Abstract
Eosinophils are a minority circulating granulocyte classically viewed as being involved in host defense against parasites and promoting allergic reactions. However, a series of new regulatory functions for these cells have been identified in the past decade. During homeostasis, eosinophils develop in the bone marrow and migrate from the blood into target tissues following an eotaxin gradient, with IL-5 being a key cytokine for eosinophil proliferation, survival and priming. In multiple target tissues, eosinophils actively regulate a variety of immune functions through their vast arsenal of granule products and cytokines, as well as direct cellular interaction with cells in proximity. The immunologic regulation of eosinophils extends from innate immunity to adaptive immunity and also involves non-immune cells. Herein, we summarize recent findings regarding novel roles of murine and human eosinophils focused on interactions with other hematopoietic cells. We also review new experimental tools available and remaining questions to uncover a greater understanding of this enigmatic cell.
Keywords: Th2 response, granular products, MBP (major basic protein), EPO (eosinophil peroxidase), EDN (eosinophil-derived neurotoxin), ECP (eosinophil cationic protein), CCR3, IL-5, asthma, eosinophil activation, fibrosis, mucosal defense and eosinophilic gastrointestinal disorders (EGID)
Eosinophils are key innate regulator/effector cells
Eosinophils represent a minor component of circulating leukocytes and are generally considered to be terminally differentiated as post-mitotic cells, yet it is now appreciated that they can be long-lived multi-faceted granulocytes involved in a variety of regulatory functions. Like other granulocytes, eosinophils develop and differentiate in the bone marrow. Under homeostasis, eosinophils are distributed in the blood, lung, thymus, uterus, adipose tissues, mammary gland, spleen and the lamina propria of the gastrointestinal (GI) tract (1), indicating a physiological function in each organ. Although eosinophils outside of the bone marrow are deemed as mature, recent evidence suggests the existence of multiple tissue-specific subtypes on the basis of distinct cell surface marker expression and functions (2–4). Driven by eosinophil-specific chemokines (primarily eotaxins) produced at baseline and markedly up-regulated after a variety of stimuli (5), mature eosinophils are recruited from the circulation into their physiological locations and inflammatory sites, respectively. The cytokine IL-5, produced primarily by Type 2 T helpers (Th2) (6) and type 2 innate helper lymphoid cells (ILC2) (7), is a crucial cytokine for eosinophil differentiation, priming and survival (8). Conversely, eosinophils also serve as a source of a variety of cytokines and growth factors closely associated with multiple immuno-modulatory functions to be discussed later. Through their vast cytokine arsenal and engagement of cell contact, eosinophils modulate immune responses through an array of interactive and orchestrated mechanisms, in trans and cis fashions, by cellular and humoral mediators, in both innate and adaptive immune responses. Recently, a burgeoning body of evidence has uncovered several underappreciated roles for eosinophils that could modulate both the adaptive and innate arms of immunity. An essential goal of this chapter is to summarize the role of eosinophils in physiological and inflammatory processes in human and small mammal models in order to identify novel pharmacological targets for specific disease management.
Eosinophils interactively regulate multiple components of adaptive immunity
Eosinophils modulate lymphocyte recruitment and homeostasis
The canonical theory for eosinophil recruitment into the GI and the lung tissue is highlighted by the “T helper – Th2 cytokine – epithelium – eosinophil chemokine” axis (9) emphasizing the influence of lymphocytes on eosinophil recruitment. Specifically, antigen-experienced local Th2 cells produce the cytokine IL-5, which promotes eosinophil production, priming and survival, and IL-13, which induces local cells to produce eosinophil-specific chemokines—the eotaxins, which attract circulating eosinophils into their niche. There are three eotaxins identified, namely eotaxin 1, 2 and 3, all of which were shown to induce eosinophilia in asthma models and some human diseases. Meanwhile, the inflammatory environment (e.g. TNF-α) prepares endothelial cells for adhesion. However, the ability of eosinophils to influence lymphocyte recruitment and activity was not appreciated until several recent studies provided alternative evidence showing a pronounced reduction of Th2 cytokines and effector T-cell recruitment in eosinophil-deficient mice, a deficit fully rescued by eosinophil re-introduction (10), suggesting that eosinophils are critical for T-cell homing in the lung. Indeed, in an Il5−/− background, the airway hyperreactivity and mucus production associated with experimental asthma were shown to be functions of both T-cell and eosinophil factors, as each alone was insufficient for induction of allergic airway inflammation (11). Therefore, at least in a rodent pulmonary system, eosinophils may interact with lymphocytes in a bidirectional manner rather than passively responding to chemotactic and priming signals. Consistent with this theory, in eosinophil-deficient ΔdblGATA-1 mice, reduced Peyer’s patch development and T helper cell cytokine production was observed, highlighting the promoting role of eosinophils on lymphocyte homeostasis (10, 12, 13). Extending this paradigm to B cells, it has recently become appreciated that bone marrow eosinophils co-localize with plasma cells during their maturation, secrete the cytokines APRIL and IL-6 and contribute to the survival of bone marrow plasma cells, whose death is augmented in eosinophil-deficient ΔdblGATA-1 mice (14). In addition, the B cell process of IgA class switching was recently found to be positively regulated by GI eosinophils in the intestinal tissue (12, 13). Finally, recent studies have uncovered a novel role for eosinophils in promoting B cell proliferation upon eosinophil activation in mice, and a positive correlation between blood eosinophil and B cell counts in humans (15). In the light of these findings, a systemic scanning of lymphocyte phenotypes and functions in eosinophil-deficient mice (not restricted to the pulmonary system) should be prioritized.
Eosinophils behave as antigen-presenting cells
Other than regulating lymphocyte recruitment and function, it is now appreciated that eosinophils have the capacity to present antigen to T cells. This topic stems from the original findings that GM-CSF–treated eosinophils have a “non-professional” antigen presentation function in vitro as shown by their capacity to induce antigen-specific T-cell clone proliferation (16). Eosinophils, after allergen exposure, express the machinery for antigen presentation and a full set of co-stimulation molecules, including MHC class II, CD80, CD86, CD9, CD28 and CD40, at the protein level (17, 18). Eosinophils labeled in the airway lumen migrate to the draining lymph node, reaching the T-cell proliferation zone, in a process that is independent of the eotaxin receptor CCR3. Moreover, these antigen-experienced eosinophils promote antigen-specific T-cell proliferation ex vivo, suggesting an antigen-presenting cell (APC) behavior. Antigen (OVA)-loaded and GM-CSF–treated eosinophils, when instilled intratracheally, promote the proliferation of adoptively transferred OVA-specific T-cell clones, which is accompanied by T-cell CD69 upregulation and IL-4 production and T cell/eosinophil co-localization in the draining lymph node (19). In a murine allergic asthma model, peripheral eosinophil recruitment into the lymph node is required for antigen-specific T-cell proliferation in situ (20), In addition to the pulmonary system, murine studies have also demonstrated the antigen-presenting capacity of eosinophils in threadworm (21) and fungus infections (22). Moreover, considering that eosinophils are potent cytokine producers and regulators of humoral immunity and are usually isolated from a population rich in professional APCs, the direct evidence for the physical T cell/eosinophil interaction (e.g. by confocal or intravital microscopy) is needed. Conceivably, eosinophil-deficient or eosinophil-specific (23) MHC II–deficient mice would serve as the best tools to substantiate this interaction.
Eosinophils, alone or via dendritic cells, drive Th2 polarization
Eosinophils are capable of driving a Th2 response in multiple ways. A crucial Th2 characteristic of eosinophils is their capacity to produce canonical Th2 cytokines (IL-4, IL-5 and IL-13) upon stimulation (1, 24). In addition, eosinophils isolated from patients with asthma may sustain Th2 polarization by maintaining a high intracellular indoleamine 2,3-dioxygenase (IDO) level, with IDO being a Th2 differentiation regulator (25). In mice, eosinophils are required for dendritic cells (DCs) and T cells to initiate Th2 inflammation in the lung (20). Emerging evidence also suggests that eosinophils suppress Th17 and Th1 responses via DC regulation, which will be further discussed below.
As a messenger between innate and adaptive immunity, eosinophils engage in direct cross-talk with DCs. Although eosinophils themselves are able to present antigen to T cells de novo, the well-recognized positive regulation by eosinophils on professional APCs should be emphasized as well. Conventional myeloid DC (mDC, immunotyped as IL-12, Toll-like receptor 2 [TLR2] and TLR4 positive) can ingest the eosinophil granule protein major basic protein (MBP) in vivo, and the physical interaction of human blood eosinophils and DCs, revealed by confocal imaging, results in DC maturation in the presence of the bacterial pathogen-associated molecular pattern (PAMP) CpG-C (26). In general, the presence of either eosinophils or DCs in tumor tissue is an indicator of positive prognosis and a negative prognosis for allograft, further suggesting the role of eosinophils in antigen presentation, either alone or in combination with the DCs (27, 28). Furthermore, the eosinophil granule protein “eosinophil derived neurotoxin” (EDN), which is a member of the RNase family, has been shown to be chemotactic factor for mDC (29), triggering mDC cytokine production (30). Moreover, EDN specifically binds to TLR2 on DCs as an endogenous ligand triggering MyD88-dependent pathway in TLR-transfected HEK293 cells (31). Following OVA immunization in vivo, co-immunization with EDN potentiates OVA-specific IgG1 (a Th2 Ig) production, but not IgG2 and IgG3. Splenocytes from TLR2+/+ mice immunized with OVA in the presence of EDN primarily produce IL-5, IL-6, IL-10 and IL-13 (Th2 cytokine signature), whereas the TLR2−/− splenocytes (lacking the signal through EDN) primarily produce interferon γ (31). Collectively, these findings indicate that specific eosinophil products can serve as Th2 adjuvants via DC regulation, at least partially contributing to the Th2-promoting role of eosinophils, and facilitate maturation of other immunocytes including the lineage commitment of eosinophils themselves (32), although more studies are needed to substantiate these novel observations.
