Abstract
Retroviral engineering of hematopoietic stem cell-derived precursor T-cells (preTs) opens the possibility of targeted T-cell transfer across human leukocyte antigen (HLA)-barriers. Alpharetroviral vectors exhibit a more neutral integration pattern thereby reducing the risk of insertional mutagenesis. Cord blood-derived CD34+ cells were transduced and differentiated into preTs in vitro. Two promoters, elongation-factor-1-short-form, and a myeloproliferative sarcoma virus variant in combination with two commonly used envelopes were comparatively assessed choosing enhanced green fluorescent protein or a third-generation chimeric antigen receptor (CAR) against CD123 as gene of interest. Furthermore, the inducible suicide gene iCaspase 9 has been validated. Combining the sarcoma virus-derived promoter with a modified feline endogenous retrovirus envelope glycoprotein yielded in superior transgene expression and transduction rates. Fresh and previously frozen CD34+ cells showed similar transduction and expansion rates. Transgene-positive cells did neither show proliferative impairment nor alteration in their lymphoid differentiation profile. The sarcoma virus-derived promoter only could express sufficient levels of iCaspase 9 to mediate dimerizer-induced apoptosis. Finally, the CD123 CAR was efficiently expressed in CD34+ cells and proved to be functional when expressed on differentiated T-cells. Therefore, the transduction of CD34+ cells with alpharetroviral vectors represents a feasible and potentially safer approach for stem cell-based immunotherapies for cancer.
Introduction
Cord blood (CB) is now a widely used source of hematopoietic stem cells (HSCs) for allogeneic hematopoietic stem cell transplantation (allo-HSCT).1,2,3 The nature of its source excludes the possibility of returning to the donor to retransplant HSCs or T-cells to treat engraftment failure or relapse of malignancy. Thus, alternative T-cell sources to enhance immune reconstitution are needed. Over the last decade, coculture systems based on Notch ligand expressing bone marrow stroma cell lines have been developed allowing the differentiation and generation of T-cell-committed precursors (preTs).4,5 These systems can be controlled to limit the differentiation of the precursors to early thymic preTs which upon cotransplantation into allogeneic recipients undergo further development in vivo including T-cell receptor (TCR) rearrangement, TCRβ-selection and both, positive and negative selection of developing thymocytes.6,7 As a result, cotransplantation of preTs allows T-cell reconstitution of an immunosuppressed host across major histocompatibility complex (MHC)-barriers without the risk for graft-versus-host-disease while maintaining predominantly host-derived antigen presenting cell chimerism.5
The antitumor effects of preTs can be enhanced through genetic engineering with either chimeric antigen receptors (CARs) or TCRs against tumor-associated antigens.8,9 However, whereas genetic engineering of mature T-cells using gammaretroviral vectors has remained demonstrably safe without serious adverse effects due to insertional mutagenesis, this remains a major safety concern when engineering HSCs and incompletely differentiated T-cells.10,11 In contrast to gammaretroviral vectors, alpharetroviral vectors have a neutral integration pattern and can be readily designed to lack strong splice signals that might interfere with cellular mRNA processing.12,13
Here, we used an alpharetroviral vector system to genetically engineer human CB-derived CD34+ HSCs that were subsequently differentiated in vitro into preTs. We comparatively assessed the myeloproliferative sarcoma virus (MPSV) and the short form of the constitutively acting elongation factor 1α (EFS) promoter system in combination with either the vesicular stomatitis virus glycoprotein (VSVG) or a modified feline endogenous retrovirus glycoprotein (RD114/TR) envelope. We show that superior transduction and expression rates of the gene of interest (GOI) are physiologically highly relevant, especially if inducible caspase 9 (iCasp9) is used as a suicide gene. We observed that transducing CB-derived CD34+ cells with the alpharetroviral construct carrying a third-generation CAR against CD123 does slightly delay the in vitro differentiation process of preTs when using the OP9-DL1 coculturing system. The transduction efficiency and expansion patterns of preTs from fresh or previously frozen CB were comparable. We further demonstrate for the first time that T-cells expressing a CAR against CD123, that had been cloned into an alpharetroviral backbone, are functional and effective against CD123-expressing target cells. Altogether, we present a novel alpharetroviral system for potential clinical use when CB-derived CD34+ cells for the generation of preTs and T-cells are to be genetically engineered.
Results
Human CB-derived CD34+ cells are differentiated into preTs in vitro
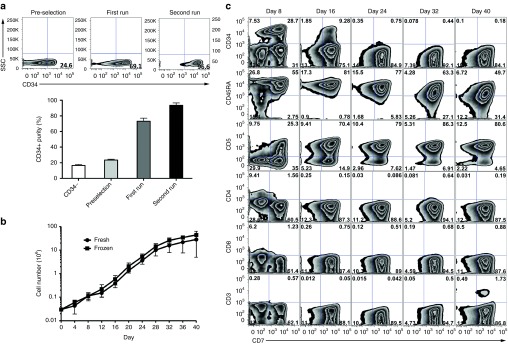
For generation of human preTs, CB was used as a source of CD34+ HSCs. CB samples were obtained from consenting mothers and CD34+ cells were isolated via two rounds of magnetic-activated cell sorting. The CD34+ purity was consistently higher (93 ± 3%) when two purification steps were performed (Figure 1a). Freshly isolated or thawed CD34+ cells were cocultured with the Notch ligand-expressing OP9-DL1 stromal cells having previously been shown to support in vitro generation of human preTs.14 The kinetics of preT growth, which were comparable for both, fresh and frozen CD34+ cells, revealed slower cell proliferation up to day 12, and a more rapid cell growth until day 28 which was followed by a plateau phase. We observed an expansion rate of up to 750-fold until day 28 (Figure 1b).
Figure 1.
Human cord blood (CB)-derived CD34+ cells are differentiated into precursor T cells (preTs) in vitro. (a) Human CB samples were obtained from consenting mothers and CD34+ cells consecutively isolated via positive selection by magnetic-activated cell sorting (MACS). Purity was determined by flow cytometry after one or two rounds of MACS (n = 2). (b) Fresh or thawed CD34+ cells were cocultured on OP9-DL1 stromal cells in a cytokine cocktail for preT differentiation. The proliferation rate of the cells was assessed every 4 days (n = 3). (c) The lymphoid phenotype of the cells was determined by flow cytometry every 8 days for a period of 40 days. Results of a representative experiment are shown.
