Abstract
Genetically evoked deficiency of collagen VII causes dystrophic epidermolysis bullosa (DEB)—a debilitating disease characterized by chronic skin fragility and progressive fibrosis. Removal of exons carrying frame-disrupting mutations can reinstate protein expression in genetic diseases. The therapeutic potential of this approach is critically dependent on gene, protein, and disease intrinsic factors. Naturally occurring exon skipping in COL7A1, translating collagen VII, suggests that skipping of exons containing disease-causing mutations may be feasible for the treatment of DEB. However, despite a primarily in-frame arrangement of exons in the COL7A1 gene, no general conclusion of the aptitude of exon skipping for DEB can be drawn, since regulation of collagen VII functionality is complex involving folding, intra- and intermolecular interactions. To directly address this, we deleted two conceptually important exons located at both ends of COL7A1, exon 13, containing recurrent mutations, and exon 105, predicted to impact folding. The resulting recombinantly expressed proteins showed conserved functionality in biochemical and in vitro assays. Injected into DEB mice, the proteins promoted skin stability. By demonstrating functionality of internally deleted collagen VII variants, our study provides support of targeted exon deletion or skipping as a potential therapy to treat a large number of individuals with DEB.
Introduction
Dystrophic Epidermolysis Bullosa (DEB) is an orphan disorder caused by mutations in the gene COL7A1 encoding collagen VII—a large extracellular protein and the main component of anchoring fibrils.1 Because anchoring fibrils are crucial for attachment of the dermal-epidermal basement membrane to the underlying papillary dermis, DEB is characterized by chronic skin fragility leading to mechanically induced blistering and formation of longstanding wounds. Further complications arise from progressive soft tissue fibrosis that, in the most severe forms of the disease, results in formation of mitten-like deformities of hands and feet, and high propensity for development of aggressive squamous cell carcinomas.2 DEB is currently incurable but development of gene-, protein-, and cell-based therapies is actively being pursued,3 with topical therapies showing the best prospect of translation into the clinics. Importantly, DEB is in its most severe forms a systemic disorder with large surfaces inaccessible to topical treatment and thus, a systemic therapy would be most beneficial for patients. However, at present, the size of the COL7A1 gene and the lack of vectors with high specific skin tropism remain the principal limitations for the development of systemic cDNA-mediated gene therapy approaches for this disease.4,5,6,7
To overcome challenges related to classical gene therapy, development of systemic exon skipping–based strategies to correct the reading frame is promising. Duchene muscular dystrophy (DMD) is a progressive neuromuscular disorder caused by mutations in the DMD gene.8 These mutations lead to diminished levels of functional dystrophin.9 Like COL7A1, the DMD gene is composed of a multitude of exons10,11 and over 60% of mutations are frame disrupting.9 Strategies targeting mutated exons have been successfully developed for DMD by using antisense oligonucleotides (AON) inducing transient dystrophin expression. In addition, more recently the Dmd reading frame was permanently restored in vivo in mice after CRISPR/Cas9-mediated excision of exon 23.12,13,14 These approaches aim to remove the disease-initiating mutation to restore the reading-frame that will promote expression of internally deleted proteins. The hope is that the resulting protein retains full or partial functionality, thereby providing protection against disease progression.15
Case reports suggest that skipping of mutated exons in some patients results in less severe DEB,16,17,18,19 supporting the potential of excision of faulty exons to treat the disease. In addition, one study provided cautious optimism of AON-based therapy for DEB.5 However, skipping of in-frame exons that carry causal mutations does not unequivocally improve symptoms. It may in fact exaggerate the disease by rendering proteins with severely impaired functionality.20 Consequently, detailed analysis on the protein level is needed to determine the functionality of internally deleted protein variants. Eventually, the benefit of exon skipping or removal therapies depends on efficacy of the therapy, functionality of the protein and diseases progression. For example, although, there is a clear and irrefutable correlation of dystrophin expression and improved outcome of the disease,15 shortage of information of the functionality of internally deleted dystrophin variants may have delayed translation of AON-based therapies for DMD into the clinics.15,21 The complex folding, the multimeric arrangement, and the multiple interaction partners of collagen VII make the effect of removal of amino acids encoded by single exons from collagen VII hard to predict. Thus, a first step for development of therapies aiming to skip or remove exons containing causal mutations is to demonstrate that collagen VII variants resulting from in-frame exon deletion remain functional. In order to do so, an efficient method to generate and to characterize the functional consequence of deletion of any in-frame exon from COL7A1 is needed.
Here, we investigated the feasibility of DEB therapies based on exon skipping or gene editing to remove exons carrying causal mutations. We confirmed that disease relevant exons in COL7A1 could be targeted by AONs. Subsequently, we designed a strategy to generate collagen VII variants lacking amino acids encoded by specific exons and to determine the level of functionality of these recombinantly expressed collagen VII variants by molecular, cellular, and in vivo assays. As proof-of-concept we chose to generate DEB patient-relevant collagen VII variants resulting from deletion of COL7A1 exon 13 or 105 (named collagen VII Δ13 and Δ105, respectively). Altogether, our study provides support that targeted removal of exons containing causal mutations can be used to treat a large number of individuals with DEB.
Results
In silico analysis of COL7A1 gene reveals that the majority of exons can be skipped or removed without disrupting the reading frame
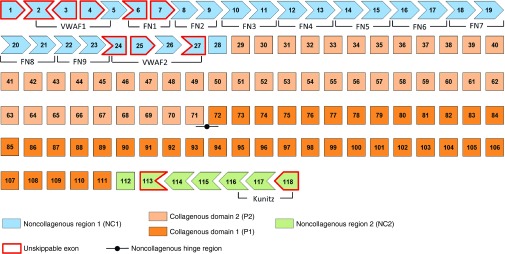
COL7A1, the gene encoding for collagen VII, is composed of 118 exons.22 In silico analysis of this gene revealed that 92% (107/118) of exons can be individually skipped without disturbing the open reading frame of COL7A1 or modifying more than one amino acid (Figure 1). Importantly, none of the single amino acid modifications leads to creation of a stop codon. Every exon coding for parts of the collagenous domains can be skipped without causing a frame-shift. Since the collagenous domain is mainly responsible of the fibrillar structure of collagen VII, it is likely that deletion of several Gly-X-Y repeats will not drastically impair protein-protein interactions but it may have structural consequences. This could be a special consideration for exons encoding a number of amino acids not dividable by three, since their removal will interrupt the Gly-X-Y repeat. However, as the collagenous domain of collagen VII is already imperfect, it is challenging to predict the outcome of deletion of such exons. All exons that cannot be removed without disturbing the open reading frame are located in the NC1 and NC2 domains of collagen VII. Importantly, both domains also contain exons that can be removed without changing more than one amino acid or creating a stop codon. The NC1 and NC2 domains are responsible for mediating skin-stabilizing binding to laminin 332 and collagen IV,23,24,25,26 and for homodimerization to initiate anchoring fibril formation,27 respectively. Since these interaction sites have not been precisely mapped, the consequences of removing exons coding for parts of the NC1 or NC2 domains cannot be easily predicted. In conclusion, the specific exon organization of COL7A1 suggests that exon-skipping therapy and gene editing to remove faulty exons are attractive therapeutic approaches for DEB. However, careful analyses of the protein variants resulting from specific exon removal are a prerequisite to fully determine the therapeutic potential of such therapies.
Figure 1.