In a murine OVA-induced asthma model, cellular trafficking of eosinophils into the lung lymphatic compartment is a prerequisite for mDC accumulation in draining lymph nodes (20) and, consequently, for allergen-specific effector T-cell proliferation. Importantly, this pulmonary Th2 enhancement through DC regulation is not MHC II (classical antigen presentation complex) or CCR7 (classical lymph node homing signal) dependent (20). Whether the DC regulation is through eosinophil cytokine secretion or cellular interaction remains to be resolved. It should also be noted that most of the studies about DC regulation are confined to the lung and use allergic asthma models; the generalization of these processes in other tissues is ripe for investigation.
Regulation of T-cell development in the thymus
The thymus is one of several organs where eosinophils are readily found under homeostatic conditions. Eosinophils migrate into the thymus during the neonatal period and wane after adolescence (33). Eosinophils are present in the thymus under homeostasis and have a unique CD11c+CD11b+CD44highMHC II lowSSChigh phenotype, distinct from circulating eosinophils. Thymus-bound eosinophils exhibit an activated phenotype, as shown by expression of several surface activation markers, including CD25, CD69 and mRNA for Th2 cytokines IL-4, IL-5, IL-13 and GM-CSF (34). Initial data suggest that eosinophils have a role in MHC I–dependent negative selection and may induce T-cell apoptosis by free radicals to facilitate negative selection. Eosinophils in the thymus are Indoleamine 2,3-dioxygenase(IDO)-positive (25) and correlate with local Th2 cytokine levels (33), suggesting a Th1/Th2-regulating role within the thymus, possibly via DC regulation.
Taken together, eosinophils actively orchestrate a chain response in adaptive immunity at earlier points than previously appreciated, as evidenced by the lymphocyte deficiency and impaired local Th2 cytokine profile seen in eosinophil-deficient mice. Notably, eosinophils also possess a robust capacity to regulate a variety of cell types in the non-adaptive immune system, which will be discussed in the following sections.
Eosinophils directly participate in innate recognition and actively regulate other aspects of non-adaptive immunity
Unique PAMP receptor repertoire for specific pathogen recognition and clearance
Despite early studies showing that eosinophils are able to engulf foreign pathogens, it is now generally accepted that they are not directly involved in cellular phagocytosis. Rather, eosinophils rapidly release (catapult) their mitochondrial DNA to confine bacterial infection in the GI mucosa (35). Furthermore, it was recently found that the eosinophil extracellular DNA trap formation after cytolysis is also accompanied by release of cell-free secretion-competent granules in a NADPH oxidase–dependent fashion (36), further strengthening the evidence of the pathogen-combating function of human eosinophils. In addition, evidence exists that eosinophils have anti-parasite activity, mediated by release of their cytotoxic granule proteins (37). The capacity of eosinophils to release anti-parasite mediators has been shown in multiple studies demonstrating the deposition of eosinophil granule proteins around parasites (38, 39). Gene deletion studies in mice have also indicated that disruption of EPO or MBP results in significantly higher worm burdens compared to wildtype mice (40). The key activating signal for promoting these responses is not agreed upon yet, but multiple lines of evidence suggest that functional Fc receptors on eosinophils (allowing mediation of antibody-dependent cellular cytotoxicity), complement receptors and several receptors for pathogen/damage-associated molecular patterns (PAMP/DAMP) may contribute (41, 42).
As to PAMP recognition, eosinophils express several functional TLR, including TLR1, 2, 3, 5, 6, 7, and 9 (43, 44), and activation of these receptors by microbes and pathogens leads to intracellular signal activation and cytokine production by eosinophils (45). These findings not only imply that eosinophils may take part in PAMP recognition and subsequent defensive processes but also suggest a potential mechanism explaining the exacerbation of allergy inflammation by bacterial/viral infection (46). Notably, human data regarding eosinophil TLR2 and TLR4 expression exhibits a certain degree of heterogeneity associated with atopic and eosinophilia status (47).
Specifically regarding viral PAMP recognition, TLR7, which recognizes viral ssRNA, is expressed in human eosinophils with intracellular signaling capacity (43). In addition to their pro-inflammatory roles in asthma, eosinophils are tightly associated with anti-viral activity in a variety of systems. EDN has been shown to possess an inhibitory effect on HIV (48), and eosinophil-tropic IL-5 transgenic mice have a more rapid, MyD88-dependant clearance of respiratory syncytial virus (RSV) compared to wild-type mice (49). Likewise, in pulmonary viral infection, eosinophils cooperate with macrophages to prevent the infection from spreading to uninfected epithelial cells (50), suggesting a positive role of eosinophils in combatting viral infections through TLRs. However, in the context of human rhinovirus (HRV), eosinophils enhanced the viral load by inhibiting epithelial interferon production (51), indicating the complexity in eosinophil functions in viral infections.
Currently, a large portion of our knowledge regarding eosinophil-viral interactions comes from RSV. At a mechanistic level, it is not clear how the virus initiates eosinophilia and how the eosinophil-viral interactions are regulated. Further insights are expected by broadening the scope to other organs/systems (such as the GI), and investigating other types of viruses may provide further depth, such as the rotavirus infection in mouse and human GI tract (52).
Eosinophil: a key orchestrator of asthma and other allergic diseases
In addition to the Th2-tilted cytokine production and DC induction discussed earlier in the adaptive immunity section, eosinophils also contribute to asthma pathogenesis by serving as a key orchestrator. Indeed, genetic variants affecting eosinophilia at least in part, such as variants in WDR36, ST2, IL33 and MYB, have been associated with morbidity of atopic asthma (53). Importantly, major eosinophil granule products are composed of four bio-active cytotoxic proteins, namely MBP, eosinophil peroxidase (EPO), eosinophil cationic protein (ECP) and EDN. In human lung, eosinophil products can be detected in macrophage intracellular compartments, contributing to macrophage activation. EPO has been shown to positively regulate macrophage phagocytosis (54). MBP has been shown to disrupt GI barrier function, induce airway smooth muscle contraction and elicit mast-cell / basophil degranulation (55). As for the interaction with lung epithelium, emerging evidence suggests that eosinophils regulate the airway epithelial cytokine profile and permeability (51). Multiple lines of evidence also suggest that eosinophils take an active role in epithelial damage and basal membrane hyper-proliferation (56, 57). Additionally, the observation that human eosinophils induce mucus production via EGF activation (58) underlines the interaction between eosinophils and asthmatic lung epithelium. Notably, with the vital role of mast cells in asthma increasingly revealed (59), the close interaction between eosinophils and mast cells are highly likely to be important as well, which will be discussed in the next sub-section.
In human and murine asthmatic lung, eosinophils are generally recognized as a contributor to airway hyperreactivity. Eosinophils are sources of IL-5 and GM-CSF, thereby promoting their own survival in an autocrine fashion (60), but the major source of IL-5 is believed to be the Th2 cells or ILC2 cells. The causal relationship between asthma and eosinophils has been best elucidated by a series of anti–IL-5 studies in human asthma. Although early anti–IL-5 studies yielded controversial results, more recent studies focusing on severe eosinophilic asthmatic phenotypes, as well as sputum eosinophil count, clearly demonstrated the efficacy of humanized anti–IL-5 therapy (mepolizumab), representing an important treatment avenue in an area of unmet clinical need (61–63). From an independent development perspective, another anti-IL-5 humanized antibody, reslizumab, also convincingly demonstrated efficacy in severe asthma management including improvements in lung function, significantly reducing the asthmatic exacerbation frequency in two independent phase III trials (64). Interestingly, the optimal effect was achieved in inadequately controlled asthma with elevated blood eosinophil counts. Of note, with the demonstrated efficacy during the late phase development, both anti-IL-5 humanized antibodies have now been FDA-approved for clinical management of asthma. (65, 66). Human eosinophils have been shown to directly activate neutrophils by releasing ENA-78/CXCL5 (67). Additionally, MBP stimulates IL-8 secretion by neutrophils, with regulation occurring at both transcriptional and post-transcriptional levels (68). These can lead to neutrophilia and neutrophil activation, which is also a critical component of asthma (69). In the presence of GM-CSF, eosinophils express basal levels of NOTCH ligand (60, 70), capable of regulating both innate and adaptive immunity, including macrophage polarization (71) and cytokine-independent, T-cell lineage commitment (72), respectively, suggesting another regulatory function of lung eosinophils in asthma.
