM TriKE group compared to the EpCAM16 BiKE and to effectors (E) plus targets (T) alone. IL-6 (b) and IL-8 (c) showed no significant differences between BiKE and TriKE. TNF-α levels were significantly higher compared to E plus T and E alone when the BiKE or the TriKE was added. However, no difference between both drugs was obvious. P values were estimated with one-way analysis of variance and presented with SD.
Proliferation
The ability of the 1615EpCAM TriKE to induce proliferation in NK cells is shown in Figure 8. When donor PBMC were exposed to TriKE, only NK cells, but not T-cells showed a proliferation-specific pattern in a flow-cytometric assay (Figure 8a). Four additional donors were tested identically to determine whether these findings were reproducible, see Supplementary Figure S1a–d. The results were identical in three of these. Some T-cell proliferation was seen in the fourth donor. However, it is clear that when exposed to TriKE, NK cells always undergo more robust proliferation than T cells. In Figure 8b, direct comparison of the EpCAM16 BiKE and the 1615EpCAM TriKE showed that the TriKE has the ability to induce proliferation and expansion, but the BiKE does not (Figure 8b). To exclude other sources of induction of proliferation, we exposed PBMCs to the TriKE, BiKE, anti-CD16 scFv alone, IL-15 alone, anti-EpCAM scFv alone, and DT2219 (a targeted toxin comprising diphtheria toxin, linked to an anti-CD22 scFv and anti-CD19 scFv). Only the TriKE and IL-15 groups induced significant NK-cell proliferation displayed by the changes in expansion index (Figure 8c), which determines the fold expansion of the cells. NK-cell survival on enriched NK cells was determined after 7 days of incubation with drugs using LIVE/DEAD fixable dye. The group with TriKE stimulation showed higher NK-cell survival while the group exposed to the BiKE contained predominantly dead cells as confirmed with forward/side scatter flow cytometry (Figure 8d) and trypan blue staining. These data indicate that besides increasing priming of the cells, the IL-15 moiety in the 1615EpCAM TriKE can also induce expansion and maintenance of the NK cells.
Figure 8.
Proliferation capabilities of 1615EpCAM TriKE. (a) Peripheral blood mononuclear cells (PBMCs) were treated with 1615EpCAM TriKE and EpCAM16 BiKE. The discrete peaks in the histograms mark successive generations of NK cells after cell division leading in a repetitive slight reduction of florescence intensity. Whereas NK cells show a typical proliferation pattern, T-cells do not. Representative of five experiments. (b) PBMCs cells were cocultured with the TriKE and the BiKE and NK cell proliferation was evaluated. Representative of five experiments. (c) PBMCs cells were cocultured with the TriKE, the BiKE, anti-CD16scFv (CD16), Interleukin (IL)-15, anti-EpCAM scFv (EpCAM) and DT2219, a targeted toxin comprised of Diphtheria enterotoxin linked to anti-CD22 and anti-CD19 scFv. Evaluation of the NK cell Expansion Index showed a significantly (P < 0.001) enhanced index in the IL-15 comprising drug as well as with IL-15 alone, marked with *, (n = 5). (d) Purified NK cells were exposed to the TriKE and the BiKE. After 7 days, a reactive dye was used to differentiate alive and dead cells. This reactive dye is able to permeate the impaired membranes of dead NK cells, resulting in more intense staining (right peak) whereas no penetration in alive cells led to a weaker staining (left peak).
Discussion
The original contribution of this work is the development of an immune engager for carcinoma therapy that simultaneously mediates ADCC and provides a self-sustaining signal inducing NK effector cell expansion and maintenance. We previously reported that the BiKE, anti-CD16 scFv spliced to anti-EpCAM scFv, promoted formation of an immune synapse between NK effector cells and EpCAM-expressing carcinoma cells resulting in cytotoxic degranulation culminating in ADCC of the target cells.13 We hypothesized that both cytotoxic activity and NK longevity would benefit by providing a costimulatory signal that enhances effector cell expansion directly at the site of immune engagement. This could best be accomplished by the addition of an agent well-suited to expanding NK cells. To facilitate selective NK expansion, modified IL-15 was cross-linked into EpCAM16 BiKE. In this study, the molecular addition of IL-15 mediated NK proliferation, sustained ADCC activity, and improved lytic degranulation and cytokine secretion. Enhanced proliferations were not observed herein with BiKE treatment alone.
Over the past years, evidence has mounted indicating that the patient's own immune system could serve a major role in eliminating tumor cells. T cells and NK cells were identified as major players in cancer defense. Most recently, studies showed that T cells, genetically modified to express chimeric antigen receptors (CARs) are powerful clinical mediators of antitumor activity.22,23 T-CARs consist of T cells transfected with a tumor recognizing scFv, costimulatory motifs, and T-cell receptor zeta. Treatment of hematologic malignancies show great promise24,25 but production of T-CARs is costly and complex. The final value of the approach is not yet fully realized. Disadvantages include the risk of cytokine toxicity and long-term persistence resulting in interaction with healthy tissue or neoplastic transformation. However, it is clear that their ability to mediate lymphocyte expansion based on the inclusion of costimulatory moieties in their construction is critical to their success. Thus, we set out to include expansion-causing costimulatory elements into the construction of bispecific ADCC molecules. 1615EpCAM is not only a powerful mediator of ADCC, but expands NK cells without the need of extracorporal genetic modification and gene therapy, which is a huge potential advantage over the T-CAR system. Because the drug is rapidly cleared, there is no need to worry that the response will be indefinitely sustained strengthening its safety profile.