In silico analysis of COL7A1 exon organization. The figure presents COL7A1 exons, organized as a jigsaw puzzle-like structure to illustrate which exons can be skipped without disrupting the open reading frame or changing more than one amino acid. Boxes with red line represent exons that cannot be skipped without evoking a reading frame shift. All such exons are located in noncollagenous domain 1 (NC1, in blue) and 2 (NC2, in green). The remaining exons correspond to the collagenous domain (in orange), which can be divided into two parts (P1 and P2) based on the presence of a noncollagenous hinge region in the middle of the protein. Localization of von Willebrand factor A (VWFA-1 and -2), FN type III and Kunitz inhibitor (Kunitz) domains are indicated as well.
Removal of amino acids encoded by exon 13 or 105 does not impair folding
To start addressing the feasibility of the above-mentioned approaches to treat DEB, we first developed a robust strategy for deletion of any exon from COL7A1 cDNA. We used this strategy to delete exon 13 and 105, respectively (Supplementary Figure S1). Exon 13 is one of the most recurrent mutated COL7A1 exons,28 and thus investigation of the functionality of collagen VII lacking the amino acids encoded by exon 13 is of high medical interest. Mutations in exon 105, which encodes for 27 amino acids, cause a severe disease manifestation.28 In addition, the two selected exons encode parts of two different functional domains, i.e., exon 13 encodes a part of the NC1 domain and exon 105 encodes a part of the carboxyl-terminal end of the collagenous domain. A deletion within the NC1 domain might impair the binding to interaction partners. Although the position-dependent effect of amino acid substitutions and deletions in collagen VII is elusive,29 it is known that the collagen VII triple helix folds from the C to the N terminus.30 Thus, in analogy with naturally occurring causal mutations for other collagens,31,32 the impact on folding after removal of amino acids in the collagenous domain can therefore be assumed to be greater the more C-terminally the deletion occurs.
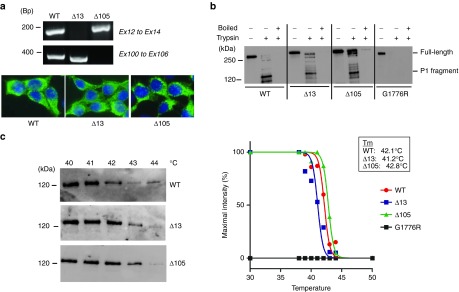
Before detailed characterization of the functionality of the internally deleted collagen VII variants resulting from exon skipping or deletion, we first tested that these exons could be efficiently targeted. Toward this end, we designed AONs against human COL7A1 exons 13 and 105 and showed that efficient skipping was feasible in cultured dermal fibroblasts (Figure 2). AONs based on the same chemistry have previously been shown to reach skin after systemic administration in concentrations sufficient to restore protein synthesis,33 and we chose fibroblasts because they would be the main targets in a systemic therapy. After having established that both exons were amenable to exon skipping, we then proceeded to construct expression vectors containing COL7A1 lacking the nucleotides corresponding to exon 13 or 105. HEK293 cells were subsequently transfected with these constructs. Reverse transcription polymerase chain reaction (RT-PCR) on RNA isolated from transfected cells first confirmed expression of COL7A1 mRNA lacking exon 13 or 105 (Figure 3a). Further, as shown in Figure 3a, immunostaining with collagen VII antibodies revealed that the deletion of exon 13 or 105 did not interfere with protein expression or secretion.
Figure 2.
Antisense oligonucleotides (AON) can induce skipping of exon 13 and 105 in dermal fibroblast. RT-PCR on RNA extracted from dermal fibroblast treated with 250 or 500 nmol/l of AONs designed against exon 13 (a) or 105 (c) shows efficient skipping of the respective exons. For both exons amplification of the region surrounding exon 13 (exon 12 to 14) or exon 105 (exon 102 to 106) reveals an additional lower band corresponding to an amplicon lacking exon 13 (144 bp) or 105 (81 pb), respectively. Sanger sequencing then confirms the precise skipping of exon 13 (b) or 105 (d). White asterisk indicates heteroduplex DNA.
Figure 3.
Collagen VII variants can form stable triple helices. (a) The upper panel shows by RT-PCR on mRNA isolated from HEK cells expressing WT, Δ13, or Δ105 collagen VII that exon 13 or 105 are absent in the Δ13 and Δ105 construct carrying cells, respectively. The lower panel shows collagen VII immunostaining of HEK 293 cells expressing WT, Δ13 or Δ105 collagen VII. (b) Limited trypsin digestion at 30 °C with or without pre-boiling of the samples confirms that deletion of exon 13 or 105 in COL7A1 does not disturb the thermal stability of the collagen VII collagenous domain. (c) Detailed thermal stability analysis reveals that the Tm of all collagen VII variants (WT, Δ13, or Δ105) is approximately 42 °C.
We then expressed wild-type (WT) and Δ13 or Δ105 collagen VII in the presence of ascorbic acid to allow stable triple helix formation and purified the proteins from the media.34 We compared the ability of collagen VII molecules to withstand proteolytic digestion with trypsin in order to gain information about the folding of the collagen VII variants, because properly folded collagen triple helices are resistant to trypsin digestion.35,36 The WT and the two collagen VII variants were protected from trypsin proteolysis at 30 °C, while boiling prior to digestion completely abolished the protection (Figure 3b). A natural collagen VII mutant carrying a glycine substitution at amino acid position 1776 (G1776R) was used as negative control, and in accordance with previous findings, showed no thermal stability at 30 °C.35 Relatively subtle reduction in the thermal stability is connected to forms of dominantly inherited DEB35,37; therefore, we more carefully evaluated the thermal stability of the triple helical structure of collagen VII by performing limited trypsin digestion along a temperature gradient.34 No significant changes were observed in the thermal stability of collagen VII Δ13 or Δ105 in comparison to the WT protein (Figure 3c). For the WT and the two collagen VII variants, we observed a mean melting temperature of around 42 °C, which is in agreement with previous experiments.35,38 Taken together, our data confirm that removal of the amino acids encoded by exon 13 or 105 does not interfere with the essential ability to fold into stable collagen VII triple helices.