Eosinophils were identified as the key factor for human asthma exacerbation (73) and lung connective tissue remodeling, which has been attributed to granule products such as MBP and cytokines such as TGF-β. Recent research in patients with asthma indicates that eosinophils actively participate in the lung tissue fibrosis and remodeling, linking eosinophils to the potential etiology that commonly leads to progressive worsening of quality of life (74) (75). In addition to the well-known fibrogenic molecule TGF-β being produced by eosinophils, in vitro evidence indicated that the eosinophil product ECP induces lung fibroblast migration (76) and stimulates them to produce TGF-β in vitro (77). Interestingly, the tissue-remodeling capacity of GI eosinophils seems to be suppressed by their surface inhibitory receptors, such as CD172a (3), implying a promising potential to suppress this adverse effect.
Eosinophil–mast-cell cross-talk
As pivotal components of allergic hypersensitivity, eosinophils and mast cells mutually potentiate each other in several allergic disorders, such as asthma and eosinophilic esophagitis (EoE) (78). While mast cells support the survival and activation of eosinophils by secreting IL-5, eosinophil MBP directly activates mast cells and basophils, triggering the release of an arsenal of allergic mediators and cytokines, including histamine and TNF-α. In the murine system, ECP and EPO also activate allergic mediator release from mast cells (1). Conversely, mast cells are the major sources of prostaglandin D2 (PGD2) (79, 80), a key inflammation mediator whose receptor, CRTH2/CD294, is robustly expressed on rodent and human eosinophils (81, 82). This transmembrane G-protein coupled receptor has the dual functions of eosinophil-activating receptor and chemotaxis receptor. Exposure of eosinophils to PGD2 induces rapid morphological changes, intracellular calcium flux, chemotaxis and cellular degranulation of human eosinophils. CRTH2 is known to be expressed on Th2 cells, whose Th2 cytokines will also promote eosinophilia (83). The eosinophil–mast-cell interaction was highlighted by a recent clinical study showing that in human patients with the allergic GI disorder EoE, eosinophils are physically coupled with mast cells as assessed by immunohistochemistry (84) and are a major source of the mast cell–supporting cytokine IL-9 (85). In vitro ultra-structural evidence also indicated that eosinophils and mast cells form cell-cell contact and exchange their products for reciprocal activation (84, 86). Importantly, anti–IL-5 therapy concomitantly reduced both eosinophils and mast cell numbers (84), indicating that eosinophils promote mastocytosis in human allergic disease. Of note, in eosinophilic gastrointestinal disorders (EGIDs, e.g. EoE), despite the fact that eosinophil migration is relatively better understood, it remains a mystery how circulating mast-cell precursors migrate into tissue and become mature/activated. The reciprocal eosinophil–mast-cell potentiation may be critical to understanding the pathogenesis of allergic disease and developing new intervention platforms for human allergic disorders.
In summary, eosinophils serve as recognition cells of certain unique PAMPs, playing a vital role in innate defense against viral, parasitic and bacterial infection. Aside from the Th2 induction and T cell/DC interactions, the regulating role of eosinophil in asthma can be reflected by its multi-faceted interaction with a myriad of cell types in the lung and by its robust tissue-remodeling capacity.
Some new and intriguing areas of research about eosinophils
Paradoxical roles in tissue destruction and repair
Eosinophils are equipped with a tissue damage–sensing system, including several histamine receptors (HR1, HR2, and HR4) (87, 88), which enables them to release multiple tissue-repairing molecules. Damaged epithelial cells from different tissue origins directly stimulate eosinophil secretion of TGF-β and fibroblast growth factor (FGF) (89). Indeed, the eosinophil is capable of producing TGF-β, TGF-α, epidermal growth factor (EGF) (90), FGF (89), platelet-derived growth factor (PDGF) (91) and vascular-endothelial growth factor (VEGF) (92), all of which have well-recognized beneficial roles in tissue repair (8). The tissue repair function of eosinophils was substantiated by a recent study showing that eosinophil IL-4 production is necessary for hepatocyte regeneration after hepatectomy or toxin injury, as this effect is abolished in eosinophil-deficient mice (93). Although it was proposed that eosinophils promote wound healing due to growth factor production (90, 94), Il5 over-expressing mice have a delayed wound healing due to augmented inflammatory responses and delayed matrix synthesis (95, 96), suggesting that the reparative function of eosinophils is tonically modulated.
Adipose tissue residential eosinophils regulate local cytokine milieu and glucose homeostasis
Recent studies indicated that eosinophils are tightly associated with alternatively activated macrophages (M2) (97, 98), whereas obesity is interpreted as an uncontrolled chronic inflammatory response associated with dysregulated macrophage populations in the adipose tissue (99). Interestingly, with eosinophils found as a residential cell type in the visceral adipose tissue under homeostasis, a recent study has uncovered unexpected, non-redundant roles of eosinophils in adipose tissue macrophage polarization and glucose metabolism (100). In murine models, eosinophils have been proven to be critical for maintaining adipose tissue M2 macrophages as a major contributor of IL-4, a key factor for M2 polarization. In the absence of eosinophils, mice are prone to develop obesity, glucose intolerance and insulin resistance (100). Notably, the eosinophilia induced by parasitic infection has been shown to enhance glucose tolerance, reinforcing a regulatory effect of eosinophils on metabolism. The field is awaiting studies performed in human adipose tissue demonstrating similar regulatory roles of eosinophils on macrophage subtypes and body metabolism.
Eosinophils may contribute to neurologic symptoms by regulating neural activity
Notably, several studies suggest a link between eosinophils and neuronal homeostasis. First, peripheral dorsal root ganglia (DRG) and airway neurons produce eotaxins to chemoattract eosinophils into their niche (101, 102). Furthermore, eosinophils are capable of producing nerve growth factor (NGF) and neurotrophins (e.g. NT-3), at the mRNA and protein level, to directly regulate neuronal activity. This constitutive activity can be further boosted by Fc receptor–mediated eosinophil activation (103). In vitro, both murine and human eosinophils promoted DRG neuron branching, an effect independent of physical contact (101) and a plausible explanation for the cutaneous nerve outgrowth and associated neurologic symptoms in atopic dermatitis. EGIDs have pronounced tissue-specific eosinophilia, with expression of several neuro-filament elements being robustly upregulated in EoE (104). Notably, a vast majority of patients with EoE experience a certain degree of neuropathy and somato-sensory alterations (105). Additionally, the neurotrophins present in the asthmatic lung promote the survival of airway eosinophils (106), forming a potential vicious cycle. It is therefore conceivable that eosinophils may directly contribute to these neuronal dysregulations. These observations may at least partially explain the neurologic hypersensitivity in a myriad of allergic diseases, such as asthma, atopic dermatitis and allergic rhinitis (107, 108), and in some eosinophilic disorders, such as EGIDs (109, 110). Although the neural hyperplasia in atopic dermatitis has been well documented, the anomalous neurologic changes in EGIDs have yet to be characterized. In a guinea pig model of asthma, it is notable that eosinophils migrate into the nerves in a CCR3-dependent fashion (102) (especially the vagal nerve) and release MBP (111), which serves as a muscarinic receptor 2 antagonist (112) that enhances the acetylcholine release and thereby exacerbates bronchoconstriction. In human asthma and EoE, eosinophils localize near nerve endings with extracellular MBP adhered to the nerve endings, suggesting a neuronal regulatory role of eosinophils (113).
The enigmatic functions of GI eosinophils
Rodent and human GI tissue harbors the largest reservoir of eosinophils compared to other anatomical compartments including blood and bone marrow. Although intestinal eosinophils are extensively present in the lamina propria of the full length of the GI tract, their function is the least understood compared to eosinophils in other organs. From a limited number of studies, eosinophils in the GI compartment seem to adopt a unique surface expression profile to accommodate their GI-specific functions (2). For reasons that are not fully comprehended, GI eosinophil turnover rate is much slower than that of the lung and blood eosinophils, as assessed by BrdU incorporation (114). It was also found that signaling through the common γ chain receptor is necessary for the longer survival of GI eosinophils (114), but the functions of these cells still remain largely unknown. Recently, utilizing the eosinophil-deficient ΔdblGATA-1 and PHIL mice, multiple research groups showed that GI eosinophils promote the generation and production of IgA-producing plasma cells in the GI tract (12, 13). These studies also identified that eosinophils have novel and profound functions in the GI tract, such as promoting IgA class-switching, enhancing intestinal mucus secretions, determining intestinal micro-biota and inducing the development of Peyer’s patches (12, 13). The functional exploration of GI eosinophils has just started. With conditional, eosinophil lineage–deficient mice and genome-wide screening methods become increasingly available, key functions of GI eosinophils in homeostasis and disease contexts, such as EGIDs, are soon to be discovered.