Genetic constructs consisting of antibodies and cytokines have been previously reported. Monoclonal antibodies linked to IL-2 were used for phase I studies focusing on solid tumors neuroblastoma/melanoma,26,27 prostate cancer,28 and ovarian cancer.29 However, clinical responses in patients were negligible and IL-2-related cytokine toxicity led to limitation of dosing and treatment. In addition, IL-2 promotes CD25+ Treg expansion and these suppressor cells cause immunosuppression and tumor progression in cancer patients.30
IL-15 is reported to have advantages over IL-2. Importantly, it does not induce Tregs. Munger et al.31 performed a side by side evaluation between IL-15 and IL-2 in a murine model. IL-2 was responsible for capillary leak syndrome that was not observed with IL-15. IL-15 shares common components with IL-2 receptor but seems to be less toxic and is a pivotal factor for regulation of NK cells.17 Beside improving activation and cytotoxicity, IL-15 regulates and initiates antiapoptotic and proliferative signals on NK cells leading to enhanced expansion and survival.32,33,34,35 These characteristics tend to be beneficial in the treatment against cancer explaining our efforts to combine it with our EpCAM16 construct.
We studied the positioning of IL-15 in the TriKE scaffold to determine its impact on activity. For these studies, we used an anti-CD33 TriKE recently reported.36 A TriKE mutant was constructed with IL-15 in the n-terminal and c-terminal position instead of the linker position. When these modifications were compared simultaneously in the same chromium release study on CD33+ HL-60 target cells, none of them attained the level of killing observed using the parental form of the drug indicating that the IL-15 modifications and orientation of IL-15 are crucial to its activity (not shown).
An argument could be made that it might be more effective to administer BiKE plus free IL-15 rather than TriKE alone because the amount of IL-15 needed for an optimal response might be different from the amount of BiKE needed, but with a TriKE, the ratio is fixed at 1:1. Thus, we compared the functional effects of BiKE plus free IL-15 to TriKE on HT-29 cells. Studies showed superior activity of TriKE on both lytic degranulation and production of IFN-γ, which are hallmarks of antitumor functionality. This seems to indicate that the IL-15 within the TriKE exerts its functionality in a different manner than separate addition of IL-15. This could be mediated by directed delivery of the TriKE to the NK/target cell synapse. We believe these findings will extend to the clinic since it is possible that the TriKE will cause IL-15 to accumulate at the site of tumor more effectively than systemic IL-15 does. Thus, a single drug is capable of doing the job of both BiKE and exogenous IL-15 without systemic IL-15 exposure.
Administration of recombinant human (rh) IL-15 was performed in a study of Conlon et al.37 in patients suffering from solid malignancies in advanced stages with 0.3, 1 and 3 µg/kg. IL-6, IL-8, and IFN-γ elevations were observed shortly after administration leading to short-term acute toxicities such as fever, hypotension, and chills. Our research also included IFN-γ production (flow cytometry based) since it is prominent in cytokine toxicity. While we found that in vitro EpCAM16 mediated IFN-γ release by NK cells in our last study,13 we were encouraged by the moderate levels induced, possibly due to our selective delivery method. Even with IL-15 stimulation, IFN-γ release was minimal and significantly lower than IL-12/IL-18-stimulated NK cells.14 IL-12/IL-18 is used as a control to induce supraphysiologic levels of IFN-γ. In this study, we performed a cytokine Luminex analysis measuring hallmark inflammatory cytokines including GM-CSF, IL-6, IL-8, TNF-α. We observed a statistically significant difference in GM-CSF secretion between BiKE and TriKE. No difference was seen in the other cytokines, implying only a moderate induction. Together, these data suggest that CD16 stimulation with IL-15 costimulation will induce antigen specific IFN-γ release and GM-CSF production that may not be at the levels placing patients at risk.