To assess in vitro development, preTs were phenotyped by flow cytometry. As shown in Figure 1c, the expression of CD34 decreased over time and disappeared by day 24. The T-cell development markers CD45RA, CD7, and CD5 increased during the culture period. A small population of cells only started to express CD3 by day 40. We did not observe the appearance of CD4- and CD8-positive cells over the whole culturing course. Of note, CD34+ CD7+ progenitor T-cells that represent the thymus-engrafting population were most prominent on day 8 and had disappeared by day 24.7
Alpharetroviral vectors containing an MPSV promoter and an RD114/TR envelope deliver enhanced gene transfer into CB-derived CD34+ HSCs
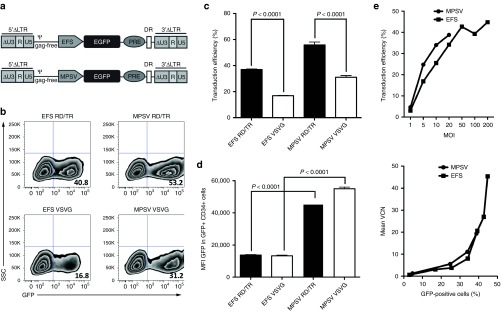
To decrease the risk of insertional mutagenesis after transduction of CD34+ cells, we used self-inactivating (SIN) alpharetroviral vectors.12,13 To improve gene transfer into CD34+ cells, we compared alpharetroviral vectors containing two different promoters, either using the EFS or the MPSV promoter. Respective retroviral particles were pseudotyped with either the VSVG or RD114/TR envelope. Enhanced green fluorescent protein (EGFP) was used as a reporter gene (Figure 2a). Using similar viral multiplicity of infection (MOI), transduction efficiency of CD34+ cells with RD114/TR- versus VSVG-pseudotyped vectors was up to twofold higher (percentage of EGFP+ cells: 55.8 ± 2.3 versus 31.1 ± 1.3 for the MPSV vectors and 37 ± 0.3 versus 16.9 ± 0.2 for the EFS vectors, respectively) (Figure 2b,c). As compared to EFS, MPSV-driven vectors resulted in up to fourfold increased transgene expression (EGFP mean fluorescence intensity: 45,484 ± 1,428 versus 13,720 ± 345 for the RD114/TR-pseudotyped and 55,035 ± 943 versus 13,274 ± 431 for the VSVG-pseudotyped vectors, respectively) (Figure 2d).
Figure 2.
Alpharetroviral vectors containing a myeloproliferative sarcoma virus (MPSV) promoter and the modified feline endogenous retrovirus envelope glycoprotein RD114/TR deliver enhanced gene transfer into cord blood (CB)-derived CD34+ hematopoietic stem cells. (a) Self-inactivating (SIN) alpharetroviral vectors containing either an MPSV or an elongation-factor-1-short-form (EFS) promoter combined with an enhanced green fluorescent protein (EGFP) reporter gene were generated. Indicated are unique 5 (U5), repeat (R), and SIN unique 3 (ΔU3) regions, long terminal repeat (LTR), packaging signal (ψ), woodchuck posttranscriptional regulatory element (PRE) and direct repeat element (DR). (b) MPSV- and EFS-driven alpharetroviral vectors were pseudotyped with either vesicular stomatitis virus glycoprotein (VSVG) or RD114/TR and used to transduce CD34+ cells using an equal multiplicity of infection (MOI) of 100. (c,d) Transduction efficiency and mean fluorescence intensity (MFI) of EGFP were determined 6 days after transduction. Representative results of two independent experiments are shown (n = 3). (e) CB CD34+ cells were transduced with an increasing MOI using alpharetroviral MPSV- or EFS-driven vectors with the EGFP gene. After 6 days, transduction efficiency was determined by flow cytometry. Mean vector copy number (VCN) was assessed using quantitative real-time PCR for detection of PRE within the vector.
As the number of viral vectors integrating into the genome can increase the risk of insertional mutagenesis, we assessed the impact of vector MOI on transduction efficiency and mean vector copy number (VCN). As shown in Figure 2e, increasing MOIs do enhance transduction efficiency of transduced human CD34+ cells. This increase is more prominent at lower MOIs and reaches a plateau level at MOIs of more than 100. There was a linear correlation between transduction efficiencies of less than 25% and VCN.15 For higher transduction efficiencies, this correlation becomes exponential. Collectively, these data suggest that at lower MOIs, an increase in transduction efficiency is associated with a proportional enhancement of VCN. Nevertheless, whereas higher MOIs are associated with modest increase in transduction efficiencies only, they result in a steep increase in VCN.
Transduction procedure and transgene positivity of human CB-derived CD34+ cells do neither impact the proliferation nor the differentiation pattern toward preTs in vitro when using EGFP as an insert only
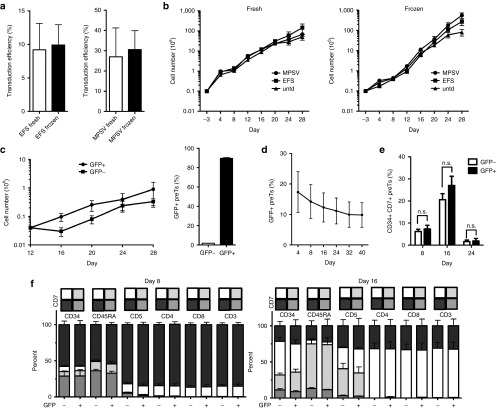
Since frozen CB CD34+ cells could be stored and used on demand, we compared transduction efficiency and proliferation rate of freshly isolated versus thawed CD34+ cells. As GOI, a cassette containing EGFP only was used. Figure 3a illustrates that transduction efficiency of CD34+ cells, transduced with either RD114/TR pseudotyped MPSV- or EFS-driven vectors, was comparable for both, fresh and thawed CD34+ cells. Curves showing proliferation kinetics of fresh and thawed CD34+ cells were nearly superimposable (Figure 3b).
Figure 3.
Transduction procedure and transgene positivity of human cord blood (CB)-derived CD34+ cells do neither impact the proliferation nor the differentiation pattern towards precursor T cells (preTs) in vitro when using enhanced green fluorescent protein (EGFP) as an insert only. (a) Fresh or thawed CB CD34+ cells were transduced with alpharetroviral vectors containing an EGFP gene serving as both, reporter and gene of interest. A multiplicity of infection of 10 was used. Transduction efficiency was determined by flow cytometry after 6 days (n = 3). Indicated are myeloproliferative sarcoma virus (MPSV) and elongation-factor-1-short-form (EFS) promoter. (b) Transduced or control untransduced (untd) preTs were cocultured with OP9-DL1 cells for 28 days. The proliferation rate of the cells was assessed every 4 days using trypanblue for determination of viability (n = 3). (c) Transduced preTs were sorted for EGFP expression (GFP+) on day 8 and consecutively continued to be cultured on OP9-DL1 cells under comparable conditions for up to 28 days. EGFP expression was determined on day 32 (24 days after sorting) (n = 3). (d) EGFP transgene expression of transduced but nonsorted preTs was determined over a period of 40 days (n = 3). (e) The percentage of nontransduced and transduced CD34+ CD7+ preTs was determined by flow cytometry on day 8, 16, and 24. (f) The phenotype of GFP- and GFP+ preTs was determined on day 8 and 16. Gray-colored bars depict subpopulations of preTs.
We sorted EGFP+ cells on day 12. As shown in Figure 3c, sorted cells expanded and remained 90% transgene positive for at least 24 days. By sorting, we enriched for transduced cells, since their content in culture slightly reduced over time (Figure 3d).
It has been shown that a subset of in vitro-generated preTs coexpressing both, CD34 and CD7, has high engraftment potential after adoptive transfer.7 As the percentage of CD34+ cells decreases with ongoing differentiation and the content of CD7+ is increasing, we asked whether transduction of CD34+ cells could possibly alter their differentiation pattern. In contrast to the construct additionally carrying a CAR against CD123 (see Figure 6), phenotype comparison of EGFP+ and EGFP− populations showed comparable differentiation phenotypes on day 8, 16 (Figure 3e,f), and 24 (data not shown) if MOIs of 10 were used.