Perspectives
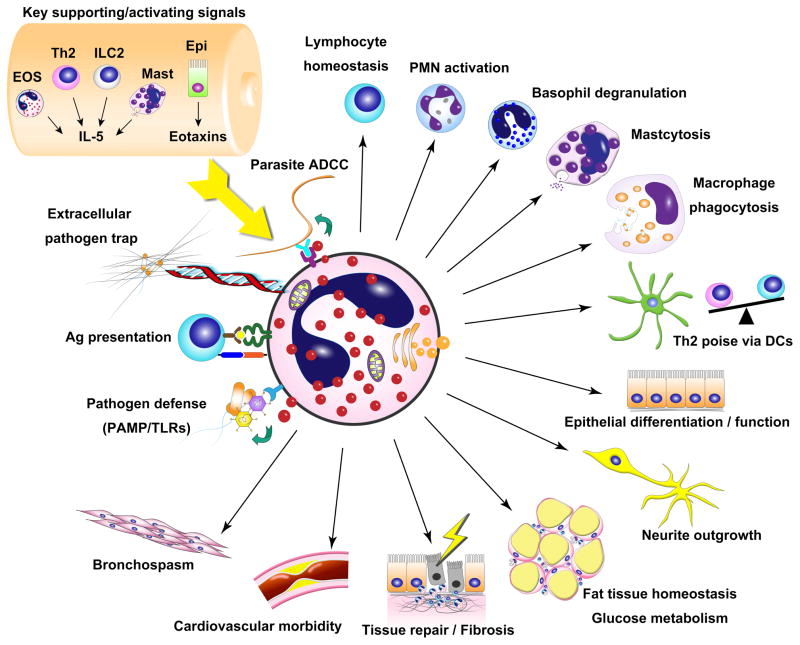
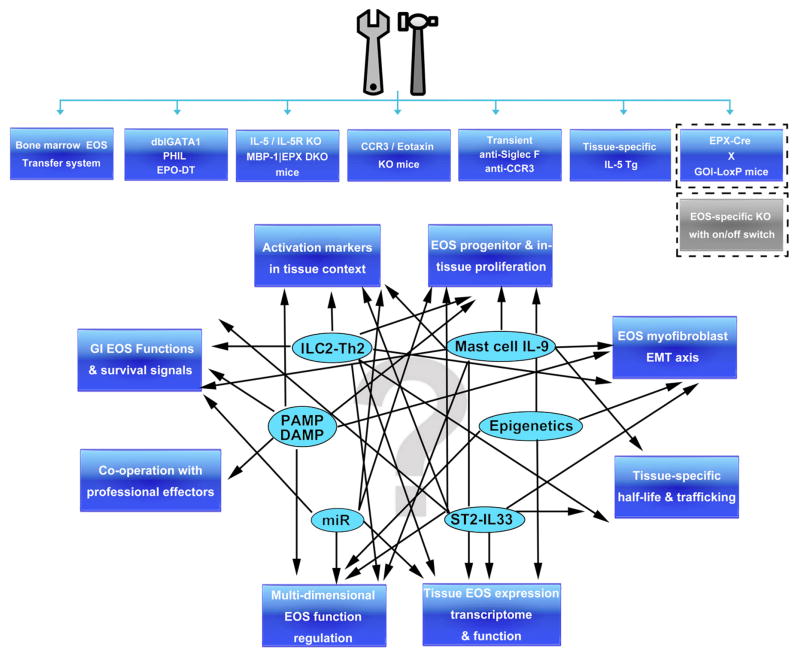
With advancing technology and accumulating knowledge, the understanding of eosinophils has changed from a simple post-mitotic cell with limited functional capacity in parasite infection and allergy to a cell type that is actively involved in orchestrating a variety of mucosal and non-mucosal immune responses at baseline and during a variety of disease responses (see Figure 1 for schematic summary illustration). With more novel functions of eosinophils being actively investigated and the increasing number of murine models available, rapid advances in further understanding this cell type are likely to occur in the near future (summarized in Figure 2). The recent generation of the EPO–diphtheria toxin eosinophil-depleted mice (115) and the MBP-1 EPX –disrupted eosinophil progenitor–deficient mice (23) is expected to provide a complementary and independent means to assess the participation of eosinophils in a variety of responses. The interaction network between eosinophils and the recently identified IL-33–ST2 axis provides an opportunity to further understand the activation of this cell type in innate allergic responses. The interplay between eosinophils and newly identified ILC2 cells (116), which produces abundant amount of IL-5 and primarily resides in non-lymphoid tissues (7), is a particularly promising new finding that may explain the key source of eosinophilopoetins and early involvement of eosinophils in acute injury responses. The recent generation of an eosinophil-specific gene disruption model, the EPO-driven Cre expression mouse (117), is likely to facilitate the investigation of underappreciated functions of eosinophils. As to advancing asthma management strategy, as illustrated by the success of the anti–IL-5 studies, eosinophil-suppressive therapy represents an effective and well-tolerated treatment that reduces the possibility of asthma exacerbation and will likely get further approval in the market and more clinical and research attention. Collectively, these studies and the implications of their findings support the likelihood for an emerging and expanding body of data concerning the function and role of eosinophils in immunity.
Figure 1. Schematic summary of eosinophil-tropic signaling and eosinophil cellular and humoral regulatory functions.
EOS, eosinophils; IL-5, interleukin 5; Th2, T helper cell type 2, ILC2, innate lymphoid cell type 2; Mast, mast cells; Epi, epithelium; ADCC, antibody-dependent cell-mediated cytotoxicity; Ag, antigen; PAMP: pathogen-associated molecular pattern; TLRs, toll-like receptors; DCs; dendritic cells; PMN, polymorphonuclear leukocyte (neutrophil).
Figure 2. Research tool summary and questions to be answered.
Although recent advances provide tremendous insight into the regulatory functions of eosinophils, important questions remain. With the increasing number of tools available (upper panel), progress in the listed areas (lower panel, outside blue boxes), in the light of key eosinophil regulation elements (inside ovals) will be interesting and crucial to understanding the still enigmatic function of eosinophils. EOS, eosinophils; EPO-DT, EPO-driven diphtheria toxin expression mice; EMT, epithelial-mesenchymal transition; DAMP, damage-associated molecular pattern; PAMP, pathogen-associated molecular pattern; miR, microRNA; GOI, gene of interest; Tg, transgenic; (D)KO, (double)knockout.
Acknowledgments
This work is supported by the NIH grants R37 A1045898, R01 AI083450, P30 DK078392 (the Digestive Diseases Research Core Center in Cincinnati), the CURED (Campaign Urging Research for Eosinophilic Disease) Foundation, the Food Allergy Research & Education (FARE), the Buckeye Foundation, and the American Partnership for Eosinophilic Disorders (APFED). The authors thank Shawna Hottinger for editorial assistance.