Impact was also reported on homeostasis of lymphocytes.37 NK cells were increased (up to threefold) and γδ- and CD8 T-cells were also increased (up to 8–10-fold). Despite measurable treatment response in patients with solid tumors,37 there may be risks associated with IL-15 administration since neutropenia, thrombopenia, and hypotension were observed.37 IL-15 may also introduce risks of carcinogenesis.38,39,40,41,42 Zambello et al. reported that IL-15 promoted proliferation in patients with lymphoproliferative disease.43,44 Furthermore, IL-15 may impact hyperplasia, angiogenesis,41 and invasion.42 We believe that the selective delivery of IL-15 may reduce these risks. EpCAM was selected for our studies because it is frequently overexpressed on carcinoma.45 Also, EpCAM expression on tumors correlates with a poor survival prognosis45,46 revealing a key role in tumor development. Other immune engagers have been developed that target EpCAM with some success. Catumaxomab, a monoclonal hybrid antibody targeting CD3 and EpCAM was approved for patients with malignant ascites with EpCAM-positive cancers and got marketing authorization for malignant ascites in 2009 (ref. 47). Based on a randomized clinical trial, Catumaxumab showed efficacy in significantly decreasing the need of ascites drainage.48 Additionally, isolated cases reported about shrinkage of distant metastasis after therapeutic intraperitoneal administration.49,50 Other studies using EpCAM-specific monoclonal antibodies showed promising results in murine models inhibiting tumor growth and inducing prolonged survival in pancreatic cancer. However, it must be noted that EpCAM is expressed on normal epithelial tissue, albeit in lower copy number. Thus, there are risks associated with nontarget reactivity.
In conclusion, BiKEs and bispecific antibodies were previously reported,13,51,52,53 but this manuscript describes a new modification to the bispecific killer engager platform that markedly enhances its therapeutic potential beyond ADCC by extending the ability of the reagent to induce NK-cell proliferation and maintenance. When constructed optimally, these new agents provide a signal that may self-sustain NK effector levels and strengthen the immune response in order to achieve superior tumor elimination, a benefit that could extend to TriKEs targeting other forms of cancer as well.
Materials and Methods
Construction of 1615EpCAM TriKE. Synthesis and assembly of hybrid genes encoding 1615EpCAM TriKE was accomplished using DNA shuffling and ligation techniques. The fully assembled 1615EpCAM gene (from the 5' end to 3'end) consisted of an NcoI restriction site, an ATG initiation codon, the VH and VL regions of human CD16 (NM3E2) derived from a phage display library produced by McCall et al.,54 a 20 amino acid segment (PSGQAGAAASESLFSNHAY), modified IL-15 (ref. 55), a 7 amino acid linker (EASGGPE), the humanized anti-EPCAM scFv from the antibody MOC-31, and finally a XhoI restriction site. The resulting 1914 bp NcoI/XhoI fragment gene was spliced into the pET21d expression vector under control of an isopropyl-β-D-thiogalactopyranoside inducible T7promoter. DNA sequencing analysis (Biomedical Genomics Center, University of Minnesota, MN) was used to verify that the gene was correct in sequence and had been cloned in frame. Other constructs used in this study, were created in a similar manner including genes for monospecific anti-CD16 scFv, and anti-EpCAM scFv.
Inclusion body isolation. Bacterial protein expression was performed with Escherichia coli strain BL21 (DE3) (Novagen, Madison, WI) by plasmid transformation. After overnight culture, bacteria were grown in 800 ml Luria broth containing 50 mg/ml carbenicillin. Induction of gene expression occurred when culture media reached an optical density (OD) 600 of 0.65 with the addition of isopropyl-β-D-thiogalactopyranoside (FischerBiotech, Fair Lawn, NJ). Two hours after induction, bacteria were harvested (from 5 l cultured media we isolated 43 g bacterial pellet). After that, pellet got homogenized in a buffer solution (50 mmol/l tris, 50 mmol/l NaCl, and 5 mmol/l Ethylenediaminetetraacetic acid (EDTA) pH 8.0), sonicated and centrifuged. Pellets were extracted with 0.3% sodium deoxycholate, 5% Triton X-100, 10% glycerin, 50 mmol/l Tris, 50 mmol/l NaCl, 5 mmol/l EDTA (pH 8.0) and washed (final pellet weight was 12.5 g).
Refolding and purification. Refolding and purification was recently described.12 Shortly, in order to refold, proteins from inclusion bodies (IB) were dissolved at 20:1 (mg wet weight/ml) in solubilization buffer (7 M Guanidine Hydrochloride, 50 mmol/l Tris, 50 mmol/l NaCl, 5 mmol/l EDTA, and 50 mmol/l DTT, pH 8.0). Following a 1-hour incubation at 37 °C, pellets were removed by centrifugation. The supernatant was diluted (20-fold) with refolding buffer (50 mmol/l Tris-HCl, 50 mmol/l NaCl, 0.8 mmol/l L-arginine, 20% glycerin, 5 mmol/l EDTA, and 1 mmol/l GSSG, pH 8.0) at 4 °C for 2 days. The buffer was removed by 10-fold dialysis against 20 mmol/l Tris-HCl, pH 9.0 in 20 mmol/l Tris-HCl, pH 9.0 (over four column volumes). SDS-PAGE analysis was performed to evaluate purity. The fusion proteins were stained with Simply Blue life Stain (Invitrogen, Carlsbad, CA). The size of our TriKE was about 68,860 Da.