Transgene expression strength mediated by the MPSV promoter determines efficacy of iCasp9-induced apoptosis and allows efficient CD123 CAR expression in preTs
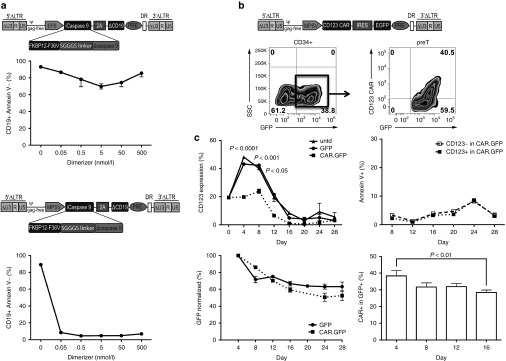
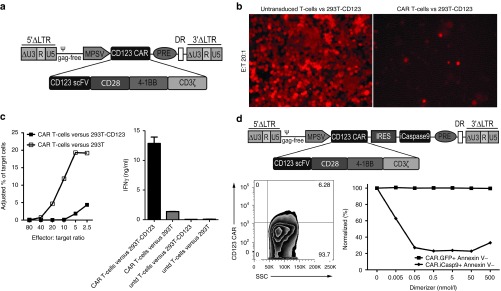
To provide an effective safety switch for adoptive transfer of preTs, we used the iCasp9 suicide gene technology. The iCasp9 cassette, which is comprised of a modified human caspase 9 gene fused to a human FK506-binding protein was additionally linked to a truncated human CD19 reporter gene via a 2A sequence and cloned into alpharetroviral vectors under the control of the EFS or MPSV promoter (Figure 4a). To assess the functionality of the constructs, Jurkat cells were transduced and enriched for CD19 transgene-positive cells by cell sorting. The dimerizing agent AP20187 was added at various concentrations to induce apoptosis and the percentage of surviving transgene positive cells (CD19+ Annexin V-) was assessed by flow cytometry 72 hours later. Whereas the majority (90%) survived up to 500 nmol/l of the dimerizing agent after transduction with the EFS-driven vector, less than 10% survived at dimerizer concentrations of 0.05 nmol/l after transduction with the MPSV-containing vector (Figure 4a). These data emphasize the physiological relevance of promoter selection for sufficient expression of the iCasp9 suicide gene.
Figure 4.
Transgene expression strength mediated by the myeloproliferative sarcoma virus (MPSV) promoter determines efficacy of inducible caspase 9 (iCasp9)-induced apoptosis and allows efficient CD123 chimeric antigen receptor (CAR) expression in precursor T cells (preTs). (a) iCasp9 containing the human FK506-binding protein (FKBP12) with an F36V mutation and a truncated ΔCD19 reporter gene was cloned into an alpharetroviral backbone under the elongation-factor-1-short-form (EFS) or MPSV promoter. Human Jurkat cells were transduced with fresh retrovirus and expanded in vitro. Transgene positive cells were sorted based on ΔCD19 expression and cultured with increasing concentrations of a dimerizing agent. Three days later, cell viability was assessed based on Annexin V staining. The percentage of transgene positive cells that did not undergo apoptosis upon dimerizer administration (CD19+ Annexin V-) was used for comparison (n = 3). (b,c) The CD123 CAR gene using an enhanced green fluorescent protein (EGFP) marker was cloned into an MPSV alpharetroviral backbone. Thawed CD34+ cells were transduced with modified feline endogenous retrovirus envelope glycoprotein (RD114/TR)-pseudotyped retroviral particles from the CAR.GFP, the GFP only construct or left untransduced (untd). At indicated time points during the OP9-DL1 coculture, preTs were assessed by flow cytometry for CD123, Annexin V, EGFP, and the CAR (n = 3). CAR.GFP groups are shown as dotted lines. Indicated are unique 5 (U5), repeat (R), and self-inactivating unique 3 (ΔU3) regions, long terminal repeat (LTR), packaging signal (ψ), internal ribosomal entry site (IRES), woodchuck posttranscriptional regulatory element (PRE) and direct repeat element (DR).
Due to this observation, we decided to use the MPSV promoter for subsequent transduction experiments. We designed a third-generation CAR as a potentially relevant GOI. It consisted of a CD123-specific single chain variable fragment (scFV) being fused to CD28 and 4-1BB costimulatory molecules and a CD3ζ signaling domain as well as an EGFP reporter gene for first functionality assays. Umbilical cord blood (UCB) CD34+ cells were transduced with this construct and EGFP expression readily detected. Upon subsequent coculture with OP9-DL1 cells, we stained for CAR expression of preTs and observed that around 40% of EGFP+ cells can be stained for the CD123 CAR (Figure 4b).
Owing to the fact that CD123 is expressed on multipotent progenitor cells, we investigated whether forced CD123 CAR expression on CD34+ cells and preTs would impact CD123 expression, viability, and transgene expression of transduced preTs. In the CAR.GFP-transduced group, CD123 expression during OP9-DL1 coculture was significantly reduced. This effect was predominantly seen between day 4 and 8 of differentiation culture suggesting some degree of fratricide (Figure 4c). Of note, CD123 expression in CAR.GFP+ preTs was more markedly decreased than in CAR.GFP- preTs (Supplementary Figure S1). No increased Annexin V binding of CD123+ as compared to CD123- preTs in the CAR.GFP-transduced preT population could be demonstrated after day 8, which makes fratricide by CD123 CAR-expressing preTs unlikely from day 8 onwards (Figure 4c). In line with this finding, the slow decline of transgene expression over time in the CAR.GFP-transduced group did not differ in its kinetics from the GFP only-transduced group allowing for comparable cell yields at the end. CAR expression of EGFP+ preTs declined as well from around 38% on day 4 to 28% on day 16. This is most likely caused by a proliferation disadvantage of the respective cell population after insertion of a relatively large gene cassette and not as a result of fratricide as shown above (Figure 4c).
Mature human T-cells expressing a third-generation CAR against CD123, or CD123 CAR and iCasp9 in combination are functional in vitro
For proof of principle experiments using a potentially relevant GOI, we evaluated the function of the new CD123 CAR and transduced human peripheral blood mononuclear cells (PBMCs) with the MPSV promoter-driven SIN alpharetroviral vector, into which our proof of principle GOI was cloned. Functionality was assessed by enzyme-linked immunosorbent assay and a fluorescence-based in vitro cytotoxicity assay. For targets, 293T cells were transduced with retroviral vectors using the human CD123 sequence which had been linked to tdTomato via an internal ribosomal entry site (IRES) cassette. Two sorting rounds ensured over 98% CD123 expression. Nontransduced 293T cells were used as controls. CD123 CAR T-cells strongly produced IFNγ upon stimulation on CD123+ target cells (Figure 5c). More importantly, these cells effectively lysed target cells expressing the CD123 antigen, as evident by a nearly complete loss of the tdTomato signal (Figure 5b). Antigen-negative target cells were spared demonstrating specific recognition of CD123 (Figure 5c). Nontransduced PBMCs mediated a weak background response only, being comparable for both, antigen-negative and antigen-positive target cells (Figure 5c).
Figure 5.