References
- 1.Rothenberg ME, Hogan SP. The eosinophil. Annu Rev Immunol. 2006;24:147–174. doi: 10.1146/annurev.immunol.24.021605.090720. [DOI] [PubMed] [Google Scholar]
- 2.Wen T, Mingler MK, Blanchard C, Wahl B, Pabst O, Rothenberg ME. The pan-B cell marker CD22 is expressed on gastrointestinal eosinophils and negatively regulates tissue eosinophilia. J Immunol. 2012;188:1075–1082. doi: 10.4049/jimmunol.1102222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Verjan Garcia N, Umemoto E, Saito Y, Yamasaki M, Hata E, Matozaki T, Murakami M, Jung YJ, Woo SY, Seoh JY, Jang MH, Aozasa K, Miyasaka M. SIRPalpha/CD172a regulates eosinophil homeostasis. J Immunol. 2011;187:2268–2277. doi: 10.4049/jimmunol.1101008. [DOI] [PubMed] [Google Scholar]
- 4.Rose CE, Jr, Lannigan JA, Kim P, Lee JJ, Fu SM, Sung SS. Murine lung eosinophil activation and chemokine production in allergic airway inflammation. Cell Mol Immunol. 2010;7:361–374. doi: 10.1038/cmi.2010.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Conroy DM, Williams TJ. Eotaxin and the attraction of eosinophils to the asthmatic lung. Respir Res. 2001;2:150–156. doi: 10.1186/rr52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Upadhyaya B, Yin Y, Hill BJ, Douek DC, Prussin C. Hierarchical IL-5 expression defines a subpopulation of highly differentiated human Th2 cells. J Immunol. 2011;187:3111–3120. doi: 10.4049/jimmunol.1101283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nussbaum JC, Van Dyken SJ, von Moltke J, Cheng LE, Mohapatra A, Molofsky AB, Thornton EE, Krummel MF, Chawla A, Liang HE, Locksley RM. Type 2 innate lymphoid cells control eosinophil homeostasis. Nature. 2013 doi: 10.1038/nature12526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hogan SP. Functional role of eosinophils in gastrointestinal inflammation. Immunol Allergy Clin North Am. 2009;29:129–140. xi. doi: 10.1016/j.iac.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Munitz A, Brandt EB, Mingler M, Finkelman FD, Rothenberg ME. Distinct roles for IL-13 and IL-4 via IL-13 receptor alpha1 and the type II IL-4 receptor in asthma pathogenesis. Proc Natl Acad Sci U S A. 2008;105:7240–7245. doi: 10.1073/pnas.0802465105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jacobsen EA, Ochkur SI, Pero RS, Taranova AG, Protheroe CA, Colbert DC, Lee NA, Lee JJ. Allergic pulmonary inflammation in mice is dependent on eosinophil-induced recruitment of effector T cells. J Exp Med. 2008;205:699–710. doi: 10.1084/jem.20071840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shen HH, Ochkur SI, McGarry MP, Crosby JR, Hines EM, Borchers MT, Wang H, Biechelle TL, O’Neill KR, Ansay TL, Colbert DC, Cormier SA, Justice JP, Lee NA, Lee JJ. A causative relationship exists between eosinophils and the development of allergic pulmonary pathologies in the mouse. J Immunol. 2003;170:3296–3305. doi: 10.4049/jimmunol.170.6.3296. [DOI] [PubMed] [Google Scholar]
- 12.Chu VT, Beller A, Rausch S, Strandmark J, Zanker M, Arbach O, Kruglov A, Berek C. Eosinophils promote generation and maintenance of immunoglobulin-A-expressing plasma cells and contribute to gut immune homeostasis. Immunity. 2014;40:582–593. doi: 10.1016/j.immuni.2014.02.014. [DOI] [PubMed] [Google Scholar]
- 13.Jung Y, Wen T, Mingler MK, Caldwell JM, Wang YH, Chaplin DD, Lee EH, Jang MH, Woo SY, Seoh JY, Miyasaka M, Rothenberg ME. IL-1beta in eosinophil-mediated small intestinal homeostasis and IgA production. Mucosal Immunol. 2015;8:930–942. doi: 10.1038/mi.2014.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chu VT, Frohlich A, Steinhauser G, Scheel T, Roch T, Fillatreau S, Lee JJ, Lohning M, Berek C. Eosinophils are required for the maintenance of plasma cells in the bone marrow. Nat Immunol. 2011;12:151–159. doi: 10.1038/ni.1981. [DOI] [PubMed] [Google Scholar]
- 15.Wong TW, Doyle AD, Lee JJ, Jelinek DF. Eosinophils regulate peripheral B cell numbers in both mice and humans. J Immunol. 2014;192:3548–3558. doi: 10.4049/jimmunol.1302241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Del Pozo V, De Andres B, Martin E, Cardaba B, Fernandez JC, Gallardo S, Tramon P, Leyva-Cobian F, Palomino P, Lahoz C. Eosinophil as antigen-presenting cell: activation of T cell clones and T cell hybridoma by eosinophils after antigen processing. Eur J Immunol. 1992;22:1919–1925. doi: 10.1002/eji.1830220736. [DOI] [PubMed] [Google Scholar]
- 17.Shi HZ, Humbles A, Gerard C, Jin Z, Weller PF. Lymph node trafficking and antigen presentation by endobronchial eosinophils. J Clin Invest. 2000;105:945–953. doi: 10.1172/JCI8945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Akuthota P, Melo RC, Spencer LA, Weller PF. MHC Class II and CD9 in human eosinophils localize to detergent-resistant membrane microdomains. Am J Respir Cell Mol Biol. 2012;46:188–195. doi: 10.1165/rcmb.2010-0335OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang HB, Ghiran I, Matthaei K, Weller PF. Airway eosinophils: allergic inflammation recruited professional antigen-presenting cells. J Immunol. 2007;179:7585–7592. doi: 10.4049/jimmunol.179.11.7585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jacobsen EA, Zellner KR, Colbert D, Lee NA, Lee JJ. Eosinophils regulate dendritic cells and Th2 pulmonary immune responses following allergen provocation. J Immunol. 2011;187:6059–6068. doi: 10.4049/jimmunol.1102299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Padigel UM, Hess JA, Lee JJ, Lok JB, Nolan TJ, Schad GA, Abraham D. Eosinophils act as antigen-presenting cells to induce immunity to Strongyloides stercoralis in mice. J Infect Dis. 2007;196:1844–1851. doi: 10.1086/522968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garro AP, Chiapello LS, Baronetti JL, Masih DT. Eosinophils elicit proliferation of naive and fungal-specific cells in vivo so enhancing a T helper type 1 cytokine profile in favour of a protective immune response against Cryptococcus neoformans infection. Immunology. 2011;134:198–213. doi: 10.1111/j.1365-2567.2011.03479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Doyle AD, Jacobsen EA, Ochkur SI, McGarry MP, Shim KG, Nguyen DT, Protheroe C, Colbert D, Kloeber J, Neely J, Shim KP, Dyer KD, Rosenberg HF, Lee JJ, Lee NA. Expression of the secondary granule proteins major basic protein 1 (MBP-1) and eosinophil peroxidase (EPX) is required for eosinophilopoiesis in mice. Blood. 2013;122:781–790. doi: 10.1182/blood-2013-01-473405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spencer LA, Szela CT, Perez SA, Kirchhoffer CL, Neves JS, Radke AL, Weller PF. Human eosinophils constitutively express multiple Th1, Th2, and immunoregulatory cytokines that are secreted rapidly and differentially. J Leukoc Biol. 2009;85:117–123. doi: 10.1189/jlb.0108058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Odemuyiwa SO, Ghahary A, Li Y, Puttagunta L, Lee JE, Musat-Marcu S, Ghahary A, Moqbel R. Cutting edge: human eosinophils regulate T cell subset selection through indoleamine 2,3-dioxygenase. J Immunol. 2004;173:5909–5913. doi: 10.4049/jimmunol.173.10.5909. [DOI] [PubMed] [Google Scholar]
- 26.Lotfi R, Lotze MT. Eosinophils induce DC maturation, regulating immunity. J Leukoc Biol. 2008;83:456–460. doi: 10.1189/jlb.0607366. [DOI] [PubMed] [Google Scholar]
- 27.Weir MR, Hall-Craggs M, Shen SY, Posner JN, Alongi SV, Dagher FJ, Sadler JH. The prognostic value of the eosinophil in acute renal allograft rejection. Transplantation. 1986;41:709–712. doi: 10.1097/00007890-198606000-00008. [DOI] [PubMed] [Google Scholar]
- 28.Lotfi R, Lee JJ, Lotze MT. Eosinophilic granulocytes and damage-associated molecular pattern molecules (DAMPs): role in the inflammatory response within tumors. J Immunother. 2007;30:16–28. doi: 10.1097/01.cji.0000211324.53396.f6. [DOI] [PubMed] [Google Scholar]
- 29.Yang D, Rosenberg HF, Chen Q, Dyer KD, Kurosaka K, Oppenheim JJ. Eosinophil-derived neurotoxin (EDN), an antimicrobial protein with chemotactic activities for dendritic cells. Blood. 2003;102:3396–3403. doi: 10.1182/blood-2003-01-0151. [DOI] [PubMed] [Google Scholar]