NK-cell isolation and purification. A histopaque gradient (Sigma-Aldrich, St. Louis, MO) and SepMate tubes (Stemcell technologies, Vancouver, Canada) were used to isolate PBMC from adult blood (Memorial Blood Center, Minneapolis, MN) of healthy volunteers were obtained enriched NK cells via negative selection using magnetic beads per the manufacturer's protocol (Stemcell technologies). Samples were obtained after informed consent and in accordance with the University of Minnesota human subjects Institutional Review Board and the Declaration of Helsinki.
Tissue culture. The following cell lines were obtained from the American Type Culture Collection: Breast cancer cell lines BT-474, SK-BR-3; prostate cancer cell lines PC-3, DU145; head-and heck cancer cell lines UMSCC-11B, NA; ovarian cancer cell line SKOV-1; colon carcinoma cell line HT-29; lung cancer cell line Calu-3; Burkitts lymphoma cell line Daudi; acute myeloid leukemia cell line HL-60; human glioblastoma cell line U87. Carcinoma and glioblastoma cell lines were grown in monolayers using tissue flasks,56 HL-60 and Daudi cell lines57 were grown in suspension. Cells were maintained in either RPMI 1640 (BT-474, SK-BR-3, PC-3, DU-145, HT-29, Daudi, HL60, Calu-3), DMEM (UMSCC-11B, NA, SK-OV-3, U87) supplemented with 10% fetal bovine serum and 2 mmol/l L-glutamine. In addition to the preceding supplements, BT-474 media contained 10 IU/ml insulin. Cells were incubated in a humidified constant 37 °C atmosphere containing 5% CO2. When cells were 90% confluent, they were passaged using trypsin-EDTA for detachment. Cell counts were conducted using a standard hemacytometer. Only cells with a viability >95%, as determined by trypan blue exclusion, were used for experiments.
Binding/blocking assay. For evaluation of binding 4 × 105 of the respective cancer cells (BT-474, PC-3, UMSCC-11B, Calu-3, Daudi, U87) were washed and incubated at 4 °C with 10 nmol/l fluorescein isothiocyate (FITC)-labeled anti-EpCAM scFv for 30 minutes. For the blocking assay 200 nmol/l FITC-labeled 1615EpCAM TriKE were added to either 500 nmol/l of anti-EpCAM scFv, or an anti-CD22-CD19 scFv construct and incubated for 30 minutes at 4 °C with HT-29 colon carcinoma cells. After washing, staining intensity was evaluated with an LSRII flow cytometer (BD Biosciences, San Jose, CA).
CD107a degranulation assay. Flow cytometry assays measuring cytolytic degranulation via CD107a expression, and IFN-γ presence were previously reported.51 PBMCs were incubated over night (37 °C, 5% CO2) in RPMI 1640 supplemented with 10% fetal calf serum and with recombinant IL-12 10 ng/ml (Peprotech, Rocky Hill, NJ) and IL-18 100 ng/ml (R&D Systems, Minneapolis, MN) as a positive control. Cells were washed in 1× PBS, treated with 30 nmol/l of 1615EpCAM TriKE or other drugs and incubated for 10 minutes at 37 °C with 5% CO2. FITC-conjugated anti-human CD107a monoclonal antibody (mAb) (LAMP-1) (BD biosciences, New Jersey, CA), was added and further incubated for 1 hour with respective target cells (BT-474, SK-BR-3, PC-3, DU-145, HT-29, HL60, UMSCC-11B, NA, SK-OV-3). GolgiStop (1:1,500) (BD Biosciences, San Jose, CA) and GolgiPlug (1:1,000) (BD Biosciences, San Jose, CA) were added and cells were further incubated for 3 hours. Cells were washed in 1× PBS and stained with PE/Cy7-conjugated anti-CD56 mAb, APC/Cy 7-conjugated anti-CD16 mAb and PE-CF594-conjugated anti-CD3 mAb (BioLegend, San Diego, CA), incubated for 15 minutes and then fixed in 2% paraformaldehyde. Then, cells were prepared for intracellular stain using permeabilization buffer (BD Biosciences, San Jose, CA). Cells were incubated with Pacific Blue-conjugated anti-human IFN-γ (BioLegend) for 20 minutes, washed and evaluated by FACS analysis using a LSRII flow cytometer (BD Biosciences, San Jose, CA). For compensation, CompBead Plus Anti-Mouse Ig, κ/Negative Control (BSA) Compensation Plus (7.5 µm) particles (BD Biosciences, New Jersey, CA) were used.
Chromium-51 release cytotoxicity assay. HT-29 target cells were labeled for 1 hour with 1μCi of 51Cr per 1 × 105 target cells at 37 °C, 5% CO2. Washing procedures were performed to remove excess 51Cr. Labeled target cells were added to the wells of 96-well round-bottom plates (5 × 103 cells). Resting effector NK cells treated with 1615EpCAM TriKE, EpCAM16 BiKE or negative controls were added to the plates. E:T ratio ranged between 20:1 and 0.08:1. The amount of 51Cr released, which corresponds to target cell death, was measured by a gamma scintillation counter, and the percent target cell lysis was calculated as follows: ((experimental lysis – spontaneous lysis)/(maximal lysis – spontaneous lysis)) × 100. To determine maximal lysis, 51Cr-labeled target cells were treated with 3% Triton X for 4 hours.