Mature human T-cells expressing a third-generation chimeric antigen receptor (CAR) against CD123, or CD123 CAR and inducible caspase 9 (iCasp9) in combination are functional in vitro. (a) Schematic figure of the MPSV.CD123 CAR construct. Self-inactivating (SIN) alpharetroviral vectors encoding for a third-generation CAR against the human surface molecule CD123 were generated. (b) Human peripheral blood mononuclear cells (PBMCs) were activated with anti-CD3 and anti-CD28 antibodies for 2 days, transduced on 2 consecutive days, and expanded for 4 more days before being used in a cytotoxicity assay. TdTomato.CD123-transduced 293T cells were used as target cells. Decreasing numbers of untransduced or CAR-transduced PBMCs were cultured with target cells in triplicates for 2 days. TdTomato expression was used as a marker for surviving target cells in immunofluorescence microscopy studies. (c) Cytotoxicity of the transduced effectors was determined by means of flow cytometry. Cell suspensions containing CAR-transduced effector cells and targets (either 293T cells only or 293T cells expressing CD123) at indicated ratios were stained for CD3. The percentage of viable CD3neg cells (surviving target cells) after incubation was used as an indirect parameter for cytotoxicity (left panel). Using an IFNγ release assay, T-cells (2 × 105) and target cells (2 × 104) were incubated at fixed effector:target ratios of 10:1 in 96-well plates. After 24 hours, supernatant was harvested and used in duplicates for an IFNγ enzyme-linked immunosorbent assay (right panel) (n = 2). Untransduced (untd) T-cells were used as control. (d) Schematic figure of the MPSV.CD123 CAR.iCasp9 vector. Human PBMCs were transduced with this bicistronic construct. Transgene positive cells with a functional CAR were identified by specific cell binding of a His Tag-labeled CD123 peptide. Transduced cells were incubated with the dimerizer for 48 hours. CAR.GFP-transduced PBMCs were used as control (n = 3). Apoptosis was quantified by staining viable transgene positive (CAR+ or GFP+) Annexin V-PBMCs. Indicated are unique 5 (U5), repeat (R), and SIN unique 3 (ΔU3) regions, long terminal repeat (LTR), packaging signal (ψ), myeloproliferative sarcoma virus promoter (MPSV), single chain variable fragment (scFV), internal ribosomal entry site (IRES), woodchuck posttranscriptional regulatory element (PRE) and direct repeat element (DR).
After evaluating iCasp9 and the CD123 CAR in separate vector constructs, we designed a clinically more relevant combined gene cassette encoding both transgenes under the control of the MPSV promoter and used it for transduction of PBMCs. After transduction, the CD123 CAR could be specifically stained by flow cytometry using a CD123 peptide. To assess functionality of this combined construct, apoptosis was induced with the dimerizer. We did not observe any effects of the dimerizer on CAR.GFP-transduced PBMCs that were used as controls demonstrating the selectivity of dimerizer-mediated induction of apoptosis. A concentration as low as 0.05 nmol/l was sufficient to induce the maximal apoptosis rates in CAR.iCasp9-transduced PBMCs (Figure 5d). These functionality data encourage the further use of alpharetroviral vector platforms for adoptive T-cell transfer studies in preclinical models of leukemia.
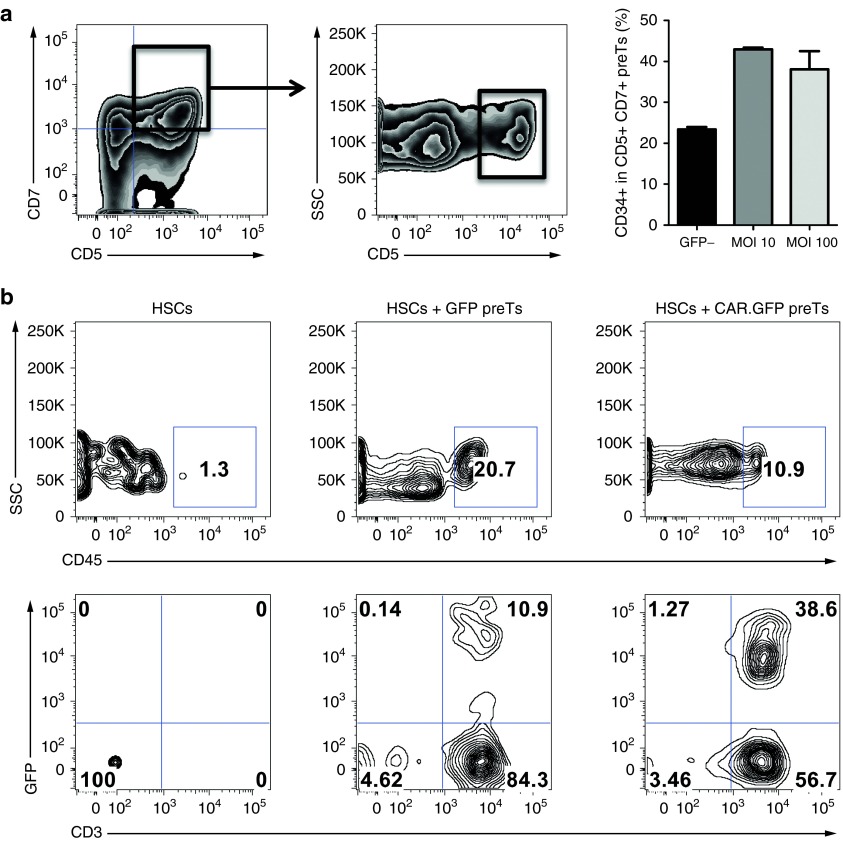
Equipping the alpharetroviral construct with the sequence of a third-generation CD123 CAR delays preT differentiation in vitro and allows for thymic engraftment and further differentiation in vivo
Since the phenotype of the preT-product has been shown to profoundly impact its thymic repopulating capacities (a prerequisite for further release of matured T-cell progeny into the periphery), we assessed the potential influence of the complete CAR-containing construct on in vitro differentiation of CD34+ cells in the OP9-DL1 coculture system by comparing our standard MOI of 10 to an MOI of 100. Nontransduced cells within the same culture were used as controls. CD34 on the early developing CD7+ CD5+ population has been demonstrated to be a sensitive differentiation marker and its coexpression facilitated robust thymic engraftment.7 Using the CD123 CAR containing construct, coexpression of CD34 by day 11 was significantly higher than on the nontransduced control population. This suggests a differentiation delay induced by transduction. No differences were seen between the lower and the higher MOI (Figure 6a). PreTs harvested by day 11 of culture were sorted by fluorescence-activated cell sorting and consecutively injected intrahepatically into irradiated (1 Gy) 4 day old baby NSG (NOD.cg-PrkdcscidIL2rgtm/Wjl/Sz) mice. Six weeks after injection, thymi were harvested and analyzed for transgene-positive CD3 expressing thymocytes of human origin (Figure 6b). Whereas control mice, that had received CB-derived HSCs only, did not show any evidence of thymic engraftment at this time point, transgene positive CD3+ thymocytes could be readily detected in both treatment groups (CD123 CAR.GFP preTs or GFP only preTs) (Figure 6b). This demonstrates the engraftment capacity of alpharetrovirally-engineered preTs.
Figure 6.