- 30.Yang D, Chen Q, Rosenberg HF, Rybak SM, Newton DL, Wang ZY, Fu Q, Tchernev VT, Wang M, Schweitzer B, Kingsmore SF, Patel DD, Oppenheim JJ, Howard OM. Human ribonuclease A superfamily members, eosinophil-derived neurotoxin and pancreatic ribonuclease, induce dendritic cell maturation and activation. J Immunol. 2004;173:6134–6142. doi: 10.4049/jimmunol.173.10.6134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang D, Chen Q, Su SB, Zhang P, Kurosaka K, Caspi RR, Michalek SM, Rosenberg HF, Zhang N, Oppenheim JJ. Eosinophil-derived neurotoxin acts as an alarmin to activate the TLR2-MyD88 signal pathway in dendritic cells and enhances Th2 immune responses. J Exp Med. 2008;205:79–90. doi: 10.1084/jem.20062027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O’Connell AE, Hess JA, Santiago GA, Nolan TJ, Lok JB, Lee JJ, Abraham D. Major basic protein from eosinophils and myeloperoxidase from neutrophils are required for protective immunity to Strongyloides stercoralis in mice. Infect Immun. 2011;79:2770–2778. doi: 10.1128/IAI.00931-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tulic MK, Sly PD, Andrews D, Crook M, Davoine F, Odemuyiwa SO, Charles A, Hodder ML, Prescott SL, Holt PG, Moqbel R. Thymic indoleamine 2,3-dioxygenase-positive eosinophils in young children: potential role in maturation of the naive immune system. Am J Pathol. 2009;175:2043–2052. doi: 10.2353/ajpath.2009.090015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Throsby M, Herbelin A, Pleau JM, Dardenne M. CD11c+ eosinophils in the murine thymus: developmental regulation and recruitment upon MHC class I-restricted thymocyte deletion. J Immunol. 2000;165:1965–1975. doi: 10.4049/jimmunol.165.4.1965. [DOI] [PubMed] [Google Scholar]
- 35.Yousefi S, Gold JA, Andina N, Lee JJ, Kelly AM, Kozlowski E, Schmid I, Straumann A, Reichenbach J, Gleich GJ, Simon HU. Catapult-like release of mitochondrial DNA by eosinophils contributes to antibacterial defense. Nat Med. 2008;14:949–953. doi: 10.1038/nm.1855. [DOI] [PubMed] [Google Scholar]
- 36.Ueki S, Melo RC, Ghiran I, Spencer LA, Dvorak AM, Weller PF. Eosinophil extracellular DNA trap cell death mediates lytic release of free secretion-competent eosinophil granules in humans. Blood. 2013;121:2074–2083. doi: 10.1182/blood-2012-05-432088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Klion AD, Nutman TB. The role of eosinophils in host defense against helminth parasites. J Allergy Clin Immunol. 2004;113:30–37. doi: 10.1016/j.jaci.2003.10.050. [DOI] [PubMed] [Google Scholar]
- 38.Kephart GM, Gleich GJ, Connor DH, Gibson DW, Ackerman SJ. Deposition of eosinophil granule major basic protein onto microfilariae of Onchocerca volvulus in the skin of patients treated with diethylcarbamazine. Lab Invest. 1984;50:51–61. [PubMed] [Google Scholar]
- 39.Mehlotra RK, Hall LR, Higgins AW, Dreshaj IA, Haxhiu MA, Kazura JW, Pearlman E. Interleukin-12 suppresses filaria-induced pulmonary eosinophilia, deposition of major basic protein and airway hyperresponsiveness. Parasite Immunol. 1998;20:455–462. doi: 10.1046/j.1365-3024.1998.00174.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Specht S, Saeftel M, Arndt M, Endl E, Dubben B, Lee NA, Lee JJ, Hoerauf A. Lack of eosinophil peroxidase or major basic protein impairs defense against murine filarial infection. Infect Immun. 2006;74:5236–5243. doi: 10.1128/IAI.00329-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bandeira-Melo C, Weller PF. Mechanisms of eosinophil cytokine release. Mem Inst Oswaldo Cruz. 2005;100(Suppl 1):73–81. doi: 10.1590/s0074-02762005000900013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Elsner J, Oppermann M, Kapp A. Detection of C5a receptors on human eosinophils and inhibition of eosinophil effector functions by anti-C5a receptor (CD88) antibodies. Eur J Immunol. 1996;26:1560–1564. doi: 10.1002/eji.1830260723. [DOI] [PubMed] [Google Scholar]
- 43.Nagase H, Okugawa S, Ota Y, Yamaguchi M, Tomizawa H, Matsushima K, Ohta K, Yamamoto K, Hirai K. Expression and function of Toll-like receptors in eosinophils: activation by Toll-like receptor 7 ligand. J Immunol. 2003;171:3977–3982. doi: 10.4049/jimmunol.171.8.3977. [DOI] [PubMed] [Google Scholar]
- 44.Mansson A, Cardell LO. Role of atopic status in Toll-like receptor (TLR)7- and TLR9-mediated activation of human eosinophils. J Leukoc Biol. 2009;85:719–727. doi: 10.1189/jlb.0808494. [DOI] [PubMed] [Google Scholar]
- 45.Wong CK, Cheung PF, Ip WK, Lam CW. Intracellular signaling mechanisms regulating toll-like receptor-mediated activation of eosinophils. Am J Respir Cell Mol Biol. 2007;37:85–96. doi: 10.1165/rcmb.2006-0457OC. [DOI] [PubMed] [Google Scholar]
- 46.Guilbert TW, Denlinger LC. Role of infection in the development and exacerbation of asthma. Expert Rev Respir Med. 2010;4:71–83. doi: 10.1586/ers.09.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Driss V, Legrand F, Hermann E, Loiseau S, Guerardel Y, Kremer L, Adam E, Woerly G, Dombrowicz D, Capron M. TLR2-dependent eosinophil interactions with mycobacteria: role of alpha-defensins. Blood. 2009;113:3235–3244. doi: 10.1182/blood-2008-07-166595. [DOI] [PubMed] [Google Scholar]
- 48.Bedoya VI, Boasso A, Hardy AW, Rybak S, Shearer GM, Rugeles MT. Ribonucleases in HIV type 1 inhibition: effect of recombinant RNases on infection of primary T cells and immune activation-induced RNase gene and protein expression. AIDS Res Hum Retroviruses. 2006;22:897–907. doi: 10.1089/aid.2006.22.897. [DOI] [PubMed] [Google Scholar]
- 49.Phipps S, Lam CE, Mahalingam S, Newhouse M, Ramirez R, Rosenberg HF, Foster PS, Matthaei KI. Eosinophils contribute to innate antiviral immunity and promote clearance of respiratory syncytial virus. Blood. 2007;110:1578–1586. doi: 10.1182/blood-2007-01-071340. [DOI] [PubMed] [Google Scholar]
- 50.Soukup JM, Becker S. Role of monocytes and eosinophils in human respiratory syncytial virus infection in vitro. Clin Immunol. 2003;107:178–185. doi: 10.1016/s1521-6616(03)00038-x. [DOI] [PubMed] [Google Scholar]
- 51.Mathur SK, Fichtinger PS, Kelly JT, Lee WM, Gern JE, Jarjour NN. Interaction between allergy and innate immunity: model for eosinophil regulation of epithelial cell interferon expression. Ann Allergy Asthma Immunol. 2013;111:25–31. doi: 10.1016/j.anai.2013.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Knipping K, McNeal MM, Crienen A, van Amerongen G, Garssen J, Van’t Land B. A gastrointestinal rotavirus infection mouse model for immune modulation studies. Virol J. 2011;8:109. doi: 10.1186/1743-422X-8-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gudbjartsson DF, Bjornsdottir US, Halapi E, Helgadottir A, Sulem P, Jonsdottir GM, Thorleifsson G, Helgadottir H, Steinthorsdottir V, Stefansson H, Williams C, Hui J, Beilby J, Warrington NM, James A, Palmer LJ, Koppelman GH, Heinzmann A, Krueger M, Boezen HM, Wheatley A, Altmuller J, Shin HD, Uh ST, Cheong HS, Jonsdottir B, Gislason D, Park CS, Rasmussen LM, Porsbjerg C, Hansen JW, Backer V, Werge T, Janson C, Jonsson UB, Ng MC, Chan J, So WY, Ma R, Shah SH, Granger CB, Quyyumi AA, Levey AI, Vaccarino V, Reilly MP, Rader DJ, Williams MJ, van Rij AM, Jones GT, Trabetti E, et al. Sequence variants affecting eosinophil numbers associate with asthma and myocardial infarction. Nat Genet. 2009;41:342–347. doi: 10.1038/ng.323. [DOI] [PubMed] [Google Scholar]
- 54.Lefkowitz DL, Lincoln JA, Howard KR, Stuart R, Lefkowitz SS, Allen RC. Macrophage-mediated candidacidal activity is augmented by exposure to eosinophil peroxidase: a paradigm for eosinophil-macrophage interaction. Inflammation. 1997;21:159–172. doi: 10.1023/a:1027366119901. [DOI] [PubMed] [Google Scholar]
- 55.Furuta GT, Nieuwenhuis EE, Karhausen J, Gleich G, Blumberg RS, Lee JJ, Ackerman SJ. Eosinophils alter colonic epithelial barrier function: role for major basic protein. Am J Physiol Gastrointest Liver Physiol. 2005;289:G890–897. doi: 10.1152/ajpgi.00015.2005. [DOI] [PubMed] [Google Scholar]
- 56.Hirata A, Motojima S, Fukuda T, Makino S. Damage to respiratory epithelium by guinea-pig eosinophils stimulated with IgG-coated Sepharose beads. Clin Exp Allergy. 1996;26:848–858. [PubMed] [Google Scholar]