Collagen VII Δ13 and Δ105 retain their ability to bind collagen IV
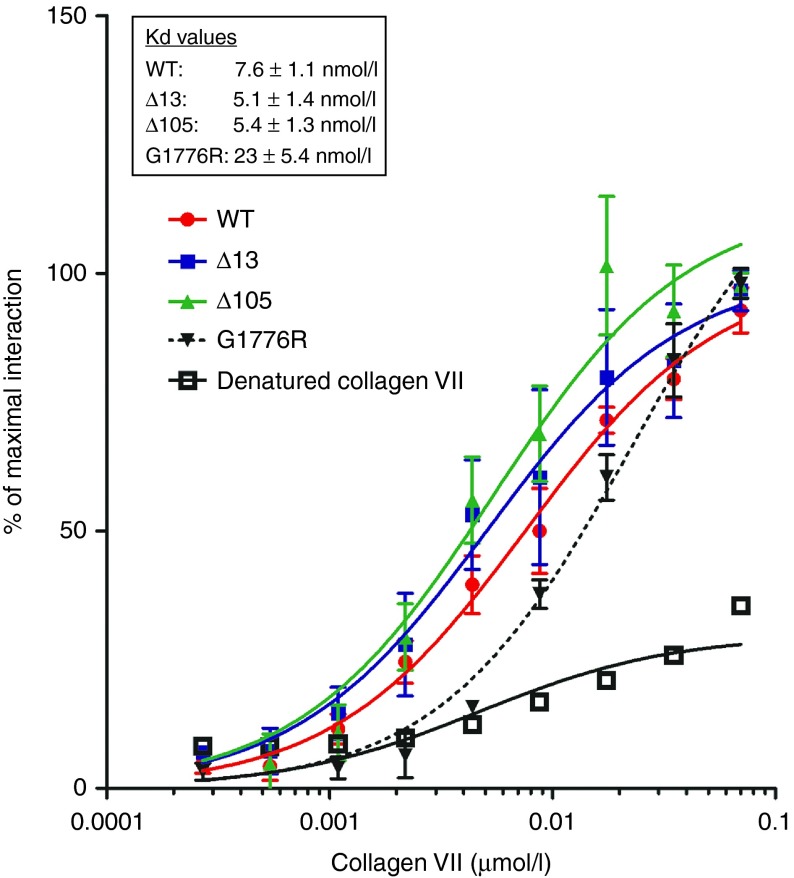
Binding of collagen VII to interaction partners in the dermal-epidermal basement membrane is essential for skin stability. It has been demonstrated that the collagen VII NC1 domain interaction with collagen IV and laminin 332 is site-specific.24 Thus, it is important to assess the ability of the collagen VII variants to interact with these two binding partners. This was especially important for Δ13 collagen VII, which lacks amino acids in the NC1 domain. We performed solid-phase binding assays to test the binding capacity of collagen VII variants. These assays revealed no significant difference between the interaction of WT, Δ13 or Δ105 collagen VII with collagen IV or laminin 332 (Figure 4 and Supplementary Figure S2). In contrast, only a weak interaction was observed with collagen VII denatured by boiling, while the interaction of the glycine substituted mutant G1776R with collagen IV was preserved but significantly reduced (WT: 7.6 nmol/l ± 1.1; Δ13: 5.1 nmol/l ± 1.4; Δ105: 5.4 nmol/l ± 1.3; G1776R: 23 nmol/l ± 5.4). Because the mutation G1776R occurs in the collagenous domain, it is not surprising that this misfolded glycine substituted mutant is still able to bind collagen IV. Nevertheless, this result also shows that mutations in the collagenous domain can affect the binding strength to the interaction partners of the NC1 domain. Importantly, these data show that Δ13 and Δ105 collagen VII retain the ability to bind the epidermal basement membrane with similar strength as WT collagen VII.
Figure 4.
Collagen VII variants retain ability to bind collagen IV. Variable concentrations of WT, denatured WT, Δ13, or Δ105 collagen VII were used (0.27 to 70 nmol/l) to analyze binding to 500 ng immobilized collagen IV. The data were normalized to the maximal signal recorded and were collected from three independent experiments. Heat denatured WT collagen VII shows a weak and nonsaturable binding and the collagen VII mutant G1776R shows a saturable interaction but with weaker affinity than for WT collagen VII. In contrast Δ13 and Δ105 collagen VII display similar Kd values as WT collagen VII indicating that binding to collagen IV is not disturbed by deletion of exon 13 or 105 from COL7A1.
Collagen VII variants support fibroblast adhesion and migration in vitro
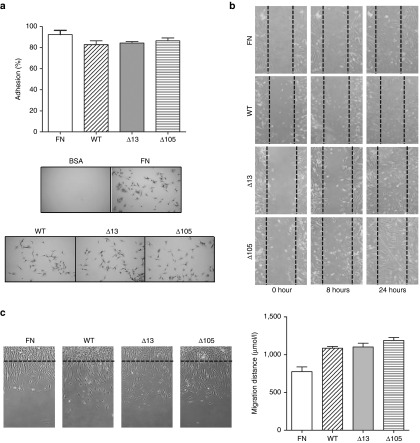
It is known that collagen VII can support keratinocyte and fibroblast adhesion and migration in vitro,39,40 and that collagen VII derived from DEB patients supports these events to a lesser extent.41 Therefore, we tested the ability of Δ13 or Δ105 collagen VII to promote cell adhesion and migration of fibroblasts. No adhesion was observed with bovine serum albumin (BSA), whereas, in agreement with previous findings, fibronectin (FN) and WT collagen VII efficiently supported fibroblast adhesion.41 Importantly, Δ13 and Δ105 collagen VII supported adhesion of fibroblasts equally well as WT collagen VII (Figure 5a). We then analyzed the effect on cell migration in in vitro wound healing and in direct cell migration assays. From both assays, it was evident that Δ13 and Δ105 collagen VII indeed supported fibroblast migration to a similar degree as WT collagen VII (Figure 5b,c). Altogether, these results show that absence of the amino acids encoded by exon 13 or 105 does not interfere with fibroblast adhesion and migration. This suggests that skipping or permanent deletion of these exons will still allow production of functional collagen VII variants in a cellular context.
Figure 5.
Collagen VII variants support fibroblast adhesion and migration. Tissue culture plates were coated with bovine serum albumin, FN, WT, Δ13, or Δ105 collagen VII to study cell adhesion and migration. (a) Fibroblast adhesion assay. Two hours after seeding, fibroblasts were fixed and stained with crystal violet. After cell lysis, quantification was performed by recording the absorbance at 550 nm. Data are expressed as the percentage of adhesion. (b) Fibroblast wound healing assay. Dotted line represents the original wound edge. (c) Direct migration assay. Dotted line represents the original border. These results suggest that the lack of amino acids resulting from deletion of exon 13 or 105 does not interfere with the ability to support fibroblast adhesion and migration.
Injection of collagen VII variants in RDEB mice leads to deposition at the dermal-epidermal junction
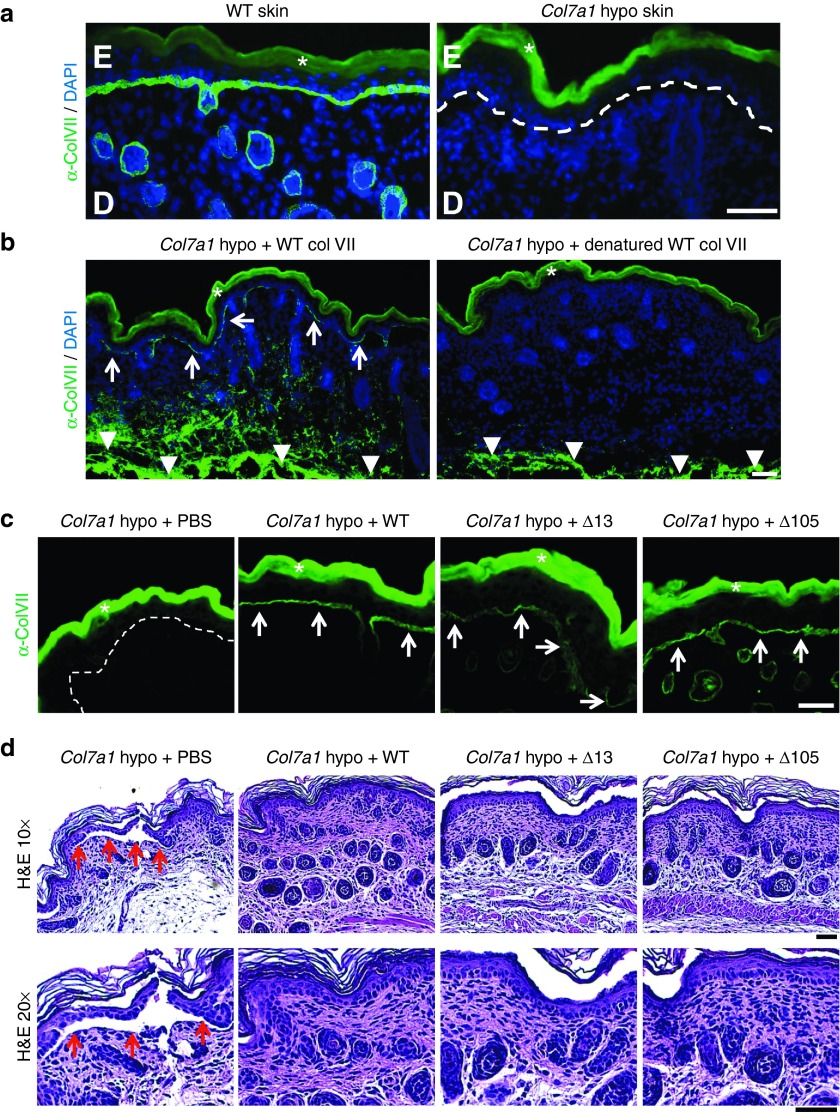
To test the in vivo functionality of the collagen VII variants, we used the Col7a1 hypomorphic mouse. This mouse maintains a residual 10% expression of WT collagen VII (Figure 6a),42 which is sufficient to rescue the mice from lethality but leads to a phenotype resembling the most severe human phenotype, i.e., generalized severe recessive DEB.42 To assess the functionality of collagen VII variants resulting from exon deletion, we intradermally injected Col7a1 hypomorphic mice with WT, Δ13 or Δ105 collagen VII. One day after the injection the mice were sacrificed and skin close to the injection site was collected, sectioned, and stained with an antibody specifically detecting human collagen VII.36 Injection of WT collagen VII resulted in deposition of collagen VII at the dermal-epidermal junction zone (DEJZ) (Figure 6b), as previously described.43 In order to discriminate unspecific retention of collagen VII, we also injected heat denatured WT collagen VII. The denatured collagen VII was present at the injection site but was unable to translocate to the DEJZ (Figure 6b). Importantly, injection of Δ13 and Δ105 collagen VII resulted in a clear deposition at the DEJZ, which was comparable to the deposition of WT collagen VII (Figure 6c—white arrows). We then investigated if injections of the collagen VII variants could promote skin stabilization. Interestingly, whereas the skin from vehicle-injected Col7a1 hypomorphic mice displayed clearly visible dermal-epidermal microblisters (Figure 6d), only very limited dermal-epidermal separation was observed after injection of WT, Δ13, and Δ105 collagen VII, respectively (Figure 6d). Although the time elapsed between the injection and the analysis (1 day) is too short to allow formation of well-developed anchoring fibrils, the injected collagen VII was nonetheless already able to promote dermal-epidermal cohesion. The analysis suggests a skin stabilizing effect of collagen VII even outside mature anchoring fibrils and shows that the primary physiological function of both internally deleted collagen VII variants is maintained in vivo.