Equipping the alpharetroviral construct with the sequence of a third-generation CD123 chimeric antigen receptor (CAR) delays precursor T cell (preT) differentiation in vitro and allows for thymic engraftment and further differentiation in vivo. (a) Cord blood (CB)-derived CD34+ cells were transduced with the CD123 CAR-containing alpharetroviral construct either using a multiplicity of infection (MOI) of 10 or 100. Enhanced green fluorescent protein (GFP) was used as reporter gene. After transduction, cells were brought into the OP9-DL1 coculture system and phenotypically analyzed on day 11. Within the GFP+ population (transduced cells) CD5+ CD7+ were gated on and assessed for CD34 coexpression. The GFP- fraction (nontransduced) served as control. CD34 coexpression was lower on nontransduced as compared to transduced CD5+ CD7+ cells (P < 0.05). No significant difference was found between MOI 10 and 100. Two independent experiments were performed. (b) 2 × 105 transgene positive preTs (either transduced with GFP only or CAR.GFP) together with 0.2 × 105 CB-derived CD34+ hematopoietic stem cells (HSCs) were intrahepatically injected into 4 day old irradiated NSG mice. Controls were injected with HSCs only. Mice were boosted with the IL-7/M25 mixture every 5 days. Thymi were harvested 6 weeks after injection, single cell suspensions were generated and analyzed by flow cytometry. The upper row shows the fraction of human-derived cells, which were obtained from the different treatment groups. After gating on this human-derived cell population, transgene-positive cells expressing CD3 can be found in the thymi of treated mice (lower row).
Discussion
Redirecting the specificity of T-cells against tumor-associated antigens by genetically enforced expression of TCRs or CARs has recently boosted the field of adoptive T-cell transfer.16,17,18 The use of second- and third-generation CARs has helped to resolve the long standing problem of insufficient in vivo T-cell persistence after transfer that was severely hampering its efficacy.19,20 Nevertheless, important obstacles for a wider application are still remaining such as the necessity to produce a T-cell product on an individualized basis making this promising treatment approach hardly economically feasible. Although recent studies have successfully reported the use of autologous T-cells, it can be difficult to obtain suitable numbers of autologous cells in heavily pretreated patients.21,22
Human preTs might serve as the basis of a prefabricated cellular product that can be given even in a third party transplant setting across complete human leukocyte antigen (HLA) barriers without evoking graft-versus-host-disease. Their need to undergo thymic selection processes after cotransfer represents a potent filter preventing the release of graft-versus-host-disease-inducing alloreactive mature T-cells.9 Alternative developments might even allow the use of post-thymic mature T-cells for multiple recipients. Elegant concepts are pursued wherein CAR-modified T-cells are subject to genetic editing and by doing so eliminating the expression of the endogenous αβ TCR.23 Whereas we and others have shown that genetically engineered preTs can give rise to a long persisting tumor-specific T-cell population in vivo—a prerequisite for efficient antitumor efficacy9—this is, to our knowledge, still to be demonstrated for TCR-edited T-cells.
In vitro generation of human preTs from UCB has proven to compare favorably to their generation from bone marrow and peripheral blood stem cells.4,14,24 T-cell potential of hematopoietic progenitors is known to decline with age as demonstrated in experiments conducted in fetal thymic organ cultures.25 Since the demonstration of thymus-independent T-cell development in vitro using delta-like-1 by TM Schmitt and JC Zúñiga-Pflücker different human stem cell sources have been used on this system. Apart from studies with human UCB cells,7,14 in vitro T-cell differentiation has been performed using embryonic stem cells,26 adult bone marrow-derived hematopoietic progenitors,24 and G-CSF-mobilized peripheral blood stem cells.27 Work done by Patel et al.28 comparing T-cell differentiation from various sources of CD34+ cells showed important differences in the kinetics and extent of proliferation prior to β-selection with a 10-fold greater expansion of CB cells as compared to bone marrow cells. Therefore, different sources of CD34+ cells are not expected to yield a product of numerical comparability. Given the availability of CB, its biological proximity to fetal tissue, and the low degree of ethical concern as compared to other fetal stem cell sources, we decided to focus our studies on CB. In a next step, we were interested whether the use of frozen CB-derived preTs would result in comparable expansion and differentiation in an OP9-DL1 coculturing system. The fact that we could demonstrate superimposable expansion curves, both of fresh and thawed CB-derived CD34+ cells, supports the concept that frozen CB cells can serve for this technology.
Decreasing thymic function in elderly bone marrow transplant (BMT) recipients has raised concerns whether the cotransfer of preTs would sufficiently give rise to a respective post-thymic mature T-cell progeny. An increasing amount of evidence suggests that the potential for extrathymic T-cell development is strongly enhanced after BMT. It has been nicely shown that the extrathymic microenvironment, importantly the mesenterial lymphatic lymph node system of the intestine, can provide the required cues to harbor and further differentiate preTs into a functional extrathymically-derived T-cell pool. These data have underlined the potential of extrathymic T-cell development for T-cell reconstitution in patients with limited thymic function.29
Especially for lentiviral constructs, although being very efficient for transducing end-differentiated T-cells, the application of stable packaging cells for economical large scale vector production has proven to be extraordinarily difficult.30,31 Therefore, for the production of genetically engineered preTs, gammaretroviral systems remain attractive since stable packaging cell lines can deliver consistency required for market authorization.32 Furthermore, transducing a relatively small number of CD34+ cells for later differentiation into much larger numbers of preTs minimizes the amount of viral supernatant needed for transduction.
For the transduction of CB-derived hematopoietic precursors, we use a new generation of alpharetroviral SIN vectors since genotoxicity will be a major concern if genetically engineered preTs will be considered for clinical use. As comparatively shown by us in a serial murine transplantation model, the use of alpharetroviral vectors can result in sustained multilineage transgene expression being comparable to lentiviral and gammaretroviral SIN vectors.12 In contrast to the latter two, alpharetroviral integration showed to be less frequently occurring close to transcription start sites, CpG islands, and potential oncogenes. Of note, the propensity of the “extragenetic” genomic integration pattern of alpharetroviruses still suggested long-term transgene expression as demonstrated in serial transplant experiments. This extremely elegant comparative analysis of Suerth and co-workers was performed using the SFFV internal promoter for all three tested vector systems thereby modeling a “worst case scenario” for both, insertional gene activation and epigenetic silencing.12,33,34,35 However, despite reported interlineage variability a trend versus lowest transgene expression rates was seen when using alpharetroviral vectors. Therefore, for the generation of genetically engineered preTs, we targeted previously enriched CB-derived CD34+ cells and comparatively assessed the use of two promoters (EFS versus MPSV), combining each with improved envelope glycoproteins, either VSVG or RD114/TR. The comparative study by Suerth and co-workers observed a reduced incidence of immortalization and a tendency for decreased fitness in immortalized cells when using alpharetroviral vectors with the GOI put under the SFFV promoter. Exchanging this internal “high risk” promoter with the EFS promoter abolished any evidence of genotoxic potential within the detection limits of their in vitro immortalization assay.