- 57.Ricciardolo FL, Di Stefano A, van Krieken JH, Sont JK, van Schadewijk A, Rabe KF, Donner CF, Hiemstra PS, Sterk PJ, Mauad T. Proliferation and inflammation in bronchial epithelium after allergen in atopic asthmatics. Clin Exp Allergy. 2003;33:905–911. doi: 10.1046/j.1365-2222.2003.01686.x. [DOI] [PubMed] [Google Scholar]
- 58.Burgel PR, Lazarus SC, Tam DC, Ueki IF, Atabai K, Birch M, Nadel JA. Human eosinophils induce mucin production in airway epithelial cells via epidermal growth factor receptor activation. J Immunol. 2001;167:5948–5954. doi: 10.4049/jimmunol.167.10.5948. [DOI] [PubMed] [Google Scholar]
- 59.Bradding P. Asthma: eosinophil disease, mast cell disease, or both? Allergy Asthma Clin Immunol. 2008;4:84–90. doi: 10.1186/1710-1492-4-2-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Radke AL, Reynolds LE, Melo RC, Dvorak AM, Weller PF, Spencer LA. Mature human eosinophils express functional Notch ligands mediating eosinophil autocrine regulation. Blood. 2009;113:3092–3101. doi: 10.1182/blood-2008-05-155937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Robinson DS, Kariyawasam HH. Mepolizumab for eosinophilic severe asthma: recent studies. Expert Opin Biol Ther. 2015;15:909–914. doi: 10.1517/14712598.2015.1041911. [DOI] [PubMed] [Google Scholar]
- 62.Ortega HG, Liu MC, Pavord ID, Brusselle GG, FitzGerald JM, Chetta A, Humbert M, Katz LE, Keene ON, Yancey SW, Chanez P Investigators M. Mepolizumab treatment in patients with severe eosinophilic asthma. N Engl J Med. 2014;371:1198–1207. doi: 10.1056/NEJMoa1403290. [DOI] [PubMed] [Google Scholar]
- 63.Pavord ID, Korn S, Howarth P, Bleecker ER, Buhl R, Keene ON, Ortega H, Chanez P. Mepolizumab for severe eosinophilic asthma (DREAM): a multicentre, double-blind, placebo-controlled trial. Lancet. 2012;380:651–659. doi: 10.1016/S0140-6736(12)60988-X. [DOI] [PubMed] [Google Scholar]
- 64.Castro M, Zangrilli J, Wechsler ME, Bateman ED, Brusselle GG, Bardin P, Murphy K, Maspero JF, O’Brien C, Korn S. Reslizumab for inadequately controlled asthma with elevated blood eosinophil counts: results from two multicentre, parallel, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet Respir Med. 2015;3:355–366. doi: 10.1016/S2213-2600(15)00042-9. [DOI] [PubMed] [Google Scholar]
- 65.Reichert JM. Antibodies to watch in 2015. MAbs. 2015;7:1–8. doi: 10.4161/19420862.2015.988944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rothenberg ME. Humanized Anti-IL-5 Antibody Therapy. Cell. 2016;165:509. doi: 10.1016/j.cell.2016.04.020. [DOI] [PubMed] [Google Scholar]
- 67.Persson T, Monsef N, Andersson P, Bjartell A, Malm J, Calafat J, Egesten A. Expression of the neutrophil-activating CXC chemokine ENA-78/CXCL5 by human eosinophils. Clin Exp Allergy. 2003;33:531–537. doi: 10.1046/j.1365-2222.2003.01609.x. [DOI] [PubMed] [Google Scholar]
- 68.Page SM, Gleich GJ, Roebuck KA, Thomas LL. Stimulation of neutrophil interleukin-8 production by eosinophil granule major basic protein. Am J Respir Cell Mol Biol. 1999;21:230–237. doi: 10.1165/ajrcmb.21.2.3647. [DOI] [PubMed] [Google Scholar]
- 69.Simpson JL, Grissell TV, Douwes J, Scott RJ, Boyle MJ, Gibson PG. Innate immune activation in neutrophilic asthma and bronchiectasis. Thorax. 2007;62:211–218. doi: 10.1136/thx.2006.061358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Singh N, Phillips RA, Iscove NN, Egan SE. Expression of notch receptors, notch ligands, and fringe genes in hematopoiesis. Exp Hematol. 2000;28:527–534. doi: 10.1016/s0301-472x(00)00146-6. [DOI] [PubMed] [Google Scholar]
- 71.Wang YC, He F, Feng F, Liu XW, Dong GY, Qin HY, Hu XB, Zheng MH, Liang L, Feng L, Liang YM, Han H. Notch signaling determines the M1 versus M2 polarization of macrophages in antitumor immune responses. Cancer Res. 2010;70:4840–4849. doi: 10.1158/0008-5472.CAN-10-0269. [DOI] [PubMed] [Google Scholar]
- 72.Bailis W, Yashiro-Ohtani Y, Fang TC, Hatton RD, Weaver CT, Artis D, Pear WS. Notch simultaneously orchestrates multiple helper T cell programs independently of cytokine signals. Immunity. 2013;39:148–159. doi: 10.1016/j.immuni.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Haldar P, Brightling CE, Hargadon B, Gupta S, Monteiro W, Sousa A, Marshall RP, Bradding P, Green RH, Wardlaw AJ, Pavord ID. Mepolizumab and exacerbations of refractory eosinophilic asthma. N Engl J Med. 2009;360:973–984. doi: 10.1056/NEJMoa0808991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Al-Muhsen S, Johnson JR, Hamid Q. Remodeling in asthma. J Allergy Clin Immunol. 2011;128:451–462. doi: 10.1016/j.jaci.2011.04.047. quiz 463–454. [DOI] [PubMed] [Google Scholar]
- 75.Venge P. The eosinophil and airway remodelling in asthma. Clin Respir J. 2010;4(Suppl 1):15–19. doi: 10.1111/j.1752-699X.2010.00192.x. [DOI] [PubMed] [Google Scholar]
- 76.Zagai U, Lundahl J, Klominek J, Venge P, Skold CM. Eosinophil cationic protein stimulates migration of human lung fibroblasts in vitro. Scand J Immunol. 2009;69:381–386. doi: 10.1111/j.1365-3083.2009.02233.x. [DOI] [PubMed] [Google Scholar]
- 77.Zagai U, Dadfar E, Lundahl J, Venge P, Skold CM. Eosinophil cationic protein stimulates TGF-beta1 release by human lung fibroblasts in vitro. Inflammation. 2007;30:153–160. doi: 10.1007/s10753-007-9032-4. [DOI] [PubMed] [Google Scholar]
- 78.Abonia JP, Blanchard C, Butz BB, Rainey HF, Collins MH, Stringer K, Putnam PE, Rothenberg ME. Involvement of mast cells in eosinophilic esophagitis. J Allergy Clin Immunol. 2010;126:140–149. doi: 10.1016/j.jaci.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.O’Sullivan S. On the role of PGD2 metabolites as markers of mast cell activation in asthma. Acta Physiol Scand Suppl. 1999;644:1–74. [PubMed] [Google Scholar]
- 80.Dahlen SE, Kumlin M. Monitoring mast cell activation by prostaglandin D2 in vivo. Thorax. 2004;59:453–455. doi: 10.1136/thx.2004.026641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kataoka N, Satoh T, Hirai A, Saeki K, Yokozeki H. Indomethacin inhibits eosinophil migration to prostaglandin D2 : therapeutic potential of CRTH2 desensitization for eosinophilic pustular folliculitis. Immunology. 2013;140:78–86. doi: 10.1111/imm.12112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kagawa S, Fukunaga K, Oguma T, Suzuki Y, Shiomi T, Sayama K, Kimura T, Hirai H, Nagata K, Nakamura M, Asano K. Role of prostaglandin D2 receptor CRTH2 in sustained eosinophil accumulation in the airways of mice with chronic asthma. Int Arch Allergy Immunol. 2011;155(Suppl 1):6–11. doi: 10.1159/000327257. [DOI] [PubMed] [Google Scholar]
- 83.Cosmi L, Annunziato F, Galli MIG, Maggi RME, Nagata K, Romagnani S. CRTH2 is the most reliable marker for the detection of circulating human type 2 Th and type 2 T cytotoxic cells in health and disease. Eur J Immunol. 2000;30:2972–2979. doi: 10.1002/1521-4141(200010)30:10<2972::AID-IMMU2972>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 84.Otani IM, Anilkumar AA, Newbury RO, Bhagat M, Beppu LY, Dohil R, Broide DH, Aceves SS. Anti-IL-5 therapy reduces mast cell and IL-9 cell numbers in pediatric patients with eosinophilic esophagitis. J Allergy Clin Immunol. 2013;131:1576–1582. doi: 10.1016/j.jaci.2013.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Osterfeld H, Ahrens R, Strait R, Finkelman FD, Renauld JC, Hogan SP. Differential roles for the IL-9/IL-9 receptor alpha-chain pathway in systemic and oral antigen-induced anaphylaxis. J Allergy Clin Immunol. 2010;125:469–476. e462. doi: 10.1016/j.jaci.2009.09.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Minai-Fleminger Y, Elishmereni M, Vita F, Soranzo MR, Mankuta D, Zabucchi G, Levi-Schaffer F. Ultrastructural evidence for human mast cell-eosinophil interactions in vitro. Cell Tissue Res. 2010;341:405–415. doi: 10.1007/s00441-010-1010-8. [DOI] [PubMed] [Google Scholar]
- 87.Numata Y, Terui T, Okuyama R, Hirasawa N, Sugiura Y, Miyoshi I, Watanabe T, Kuramasu A, Tagami H, Ohtsu H. The accelerating effect of histamine on the cutaneous wound-healing process through the action of basic fibroblast growth factor. J Invest Dermatol. 2006;126:1403–1409. doi: 10.1038/sj.jid.5700253. [DOI] [PubMed] [Google Scholar]