Figure 6.
Collagen VII variants can be deposited at the dermal-epidermal junction in RDEB mice. (a) Skin tissue sections from WT and Col7a1 hypomorphic mice immunostained for collagen VII (green). While WT skin exhibits a strong signal for collagen VII at the dermal-epidermal junction zone, minimal collagen VII deposition is seen in Col7a1 hypomorphic mice (white dotted line). (b) Skin sections stained for human collagen VII (green) after intradermal injection of Col7a1 hypomorphic mice with 25 µg of WT collagen VII and heat denatured WT collagen VII. The staining reveals that only correctly folded collagen VII is able to translocate to the dermal-epidermal junction after injection (white arrows). Note that the injected collagen VII is clearly visible in deeper dermal areas in both skin sections (white arrowheads). (c) Skin sections one day after intradermal injection of Col7a1 hypomorphic mice with 10 µg of WT, Δ13, and Δ105 collagen VII stained for human collagen VII (green). The staining reveals that deletion of amino acids encoded by exon 13 or 105 does not affect the ability of collagen VII to translocate to the dermal-epidermal junction zone compared to WT collagen VII (white arrows). (d) H&E staining of skin sections as in (c). The histological analysis reveals that collagen VII variants lacking amino acids encoded by exon 13 or 105 promote dermal-epidermal stability equally well as WT collagen VII. While vehicle injected mice show areas of dermal-epidermal separation (red arrows), such separations are not visible in mice which had received 10 µg of WT, Δ13, or Δ105 collagen VII. Bar = 50 µm, D = dermis, E = epidermis, in a and b nuclei visualized by 4′,6-diamidino-2-phenylindole (blue). White asterisks indicate autofluorescence of the epidermis.
Discussion
One approach to treat genetic diseases is to restore protein expression by removing exons carrying causal mutations, either via excision on the DNA level or via exon skipping-based therapies on the pre-mRNA level. The unique gene structure of COL7A1 makes development of such therapies to treat DEB highly interesting. We here provide solid evidence that collagen VII can withstand deletion of amino acids provided by individual exons without significant impairment of functionality. Recently, it was reported that natural skipping of exon 15 resulted in drastic attenuation of the phenotypic severity of DEB.19 A milder phenotype was also observed for natural skipping of exon 87 (refs. 16,17) and exon 19 (ref. 18) Although removal of some exons will certainly lead to nonfunctional collagen VII, these observations, together with our findings, provide evidence for the feasibility of exon skipping or exon removal therapy to treat DEB.
Among the options that are available to achieve therapeutic exon skipping for DEB, development of AON therapy would likely result in the fastest translation into use in the clinics. We here show that AONs can be designed against exons 13 and 105 to robustly promote exon skipping. Nevertheless, the development of AON therapies is still hampered by low cellular uptake and exon skipping efficiency.44 However, it has been shown that they can reach the skin after systemic delivery in concentrations sufficient to restore protein synthesis.33 It also has to be considered that the effect of AONs is transient and limited by the protein lifetime. (Bornert, O, Peking, P, Bremer, J, Koller, U, van den Akker, PC, Aartsma-Rus, A, et al. (2016). RNA-based therapies for genodermatoses. Submitted.). In the case of collagen VII the in vivo half-life is around 1 month indicating that frequent treatment cycles with AONs would be needed for significant protection against frictional challenges.45 To overcome these challenges, development of permanent exon skipping-based strategies are of high interest. U7 small nuclear RNA (snRNA), usually involved in histone pre-mRNA 3'-end processing, can be used as a splicing modulator.46,47,48 Very recently, permanent restoration of the Dmd reading frame resulting in dystrophin expression was achieved in vivo in a Duchenne mouse model. In the mouse, AAV-mediated muscular delivery of CRISPR/Cas9 allowed excision of exon 23 that harbored the causal mutation.12,13,14 It is a clear asset that CRISPR/Cas9-mediated gene editing promotes permanent repair, which would limit treatment cycles. However, multiple safety concerns surround the approach and there is a shortage of suitable vectors with high skin tropism. Nevertheless, the development of gene-editing techniques for permanent restoration of collagen VII expression should be considered as an attractive therapy approach for DEB. Thus, there are multiple options to remove faulty messages encoded by exon sequences, and although these techniques still need improvement and optimization, we do not view them as the principal limitation of exon skipping or exon removal strategies. What will ultimately determine therapeutic success are the protein intrinsic properties. The internally deleted proteins resulting from these efforts need to remain highly functional. Therefore, we believe that it is imperative to analyze the functionality of such protein variants before endeavoring into optimization of exon skipping or gene editing of specific exons.
A potential limitation of restored protein expression by therapeutic removal or skipping of exons carrying mutations is neutralizing immune reactions that may nullify the therapeutic benefit. As for all therapies aiming to restore protein expression in a genetic disorder, special attention needs to be placed on the immunological response from the host to the de novo expressed protein. This is especially important for collagen VII, which is known to be highly antigenic.49 However, it has become evident that a large number of individuals with generalized severe DEB are mosaic and carry revertant skin patches that express collagen VII.50 Thus, the immune system of many of the prospective patients for these therapies has already encountered collagen VII, and accordingly immunogenicity to collagen VII may not be an issue. Still, it is conceivable that skipping or permanent removal of specific exons, especially in the NC1 domain can create new highly antigenic epitopes. These concerns can only be reliably addressed directly in patients.