Luminex. For analysis of chemokines and cytokines, purified NK cells from six healthy volunteers were coincubated in 96-well plates for 24 hours with HT-29 colon carcinoma cells at a 2:1 E:T ratio and the respective drug in a concentration of 50 nmol/l at 37 °C, 5% CO2. After a 24-hour incubation time, cells were centrifuged and supernatants were collected and stored at −80 °C until being analyzed. GM-CSF, IL-6, IL-8, and TNF-α (R&D Systems, Minneapolis, MN) were determined using the Luminex system (MAGPIX, Luminex, Austin, TX). Values represent pg/ml and were interpolated from standard curves of the recombinant human proteins by using Xponent 4.2 software (Luminex).
Proliferation and viability assays. PBMCs or enriched NK cells from healthy donors were labeled with CellTrace Violet Cell Proliferation Dye (Invitrogen, Carlsbad, CA) according to the manufacturer's protocol. After labeling, cells were cultured with 50 nmol/l concentrations of the respective drugs. Cells were harvested after 7 days, stained for viability with Live/Dead reagent (Invitrogen) and surface stained for anti-CD56 PE/Cy7 (Biolegend) and anti-CD3 PE-CF594 (BD Biosciences, Franklin Lakes, NJ) to gate on the viable CD3-CD56+ population. Data were analyzed with FlowJo software version 7.6.5. (Flowjo enterprise LCC, Ashland, OR).
Statistical Analyses. Data are presented as mean ± standard deviation. Differences between two groups were analyzed by Student's t-test or one-way-analysis of variance. Analysis and presentation of data was done with Graphpad prism 5 (GraphPad Software, La Jolla, CA).
SUPPLEMENTARY MATERIAL Figure S1. Peripheral blood mononuclear cells from 4 additional donors were collected and treated with the IL-15 containing drug 1615EpCAM.
Acknowledgments
We acknowledge the excellent technical assistance of Deborah Todhunter, Andy Sicheneder, Sami Chu, and Seunguk Oh. This work was supported in part by the US Public Health Service Grants R01-CA36725, R01-CA72669, P01-CA65493, P01-CA111412 and R35 CA197292 awarded by the NCI and the NIAID, DHHS. It was also supported by an NIH Research Evaluation and Commercialization Hub (REACH) Award (U01), the Mayo Partnership Award, the Lion Fund, William Lawrence and Blanche Hughes Fund, the Randy Shaver Foundation, the Atwater Cancer Drug Development Award, a CETI translational award from the University of Minnesota Masonic Cancer Center (D.A.V.) and the Deutsche Krebshilfe (111548) (J.U.S.). D.A.V. and J.S.M. are members of the Oxis Biotech Scientific Advisory Board and hold equity in the company. This relationship has been reviewed and managed by the University of Minnesota in accordance with its conflict of interest policies.
Supplementary Material
References
- Gastl, G, Spizzo, G, Obrist, P, Dünser, M and Mikuz, G (2000). Ep-CAM overexpression in breast cancer as a predictor of survival. Lancet 356: 1981–1982. [DOI] [PubMed] [Google Scholar]
- Gutzmer, R, Li, W, Sutterwala, S, Lemos, MP, Elizalde, JI, Urtishak, SL et al. (2004). A tumor-associated glycoprotein that blocks MHC class II-dependent antigen presentation by dendritic cells. J Immunol 173: 1023–1032. [DOI] [PubMed] [Google Scholar]
- Münz, M, Kieu, C, Mack, B, Schmitt, B, Zeidler, R and Gires, O (2004). The carcinoma-associated antigen EpCAM upregulates c-myc and induces cell proliferation. Oncogene 23: 5748–5758. [DOI] [PubMed] [Google Scholar]
- Ensinger, C, Kremser, R, Prommegger, R, Spizzo, G and Schmid, KW (2006). EpCAM overexpression in thyroid carcinomas: a histopathological study of 121 cases. J Immunother 29: 569–573. [DOI] [PubMed] [Google Scholar]
- Trzpis, M, McLaughlin, PM, de Leij, LM and Harmsen, MC (2007). Epithelial cell adhesion molecule: more than a carcinoma marker and adhesion molecule. Am J Pathol 171: 386–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munz, M, Baeuerle, PA and Gires, O (2009). The emerging role of EpCAM in cancer and stem cell signaling. Cancer Res 69: 5627–5629. [DOI] [PubMed] [Google Scholar]
- Gostner, JM, Fong, D, Wrulich, OA, Lehne, F, Zitt, M, Hermann, M et al. (2011). Effects of EpCAM overexpression on human breast cancer cell lines. BMC Cancer 11: 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita, T, Budhu, A, Forgues, M and Wang, XW (2007). Activation of hepatic stem cell marker EpCAM by Wnt-beta-catenin signaling in hepatocellular carcinoma. Cancer Res 67: 10831–10839. [DOI] [PubMed] [Google Scholar]