Engineering CB-derived CD34+ cells, we observe a significantly enhanced gene expression strength and transduction efficiency when using the MPSV promoter in combination with the RD114/TR envelope glycoprotein for pseudotyping the particles. Importantly, these differences were physiologically relevant when iCasp9 as a suicide gene was put under the respective promoter control. Adding the clinically applicable apoptosis-inducing dimerizer AP20187 in vitro, target cells containing the GOI under the control of the MPSV promoter only underwent rapid apoptosis at concentrations of the dimerizer being relevant for clinical studies.36 Therefore, for further experiments (Figure 4b,5,6) alpharetroviral constructs containing the MPSV promoter were used only. In these studies, we could demonstrate that transduction had no impact on preT expansion rates regardless of whether CD34+ cells were used either freshly isolated or after storage in liquid nitrogen. This has important implications when evaluating a cell product with a potential for “off the shelf” use. Last, transduction of CB-derived CD34+ cells did not seem to influence the differentiation pattern along the different stages of preT development in vitro. However, this was the case only when EGFP alone was used as GOI. Adding a third-generation CD123 CAR to the construct caused maturation delay in vitro as demonstrated by a longer persistence of CD34 expression on CD5+ CD7+ early preTs. This is relevant since the human CD34+ CD7+ cellular equivalent to murine DN2 cells has the highest potential of thymic seeding after cotransfer and will consecutively give rise to a fully functional host MHC-restricted T-cell population.5,14,37
With CD123 being expressed on hematopoietic precursor cells, one might hypothesize that forced CD123 CAR expression on CD34+ cells and their further differentiated progeny might cause fratricide among CD123-expressing cells during in vitro culture. Indeed, this seems to be the case early in the differentiation process. However, this fratricide showed to be incomplete and decreases over time, most likely caused by decreasing levels of CD123 expression upon differentiation and an incomplete killing machinery of preTs. In this context, one needs to state, that some functions of an introduced construct can be functionally assessed only after preTs have undergone final maturation in the recipient. This holds specifically true for genes interfering with T-cell effector mechanism such as TCRs and CARs. It was therefore important to demonstrate in a humanized mouse model that the engineered preTs had indeed thymus engrafting potential with consecutive further T-cell developmental capacities in vivo.
Altogether, we apply a novel and improved alpharetroviral vector system for the generation of genetically engineered preTs. As proof of principle, we cloned three different genes of interest, either EGFP, the suicide gene iCasp9 or a CAR against CD123 into this construct. If once considered for clinical use, this system might have the potential to make engineering of preTs safer and economically feasible.
Materials and Methods
Primary samples and cell lines. Human UCB samples (approximately 50 ml/sample), not eligible for banking, were obtained after written, informed consent by the child's mother. Procedures for the use of UCB for this study were reviewed and approved by the medical ethics committee of Hannover Medical School. CB mononuclear cells were isolated using Ficoll density centrifugation and CD34 selection was performed using a CD34 microbead kit (Miltenyi Biotec, Bergisch Gladbach, Germany) according to the manufacturer's instructions. Purity of CD34+ cells was higher than 95% as determined by postenrichment flow cytometric analysis. CD34+ cells were cryopreserved in 50% fetal calf serum, 40% αMinimum Essential Medium, and 10% dimethylsulfoxide.
OP9-DL1 cells were cultured in αMinimum Essential Medium containing 20% heat-inactivated fetal calf serum. UCB CD34+ cells were stimulated for 36 hours in X-VIVO 10 medium containing the following human cytokines: stem cell factor (SCF, 100 ng/ml), thrombopoietin (TPO, 100 ng/ml), and FMS-like tyrosine kinase 3 ligand (Flt3L, 100 ng/ml) (all PeproTech, Rocky Hill, NJ). Stimulated CD34+ cells were transduced on Retronectin-coated 24-well plates. Cells were then transferred on 90% confluent OP9-DL1 cell monolayers containing 20% fetal calf serum, SCF (20 ng/ml), TPO (20 ng/ml; until day 24), Flt3L (10 ng/ml), and interleukin 7 (10 ng/ml). Every 4 days, preTs were harvested, passed through a 70 µm filter, and transferred to new OP9-DL1 cell monolayers.
Human embryonic kidney 293T cells and the fibrosarcoma cell line HT1080 were cultured in Dulbecco's modified Eagle's medium supplemented with 10% fetal calf serum. TdTomato.CD123-expressing 293T cells were generated by transduction with a gammaretroviral vector encoding tdTomato and CD123 linked by an IRES sequence.
Mice. Animals in the experiments were used under protocols approved by the State Government of Lower Saxony, Germany. NOD.cg-PrkdcscidIL2rgtm/Wjl/Sz (NSG) mice were purchased from Charles River, housed, and bred in a pathogen free facility.
Hematopoietic cell transplantation. PreTs in combination with UCB-derived CD34+ HSCs were intrahepatically injected in 4 day old irradiated NSG mice as previously described.7 In brief, transgene-positive preTs were sorted on day 11 of OP9-DL1 coculture. 2 × 105 preTs together with 0.2 × 105 HSCs were resuspended in 30 μl PBS containing recombinant human interleukin 7 (rhIL-7) (2.5 μg) and the IL-7 antibody M25 (0.5 μg) and consecutively injected intrahepatically into irradiated (1 Gy) newborn mice. Controls were injected with HSCs alone. Mice were boosted with the IL-7/M25 mixture every 5 days. Thymi were harvested 6 weeks after injection for flow cytometric analysis.
Vector construction and cloning. We utilized SIN alpharetroviral vectors containing an MPSV or EFS promoter and a woodchuck posttranscriptional regulatory element (PRE).38 EGFP was cloned into the constructs and expressed via an IRES sequence. Cloning details are available on request.
Inducible caspase 9 was kindly provided by Prof. Malcolm K. Brenner, Baylor College of Medicine, Houston, TX, USA. It is comprised of a mutated human FK506-binding protein fused via an SGGGS linker to human caspase 9, and was linked by a 2A sequence to truncated human CD19 (▵CD19). The dimerizer agent (Clontech, Palo Alto, CA) is a synthetic nontoxic FK506 analog that upon administration leads to aggregation and activation of inducible caspase 9 monomers and eventually induction of apoptosis.
A third-generation CD123-specific CAR containing the codon-optimized sequences for a CD123-specific scFV, the transmembrane region of the human CD28 molecule, the costimulatory signaling endodomains of CD28 and 4-1BB, and the CD3ζ signaling domain, was cloned into a SIN alpharetroviral backbone. EGFP or iCasp9 linked by an IRES sequence was cloned downstream of the CAR cassette.
Cell transduction. For transient viral vector production, 293T cells were transfected using a calcium phosphate transfection kit (Sigma Aldrich, Steinheim, Germany) with MPSV or EFS constructs. They were combined with plasmids encoding gag/pol and either the RD114/TR (described previously, provided by Prof. Els Verhoeyen, Lyon, France) or the VSVG envelope.39 Retroviral supernatant was either freshly used or concentrated by ultra-centrifugation, immediately frozen in dry ice, and stored at −80 °C for further usage. The fibrosarcoma cell line HT1080 was used for determining the viral titer.
Before transduction, CD34+ cells were prestimulated for 36 hours in X-VIVO 10 serum free medium (Lonza, Basel, Switzerland) at a maximal density of 0.6 × 106/ml in the presence of SCF, TPO, and Flt3L (all 100 ng/ml; PeproTech).
For the transduction of Jurkat cells, PBMCs and CB-derived CD34+ cells, 24-well plates were coated with Retronectin (Takara, Otsu, Japan) overnight at 4 °C. Retroviral supernatant was added and plates were spin-oculated (490×g, 1 hour, 4 °C) to facilitate retrovirus binding to Retronectin. Subsequently, retroviral supernatant was removed and up to 15 × 104 cells were added per well.