Based on the results presented in this paper, we can conclude that skipping or excision on the DNA level of exon 13 or 105 will lead to production of collagen VII variants with retained in vivo functionality. Because DEB is a familial disease, development of skipping or gene-editing strategies to remove specific exons will only be beneficial for a small subset of patients, making these approaches a type of personal medicine. In comparison to traditional therapy, personal medicine is highly challenging because adaptation to each patient phenotype and/or genotype is an inescapable prerequisite. In order to optimize cost and time for the development of such therapy, a generic strategy is first needed to study the theoretical effect on the target, i.e., gene or protein. By a combination of molecular, cellular, and in vivo experiments, we have developed a robust procedure for the characterization of collagen VII variants resulting from lack of faulty exons in COL7A1. Our method can be used as a screening assay to test the effect of any exon deletion from COL7A1. Importantly, this method could also be interesting as a tool to delineate the collagen VII interactome.
To conclude, we here show by detailed analysis of recombinantly expressed internally deleted collagen VII variants that exon skipping–based therapy or similar gene editing approaches should be considered highly attractive options for treatment of DEB.
Materials and Methods
Ethics statement. Studies using patient material were approved by the Ethics committee of the University of Freiburg approval number 45/03-110631. All animal experiments were approved by the regional ethics review board (Regierungspräsidium Freiburg), ethical approval number G11/70 and G14/93. The mice were housed in a clean facility with water, food, and supportive nutrition ad libitum.
Antibodies. The following primary antibodies were used: rabbit anti-NC2-10 for collagen VII P1 fragment and rabbit anti-LH7.2 for collagen VII NC1 domain.27,32 Secondary antibodies were Alexa Fluor 488-conjugated goat anti rabbit (Invitrogen, Darmstadt, Germany) and horseradish peroxidase (HRP)-conjugated goat anti-rabbit (Jackson Immuno Research Laboratories, Newmarket, UK).
Deletion of exon in COL7A1. Extraction of cDNA encoding collagen VII from pcDNA3.1 was performed by digestion with NotI (5') and EcoRI (3'). Small fragments of COL7A1 cDNA were obtained by digestion with PciI, SacII, or HincII. Fragments 3'-PciI, SacII-HincII, and HincII-5' from COL7A1 cDNA containing respectively exon 1 to 25, 57 to 101 and 103 to 117 were subcloned into pBlueScript or pUC19 vectors. Primers Exon-13-Forward (5′-TGCGCAGCACCCAGGAGCCGGAAACTCCACTT-3′) and PciI-reverse-primer (5′-ACATGTATCTGTGAGCTGTGACC-3′) were used to amplify region after exon 13, and primers Exon-13-reverse (5′-GTGGAGTTTCCGGCTCCTGGGTGCTGCGC-3′) and M13FR (5′-GTTTTCCCAGTCACGAC-3′) the region before exon 13. Primers Exon-105-forward (5′-GGATCCCTGGTGACCCGGGTGAACGGGGAGTGAAGGGA-3′) with M13RP (5′-CAGGAAACAGCTATGAC-3′) and Exon-105-reverse (5′-CGGGTCACCAGGGATCCCTGCTGCACCAGGTTGACCCT-3′) with M13FR (5′-GTTTTCCCAGTCACGAC-3′) were used to respectively amplify regions after and before exon 105. Overlap PCR was carried out with primers M13FP and M13RP (exon 105) or M13FP and PciI-reverse-primer (exon 13). PCR products lacking exon 13 or 105 were ligated together with the rest of COL7A1 cDNA in pcDNA3.1 with a ratio of 1 to 1 by using T4 DNA ligase (Thermo Fisher Scientific, Waltham, MA). All PCR reactions were performed with Phusion High Fidelity polymerase (Thermo Fisher Scientific, Waltham, MA) and the constructs were sequenced by Sanger sequencing (GATC Biotech, Konstanz, Germany). All Enzymes were purchased from Thermo Fisher Scientific and used accordingly to the manufacturer's instruction. Plasmids were purified with NucleoSpin. Plasmid and DNA products were extracted from agarose gel with NucleoSpin gel and PCR clean-up (Macherey Nagel, Düren, Germany).
Cell culture and transfection. Fibroblasts or HEK-293 cells were cultured in DMEM/F12 (Life Technologies, Carlsbad, CA) medium containing 10% fetal calf serum (Biochrome, Berlin, Germany), supplemented with 200 mmol/l L-glutamine, and 10 mg/ml penicillin-streptomycin (both Life Technologies, Carlsbad, CA). HEK-293 cells were transfected with 28 μg of cDNA per 10-cm dish using lipofectamine 2000 (Sigma-Aldrich, St. Louis, MO) for COL7A1 WT and lacking exon 13 or 105 in pcDNA3.1. Cells containing the transfected plasmid were selected with phleomycin (InvivoGen, San Diego, CA) for at least 72 hours. Expression of WT, Δ13, or Δ105 collagen VII was performed in serum-free Dulbecco's Modified Eagle Medium (DMEM) supplemented with 50 μg/ml ascorbic acid (Fluka, Buchs, Switzerland). Medium was collected after 48 hours and cleared by centrifugation at 3,000 rpm for 10 minutes prior purification of recombinant collagen VII by precipitation with ammonium sulfate (Roth, Karlsruhe, Germany). A concentration of 25% of ammonium sulfate was added to the cleared medium overnight at 4 °C under gentle agitation. Precipitated proteins were collected by high speed centrifugation at 14,000 rpm for 30 minutes at 4 °C and resuspended in tris-buffered saline (TBS) buffer (50 mmol/l Tris-HCl (Roth) pH 7.4 and 150 mmol/l NaCl (Roth)) supplemented with 10 mmol/l ethylenediaminetetraacetic acid (Serva, Heidelberg, Germany) and 1 mmol/l Pefabloc (Sigma-Aldrich, St. Louis, MO). Proteins were used immediately or stored at −70 °C for long term.
AON design and cell treatment. For design of AONs with a combined maximal predicted splicing specificity and in vivo efficiency, we used m-fold and HSF softwares, together with Tm calculation and RNA structure prediction according to established guidelines.51 After in silico analyses, we selected AONs with the sequence 5′-GCCUGAACGUCAUCCAAGUCG-3′ and 5′-CUCCUUUUUCUCCUCGGAUACCAGG-3′ to target 13 and 105, respectively. A nonspecific AON 5′-GCUUUUCUUUUAGUUGCUGC-3′ was used as negative control and with the addition of a 5′-FAM 537.46 fluorescent label as positive control. All AONs comprise 2′ O-methyl-modified bases and phosphorothioate linkages, and were synthesized and purified by reverse-phase high-performance liquid chromatography (Eurogentec BV, Liège, Belgium). Primary normal human dermal fibroblasts were transfected with AON using Lipofectamine-2000 (LF) (Invitrogen, Carlsbad, CA). The lipid-AON complex formation was optimized to a weight:weight ratio of 1:1. Prior to transfection, cells were grown to 70–80% confluence, washed, and fresh Opti-MEM (Gibco, Waltham, MA) medium was added to the wells. Lipid-AON complexes were formed according to the manufacturer's instructions and drop-wise added to the cells to a final concentration of 250 or 500 nmol/l of AONs in the medium. After 6 hours of incubation at 37 °C and 5% CO2, the medium was removed, cells were washed, and complete culture medium was added.