- Ströhlein, MA, Lordick, F, Rüttinger, D, Grützner, KU, Schemanski, OC, Jäger, M et al. (2011). Immunotherapy of peritoneal carcinomatosis with the antibody catumaxomab in colon, gastric, or pancreatic cancer: an open-label, multicenter, phase I/II trial. Onkologie 34: 101–108. [DOI] [PubMed] [Google Scholar]
- Bargou, R, Leo, E, Zugmaier, G, Klinger, M, Goebeler, M, Knop, S et al. (2008). Tumor regression in cancer patients by very low doses of a T cell-engaging antibody. Science 321: 974–977. [DOI] [PubMed] [Google Scholar]
- Barrett, DM, Teachey, DT and Grupp, SA (2014). Toxicity management for patients receiving novel T-cell engaging therapies. Curr Opin Pediatr 26: 43–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmohl, JU, Gleason, MK, Dougherty, PR, Miller, JS and Vallera, DA (2015). Heterodimeric bispecific single chain variable fragments (scFv) killer engagers (BiKEs) enhance NK-cell activity against CD133+ colorectal cancer cells. Target Oncol (epub ahead of print). doi: 10.1007/s11523-015-0391-8 [DOI] [PMC free article] [PubMed]
- Vallera, DA, Zhang, B, Gleason, MK, Oh, S, Weiner, LM, Kaufman, DS et al. (2013). Heterodimeric bispecific single-chain variable-fragment antibodies against EpCAM and CD16 induce effective antibody-dependent cellular cytotoxicity against human carcinoma cells. Cancer Biother Radiopharm 28: 274–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadakis, KA, Prehn, JL, Landers, C, Han, Q, Luo, X, Cha, SC et al. (2004). TL1A synergizes with IL-12 and IL-18 to enhance IFN-gamma production in human T cells and NK cells. J Immunol 172: 7002–7007. [DOI] [PubMed] [Google Scholar]
- Gleason, MK, Ross, JA, Warlick, ED, Lund, TC, Verneris, MR, Wiernik, A et al. (2014). CD16xCD33 bispecific killer cell engager (BiKE) activates NK cells against primary MDS and MDSC CD33+ targets. Blood 123: 3016–3026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alderson, KL and Sondel, PM (2011). Clinical cancer therapy by NK cells via antibody-dependent cell-mediated cytotoxicity. J Biomed Biotechnol 2011: 379123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson, WE, Giri, JG, Lindemann, MJ, Linett, ML, Ahdieh, M, Paxton, R et al. (1994). Interleukin (IL) 15 is a novel cytokine that activates human natural killer cells via components of the IL-2 receptor. J Exp Med 180: 1395–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehniger, TA, Shah, MH, Turner, MJ, VanDeusen, JB, Whitman, SP, Cooper, MA et al. (1999). Differential cytokine and chemokine gene expression by human NK cells following activation with IL-18 or IL-15 in combination with IL-12: implications for the innate immune response. J Immunol 162: 4511–4520. [PubMed] [Google Scholar]
- Cooper, MA, Fehniger, TA, Turner, SC, Chen, KS, Ghaheri, BA, Ghayur, T et al. (2001). Human natural killer cells: a unique innate immunoregulatory role for the CD56(bright) subset. Blood 97: 3146–3151. [DOI] [PubMed] [Google Scholar]
- Lanier, LL, Le, AM, Civin, CI, Loken, MR and Phillips, JH (1986). The relationship of CD16 (Leu-11) and Leu-19 (NKH-1) antigen expression on human peripheral blood NK cells and cytotoxic T lymphocytes. J Immunol 136: 4480–4486. [PubMed] [Google Scholar]
- Caligiuri, MA, Zmuidzinas, A, Manley, TJ, Levine, H, Smith, KA and Ritz, J (1990). Functional consequences of interleukin 2 receptor expression on resting human lymphocytes. Identification of a novel natural killer cell subset with high affinity receptors. J Exp Med 171: 1509–1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finney, HM, Akbar, AN and Lawson, AD (2004). Activation of resting human primary T cells with chimeric receptors: costimulation from CD28, inducible costimulator, CD134, and CD137 in series with signals from the TCR zeta chain. J Immunol 172: 104–113. [DOI] [PubMed] [Google Scholar]
- Imai, C, Mihara, K, Andreansky, M, Nicholson, IC, Pui, CH, Geiger, TL et al. (2004). Chimeric receptors with 4-1BB signaling capacity provoke potent cytotoxicity against acute lymphoblastic leukemia. Leukemia 18: 676–684. [DOI] [PubMed] [Google Scholar]