Flow cytometry. The following fluorochrome-conjugated antibodies were purchased from BioLegend (San Diego, CA): CD3 (PerCPCy5.5), CD4 (Brilliant Violet 570), CD5 (Brilliant Violet 421), CD8 (PE), CD34 (PECy7), CD45RA (APCCy7), CD123 (APC), Annexin V (PE) or BD Biosciences (San Jose, CA): CD7 (APC), CD19 (PE), and Annexin V (APC). For CD123 CAR staining, human IL3 receptor α/CD123 protein with a His Tag (Sino Biological, Beijing, China) and an anti-His Tag antibody (APC) (R&D Systems, Minneapolis, MN) were used. Data were acquired using a FACSCanto or LSRII (BD Biosciences) and analyzed using FlowJo software (TreeStar, Ashland, OR). Untransduced cells were used as control.
q-RT-PCR for determination of vector copy numbers. Genomic DNA was isolated from transduced CD34+ cells and vector copy numbers determined by using the TaqMan system (Qiagen, Hilden, Germany). Quantitative PCR was performed on an Applied Biosystems (Darmstadt, Germany) Step One Plus real-time PCR.13 The primers are specific for the vector PRE and the PTBP2 intron.40 Vector copy numbers of CD34+ cell samples were analyzed as previously described.41
Induction of apoptosis. Cells transduced with vectors containing the iCasp9 and the ΔCD19 gene were sorted for CD19 using a FACSAria cell sorter (BD Biosciences). AP20187 (Clontech) was added at increasing concentrations. After 48–72 hours, cells were stained with Annexin V (APC or PE) and analyzed within 1 hour by flow cytometry for apoptotic cells. Alternatively, a construct encoding CD123 CAR and iCasp9 was used.
Generation of CAR T-cells and cytotoxicity assay. For the assessment of the CAR functionality in vitro, human PBMCs were isolated from blood samples of healthy volunteers using Ficoll-Paque PLUS reagent (GE Healthcare, Uppsala, Sweden) and were activated for 2 days with anti-CD3 antibody (50 ng/ml), anti-CD28 antibody (500 ng/ml) and IL-2 (25 U/ml). Cells were transduced on 2 consecutive days with alpharetroviral supernatant containing the CD123 CAR vector. After further expansion for 4 days, effector and target cells (293T cells expressing CD123 and tdTomato linked via an IRES sequence) were cocultured at indicated ratios for 2 days. Cytotoxicity was assessed by fluorescence microscopy or flow cytometry.
Enzyme-linked immunosorbent assay (ELISA). T-cells (2 × 105) and target cells (2 × 104) were incubated (effector:target ratio of 10:1) in V-bottom 96-well plates in the presence of IL-2 (25 U/ml) and IL-7 (5 ng/ml). After 24 hours, the culture supernatant was harvested and used in duplicates for an IFNγ ELISA (BioLegend).
Statistical analysis. Unless specified in the text, data were presented as mean ± standard error of the mean. The Student's t-test was used to determine the statistical significance of differences between samples. P values <0.05 were considered to be statistically significant.
SUPPLEMENTARY MATERIAL Figure S1. CD123 expression is most prominently reduced in CAR.GFP+ preTs.
Acknowledgments
The authors would like to kindly thank the midwives and doctors of the Marienkrankenhaus Hamburg for their assistance. This work was supported by the StrucMed program of Hannover Medical School, the Deutsche Forschungsgemeinschaft (Cluster of Excellence REBIRTH, SFB738), and a grant from the German Federal Ministry of Education and Research (reference number: 01EO1302, M.H., A.S. and M.G.S.). The authors declare no conflict of interest.
Supplementary Material
References
- Lewis, ID and Verfaillie, CM (2000). Multi-lineage expansion potential of primitive hematopoietic progenitors: superiority of umbilical cord blood compared to mobilized peripheral blood. Exp Hematol 28: 1087–1095. [DOI] [PubMed] [Google Scholar]
- Gluckman, E, Rocha, V, Boyer-Chammard, A, Locatelli, F, Arcese, W, Pasquini, R et al. (1997). Outcome of cord-blood transplantation from related and unrelated donors. Eurocord Transplant Group and the European Blood and Marrow Transplantation Group. N Engl J Med 337: 373–381. [DOI] [PubMed] [Google Scholar]
- Barker, JN and Wagner, JE (2003). Umbilical-cord blood transplantation for the treatment of cancer. Nat Rev Cancer 3: 526–532. [DOI] [PubMed] [Google Scholar]
- Schmitt, TM and Zúñiga-Pflücker, JC (2002). Induction of T cell development from hematopoietic progenitor cells by delta-like-1 in vitro. Immunity 17: 749–756. [DOI] [PubMed] [Google Scholar]
- Zakrzewski, JL, Kochman, AA, Lu, SX, Terwey, TH, Kim, TD, Hubbard, VM et al. (2006). Adoptive transfer of T-cell precursors enhances T-cell reconstitution after allogeneic hematopoietic stem cell transplantation. Nat Med 12: 1039–1047. [DOI] [PubMed] [Google Scholar]
- Spits, H (2002). Development of alphabeta T cells in the human thymus. Nat Rev Immunol 2: 760–772. [DOI] [PubMed] [Google Scholar]
- Awong, G, Singh, J, Mohtashami, M, Malm, M, La Motte-Mohs, RN, Benveniste, PM et al. (2013). Human proT-cells generated in vitro facilitate hematopoietic stem cell-derived T-lymphopoiesis in vivo and restore thymic architecture. Blood 122: 4210–4219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakrzewski, JL, Suh, D, Markley, JC, Smith, OM, King, C, Goldberg, GL et al. (2008). Tumor immunotherapy across MHC barriers using allogeneic T-cell precursors. Nat Biotechnol 26: 453–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoseini, SS, Hapke, M, Herbst, J, Wedekind, D, Baumann, R, Heinz, N et al. (2015). Inducible T-cell receptor expression in precursor T cells for leukemia control. Leukemia 29: 1530–1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacein-Bey-Abina, S, Hauer, J, Lim, A, Picard, C, Wang, GP, Berry, CC et al. (2010). Efficacy of gene therapy for X-linked severe combined immunodeficiency. N Engl J Med 363: 355–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newrzela, S, Cornils, K, Li, Z, Baum, C, Brugman, MH, Hartmann, M et al. (2008). Resistance of mature T cells to oncogene transformation. Blood 112: 2278–2286. [DOI] [PubMed] [Google Scholar]
- Suerth, JD, Maetzig, T, Brugman, MH, Heinz, N, Appelt, JU, Kaufmann, KB et al. (2012). Alpharetroviral self-inactivating vectors: long-term transgene expression in murine hematopoietic cells and low genotoxicity. Mol Ther 20: 1022–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suerth, JD, Maetzig, T, Galla, M, Baum, C and Schambach, A (2010). Self-inactivating alpharetroviral vectors with a split-packaging design. J Virol 84: 6626–6635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awong, G, Herer, E, Surh, CD, Dick, JE, La Motte-Mohs, RN and Zúñiga-Pflücker, JC (2009). Characterization in vitro and engraftment potential in vivo of human progenitor T cells generated from hematopoietic stem cells. Blood 114: 972–982. [DOI] [PubMed] [Google Scholar]