For the analysis of exon skipping on the RNA level, RNA was isolated 48 hours after transfection using the RNeasy Micro Kit (Qiagen). The medium was removed from the wells prior to addition of the lysis buffer. The lysates were collected in 1.5 ml tubes, vortexed for 1 minute, flash frozen in liquid nitrogen, and stored at −80 °C before RNA isolation was performed according to the manufacturer's instructions. Following RNA isolation, RNA was immediately reverse transcribed using Superscript-III (Invitrogen) reverse transcriptase. Reverse transcription was followed by PCR analysis of exon 13 and 105 of the COL7A1 gene using nested PCR and the following primers:
First forward exon 13: 5′-GGTGGTACTGCCCTCTGATG-3′
First reverse exon 13: 5′-TCCGTTCGAGCCACGATGAC-3′
Nested forward exon 13: 5′-CCGCCTCACACTCTACACTC-3′
Nested reverse exon 13: 5′-AGCCACCTGGTAGGTGGTTC-3′
First forward exon 105: 5′-TCAGCTGTGATCCTGGGGCCT-3′
First reverse exon 105: 5′-AGGGCAGCAAGGGAGAGCCT-3′
Nested forward exon 105: 5′-AGGGCAGCAAGGGAGAGCCT-3′
Nested reverse exon 105: 5′-TTTGTGTCCTGCCAGCCCGG-3′
Sanger sequencing of amplicons was performed by GATC.
Immunofluorescence staining. Cells on cover slips were fixed with ice-cold acetone for 5 minutes. After air-drying, cells were washed with phosphate-buffered saline (PBS) and blocked with 0.1% BSA in PBS for 20 minutes. Cells were then incubated with the rabbit anti collagen VII LH7.2 antibody diluted at 1:2,000 in PBS with 0.1% BSA (Santa Cruz, Dallas, TX) for 1 hour. After washing with PBS, 1:1,000 diluted anti-rabbit Alexa488 antibody was added for 30 minutes. Nuclei were stained with 4′,6-diamidino-2-phenylindole for 2 minutes; coverslips were wased and embedded in fluorescence mounting medium (Dako, Hamburg, Germany) for examination. Images were acquired with an Axiocam MRm camera attached to a Zeiss Axio Imager A1 fluorescence microscope (Carl Zeiss, Jena, Germany), processed using Axiovision 4.8 and ZEN2009 software (Carl Zeiss).
RNA isolation and RT-PCR. RNA was isolated from cells with NucleoSpin RNA isolation kit (Macherey-Nagel, Düren, Germany) according to the manufacturer's instructions and transcribed to cDNA with First Strand cDNA Synthesis Kit (Thermo Fisher Scientific). PCRs were performed with primers surrounding exon 13 or 105 and analyzed on a 2% agarose gel. The following primers were used: 5′-CATTGTGCGCAGCACCC-3′ and 5′-AGCAAGTGGAGTTTCCGGC-3′ for exon 13, 5′-AGACTCAGCTGTGATCCTGG-3′ and 5′-CCCTTCACTCCCCGTTCAC-3′ for exon 105 (Biomer, Ulm, Germany).
Western blotting. Samples were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis and electroblotted onto nitrocellulose membranes (Millipore, Darmstadt, Germany). Blocking buffer (50 mmol/l Tris-HCl pH 7.4, 150 mmol/l NaCl, 0.05% Tween-20 with 5% non-fat milk) was used to block the membranes. Blots were then incubated with the rabbit anti collagen VII antibodies LH7.2 or NC2-10 in blocking buffer overnight at 4 °C. After extensive wash with TBS-tween buffer, HRP-conjugated goat anti-rabbit antibody was added for 1 hour. Enhanced chemiluminescence prime reagent (GE Healthcare, Freiburg, Germany) was used to develop blots and pictures were captured using a Fusion SL system (Peqlab, Erlangen, Germany). Quantification was performed with Image J (NIH, Washington, DC) and data were analyzed with GraphPad Prism software (Graphpad, La Jolla, CA).
Trypsin digestion assays. Triple helical folding of collagen VII was assessed by limited trypsin digestion with or without preboiling of collagen VII as previously described.34,36 Boiled collagen VII was used as positive control as boiling prior trypsin digestion completely disrupts the triple helix and abolishes the trypsin resistance of the collagen VII collagenous domain.
Solid-phase interaction. Five hundred nanograms of collagen IV (Sigma-Aldrich) were used to coat Nunc Maxsorp 96-well plates overnight at 4 °C. The next morning, wells were blocked with 0.1% BSA in PBS for 1 hour and various concentrations of precipitated collagen VII in TBS were then added for 1 hour. After extensive wash with PBS, 1:1,000 diluted rabbit LH7.2 antibody was added for 1 hour at room temperature. Wells were washed three times with PBS and HRP-conjugated goat anti-rabbit antibody diluted at 1:10,000 was added. Finally, SigmaFast OPD tablet (Sigma-Aldrich) was used for detection of peroxidase activity and plate was read at 450 nm on infinite M200 plate reader (Tecan, Männedorf, Switzerland).
Cells adhesion and migration assays. Nunc F Untreated 96-wells plates were used for adhesion assays (Thermo Fisher Scientific). Wells were coated with 100 ng of BSA, FN, WT collagen VII, Δ13 or Δ105 collagen VII in PBS. After overnight incubation at 4 °C, wells were blocked with 0.1% BSA in PBS for 1 hour. Then 100,000 fibroblasts were seeded per well and the plate was incubated for 2 hours at 37 °C. After washing with PBS, fibroblasts were fixed with 100% methanol for 5 minutes and stained with 0.1% crystal violet for 20 minutes. Pictures were taken after extensive wash with PBS and cells lysis was performed with 1% acetic acid. Quantification was made by reading the absorbance at 550 nm and data were analyzed using GraphPad Prism software.
Migration assays were performed by using silicone inserts (ibidi, Martinsried, Germany). The dishes were first coated with BSA, FN, WT collagen VII, Δ13 or Δ105 collagen VII in PBS as describe above. After seeding of 100,000 fibroblasts, plates were incubated at 37 °C until confluence. The insert was removed and pictures were taken at different time points to follow cell migration. Quantification was made using Image J and data were analyzed with the GraphPad Prism software.
Collagen VII injection in Col7a1 hypomorphic mice. Col7a1 hypomorphic mice were identified by hemorrhagic blisters at mechanically challenged sites, as previously described.37 Prior to injections, the back skin of Col7a1 hypomorphic mice was carefully cleaned and disinfected. 10 or 25 µg of WT, Δ13 or Δ105 collagen VII were diluted in 30 µl vehicle and injected intradermally with a 27 G needle (B.Braun Melsungen, Germany). 24 hours after injection mice were sacrificed and skin samples were taken for further analysis. Skin sections were fixed in acetone, blocked, and stained with primary and secondary antibodies, counterstained with 4′,6-diamidino-2-phenylindole, and mounted in Fluorescence Mounting Medium (Dako). Images were acquired with an Axiocam MRm camera attached to a Zeiss Axio Imager A1 fluorescence microscope (Carl Zeiss, Jena, Germany) and processed using the Axiovision 4.9 and the ZEN2012 softwares (Carl Zeiss). For H&E stainings the skin was fixed in neutral buffered ready-to-use 10% formalin solution (Sigma-Aldrich) and embedded in paraffin. The sections were stepwise deparaffinized, rehydrated, and stained with hematoxylin (Sigma-Aldrich) for 2.5 minutes, thereafter they were decolorized in acid alcohol for 2 seconds and the pH was increased by tap water for 10 minutes. The sections were counterstained with 0.1% eosin (Sigma-Aldrich) for 30 seconds and dehydrated through increasing alcohol concentrations. Eventually, sections were embedded in mounting medium (Sigma-Aldrich).