- Grupp, SA, Kalos, M, Barrett, D, Aplenc, R, Porter, DL, Rheingold, SR et al. (2013). Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med 368: 1509–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinz, K, Liu, H, Golightly, M, Jares, A, Lan, F, Zieve, GW et al. (2016). Preclinical targeting of human T-cell malignancies using CD4-specific chimeric antigen receptor (CAR)-engineered T cells. Leukemia 30: 701–707. [DOI] [PubMed] [Google Scholar]
- Osenga, KL, Hank, JA, Albertini, MR, Gan, J, Sternberg, AG, Eickhoff, J et al.; Children's Oncology Group. (2006). A phase I clinical trial of the hu14.18-IL2 (EMD 273063) as a treatment for children with refractory or recurrent neuroblastoma and melanoma: a study of the Children's Oncology Group. Clin Cancer Res 12: 1750–1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King, DM, Albertini, MR, Schalch, H, Hank, JA, Gan, J, Surfus, J et al. (2004). Phase I clinical trial of the immunocytokine EMD 273063 in melanoma patients. J Clin Oncol 22: 4463–4473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko, YJ, Bubley, GJ, Weber, R, Redfern, C, Gold, DP, Finke, L et al. (2004). Safety, pharmacokinetics, and biological pharmacodynamics of the immunocytokine EMD 273066 (huKS-IL2): results of a phase I trial in patients with prostate cancer. J Immunother 27: 232–239. [DOI] [PubMed] [Google Scholar]
- Connor, JP, Felder, M, Hank, J, Harter, J, Gan, J, Gillies, SD et al. (2004). Ex vivo evaluation of anti-EpCAM immunocytokine huKS-IL2 in ovarian cancer. J Immunother 27: 211–219. [DOI] [PubMed] [Google Scholar]
- Yamamoto, T, Yanagimoto, H, Satoi, S, Toyokawa, H, Hirooka, S, Yamaki, S et al. (2012). Circulating CD4+CD25+ regulatory T cells in patients with pancreatic cancer. Pancreas 41: 409–415. [DOI] [PubMed] [Google Scholar]
- Munger, W, DeJoy, SQ, Jeyaseelan, R Sr, Torley, LW, Grabstein, KH, Eisenmann, J et al. (1995). Studies evaluating the antitumor activity and toxicity of interleukin-15, a new T cell growth factor: comparison with interleukin-2. Cell Immunol 165: 289–293. [DOI] [PubMed] [Google Scholar]
- Ranson, T, Vosshenrich, CA, Corcuff, E, Richard, O, Müller, W and Di Santo, JP (2003). IL-15 is an essential mediator of peripheral NK-cell homeostasis. Blood 101: 4887–4893. [DOI] [PubMed] [Google Scholar]
- Huntington, ND, Legrand, N, Alves, NL, Jaron, B, Weijer, K, Plet, A et al. (2009). IL-15 trans-presentation promotes human NK cell development and differentiation in vivo. J Exp Med 206: 25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldmann, TA (2014). Interleukin-15 in the treatment of cancer. Expert Rev Clin Immunol 10: 1689–1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basak, GW, Zapala, L, Wysocki, PJ, Mackiewicz, A, Jakóbisiak, M and Lasek, W (2008). Interleukin 15 augments antitumor activity of cytokine gene-modified melanoma cell vaccines in a murine model. Oncol Rep 19: 1173–1179. [PubMed] [Google Scholar]
- Vallera, DA, Felices, M, McElmurry, RT, McCullar, V, Zhou, X, Schmohl, J et al. (2016). IL-15 trispecific killer engagers (TriKEs) make natural killer cells specific to CD33+ targets while also inducing in vivo expansion, and enhanced function. Clin Cancer Res (epub ahead of print). doi: 10.1158/1078-0432.CCR-15-2710 [DOI] [PMC free article] [PubMed]
- Conlon, KC, Lugli, E, Welles, HC, Rosenberg, SA, Fojo, AT, Morris, JC et al. (2015). Redistribution, hyperproliferation, activation of natural killer cells and CD8 T cells, and cytokine production during first-in-human clinical trial of recombinant human interleukin-15 in patients with cancer. J Clin Oncol 33: 74–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodge, DL, Yang, J, Buschman, MD, Schaughency, PM, Dang, H, Bere, W et al. (2009). Interleukin-15 enhances proteasomal degradation of bid in normal lymphocytes: implications for large granular lymphocyte leukemias. Cancer Res 69: 3986–3994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehniger, TA, Suzuki, K, VanDeusen, JB, Cooper, MA, Freud, AG and Caligiuri, MA (2001). Fatal leukemia in interleukin-15 transgenic mice. Blood Cells Mol Dis 27: 223–230. [DOI] [PubMed] [Google Scholar]
- Khawam, K, Giron-Michel, J, Gu, Y, Perier, A, Giuliani, M, Caignard, A et al. (2009). Human renal cancer cells express a novel membrane-bound interleukin-15 that induces, in response to the soluble interleukin-15 receptor alpha chain, epithelial-to-mesenchymal transition. Cancer Res 69: 1561–1569. [DOI] [PubMed] [Google Scholar]