- Fehse, B, Kustikova, OS, Bubenheim, M and Baum, C (2004). Pois(s)on–it's a question of dose. Gene Ther 11: 879–881. [DOI] [PubMed] [Google Scholar]
- Morgan, RA, Dudley, ME, Wunderlich, JR, Hughes, MS, Yang, JC, Sherry, RM et al. (2006). Cancer regression in patients after transfer of genetically engineered lymphocytes. Science 314: 126–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris, E, Hart, D, Gao, L, Tsallios, A, Xue, SA, and Stauss, H (2006). Generation of tumor-specific T-cell therapies. Blood reviews 20: 61–69. [DOI] [PubMed] [Google Scholar]
- Brentjens, RJ, Latouche, JB, Santos, E, Marti, F, Gong, MC, Lyddane, C et al. (2003). Eradication of systemic B-cell tumors by genetically targeted human T lymphocytes co-stimulated by CD80 and interleukin-15. Nat Med 9: 279–286. [DOI] [PubMed] [Google Scholar]
- Bridgeman, JS, Hawkins, RE, Hombach, AA, Abken, H and Gilham, DE (2010). Building better chimeric antigen receptors for adoptive T cell therapy. Curr Gene Ther 10: 77–90. [DOI] [PubMed] [Google Scholar]
- Sadelain, M, Brentjens, R and Rivière, I (2013). The basic principles of chimeric antigen receptor design. Cancer Discov 3: 388–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter, DL, Roth, MS, McGarigle, C, Ferrara, JL and Antin, JH (1994). Induction of graft-versus-host disease as immunotherapy for relapsed chronic myeloid leukemia. N Engl J Med 330: 100–106. [DOI] [PubMed] [Google Scholar]
- Brentjens, RJ, Davila, ML, Riviere, I, Park, J, Wang, X, Cowell, LG et al. (2013). CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci Transl Med 5: 177ra38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torikai, H, Reik, A, Liu, PQ, Zhou, Y, Zhang, L, Maiti, S et al. (2012). A foundation for universal T-cell based immunotherapy: T cells engineered to express a CD19-specific chimeric-antigen-receptor and eliminate expression of endogenous TCR. Blood 119: 5697–5705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Smedt, M, Hoebeke, I and Plum, J (2004). Human bone marrow CD34+ progenitor cells mature to T cells on OP9-DL1 stromal cell line without thymus microenvironment. Blood Cells Mol Dis 33: 227–232. [DOI] [PubMed] [Google Scholar]
- Offner, F, Kerre, T, De Smedt, M and Plum, J (1999). Bone marrow CD34 cells generate fewer T cells in vitro with increasing age and following chemotherapy. Br J Haematol 104: 801–808. [DOI] [PubMed] [Google Scholar]
- Schmitt, TM, de Pooter, RF, Gronski, MA, Cho, SK, Ohashi, PS and Zúñiga-Pflücker, JC (2004). Induction of T cell development and establishment of T cell competence from embryonic stem cells differentiated in vitro. Nat Immunol 5: 410–417. [DOI] [PubMed] [Google Scholar]
- Snauwaert, S, Verstichel, G, Bonte, S, Goetgeluk, G, Vanhee, S, Van Caeneghem, Y et al. (2014). In vitro generation of mature, naive antigen-specific CD8(+) T cells with a single T-cell receptor by agonist selection. Leukemia 28: 830–841. [DOI] [PubMed] [Google Scholar]
- Patel, E, Wang, B, Lien, L, Wang, Y, Yang, LJ, Moreb, JS et al. (2009). Diverse T-cell differentiation potentials of human fetal thymus, fetal liver, cord blood and adult bone marrow CD34 cells on lentiviral Delta-like-1-modified mouse stromal cells. Immunology 128(1 Suppl): e497–e505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland, AM, Zakrzewski, JL, Tsai, JJ, Hanash, AM, Dudakov, JA, Smith, OM et al. (2012). Extrathymic development of murine T cells after bone marrow transplantation. J Clin Invest 122: 4716–4726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda, Y, Takeuchi, Y, Martin, F, Cosset, FL, Mitrophanous, K and Collins, M (2003). Continuous high-titer HIV-1 vector production. Nat Biotechnol 21: 569–572. [DOI] [PubMed] [Google Scholar]
- Throm, RE, Ouma, AA, Zhou, S, Chandrasekaran, A, Lockey, T, Greene, M et al. (2009). Efficient construction of producer cell lines for a SIN lentiviral vector for SCID-X1 gene therapy by concatemeric array transfection. Blood 113: 5104–5110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suerth, JD, Schambach, A and Baum, C (2012). Genetic modification of lymphocytes by retrovirus-based vectors. Curr Opin Immunol 24: 598–608. [DOI] [PubMed] [Google Scholar]
- Zychlinski, D, Schambach, A, Modlich, U, Maetzig, T, Meyer, J, Grassman, E et al. (2008). Physiological promoters reduce the genotoxic risk of integrating gene vectors. Mol Ther 16: 718–725. [DOI] [PubMed] [Google Scholar]
- Zhang, F, Thornhill, SI, Howe, SJ, Ulaganathan, M, Schambach, A, Sinclair, J et al. (2007). Lentiviral vectors containing an enhancer-less ubiquitously acting chromatin opening element (UCOE) provide highly reproducible and stable transgene expression in hematopoietic cells. Blood 110: 1448–1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein, S, Ott, MG, Schultze-Strasser, S, Jauch, A, Burwinkel, B, Kinner, A et al. (2010). Genomic instability and myelodysplasia with monosomy 7 consequent to EVI1 activation after gene therapy for chronic granulomatous disease. Nat Med 16: 198–204. [DOI] [PubMed] [Google Scholar]
- Di Stasi, A, Tey, SK, Dotti, G, Fujita, Y, Kennedy-Nasser, A, Martinez, C et al. (2011). Inducible apoptosis as a safety switch for adoptive cell therapy. N Engl J Med 365: 1673–1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Motte-Mohs, RN, Herer, E and Zúñiga-Pflücker, JC (2005). Induction of T-cell development from human cord blood hematopoietic stem cells by Delta-like 1 in vitro. Blood 105: 1431–1439. [DOI] [PubMed] [Google Scholar]
- Gerull, S, Beard, BC, Peterson, LJ, Neff, T and Kiem, HP (2007). In vivo selection and chemoprotection after drug resistance gene therapy in a nonmyeloablative allogeneic transplantation setting in dogs. Hum Gene Ther 18: 451–456. [DOI] [PubMed] [Google Scholar]
- Sandrin, V, Boson, B, Salmon, P, Gay, W, Nègre, D, Le Grand, R et al. (2002). Lentiviral vectors pseudotyped with a modified RD114 envelope glycoprotein show increased stability in sera and augmented transduction of primary lymphocytes and CD34+ cells derived from human and nonhuman primates. Blood 100: 823–832. [DOI] [PubMed] [Google Scholar]
- Rahman, L, Bliskovski, V, Kaye, FJ and Zajac-Kaye, M (2004). Evolutionary conservation of a 2-kb intronic sequence flanking a tissue-specific alternative exon in the PTBP2 gene. Genomics 83: 76–84. [DOI] [PubMed] [Google Scholar]
- Pfaffl, MW (2001). A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29: e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.