Statistical analysis. Statistical analysis was performed with the GraphPad Prism software using Student's unpaired t-test, a P value of < 0.05 was considered statistically significant.
SUPPLEMENTARY MATERIAL Figure S1. Generic strategy for exon deletion in COL7A1 cDNA. Figure S2. Collagen VII variants retain ability to bind laminin 332.
Acknowledgments
We want to thank Annemieke Aartsma-Rus for valuable advice and critical reading of the manuscript. The work was supported by a grant from the German Federal Ministry for Education and Research, BMBF, under the frame of Erare-2, the ERA-Net for Research on Rare Diseases, project 01GM1310 (SpliceEB) and from the Deutsche Forsschungsgemeinschaft, NY90/2-1, NY90/3-2 to A.N. P.Cvd.A. was supported by a Clinical Fellowship grant (90715614) from the Netherlands Organisation for Health Research and Development (ZonMW).
Supplementary Material
References
- Bruckner-Tuderman, L (2010). Dystrophic epidermolysis bullosa: pathogenesis and clinical features. Dermatol Clin 28: 107–114. [DOI] [PubMed] [Google Scholar]
- Mittapalli, VR, Madl, J, Löffek, S, Kiritsi, D, Kern, JS, Römer, W et al. (2016). Injury-driven stiffening of the dermis expedites skin carcinoma progression. Cancer Res 76: 940–951. [DOI] [PubMed] [Google Scholar]
- Vanden Oever, MJ and Tolar, J (2014). Advances in understanding and treating dystrophic epidermolysis bullosa. F1000Prime Rep 6: 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto, M, Sawamura, D, Ito, K, Abe, M, Nishie, W, Sakai, K et al. (2006). Fibroblasts show more potential as target cells than keratinocytes in COL7A1 gene therapy of dystrophic epidermolysis bullosa. J Invest Dermatol 126: 766–772. [DOI] [PubMed] [Google Scholar]
- Goto, M, Sawamura, D, Nishie, W, Sakai, K, McMillan, JR, Akiyama, M et al. (2006). Targeted skipping of a single exon harboring a premature termination codon mutation: implications and potential for gene correction therapy for selective dystrophic epidermolysis bullosa patients. J Invest Dermatol 126: 2614–2620. [DOI] [PubMed] [Google Scholar]
- Lanuti, EL, Wikramanayake, TC and Kirsner, RS (2011). Overcoming obstacles for gene therapy for recessive dystrophic epidermolysis bullosa. J Invest Dermatol 131: 5. [DOI] [PubMed] [Google Scholar]
- Sallach, J, Di Pasquale, G, Larcher, F, Niehoff, N, Rübsam, M, Huber, A et al. (2014). Tropism-modified AAV vectors overcome barriers to successful cutaneous therapy. Mol Ther 22: 929–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery, AE (2002). The muscular dystrophies. Lancet 359: 687–695. [DOI] [PubMed] [Google Scholar]
- Hoffman, EP, Brown, RH Jr and Kunkel, LM (1987). Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell 51: 919–928. [DOI] [PubMed] [Google Scholar]
- Roberts, RG, Coffey, AJ, Bobrow, M and Bentley, DR (1993). Exon structure of the human dystrophin gene. Genomics 16: 536–538. [DOI] [PubMed] [Google Scholar]
- Roberts, RG, Gardner, RJ and Bobrow, M (1994). Searching for the 1 in 2,400,000: a review of dystrophin gene point mutations. Hum Mutat 4: 1–11. [DOI] [PubMed] [Google Scholar]
- Long, C, Amoasii, L, Mireault, AA, McAnally, JR, Li, H, Sanchez-Ortiz, E et al. (2016). Postnatal genome editing partially restores dystrophin expression in a mouse model of muscular dystrophy. Science 351: 400–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson, CE, Hakim, CH, Ousterout, DG, Thakore, PI, Moreb, EA, Castellanos Rivera, RM et al. (2016). In vivo genome editing improves muscle function in a mouse model of Duchenne muscular dystrophy. Science 351: 403–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabebordbar, M, Zhu, K, Cheng, JK, Chew, WL, Widrick, JJ, Yan, WX et al. (2016). In vivo gene editing in dystrophic mouse muscle and muscle stem cells. Science 351: 407–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu, QL, Cirak, S and Partridge, T (2014). What can we learn from clinical trials of exon skipping for DMD? Mol Ther Nucleic Acids 3: e152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koga, H, Hamada, T, Ishii, N, Fukuda, S, Sakaguchi, S, Nakano, H et al. (2011). Exon 87 skipping of the COL7A1 gene in dominant dystrophic epidermolysis bullosa. J Dermatol 38: 489–492. [DOI] [PubMed] [Google Scholar]
- Saito, M, Masunaga, T and Ishiko, A (2009). A novel de novo splice-site mutation in the COL7A1 gene in dominant dystrophic epidermolysis bullosa (DDEB): specific exon skipping could be a prognostic factor for DDEB pruriginosa. Clin Exp Dermatol 34: e934–e936. [DOI] [PubMed] [Google Scholar]
- McGrath, JA, Ashton, GH, Mellerio, JE, Salas-Alanis, JC, Swensson, O, McMillan, JR et al. (1999). Moderation of phenotypic severity in dystrophic and junctional forms of epidermolysis bullosa through in-frame skipping of exons containing non-sense or frameshift mutations. J Invest Dermatol 113: 314–321. [DOI] [PubMed] [Google Scholar]
- Schwieger-Briel, A, Weibel, L, Chmel, N, Leppert, J, Kernland-Lang, K, Grüninger, G et al. (2015). A COL7A1 variant leading to in-frame skipping of exon 15 attenuates disease severity in recessive dystrophic epidermolysis bullosa. Br J Dermatol 173: 1308–1311. [DOI] [PubMed] [Google Scholar]
- Toh, ZY, Thandar Aung-Htut, M, Pinniger, G, Adams, AM, Krishnaswarmy, S, Wong, BL et al. (2016). Deletion of dystrophin in-frame exon 5 leads to a severe phenotype: guidance for exon skipping strategies. PLoS One 11: e0145620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aartsma-Rus, A, Ferlini, A, Goemans, N, Pasmooij, AM, Wells, DJ, Bushby, K et al. (2014). Translational and regulatory challenges for exon skipping therapies. Hum Gene Ther 25: 885–892. [DOI] [PubMed] [Google Scholar]
- Christiano, AM, Hoffman, GG, Chung-Honet, LC, Lee, S, Cheng, W, Uitto, J et al. (1994). Structural organization of the human type VII collagen gene (COL7A1), composed of more exons than any previously characterized gene. Genomics 21: 169–179. [DOI] [PubMed] [Google Scholar]
- Rousselle, P, Keene, DR, Ruggiero, F, Champliaud, MF, Rest, M and Burgeson, RE (1997). Laminin 5 binds the NC-1 domain of type VII collagen. J Cell Biol 138: 719–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, M, Marinkovich, MP, Veis, A, Cai, X, Rao, CN, O'Toole, EA et al. (1997). Interactions of the amino-terminal noncollagenous (NC1) domain of type VII collagen with extracellular matrix components. A potential role in epidermal-dermal adherence in human skin. J Biol Chem 272: 14516–14522. [DOI] [PubMed] [Google Scholar]
- Chen, M, Marinkovich, MP, Jones, JC, O'Toole, EA, Li, YY and Woodley, DT (1999). NC1 domain of type VII collagen binds to the beta3 chain of laminin 5 via a unique subdomain within the fibronectin-like repeats. J Invest Dermatol 112: 177–183. [DOI] [PubMed] [Google Scholar]