- Kuniyasu, H, Ohmori, H, Sasaki, T, Sasahira, T, Yoshida, K, Kitadai, Y et al. (2003). Production of interleukin 15 by human colon cancer cells is associated with induction of mucosal hyperplasia, angiogenesis, and metastasis. Clin Cancer Res 9: 4802–4810. [PubMed] [Google Scholar]
- Kuniyasu, H, Oue, N, Nakae, D, Tsutsumi, M, Denda, A, Tsujiuchi, T et al. (2001). Interleukin-15 expression is associated with malignant potential in colon cancer cells. Pathobiology 69: 86–95. [DOI] [PubMed] [Google Scholar]
- Trentin, L, Cerutti, A, Zambello, R, Sancretta, R, Tassinari, C, Facco, M et al. (1996). Interleukin-15 promotes the growth of leukemic cells of patients with B-cell chronic lymphoproliferative disorders. Blood 87: 3327–3335. [PubMed] [Google Scholar]
- Zambello, R, Facco, M, Trentin, L, Sancetta, R, Tassinari, C, Perin, A et al. (1997). Interleukin-15 triggers the proliferation and cytotoxicity of granular lymphocytes in patients with lymphoproliferative disease of granular lymphocytes. Blood 89: 201–211. [PubMed] [Google Scholar]
- Baeuerle, PA and Gires, O (2007). EpCAM (CD326) finding its role in cancer. Br J Cancer 96: 417–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spizzo, G, Went, P, Dirnhofer, S, Obrist, P, Simon, R, Spichtin, H et al. (2004). High Ep-CAM expression is associated with poor prognosis in node-positive breast cancer. Breast Cancer Res Treat 86: 207–213. [DOI] [PubMed] [Google Scholar]
- Seimetz, D, Lindhofer, H and Bokemeyer, C (2010). Development and approval of the trifunctional antibody catumaxomab (anti-EpCAM x anti-CD3) as a targeted cancer immunotherapy. Cancer Treat Rev 36: 458–467. [DOI] [PubMed] [Google Scholar]
- Heiss, MM, Murawa, P, Koralewski, P, Kutarska, E, Kolesnik, OO, Ivanchenko, VV et al. (2010). The trifunctional antibody catumaxomab for the treatment of malignant ascites due to epithelial cancer: Results of a prospective randomized phase II/III trial. Int J Cancer 127: 2209–2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrelli, F, Borgonovo, K, Lonati, V, Elia, S and Barni, S (2013). Regression of liver metastases after treatment with intraperitoneal catumaxomab for malignant ascites due to breast cancer. Target Oncol 8: 291–294. [DOI] [PubMed] [Google Scholar]
- Bezan, A, Hohla, F, Meissnitzer, T and Greil, R (2013). Systemic effect of catumaxomab in a patient with metastasized colorectal cancer: a case report. BMC Cancer 13: 618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleason, MK, Verneris, MR, Todhunter, DA, Zhang, B, McCullar, V, Zhou, SX et al. (2012). Bispecific and trispecific killer cell engagers directly activate human NK cells through CD16 signaling and induce cytotoxicity and cytokine production. Mol Cancer Ther 11: 2674–2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein, C, Kellner, C, Kügler, M, Reiff, N, Mentz, K, Schwenkert, M et al. (2010). Novel conjugates of single-chain Fv antibody fragments specific for stem cell antigen CD123 mediate potent death of acute myeloid leukaemia cells. Br J Haematol 148: 879–889. [DOI] [PubMed] [Google Scholar]
- Singer, H, Kellner, C, Lanig, H, Aigner, M, Stockmeyer, B, Oduncu, F et al. (2010). Effective elimination of acute myeloid leukemic cells by recombinant bispecific antibody derivatives directed against CD33 and CD16. J Immunother 33: 599–608. [DOI] [PubMed] [Google Scholar]
- McCall, AM, Adams, GP, Amoroso, AR, Nielsen, UB, Zhang, L, Horak, E et al. (1999). Isolation and characterization of an anti-CD16 single-chain Fv fragment and construction of an anti-HER2/neu/anti-CD16 bispecific scFv that triggers CD16-dependent tumor cytolysis. Mol Immunol 36: 433–445. [DOI] [PubMed] [Google Scholar]
- Zhu, X, Marcus, WD, Xu, W, Lee, HI, Han, K, Egan, JO et al. (2009). Novel human interleukin-15 agonists. J Immunol 183: 3598–3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogh, J, Fogh, JM and Orfeo, T (1977). One hundred and twenty-seven cultured human tumor cell lines producing tumors in nude mice. J Natl Cancer Inst 59: 221–226. [DOI] [PubMed] [Google Scholar]
- Klein, E, Klein, G, Nadkarni, JS, Nadkarni, JJ, Wigzell, H and Clifford, P (1968). Surface IgM-kappa specificity on a Burkitt lymphoma cell in vivo and in derived culture lines. Cancer Res 28: 1300–1310. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.