- 88.Reher TM, Neumann D, Buschauer A, Seifert R. Incomplete activation of human eosinophils via the histamine H4-receptor: evidence for ligand-specific receptor conformations. Biochem Pharmacol. 2012;84:192–203. doi: 10.1016/j.bcp.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 89.Stenfeldt AL, Wenneras C. Danger signals derived from stressed and necrotic epithelial cells activate human eosinophils. Immunology. 2004;112:605–614. doi: 10.1111/j.1365-2567.2004.01906.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Todd R, Donoff BR, Chiang T, Chou MY, Elovic A, Gallagher GT, Wong DT. The eosinophil as a cellular source of transforming growth factor alpha in healing cutaneous wounds. Am J Pathol. 1991;138:1307–1313. [PMC free article] [PubMed] [Google Scholar]
- 91.Ohno I, Nitta Y, Yamauchi K, Hoshi H, Honma M, Woolley K, O’Byrne P, Dolovich J, Jordana M, Tamura G, et al. Eosinophils as a potential source of platelet-derived growth factor B-chain (PDGF-B) in nasal polyposis and bronchial asthma. Am J Respir Cell Mol Biol. 1995;13:639–647. doi: 10.1165/ajrcmb.13.6.7576701. [DOI] [PubMed] [Google Scholar]
- 92.Horiuchi T, Weller PF. Expression of vascular endothelial growth factor by human eosinophils: upregulation by granulocyte macrophage colony-stimulating factor and interleukin-5. Am J Respir Cell Mol Biol. 1997;17:70–77. doi: 10.1165/ajrcmb.17.1.2796. [DOI] [PubMed] [Google Scholar]
- 93.Goh YP, Henderson NC, Heredia JE, Red Eagle A, Odegaard JI, Lehwald N, Nguyen KD, Sheppard D, Mukundan L, Locksley RM, Chawla A. Eosinophils secrete IL-4 to facilitate liver regeneration. Proc Natl Acad Sci U S A. 2013;110:9914–9919. doi: 10.1073/pnas.1304046110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Elovic AE, Gallagher GT, Kabani S, Galli SJ, Weller PF, Wong DT. Lack of TGF-alpha and TGF-beta 1 synthesis by human eosinophils in chronic oral ulcers. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1996;81:672–681. doi: 10.1016/s1079-2104(96)80073-4. [DOI] [PubMed] [Google Scholar]
- 95.Yang J, Torio A, Donoff RB, Gallagher GT, Egan R, Weller PF, Wong DT. Depletion of eosinophil infiltration by anti-IL-5 monoclonal antibody (TRFK-5) accelerates open skin wound epithelial closure. Am J Pathol. 1997;151:813–819. [PMC free article] [PubMed] [Google Scholar]
- 96.Leitch VD, Strudwick XL, Matthaei KI, Dent LA, Cowin AJ. IL-5-overexpressing mice exhibit eosinophilia and altered wound healing through mechanisms involving prolonged inflammation. Immunol Cell Biol. 2009;87:131–140. doi: 10.1038/icb.2008.72. [DOI] [PubMed] [Google Scholar]
- 97.Mills CD. M1 and M2 Macrophages: Oracles of Health and Disease. Crit Rev Immunol. 2012;32:463–488. doi: 10.1615/critrevimmunol.v32.i6.10. [DOI] [PubMed] [Google Scholar]
- 98.Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest. 2012;122:787–795. doi: 10.1172/JCI59643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Fujisaka S, Usui I, Bukhari A, Ikutani M, Oya T, Kanatani Y, Tsuneyama K, Nagai Y, Takatsu K, Urakaze M, Kobayashi M, Tobe K. Regulatory mechanisms for adipose tissue M1 and M2 macrophages in diet-induced obese mice. Diabetes. 2009;58:2574–2582. doi: 10.2337/db08-1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wu D, Molofsky AB, Liang HE, Ricardo-Gonzalez RR, Jouihan HA, Bando JK, Chawla A, Locksley RM. Eosinophils sustain adipose alternatively activated macrophages associated with glucose homeostasis. Science. 2011;332:243–247. doi: 10.1126/science.1201475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Foster EL, Simpson EL, Fredrikson LJ, Lee JJ, Lee NA, Fryer AD, Jacoby DB. Eosinophils increase neuron branching in human and murine skin and in vitro. PLoS One. 2011;6:e22029. doi: 10.1371/journal.pone.0022029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Fryer AD, Stein LH, Nie Z, Curtis DE, Evans CM, Hodgson ST, Jose PJ, Belmonte KE, Fitch E, Jacoby DB. Neuronal eotaxin and the effects of CCR3 antagonist on airway hyperreactivity and M2 receptor dysfunction. J Clin Invest. 2006;116:228–236. doi: 10.1172/JCI25423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kobayashi H, Gleich GJ, Butterfield JH, Kita H. Human eosinophils produce neurotrophins and secrete nerve growth factor on immunologic stimuli. Blood. 2002;99:2214–2220. doi: 10.1182/blood.v99.6.2214. [DOI] [PubMed] [Google Scholar]
- 104.Wen T, Stucke EM, Grotjan TM, Kemme KA, Abonia JP, Putnam PE, Franciosi JP, Garza JM, Kaul A, King EC, Collins MH, Kushner JP, Rothenberg ME. Molecular Diagnosis of Eosinophilic Esophagitis by Gene Expression Profiling. Gastroenterology. 2013 doi: 10.1053/j.gastro.2013.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Noel RJ, Putnam PE, Rothenberg ME. Eosinophilic esophagitis. N Engl J Med. 2004;351:940–941. doi: 10.1056/NEJM200408263510924. [DOI] [PubMed] [Google Scholar]
- 106.Hahn C, Islamian AP, Renz H, Nockher WA. Airway epithelial cells produce neurotrophins and promote the survival of eosinophils during allergic airway inflammation. J Allergy Clin Immunol. 2006;117:787–794. doi: 10.1016/j.jaci.2005.12.1339. [DOI] [PubMed] [Google Scholar]
- 107.Sanico AM, Koliatsos VE, Stanisz AM, Bienenstock J, Togias A. Neural hyperresponsiveness and nerve growth factor in allergic rhinitis. Int Arch Allergy Immunol. 1999;118:154–158. doi: 10.1159/000024054. [DOI] [PubMed] [Google Scholar]
- 108.Peters EM, Liezmann C, Spatz K, Daniltchenko M, Joachim R, Gimenez-Rivera A, Hendrix S, Botchkarev VA, Brandner JM, Klapp BF. Nerve growth factor partially recovers inflamed skin from stress-induced worsening in allergic inflammation. J Invest Dermatol. 2011;131:735–743. doi: 10.1038/jid.2010.317. [DOI] [PubMed] [Google Scholar]
- 109.Furuta GT, Forbes D, Boey C, Dupont C, Putnam P, Roy S, Sabra A, Salvatierra A, Yamashiro Y, Husby S Eosinophilic Gastrointestinal Diseases Working G. Eosinophilic gastrointestinal diseases (EGIDs) J Pediatr Gastroenterol Nutr. 2008;47:234–238. doi: 10.1097/MPG.0b013e318181b1c3. [DOI] [PubMed] [Google Scholar]
- 110.Blanchard C, Rothenberg ME. Basic pathogenesis of eosinophilic esophagitis. Gastrointest Endosc Clin N Am. 2008;18:133–143. x. doi: 10.1016/j.giec.2007.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Jacoby DB, Costello RM, Fryer AD. Eosinophil recruitment to the airway nerves. J Allergy Clin Immunol. 2001;107:211–218. doi: 10.1067/mai.2001.112940. [DOI] [PubMed] [Google Scholar]
- 112.Yost BL, Gleich GJ, Fryer AD. Ozone-induced hyperresponsiveness and blockade of M2 muscarinic receptors by eosinophil major basic protein. J Appl Physiol. 1999;87:1272–1278. doi: 10.1152/jappl.1999.87.4.1272. [DOI] [PubMed] [Google Scholar]
- 113.Kita H. Eosinophils: multifaceted biological properties and roles in health and disease. Immunol Rev. 2011;242:161–177. doi: 10.1111/j.1600-065X.2011.01026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Carlens J, Wahl B, Ballmaier M, Bulfone-Paus S, Forster R, Pabst O. Common gamma-chain-dependent signals confer selective survival of eosinophils in the murine small intestine. J Immunol. 2009;183:5600–5607. doi: 10.4049/jimmunol.0801581. [DOI] [PubMed] [Google Scholar]
- 115.Matsuoka K, Shitara H, Taya C, Kohno K, Kikkawa Y, Yonekawa H. Novel basophil- or eosinophil-depleted mouse models for functional analyses of allergic inflammation. PLoS One. 2013;8:e60958. doi: 10.1371/journal.pone.0060958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Moro K, Yamada T, Tanabe M, Takeuchi T, Ikawa T, Kawamoto H, Furusawa J, Ohtani M, Fujii H, Koyasu S. Innate production of T(H)2 cytokines by adipose tissue-associated c-Kit(+)Sca-1(+) lymphoid cells. Nature. 2010;463:540–544. doi: 10.1038/nature08636. [DOI] [PubMed] [Google Scholar]
- 117.Doyle AD, Jacobsen EA, Ochkur SI, Willetts L, Shim K, Neely J, Kloeber J, Lesuer WE, Pero RS, Lacy P, Moqbel R, Lee NA, Lee JJ. Homologous recombination into the eosinophil peroxidase locus generates a strain of mice expressing Cre recombinase exclusively in eosinophils. J Leukoc Biol. 2013;94:17–24. doi: 10.1189/jlb.0213089. [DOI] [PMC free article] [PubMed] [Google Scholar]