- Brittingham, R, Uitto, J and Fertala, A (2006). High-affinity binding of the NC1 domain of collagen VII to laminin 5 and collagen IV. Biochem Biophys Res Commun 343: 692–699. [DOI] [PubMed] [Google Scholar]
- Bruckner-Tuderman, L, Nilssen, O, Zimmermann, DR, Dours-Zimmermann, MT, Kalinke, DU, Gedde-Dahl, T Jr et al. (1995). Immunohistochemical and mutation analyses demonstrate that procollagen VII is processed to collagen VII through removal of the NC-2 domain. J Cell Biol 131: 551–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Akker, PC, Jonkman, MF, Rengaw, T, Bruckner-Tuderman, L, Has, C, Bauer, JW et al. (2011). The international dystrophic epidermolysis bullosa patient registry: an online database of dystrophic epidermolysis bullosa patients and their COL7A1 mutations. Hum Mutat 32: 1100–1107. [DOI] [PubMed] [Google Scholar]
- Lunstrum, GP, Sakai, LY, Keene, DR, Morris, NP and Burgeson, RE (1986). Large complex globular domains of type VII procollagen contribute to the structure of anchoring fibrils. J Biol Chem 261: 9042–9048. [PubMed] [Google Scholar]
- Hammami-Hauasli, N, Schumann, H, Raghunath, M, Kilgus, O, Lüthi, U, Luger, T et al. (1998). Some, but not all, glycine substitution mutations in COL7A1 result in intracellular accumulation of collagen VII, loss of anchoring fibrils, and skin blistering. J Biol Chem 273: 19228–19234. [DOI] [PubMed] [Google Scholar]
- Bateman, JF, Moeller, I, Hannagan, M, Chan, D and Cole, WG (1992). Characterization of three osteogenesis imperfecta collagen alpha 1(I) glycine to serine mutations demonstrating a position-dependent gradient of phenotypic severity. Biochem J 288 (Pt 1): 131–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myllyharju, J and Kivirikko, KI (2004). Collagens, modifying enzymes and their mutations in humans, flies and worms. Trends Genet 20: 33–43. [DOI] [PubMed] [Google Scholar]
- Heemskerk, H, de Winter, C, van Kuik, P, Heuvelmans, N, Sabatelli, P, Rimessi, P et al. (2010). Preclinical PK and PD studies on 2'-O-methyl-phosphorothioate RNA antisense oligonucleotides in the mdx mouse model. Mol Ther 18: 1210–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyström, A, Bruckner-Tuderman, L and Kern, JS (2013). Cell- and protein-based therapy approaches for epidermolysis bullosa. Methods Mol Biol 961: 425–440. [DOI] [PubMed] [Google Scholar]
- Fritsch, A, Spassov, S, Elfert, S, Schlosser, A, Gache, Y, Meneguzzi, G et al. (2009). Dominant-negative effects of COL7A1 mutations can be rescued by controlled overexpression of normal collagen VII. J Biol Chem 284: 30248–30256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühl, T, Mezger, M, Hausser, I, Handgretinger, R, Bruckner-Tuderman, L and Nyström, A (2015). High local concentrations of intradermal MSCs restore skin integrity and facilitate wound healing in dystrophic epidermolysis bullosa. Mol Ther 23: 1368–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyström, A, Buttgereit, J, Bader, M, Shmidt, T, Ozcelik, C, Hausser, I et al. (2013). Rat model for dominant dystrophic epidermolysis bullosa: glycine substitution reduces collagen VII stability and shows gene-dosage effect. PLoS One 8: e64243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mecklenbeck, S, Compton, SH, Mejía, JE, Cervini, R, Hovnanian, A, Bruckner-Tuderman, L et al. (2002). A microinjected COL7A1-PAC vector restores synthesis of intact procollagen VII in a dystrophic epidermolysis bullosa keratinocyte cell line. Hum Gene Ther 13: 1655–1662. [DOI] [PubMed] [Google Scholar]
- Chen, M, Costa, FK, Lindvay, CR, Han, YP and Woodley, DT (2002). The recombinant expression of full-length type VII collagen and characterization of molecular mechanisms underlying dystrophic epidermolysis bullosa. J Biol Chem 277: 2118–2124. [DOI] [PubMed] [Google Scholar]
- Nyström, A, Velati, D, Mittapalli, VR, Fritsch, A, Kern, JS and Bruckner-Tuderman, L (2013). Collagen VII plays a dual role in wound healing. J Clin Invest 123: 3498–3509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodley, DT, Hou, Y, Martin, S, Li, W and Chen, M (2008). Characterization of molecular mechanisms underlying mutations in dystrophic epidermolysis bullosa using site-directed mutagenesis. J Biol Chem 283: 17838–17845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritsch, A, Loeckermann, S, Kern, JS, Braun, A, Bösl, MR, Bley, TA et al. (2008). A hypomorphic mouse model of dystrophic epidermolysis bullosa reveals mechanisms of disease and response to fibroblast therapy. J Clin Invest 118: 1669–1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remington, J, Wang, X, Hou, Y, Zhou, H, Burnett, J, Muirhead, T et al. (2009). Injection of recombinant human type VII collagen corrects the disease phenotype in a murine model of dystrophic epidermolysis bullosa. Mol Ther 17: 26–33.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siva, K, Covello, G and Denti, MA (2014). Exon-skipping antisense oligonucleotides to correct missplicing in neurogenetic diseases. Nucleic Acid Ther 24: 69–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühl, T, Mezger, M, Hausser, I, Guey, LT, Handgretinger, R, Bruckner-Tuderman, L et al. (2016). Collagen VII half-life at the dermal-epidermal junction zone: implications for mechanisms and therapy of genodermatoses. J Invest Dermatol 136: 1116–1123. [DOI] [PubMed] [Google Scholar]
- Gorman, L, Suter, D, Emerick, V, Schümperli, D and Kole, R (1998). Stable alteration of pre-mRNA splicing patterns by modified U7 small nuclear RNAs. Proc Natl Acad Sci USA 95: 4929–4934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suter, D, Tomasini, R, Reber, U, Gorman, L, Kole, R and Schümperli, D (1999). Double-target antisense U7 snRNAs promote efficient skipping of an aberrant exon in three human beta-thalassemic mutations. Hum Mol Genet 8: 2415–2423. [DOI] [PubMed] [Google Scholar]
- Le Guiner, C, Montus, M, Servais, L, Cherel, Y, Francois, V, Thibaud, JL et al. (2014). Forelimb treatment in a large cohort of dystrophic dogs supports delivery of a recombinant AAV for exon skipping in Duchenne patients. Mol Ther 22: 1923–1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pendaries, V, Gasc, G, Titeux, M, Leroux, C, Vitezica, ZG, Mejía, JE et al. (2010). Immune reactivity to type VII collagen: implications for gene therapy of recessive dystrophic epidermolysis bullosa. Gene Ther 17: 930–937. [DOI] [PubMed] [Google Scholar]
- Kiritsi, D, Garcia, M, Brander, R, Has, C, Meijer, R, Jose Escámez, M et al. (2014). Mechanisms of natural gene therapy in dystrophic epidermolysis bullosa. J Invest Dermatol 134: 2097–2104. [DOI] [PubMed] [Google Scholar]
- Aartsma-Rus, A (2012). Overview on AON design. Methods Mol Biol 867: 117–129. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.